Abstract
Background
The aim of this study was to determine the clinical benefit of transhepatic arterial chemoembolization (TACE) with or without recombinant human adenovirus type 5 (H101) administration for the treatment of patients with hepatocellular carcinoma (HCC).
Methods
Tumor response, progression-free survival (PFS), and overall survival(OS) were retrospectively evaluated in consecutive patients with unresectable HCC who received TACE with or without H101 between April 2012 and April 2013.
Results
Patients with unresectable HCC were treated with transarterial injection of H101 with TACE (H101 group, n = 87) or TACE alone (control group, n = 88). Clinicopathological features were similar between the groups. Treatment response was significantly different between the groups (P = 0.01). In the H101 group, 25 patients demonstrated a complete response (CR, 28.7 %); 28 patients, a partial response (PR, 32.2 %); 23 patients, stable disease (SD, 26.4 %); and 11 patients, progressive disease (PD, 12.6 %). In the control group, 13 patients demonstrated CR (14.8 %); 19, PR (21.6 %); 34, SD (38.6 %); and 22, PD (25 %). OS and PFS was also significantly different between the groups. In the H101 group, median OS and PFS were 12.8 and 10.49 months, whereas in the control group they were 11.6 and 9.72 months, respectively (OS: P = 0.046; PFS: P = 0.044).
Conclusion
In patients with unresectable HCC, H101 combined with TACE improves OS, PFS and treatment response compared with TACE alone.
Electronic supplementary material
The online version of this article (doi:10.1186/s12885-015-1715-x) contains supplementary material, which is available to authorized users.
Keywords: Hepatocellular carcinoma (HCC), Transhepatic arterial chemoembolization (TACE), H101, Tumor response
Background
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related death worldwide [1]. Less than 20 % of patients with HCC are eligible for potentially curative liver transplantation or surgical resection [2]. Worldwide, transhepatic arterial chemoembolization (TACE) is regarded as the best palliative treatment for unresectable HCC and has been shown to provide a clinical survival benefit [2], albeit with poor prognosis [3] suggesting that additional strategies are needed to improve patient prognosis.
Gene therapy, especially oncolytic viral therapy, is a promising treatment for liver tumors and is being increasingly used in the clinic with favorable results [4]. H101 is a recombinant human type-5 adenovirus (Ad5) in which the gene encoding the 55 kDa E1B protein responsible for p53-binding and inactivation has been deleted to confer p53-selective replication of oncolytic viruses inducing accumulation of p53 leading to direct and selective cytotoxicity in tumor cells during replication [5]. The H101 virus produced by Shanghai Sunway Biotech also contains a deletion of a 78.3–85.8 μm gene segment in the E3 region. The E3 region is responsible for the inhibition of host immunity, which enhances virus replication and spread in tumor cells [6].
Previous studies evaluating the safety of H101 as a direct injection [7] or transarterial infusion combined with TACE [8, 9], but the result were insufficient because of the small patient numbers (10,27,1), moreover, without a control group. While this large sample-sized study has enrolled 87 patients treated by H101 with a control group(n = 88), aimed to demonstrate the effect for unresectable HCCs. In the current study, treatment-related tumor response, overall survival(OS) and progression free survival (PFS) rates between H101 plus TACE and TACE alone were compared as the primary endpoints. The secondary endpoint included an assessment of treatment-related adverse events (AEs).
Methods
Patient selection
This retrospective study was approved by the ethics committee of Sun Yat-Sen University Cancer Center, and was performed in accordance with the Helsinki Declaration of 1975, as revised in 1983. From April 2012 to April 2013, 367 consecutive patients with unresectable HCC who underwent TACE, with or without transarterial injection of H101, at the Sun Yat-Sen University Cancer Center were enrolled. The diagnosis of HCC was based on non-invasive criteria according to the recommendation of the European Association for the Study of the Liver (EASL) and the European Organization for Research and Treatment of Cancer (EORTC) [10]. The definition of surgical unresectability was as follows: (1) Child-Pugh classification B; (2) ≥3 tumor nodules of any size; and (3) the inability to ensure adequate function of the postresection liver volume. Eligibility criteria included: (1) no previous treatment for HCC before TACE; (2) adequate hematological function (Child-Pugh A or Child-Pugh B); (3) adequate renal function (serum creatinine < 140 μmol/L, and serum blood urea nitrogen < the upper limit of normal). Exclusion criteria included: (1) previous resection or ablation before TACE, (2) prior bland embolization; and (3) if the patient had received therapy with more than one type of embolic agent or transcatheter therapy. Patients who met the criteria provided written informed consent for the study.
Treatment procedures
For each modality, a uniform treatment protocol was followed. TACE was performed through the femoral artery with use of the Seldinger technique with local anesthesia as previously reported [11]. The chemotherapeutic agents were infused into the hepatic artery supplying the tumor(s). Conventional chemoembolization was performed by administering carboplatin 300 mg (Bristol-Myers Squibb, NY, USA). Thereafter, chemolipiodolization was performed using epirubicin 50 mg (Pharmorubicin, Pfizer, Wuxi, China), and mitomycin 6 mg (Zhejiang Hisun Pharmaceutical Co. Ltd., Taizhou, China) mixed with 5 mL of lipiodol (Lipiodol Ultra-Fluide; Andre Guerbet Laboratories, France).
H101 was administered via the catheter into the hepatic artery supplying the tumor(s). A total of 1.0 × 1012 virus particles in 10 mL 0.9 % sodium chloride were administered. Sterile purified viral lots were produced for human clinical use by Shanghai Sunway Biotech (Shanghai, China), and tested for the titer, sterility, and general safety by the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
Follow-up
Antitumor efficacy was evaluated by computed tomography/magnetic resonance imaging (CT/MRI) at 1 month post-treatment and every 3–4 months thereafter. Further treatments were based on clinical evaluation, laboratory values, and imaging response. Tumor response according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) guidelines [12] was independently assessed in a blinded manner by 3 qualified radiologists. When a difference of opinion occurred, a consensus was obtained through discussion.
Liver function tests, ascites, and encephalopathy were monitored during follow-up visits to assess for liver failure. Clinical AEs were graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v4.0 criteria [13].
Statistical analysis
The statistical significance of the difference between the means of continuous variables was determined using the independent t-test. A P-value of 0.05 was considered to be statistically significant. The chi-squared test was used to compare categorical variables. The Kaplan–Meier method was used to estimate OS and PFS.
Results
Patient demographics and clinical characteristics were similar between the groups and are shown in Table 1. From April 2012 to April 2013, 187 patients with unresectable HCC were treated with TACE plus H101 met the inclusion criteria (H101 group) (Additional file 1: Figure S1) . In the same period, 88 patients with unresectable HCC underwent conventional TACE alone met the inclusion criteria (control group).
Table 1.
Patient demographics and characteristics
| Overall | H101 | Control | P-Value | Overall Survival(%) | Median survival(mo) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1-yr | 2-yr | P-Value | ExpB (Hazard Ratio ,95 % CI) | P-Value | ||||||
| Gender | 0.305 | 0.302 | ||||||||
| Male | 159 | 81 | 78 | 67 | 52 | 13.0 | ||||
| Female | 16 | 6 | 10 | 60 | 30 | 11.2 | ||||
| Age | 0.948 | 0.100 | ||||||||
| Median | 55.0 | 55.0 | 54.5 | |||||||
| < 60 | 114 | 59 | 55 | 68 | 49 | 12.5 | ||||
| ≥ 60 | 61 | 28 | 33 | 68 | 56 | 13.0 | ||||
| Alpha-foetoprotein(ng/ml) | 0.316 | |||||||||
| 307.2 | 269.1 | 307.2 | ||||||||
| Alpha-foetoprotein(ng/ml) | 0.947 | 0.06 | 1.669(1.178–2.366) | 0.004 | ||||||
| ≤ 20 | 53(30.1 %) | 27(30.7) | 26(29.5) | 89 | 81 | 17.6 | ||||
| 20–400 | 42(23.9 %) | 20(22.7) | 22(25.0) | 60 | 45 | 13.9 | ||||
| ≥ 400 | 80(45.5 %) | 40(45.5) | 40(45.5) | 52 | 32 | 9.1 | ||||
| Child Pugh grade | 0.820 | 0.007 | 2.132(1.138–3.995) | 0.018 | ||||||
| A | 154(88.0 %) | 76(87.3 %) | 78(88.6 %) | 70 | 55 | 13.3 | ||||
| B | 21(12.0 %) | 11(12.6) | 10(11.3) | 38 | 25 | 7.7 | ||||
| ALB(g/L) | 0.228 | 0.412 | ||||||||
| Median | 40.0 | 39.6 | 40.2 | |||||||
| ≥ 35 | 131 | 61 | 70 | 0.166 | 62 | 50 | 11.7 | |||
| < 35 | 44 | 26 | 18 | 67 | 52 | 13.3 | ||||
| Tbil(U/L) | 0.386 | 0.003 | ||||||||
| Median | 16.4 | 16.1 | 16.8 | |||||||
| < 20 | 119 | 61 | 58 | 0.628 | 75 | 56 | 13.7 | |||
| ≥ 20 | 56 | 26 | 30 | 48 | 40 | 8.0 | ||||
| Virus infection | 0.970 | 0.101 | ||||||||
| none | 15 | 7 | 8 | 88 | 66 | 13.6 | ||||
| HBV | 158 | 79 | 79 | 63 | 51 | 12.6 | ||||
| HCV | 2 | 1 | 1 | 50 | 0 | 13.9 | ||||
| Platelet count (10E9/L) | 0.630 | 0.676 | ||||||||
| Median | 167.0 | 179.2 | 154.5 | |||||||
| < 100 | 31 | 11 | 20 | 0.112 | 63 | 46 | 12.2 | 0.330(0.141–0.773) | 0.011 | |
| ≥ 100 | 144 | 76 | 68 | 67 | 53 | 12.8 | ||||
| No. of tumours | 1.000 | <0.001 | 2.024(1.127–3.633) | 0.018 | ||||||
| ≤ 3 | 127 | 69 | 70 | 74 | 57 | 13.7 | ||||
| > 3 | 36 | 18 | 18 | 38 | 30 | 7.6 | ||||
| Tumour size (cm) | 0.730 | 0.028 | 2.936(1.297–6.650) | 0.010 | ||||||
| ≤ 5 | 45 | 21 | 24 | 91 | 75 | 18.1 | ||||
| > 5 | 130 | 66 | 64 | 56 | 42 | 9.8 | ||||
| Anti-HBV therapy | 0.197 | 0.424 | ||||||||
| Yes | 56 | 32 | 24 | 60 | 54 | 13.3 | ||||
| No | 119 | 55 | 64 | 69 | 51 | 9.65 | ||||
| H101 | 0.046 | 0.042 | 0.593(0.353–0.995) | 0.048 | ||||||
| Yes | 87 | 87 | 0 | 69 | 60 | 12.8 | ||||
| No | 88 | 0 | 88 | 60 | 44 | 11.6 | ||||
| BCLC stage | 0.453 | 0.001 | 2.168(1.322–3.557) | 0.002 | ||||||
| A3 | 1 | 0 | 1 | |||||||
| A4 | 17 | 10 | 7 | 94 | 94 | 17.0 | ||||
| B | 108 | 50 | 58 | 72 | 56 | 12.96 | ||||
| C | 49 | 27 | 22 | 52 | 23 | 6.96 | ||||
AFP alpha fetoprotein, ALB serum albumin, HBV hepatitis B virus, HCV hepatitis C virus, TBIL total bilirubin, PVT portal vein thrombosis
Tumor response is shown in Table 2 significant difference was noted in tumor response between the two groups (Table 2). Furthermore, subgroup analysis according to treatment response showed that the number of each response type was significantly different between the groups (Table 2). In general, patients in the H101 group responded better to treatment compared with those who received TACE alone.
Table 2.
Treatment response of H101 group and control group
| Overall | H101(none + Anti-HBV Therapy,P = 0.162) | Control | P value | |
|---|---|---|---|---|
| 0.010 | ||||
| CR | 38(21.7 %) | 25 (28.7 %)(16 + 9) | 13(14.8 %) | 0.017 |
| PR | 47(26.9 %) | 28(32.2 %)(21 + 7) | 19(21.6 %) | 0.172 |
| SD | 57(32.6 %) | 23(26.4 %)(14 + 9) | 34(38.6 %) | 0.107 |
| PD | 33(18.9 %) | 11(12.6 %)(4 + 7) | 22(25 %) | 0.011 |
All patients enrolled in H101 group were screened to sort out cases with anti-HBV therapy or without anti-HBV therapy.
None: Patients treated by H101 without anti-HBV therapy.
Anti-HBV Therapy: Patients treated by H101 with anti-HBV therapy session.
The majority of the patients (90.2 %) tested positive for hepatitis B virus (HBV) and some patients received anti-HBV agents that could potentially confound the beneficial effects of H101 as antiviral agents. To determine the effect of anti-HBV treatment on H101, patients were stratified by anti-HBV therapy administration. As shown in Table 2B, there was no significant difference in treatment response between the two subgroups.
Significant positive correlations have been reported between lipiodol accumulation observed on CT images and necrosis in resected tumors examined after TACE, and, therefore, intratumor lipiodol accumulation is regarded as an indicator of necrosis [14, 15]. The degree of lipiodol retention for all patients is presented in Table 3. There were significant differences in lipiodol accumulation between the two treatment groups (P = 0.002).
Table 3.
Tumor response
| Overall | H101 | Control | P value | |
|---|---|---|---|---|
| Alpha-fetoprotein(ng/ml) reduce | 0.448 | |||
| ≥ 20 % | 77 | 41 | 36 | |
| < 20 % | 98 | 46 | 52 | |
| Lipiodol retention | 0.002 | |||
| None | 20 | 7 | 13 | |
| Partial | 132 | 61 | 71 | |
| Complete | 23 | 19 | 4 |
CR complete response, PR partial response, SD stable disease, PD progressive disease
Blood samples for laboratory analysis were collected before and 1–2 days after TACE for each patient (Table 4). There were no significant differences in clinical parameters between the two groups with the exception of a significant increase in white blood cell count in the H101 group compared with the control group (P = 0.001). Post-treatment AEs are shown in Table 4 Fever was significantly higher in the H101 group compared with the control group (P = 0.023). No grade 4 clinical toxicity or procedure-related deaths (30 days) due to liver failure were experienced in either group. There were no major complications or grade 3–4 liver toxicities within the first post-treatment month. The overall frequency of treatment-emergent AEs was not significantly different between the groups (P = 0.263).
Table 4.
Clinical adverse effects
| Overall | H101 | Control | P value | |
|---|---|---|---|---|
| Fever | 0.023 | |||
| > 38.5 °C | 55.4 % | 64.4 % | 46.6 % | |
| ≤ 38.5 °C | 44.6 % | 35.6 % | 53.4 % | |
| Pain | 0.875 | |||
| Yes | 65.1 % | 64.4 % | 65.9 % | |
| No | 34.9 % | 35.6 % | 34.1 % | |
| Ascites | 0.864 | |||
| Yes | 25.7 % | 26.4 % | 25 % | |
| No | 74.3 % | 73.6 % | 75 % | |
| Acute renal failure | ||||
| Yes | 5.1 % | 5.7 % | 4.5 % | 0.896 |
| No | 94.9 % | 94.3 % | 95.5 % | |
| Encephalopathy | ||||
| Yes | 0 | 0 | 0 | |
| No | 100 % | 100 % | 100 % | |
| White Blood Cell | ||||
| Before TACE | 5.7 | 6.0 | 5.5 | 0.369 |
| After TACE | 7.64 | 7.0 | 8.84 | 0.991 |
| Elevation | 1.61 | 0.5 | 2.97 | 0.001 |
| PLT | ||||
| Before | 167.0 | 179.2 | 154.5 | 0.186 |
| After | 113.0 | 122.8 | 106.1 | 0.258 |
| Elevation | 49.0 | 52.7 | 48.4 | 0.480 |
| ALT | ||||
| Before | 41.5 | 43.2 | 41.4 | 0.371 |
| After | 167.1 | 153.0 | 200.9 | 0.405 |
| Elevation | 103.1 | 88.1 | 118.4 | 0.480 |
| AST | ||||
| Before | 50.9 | 57.1 | 46.4 | 0.249 |
| After | 221.3 | 225.4 | 213.5 | 0.993 |
| Elevation | 154.2 | 141.2 | 162.0 | 0.863 |
| TBIL | ||||
| Before | 16.3 | 16.1 | 16.8 | 0.657 |
| After | 30.4 | 28.3 | 31.8 | 0.162 |
| Elevation | 12.9 | 12.4 | 13.25 | 0.413 |
| ALB | ||||
| Before | 40.0 | 38.2 | 41.2 | 0.161 |
| After | 35.4 | 34.1 | 37.0 | 0.314 |
| Elevation | 4.1 | 3.6 | 4.4 | 0.226 |
PLT platelet count, ALB albumin, ALT alanine aminotransferase, AST aspartate aminotransferase, PLT platelet, TACE transhepatic arterial chemoembolization, TBIL total bilirubin
The median OS time during follow-up was 12.8 months (mean ± SD: 12.95 ± 8.36 months) in the H101 group and 11.6 months (mean ± SD: 12.87 ± 8.28 months) in the control group. 24 patients (27.6 %) in the H101 group and 41 (46.6 %) in the control group expired. The causes of death included liver disease progression (46/65, 70.8 %), upper gastrointestinal hemorrhage (7/65, 10.8 %), encephalopathy (7/65, 10.8 %), and peritonitis and pneumonia (6/65, 9.2 %). There were no treatment-related deaths. The cumulative OS rates at 1 and 2 years were significantly different and were 69 and 60 % in the H101 group, respectively, 60 and 44 % in the control group, respectively (P = 0.046, Fig. 1). Univariate analysis by Cox-regression revealed 6 prognostic factors affecting OS: Child-Pugh grade (grade A vs. grade B, P = 0.007), total bilirubin (<20 vs. ≥20 U/L, P = 0.003), BCLC(Barcelona Clinic Liver Cancer )stage (P = 0.001), tumor number (≤3 vs. >3, P < 0.001), tumor size (≤5 cm vs. >5 cm, P = 0.028), and H101 (P = 0.042). Multivariate analysis by cox-regression revealed that AFP (P = 0.004), CHILD PUGH grade (P = 0.018), BCLC stage(P = 0.002), Platelet count (PLT)(P = 0.011), the number of tumors (P = 0.018), tumor size(P = 0.010)and H101 (P = 0.048) were independent prognostic factors of OS.
Fig. 1.
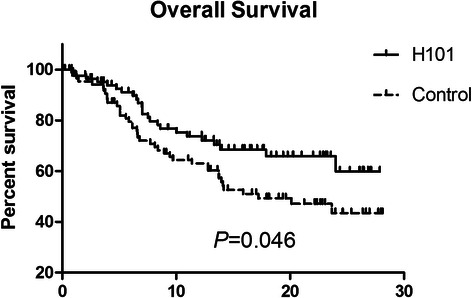
The Kaplan–Meier survival analysis comparing the overall survival from the first transcatheter therapy of advanced stage HCC patients who underwent TACE combined with H101 (H101 group, n = 87) and TACE alone (control group, n = 88).
After the first post-treatment review, all 175 patients were assigned as CR, PR, SD, or PD according to mRECIST criteria. In total, 142 patients in both groups (H101: 74; control: 68) were judged as CR, PR, or SD. During follow-up, progression free survival was observed in these 142 patients. The median time to progression for the H101 and control groups were significantly different at 10.49 and 9.72 months, respectively (P = 0.044, Fig. 2).
Fig. 2.
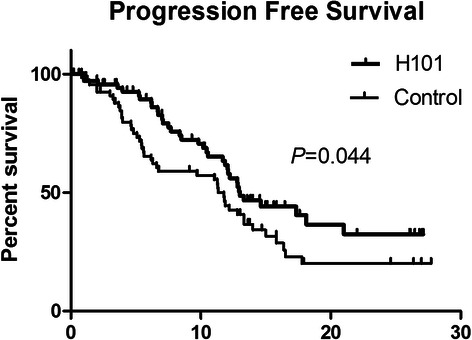
The Kaplan–Meier survival analysis for progression-free survival of the 74 patients with unresectable HCC who underwent TACE combined with H101 and the 68 patients with unresectable HCC who underwent TACE alone.
In univariate analysis by cox-regression, 3 prognostic factors affecting tumor progression were identified: tumor number (P = 0.002), tumor size (P = 0.041), and treatment modality (H101; P = 0.036; Table 5). Multivariate analysis identified 4 prognostic factors as independent predictors of progression: the number of tumors (P = 0.001), tumor size (P = 0.041), Child-Pugh grade (P = 0.050) and treatment modality (H101) (P = 0.017, Table 5).
Table 5.
Univariate and Multivariate analysis of PFS
| Cases | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| 1-yr survival rate(%) | 2-yr survival rate(%) | Median survival(mo) | P-Value | ExpB (Hazard Ratio ,95 % CI) | P-Value | ||
| Gender | |||||||
| Male | 130 | 47 | 26 | 10.79 | 0.331 | ||
| Female | 12 | 37 | 0 | 6.11 | |||
| Age | |||||||
| < 60 | 92 | 49 | 29 | 10.49 | 0.180 | ||
| ≥ 60 | 50 | 40 | 21 | 9.35 | |||
| Alpha-foetoprotein(ng/ml) | |||||||
| ≤ 20 | 42 | 50 | 44 | 11.99 | 0.445 | ||
| 20–400 | 37 | 35 | 8 | 7.03 | |||
| ≥ 400 | 63 | 51 | 28 | 8.37 | |||
| Child Pugh grade | 0.047 | 2.852(1.002–8.293) | 0.050 | ||||
| A | 133 | 48 | 28 | 10.56 | |||
| B | 9 | 22 | 0 | 7.07 | |||
| ALB(g/L) | 0.307 | ||||||
| ≥ 35 | 108 | 46 | 20 | 10.49 | |||
| < 35 | 34 | 47 | 47 | 10.25 | |||
| Tbil(U/L) | 0.429 | ||||||
| < 20 | 104 | 49 | 23 | 11.18 | |||
| ≥ 20 | 38 | 37 | 32 | 6.21 | |||
| Platelet count (10E9/L) | |||||||
| < 100 | 19 | 74 | 32 | 13.57 | 0.144 | ||
| ≥ 100 | 123 | 42 | 25 | 9.83 | |||
| No. of tumours | 0.034 | 3.992(1.978–8.057) | 0.001 | ||||
| ≤ 3 | 118 | 51 | 28 | 11.04 | |||
| > 3 | 24 | 21 | 14 | 7.6 | |||
| Tumour size (cm) | 0.988 | 2.667(1.041–6.832) | 0.041 | ||||
| ≤ 5 | 35 | 39 | 24 | 11.37 | |||
| > 5 | 107 | 48 | 25 | 8.37 | |||
| Virus infection | 0.079 | ||||||
| none | 14 | 59 | 59 | 13.05 | |||
| HBV | 126 | 43 | 24 | 10.25 | |||
| HCV | 2 | 50 | 0 | 11.30 | |||
| Anti-virus therapy | 0.951 | ||||||
| Yes | 33 | 44 | 30 | 8.37 | |||
| No | 95 | 43 | 22 | 10.56 | |||
| H101 | 0.051 | 0.461(0.244–0.870) | 0.017 | ||||
| Yes | 74 | 51 | 32 | 10.49 | |||
| No | 68 | 41 | 20 | 9.72 | |||
Discussion
The main purpose of this study was to compare the outcomes of patients with late stage HCC treated with two different methods of chemoembolization: a conventional method with commonly used protocols, and one using H101 virus. The data revealed that transcatheter therapy with H101 provided a significant tumor response and survival advantage over treatment with conventional chemoembolization (TACE alone) in patients with unresectable HCC.
H101 is an E1B-55 K-/E3B-deleted adenovirus [16], which has been used as an anticancer agent with the goal of restricting replication to p53-mutated neoplasm, sparing p53 wild-type human tissues. Preclinical studies have confirmed the anticancer activity of the H101 virus [17]. Clinical studies demonstrated the tolerability and anti-tumor efficacy of this agent as a monotherapy in patients with head and neck cancer [18, 19]. Different studies have compared the efficacy and safety of multiple routes of H101 administration in patients with HCC or liver tumors including hepatic arterial administration [20, 21],intravenous injection, and ultrasound-guided intratumoral injection [7, 22, 23]. Overall, H101 was safe when administered intratumorally, intraperitoneally, intraarterially, or intravenously at doses up to 3 × 1011 pfu [8, 24].
In this study, a significant difference in response rate was noted between the H101 and control groups. Radiologically, tumor response as determined by mRECIST was shown as obvious volume shrinkage and large areas of necrosis in tumor. The response rate of the control group was similar to that reported in previous studies of our department [25]. In the H101 group, greater improvements were seen especially with regard to CR and PD which may suggest more complete necrosis in the tumor and less lost-control. The mechanism behind the increased efficacy of H101 is not clear but may suggested as follows: 1)H101 is a p53-mutated specific agent, and up to 30–50 % [26] HCCs were found mutated or lost of p53. 2) Pei et al. [27] showed that HCC cells expressed high levels of inhibitor of apoptosis proteins, and were resistant to tumor necrosis factor (TNF)-related apoptosis while E1B-55 K-deleted oncolytic adenovirus showed partial antitumoural efficacy in the BEL7404 xenograft tumour model. 3)H101 has synergistic effect while combined with chemotherapy, and the enhanced antitumor effect was demonstrated in Hep3B (p53-null) and HepG2 (p53-wt) in vitro and in vivo [28].
The OS and PFS rate was significantly different between the two treatment modalities, and results from cox-regression showed H101 were the independent prognostic factor for these late stage HCC patients. These results coordinate with the response advantage of H101, demonstrate the survival advantage for HCCs. However, as generally accepted, beside tumor burden, overall survival in unresectable HCCs is affected by multiple reasons. First, OS in patients with HCC is greatly affected by the degree of liver dysfunction, and patients with Child-Pugh B liver function usually have poor survival regardless of the treatment regimen [29]. In many cases, liver function did not reflect the tumor response and in some patients liver function actually worsened with tumor shrinkage. In this study, most patients had a liver function status graded as Child-Pugh A(88 %), 21 Child-Pugh B cases were nearly even in the two groups(10:11), the bias to overall survival was insignificant. Second, TACE was the initial treatment for these patients, most of whom received subsequent treatments including resection, ablation, repeated TACE, and systemic therapies, or best supportive care. As our previous prospective clinical trial demonstrated, subsequent treatments can influence OS, especially for patients with large and multiple HCC at diagnosis; surgical resection for patients who responded well to TACE significantly prolonged survival, when compared to those who refused surgery [30]. In this study, patients whose tumor was downstaged were offered radical treatment including 36 patients for surgical resection and 29 for local ablation, most of which was CR, PR and some of SD, but without PD patient. These subsequent treatments most likely improved OS, which would enhance the advantage of tumor response.
More than 80 % of patients with HCC in Asia are hepatitis B virus positive, and most are receiving anti-viral therapy [31]. This could confound the results of any evaluation of H101 because it is a recombinant adenovirus and anti-viral therapy has the potential to prevent H101 replication. However, to the best of our knowledge, this has not been previously investigated. The most employed anti-viral agents for hepatitis B in our patients were lamivudine (35.5 %), adefovir dipivoxil (14.7 %), and entecavir (42.3 %). There are no reported studies demonstrating any potential interaction of these agents with adenovirus. Moreover, stratification of our patient data to those receiving anti-viral therapy or not, did not reveal any significant effect of antiviral therapy on H101 efficacy in terms of tumor response or OS and PFS.
Other than efficacy, safety and adverse events are important aspects to consider in patients undergoing viral therapy. The first case of a patient dying as a result of gene therapy was reported in 1999 by Marshall [32]. The patient, a relatively fit 18-year-old male with an inherited enzyme deficiency, received a dose of 4 × 1013 pfu of a replication-deficient adenovirus expressing the ornithine transcarbamylase gene. Less than 24 h later, he experienced hyperammonemia, acute respiratory distress syndrome, disseminated intravascular coagulation, and suffered multiorgan system failure. He died 4 days later, which questioned the safety of adenovirus for gene therapy [33, 34]. However, subsequent studies have found no mortality associated with adenoviral vector therapy and any complications are usually mild and reversible [8, 35], suggesting that the case reported by Marshall et al., may be a sporadic case of accidental death. In this study, no patients died and all AEs were reversible. The complication rates between H101 group and TACE alone (control) group were similar. Child-Pugh class A and B patients did not experience any major complications after treatment with H101, but did experience liver failure after treatment, but there was no statistically significant difference in liver toxicity at 1–2 months between the treatment groups. Increases in liver enzymes and total bilirubin levels and decreases in serum albumin levels were mild and not significantly different between the treatment groups. However, frequent high fever (P = 0.023) and an increase in the white blood cell count (P = 0.001) were apparent in the H101 group, which might be explained by the immune activation. Previous studies have noted an increase in inflammatory cytokine generation and fever after hepatic arterial infusion of adenovirus [36]. Interestingly, Lu et al., [36] found that during H101 injection, the efficacy was significantly higher in those who had fever than that in those who did not, suggesting that virus infection may activate the host immune system and the elevated cell-mediated immunity may play a role in the tumor regression. In this study, subgroup analysis based on fever did not reveal any differences between fever and treatment efficacy (OS, P = 0.109; PFS, P = 0.221).
This study has several limitations including its retrospective nature. As a case-controlled study, the survival benefit demonstrated must be considered preliminary and further prospective, randomized-controlled, long-term studies are needed to confirm our results.
Conclusion
Transcatheter H101 therapy in combination with TACE for patients with unresectable HCC may provide a survival(OS and PFS) and tumor response advantage over treatment with conventional TACE alone.
Acknowledgements
We would like to thank all participants for their support in this study. This study was not supported by funding.
Abbreviations
- TACE
Transarterial chemoembolization
- HCC
Hepatocellular carcinoma
- H101
Recombinant human adenovirus type 5
- PFS
Progression-free survival
- OS
Overall survival
- CR
Complete response
- PR
Partial response
- SD
Stable disease
- PD
Progressive disease
- AEs
Adverse events
- EASL
European Association for the Study of the Liver
- EORTC
European Organization for research and treatment of cancer
- CT/MRI
Computed tomography/magnetic resonance imaging
- mRECIST
Modified response evaluation criteria in solid tumors
- NCI
National cancer institute
- CTCAE
Common terminology criteria for adverse events
- HBV
hepatitis B virus
- BCLC
Barcelona clinic liver cancer
- PLT
Platelet count
Additional file
Flow diagram and randomization of study population. (DOCX 15 kb)
Footnotes
Xiao-jun Lin and Xiang-ming Lao contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XJL carried out the data collecting, analysing, literature reviewing and participated in writing the manuscript. XJL and XML carried out the operation procedure. HY carried out the image diagnose working, participated in the design of the study and performed the statistical analysis. SPL participated in the medical information consult. QJL conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Xiao-jun Lin, Email: linxj@sysucc.org.cn.
Qi-jiong Li, Phone: +86-20-87343114, Email: liqj@sysucc.org.cn.
Xiang-ming Lao, Email: laoxm@sysucc.org.cn.
Han Yang, Email: yanghan@sysucc.org.cn.
Sheng-ping Li, Email: lishp@sysucc.org.cn.
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Guan YS, Liu Y. Interventional treatments for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2006;5(4):495–500. [PubMed] [Google Scholar]
- 3.Lubienski A, Simon M, Lubienski K, Gellissen J, Hoffmann RT, Jakobs TF, Helmberger T. [Update on chemoinfusion and chemoembolization treatments] Radiologe. 2007;47(12):1097. doi: 10.1007/s00117-007-1587-4. [DOI] [PubMed] [Google Scholar]
- 4.Chang JF, Chen PJ, Sze DY, Reid T, Bartlett D, Kirn DH, Liu TC. Oncolytic virotherapy for advanced liver tumours. J Cell Mol Med. 2009;13(7):1238–1247. doi: 10.1111/j.1582-4934.2008.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Blockage by adenovirus E4orf6 of transcriptional activation by the p53 tumor suppressor. Science. 1996;272(5267):1470–1473. doi: 10.1126/science.272.5267.1470. [DOI] [PubMed] [Google Scholar]
- 6.Benedict CA, Norris PS, Prigozy TI, Bodmer JL, Mahr JA, Garnett CT, Martinon F, Tschopp J, Gooding LR, Ware CF. Three adenovirus E3 proteins cooperate to evade apoptosis by tumor necrosis factor-related apoptosis-inducing ligand receptor-1 and −2. J Biol Chem. 2001;276(5):3270–3278. doi: 10.1074/jbc.M008218200. [DOI] [PubMed] [Google Scholar]
- 7.Habib N, Salama H, Abd El Latif Abu Median A, Isac Anis I, Abd Al Aziz RA, Sarraf C, Mitry R, Havlik R, Seth P, Hartwigsen J, et al. Clinical trial of E1B-deleted adenovirus (dl1520) gene therapy for hepatocellular carcinoma. Cancer Gene Ther. 2002;9(3):254–259. doi: 10.1038/sj.cgt.7700431. [DOI] [PubMed] [Google Scholar]
- 8.Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J, Randlev B, Heise C, Uprichard M, Hatfield M, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62(21):6070–6079. [PubMed] [Google Scholar]
- 9.He Q, Liu Y, Zou Q, Guan YS. Transarterial injection of H101 in combination with chemoembolization overcomes recurrent hepatocellular carcinoma. World J Gastroenterol. 2011;17(18):2353–2355. doi: 10.3748/wjg.v17.i18.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cance EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, Shi M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18(2):413–420. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 12.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 13.Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13(9 Pt 2):S211–221. doi: 10.1016/S1051-0443(07)61789-8. [DOI] [PubMed] [Google Scholar]
- 14.Okusaka T, Okada S, Ishii H, Ikeda M, Nakasuka H, Nagahama H, Iwata R, Furukawa H, Takayasu K, Nakanishi Y, et al. Transarterial chemotherapy with zinostatin stimalamer for hepatocellular carcinoma. Oncology. 1998;55(4):276–283. doi: 10.1159/000011863. [DOI] [PubMed] [Google Scholar]
- 15.Okusaka T, Okada S, Ueno H, Ikeda M, Yoshimori M, Shimada K, Yamamoto J, Kosuge T, Yamasaki S, Iwata R, et al. Evaluation of the therapeutic effect of transcatheter arterial embolization for hepatocellular carcinoma. Oncology. 2000;58(4):293–299. doi: 10.1159/000012115. [DOI] [PubMed] [Google Scholar]
- 16.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274(5286):373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 17.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3(6):639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 18.Ganly I, Kirn D, Eckhardt G, Rodriguez GI, Soutar DS, Otto R, Robertson AG, Park O, Gulley ML, Heise C, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6(3):798–806. [PubMed] [Google Scholar]
- 19.Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, Kuhn J, McCarty T, Landers S, Blackburn A, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19(2):289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 20.Makower D, Rozenblit A, Kaufman H, Edelman M, Lane ME, Zwiebel J, Haynes H, Wadler S. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin Cancer Res. 2003;9(2):693–702. [PubMed] [Google Scholar]
- 21.Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther. 2001;8(2):89–98. doi: 10.1038/sj.gt.3301377. [DOI] [PubMed] [Google Scholar]
- 22.Habib NA, Sarraf CE, Mitry RR, Havlik R, Nicholls J, Kelly M, Vernon CC, Gueret-Wardle D, El-Masry R, Salama H, et al. E1B-deleted adenovirus (dl1520) gene therapy for patients with primary and secondary liver tumors. Hum Gene Ther. 2001;12(3):219–226. doi: 10.1089/10430340150218369. [DOI] [PubMed] [Google Scholar]
- 23.Xia ZJ, Chang JH, Zhang L, Jiang WQ, Guan ZZ, Liu JW, Zhang Y, Hu XH, Wu GH, Wang HQ, et al. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng. 2004;23(12):1666–1670. [PubMed] [Google Scholar]
- 24.Reid T, Galanis E, Abbruzzese J, Sze D, Andrews J, Romel L, Hatfield M, Rubin J, Kirn D. Intra-arterial administration of a replication-selective adenovirus (dl1520) in patients with colorectal carcinoma metastatic to the liver: a phase I trial. Gene Ther. 2001;8(21):1618–1626. doi: 10.1038/sj.gt.3301512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong C, Guo RP, Li JQ, Shi M, Wei W, Chen MS, Zhang YQ. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135(10):1437–1445. doi: 10.1007/s00432-009-0588-2. [DOI] [PubMed] [Google Scholar]
- 26.Tabor E. Tumor suppressor genes, growth factor genes, and oncogenes in hepatitis B virus-associated hepatocellular carcinoma. J Med Virol. 1994;42(4):357–365. doi: 10.1002/jmv.1890420406. [DOI] [PubMed] [Google Scholar]
- 27.Pei Z, Chu L, Zou W, Zhang Z, Qiu S, Qi R, Gu J, Qian C, Liu X. An oncolytic adenoviral vector of Smac increases antitumor activity of TRAIL against HCC in human cells and in mice. Hepatology. 2004;39(5):1371–1381. doi: 10.1002/hep.20203. [DOI] [PubMed] [Google Scholar]
- 28.Vollmer CM, Ribas A, Butterfield LH, Dissette VB, Andrews KJ, Eilber FC, Montejo LD, Chen AY, Hu B, Glaspy JA, et al. p53 selective and nonselective replication of an E1B-deleted adenovirus in hepatocellular carcinoma. Cancer Res. 1999;59(17):4369–4374. [PubMed] [Google Scholar]
- 29.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37(2):429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 30.Luo J, Peng ZW, Guo RP, Zhang YQ, Li JQ, Chen MS, Shi M. Hepatic resection versus transarterial lipiodol chemoembolization as the initial treatment for large, multiple, and resectable hepatocellular carcinomas: a prospective nonrandomized analysis. Radiology. 2011;259(1):286–295. doi: 10.1148/radiol.10101072. [DOI] [PubMed] [Google Scholar]
- 31.Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30(1):3–16. doi: 10.1055/s-0030-1247128. [DOI] [PubMed] [Google Scholar]
- 32.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286(5448):2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 33.Beardsley T. Gene therapy setback. Sci Am. 2000;282(2):36–37. doi: 10.1038/scientificamerican0200-36. [DOI] [PubMed] [Google Scholar]
- 34.Jenks S. Gene therapy death--“everyone has to share in the guilt”. J Natl Cancer Inst. 2000;92(2):98–100. doi: 10.1093/jnci/92.2.98. [DOI] [PubMed] [Google Scholar]
- 35.Yuan ZY, Zhang L, Li S, Qian XZ, Guan ZZ. [Safety of an E1B deleted adenovirus administered intratumorally to patients with cancer] Ai Zheng. 2003;22(3):310–313. [PubMed] [Google Scholar]
- 36.Lu W, Zheng S, Li XF, Huang JJ, Zheng X, Li Z. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: a pilot phase II clinical trial. World J Gastroenterol. 2004;10(24):3634–3638. doi: 10.3748/wjg.v10.i24.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]


