Key Points
Genetic- or compound CB-839–induced GAC inhibition reduces OXPHOS and has antileukemic activity in AML.
GAC inhibition synergizes with BCL-2 inhibition by compound ABT-199.
Abstract
Cancer cells require glutamine to adapt to increased biosynthetic activity. The limiting step in intracellular glutamine catabolism involves its conversion to glutamate by glutaminase (GA). Different GA isoforms are encoded by the genes GLS1 and GLS2 in humans. Herein, we show that glutamine levels control mitochondrial oxidative phosphorylation (OXPHOS) in acute myeloid leukemia (AML) cells. Glutaminase C (GAC) is the GA isoform that is most abundantly expressed in AML. Both knockdown of GLS1 expression and pharmacologic GLS1 inhibition by the drug CB-839 can reduce OXPHOS, leading to leukemic cell proliferation arrest and apoptosis without causing cytotoxic activity against normal human CD34+ progenitors. Strikingly, GLS1 knockdown dramatically inhibited AML development in NSG mice. The antileukemic activity of CB-839 was abrogated by both the expression of a hyperactive GACK320A allele and the addition of the tricarboxyclic acid cycle product α-ketoglutarate, indicating the critical function of GLS1 in AML cell survival. Finally, glutaminolysis inhibition activated mitochondrial apoptosis and synergistically sensitized leukemic cells to priming with the BCL-2 inhibitor ABT-199. These findings show that targeting glutamine addiction via GLS1 inhibition offers a potential novel therapeutic strategy for AML.
Introduction
Cancer cell survival is dependent on sustained biosynthetic activities that are promoted by increased uptake of nutrients, such as glutamine. The energy production required for metabolism depends on glycolysis rather than mitochondrial oxidative phosphorylation (OXPHOS), which can lead to lactate production from glucose (known as the Warburg effect).1 However, the functions of mitochondria in cancer cells remain intact,2 allowing tricarboxyclic acid (TCA) cycle intermediates to “feed” biosynthetic pathways. Consequently, cancer cells can become “addicted” to glutaminolysis (a limiting step in the TCA cycle) because glutamine represents a major source of carbon molecules that can sustain tumor growth-facilitating metabolic pathways.3 The gatekeeper enzyme of glutaminolysis is glutaminase (GA), which catalyzes the hydrolysis of glutamine to glutamate. In a second step, glutamate dehydrogenase (GDH) or transaminases produce α-ketoglutarate (αKG) from glutamate to feed the TCA cycle. In mammalian cells, the GA family includes 2 isoforms encoded by GLS, kidney (K-type) glutaminase (KGA) and glutaminase C (GAC), which are generally known as GLS1.4,5 Expression of KGA and GAC are increased in many types of cancers compared with normal tissues, and the targeted inhibition of these enzymes has been shown to exert antitumor effects.6-9 Moreover, GLS2 encodes 2 liver (L-type) isoenzymes, LGA and GAB, which are collectively known as GLS2.10-12 GLS2 is a p53 target gene that carries out tumor suppressive functions.13,14
We previously showed that targeting glutamine uptake can result in robust antileukemic responses in acute myeloid leukemia (AML), and we proposed the involvement of the mTORC1 signaling pathway downstream of glutamine addiction in this context.15 However, little is known about mitochondrial metabolism in AML. Primary AML cells have an increased mitochondrial mass and oxygen consumption rate compared with normal hematopoietic cells, and targeting mitochondrial translation has antileukemic effects.16 The inhibition of mitochondrial respiratory chain complex I by metformin can reduce oxygen consumption and induce cytotoxicity in AML.17 Moreover, leukemic stem cells, which are characterized by low reactive oxygen species, are addicted to OXPHOS rather than glycolysis, as OXPHOS targeting using BCL-2 inhibitors results in apoptotic cell death that cannot be compensated for by increased glycolysis.18
Herein, we report that GLS1, especially the GAC isoform, is the major GA isoform expressed at the protein level in AML. Using both genetic and pharmacologic approaches, we show that GLS1 inhibition can reduce the oxygen consumption rate (OCR), leading to cell proliferation inhibition and apoptosis. In a systemic mouse xenotransplantation model of AML, doxycycline-inducible GLS1 knockdown dramatically reduced AML development and extended survival. Finally, inhibition of glutaminolysis could activate a caspase-dependent mitochondrial apoptotic pathway, and BCL-2 inhibition could synergize with compound CB-839, a GLS1 inhibitor. Our findings show that targeting glutaminolysis represents a novel strategy for exploiting the glutamine addiction of AML cells as a therapeutic modality.
Materials and methods
Primary human samples
Patients and healthy donors provided written informed consent in accordance with the Declaration of Helsinki, and approval was obtained from the Cochin Hospital Institutional Ethic Committee. Bone marrow (BM) or peripheral blood (PB) samples with >70% blast cell content were obtained from 31 patients with newly diagnosed AML; patient characteristics are provided in supplemental Table 1 available on the Blood Web site. The CD34+ fraction, which is enriched in hematopoietic progenitor cells (HPCs), from BM healthy donors or umbilical cord blood were purified using MIDI MACS immunoaffinity columns (Milteny Biotech, Bergish Badgash, Germany).
Cell lines and reagents
We used MOLM-14, MV4-11, MOLM-13, OCI-AML2, OCI-AML3, HL60, K562, HEL, THP-1, U937, and KG1A AML cell lines; the main characteristics of these cells are listed in supplemental Table 2. Cells were cultured in RPMI with glutamine (Gibco 61870; Life Technologies, Saint Aubin, France) or glutamine-free RPMI (Gibco 21870), supplemented with 10% dialyzed fetal bovine serum with or without 4 mM glutamine, as indicated. CB-839 was provided by Calithera Bioscience (San Francisco, CA). Compound 968 was obtained from Merck Millipore (Darmstadt, Germany). Bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl) ethyl sulfide (BPTES) (SML0601) and dimethyl-αKG (34963-1) were obtained from Sigma-Aldrich (St Louis, MO). Z-IETD-FMK (CASPASE-8 inhibitor) was from R&D Systems (Minneapolis, MN), and the Q-VD-OPh pan-caspase inhibitor (SML0063) was from Sigma-Aldrich. ABT-199 (GDC-0199) was from Selleck Chemical (Houston, TX).
Western blotting
Whole cell extracts and western blotting were performed as previously described.15 Antibodies against poly(ADP-ribose)polymerase (PARP), cleaved-CASPASE-3, CASPASE-8, p21, and p27 were obtained from Cell Signaling Technology (Beverly, MA); anti-β-ACTIN and anti-GLS2 (HPA038608) antibodies were from Sigma-Aldrich. Anti-GAC (19958-1-AP) and anti-GLS1 (20170-1-AP) antibodies were purchased from Proteintech (Manchester, United Kingdom). Anti-P85 antibody was raised in-house.
Proliferation assays
Cells were seeded at 5 × 104/mL on day 0 and counted manually using trypan blue staining after 3, 5, and 7 days.
Apoptosis assay
Apoptosis was quantified by Annexin V-phycoerythrin (Becton Dickinson Biosciences, Le Pont De Claix, France) or tetramethylrhodamine, ethyl ester (TMRE) (ab113852; Abcam, Paris, France) staining by flow cytometry.
Lentiviral vectors
GLS1 (TCRN0000045630 [shRNA#5] and TCRN0000045632 [shRNA#7]) and nontargeted (control) short hairpin (sh)RNAs were purchased from Open Biosystems and cloned into a pLKO-Tet-On–inducible or pLKO.1 plasmid (plasmids #2191519 and #845320; Addgene, Cambridge, MA). The GACK320A and GACWT alleles were cloned from pcDNA hGAC vectors21 into the pInducer plasmid.22
Metabolic assays
Intracellular ATP levels were quantified using a luminescence-based cellular assay (CellTiter-Glo; Promega, Madison, WI). Oxygen consumption was measured using a Seahorse XF96 extracellular flux analyzer as previously reported.23 Briefly, 2 × 105 cells per condition were seeded in 96-well XF96 well plates coated with BD Cell-Tak (Becton Dickinson Biosciences) and loaded with serum-free unbuffered Dulbecco’s modified Eagle’s medium with (102365-100) or without (102353-100) glutamine. After 1-hour incubation at 37°C without CO2, steady-state and postintervention analyses were performed. At different experimental times, the wells were injected with increasing concentrations of carbonyl cyanide m-chlorophenylhydrazone (CCCP), an uncoupler that raises the OCR to its theoretical maximum. Finally, antimycin A was injected to inhibit the flux of electrons through complex III to shut down the OCR because no further oxygen could be consumed. Glutamate, malate, and citrate concentrations were measured as previously reported.24
Xenotransplantation of human leukemic cells in NSG mice
Adult mice (6-8 weeks old) were treated with 20 mg/kg busulfan (Busilvex, Pierre Fabre, France) by intraperitoneal administration 24 hours before injection of leukemic cells. Cultured AML cell lines (MOLM-14 shCTR, MOLM-14 shGLS1, OCI-AML2 shCTR, OCI-AML2 shGLS1) were washed in phosphate-buffered saline (PBS) and suspended in PBS at a final concentration of 2 million cells per 200 μL PBS per mouse for intravenous injection. Xenograft tumors were generated by injecting AML cells (in 200 μL PBS) in the tail vein of NSG mice.25 Immediately after cell injection, doxycycline (200 µg/mL + 1% sucrose) was added to drinking water for inducible shRNA expression. Drinking water was changed 2 times a week. Nine mice were injected for each group with the different AML cell lines. Daily monitoring of mice for symptoms of disease (ruffled coat, hunched back, weakness, and reduced motility) determined the time of killing for injected animals with signs of distress. All experiments were conducted in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International.
Calculating of compounds synergistic effects
CB-839 was tested in combination with the BH3-mimetic ABT-199 using different doses. Relative induction of cell apoptosis was calculated for every dose combination. Using the Chalice software,26 the response of the combination was compared with its single agents against the widely used Loewe model for drug-with-itself dose additivity. Excess inhibition compared with additivity can be plotted as a full dose matrix chart to visualize the drug concentrations where synergies occur.
Statistical analysis
All data are expressed as means with standard deviation. Statistically significant differences between experimental groups were determined using the Student t test. Data were analyzed using Prism software (GraphPad, La Jolla, CA). Independent experiments carried out in AML cell lines were each performed ≥3 times.
Results
Glutamine controls mitochondrial OXPHOS in AML
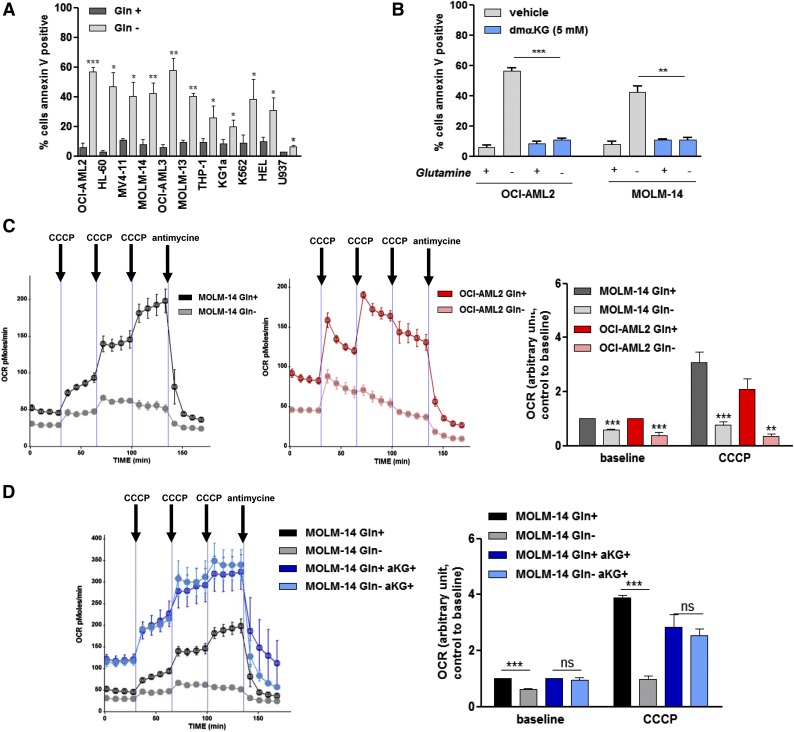
We previously reported that the inhibition of glutamine uptake exerts antileukemic effects in AML.15 In our present study, we extended this previous observation to a panel of 11 AML cell lines that were cultured with or without glutamine. Glutamine removal significantly induced apoptosis in all of the AML cell lines tested (Figure 1A). Because glutamine feeds the TCA cycle in many cancers, we hypothesized that glutamine removal-induced apoptosis that resulted from TCA cycle inhibition. Accordingly, the TCA cycle intermediate αKG could prevent glutamine removal-induced apoptosis in the OCI-AML2 and MOLM-14 cell lines (Figure 1B). Across 6 different AML cell lines, we observed a rapid and significant reduction in intracellular ATP content on glutamine removal (supplemental Figure 1). When the OCI-AML2 and MOLM-14 cell lines were cultured with or without glutamine, we observed a significant reduction in the OCR using a Seahorse XF96 analyzer, which indicates OXPHOS inhibition in glutamine-deprived AML cells (Figure 1C). This effect on OCR was observed both at baseline and after injecting CCCP, an uncoupler that raised OCR to it maximal rate. The effect of glutamine deprivation on OCR could be blunted by coincubation with αKG in all of these cell lines (Figure 1D). These findings indicate that AML cells are addicted to the glutamine-dependent TCA cycle.
Figure 1.
Glutamine controls mitochondrial OXPHOS in AML. (A) A total of 11 AML cell lines were cultured with or without Gln (4 mM) for 48 hours. Apoptosis was quantified by flow cytometric analysis of Annexin V binding. (B) OCI-AML2 and MOLM-14 cell lines were cultured for 48 hours with or without Gln (4 mM) and αKG (5 mM) as indicated, and apoptosis was quantified based on Annexin V binding. (C) OCI-AML2 and MOLM-14 cell lines were cultured for 48 hours with or without Gln (4 mM), and the OCR was measured using a Seahorse XF96 extracellular flux analyzer, under both basal conditions and after the addition of increasing doses of CCCP (0.25, 0.5, and 0.5 µM) and antimycine (10 µM), as indicated. Histograms show data that are representative of 3 independent experiments at baseline or after CCCP (cumulative dose, 1 µM). (D) MOLM-14 cell lines were cultured for 48 hours with or without Gln (4 mM) and αKG (5 mM) as indicated, and the OCR was measured as in C. Histograms show data that are representative of 3 independent experiments. *P < .05, **P < .01, ***P < .001.
GAC protein is prominently expressed in AML and modulates the OCR
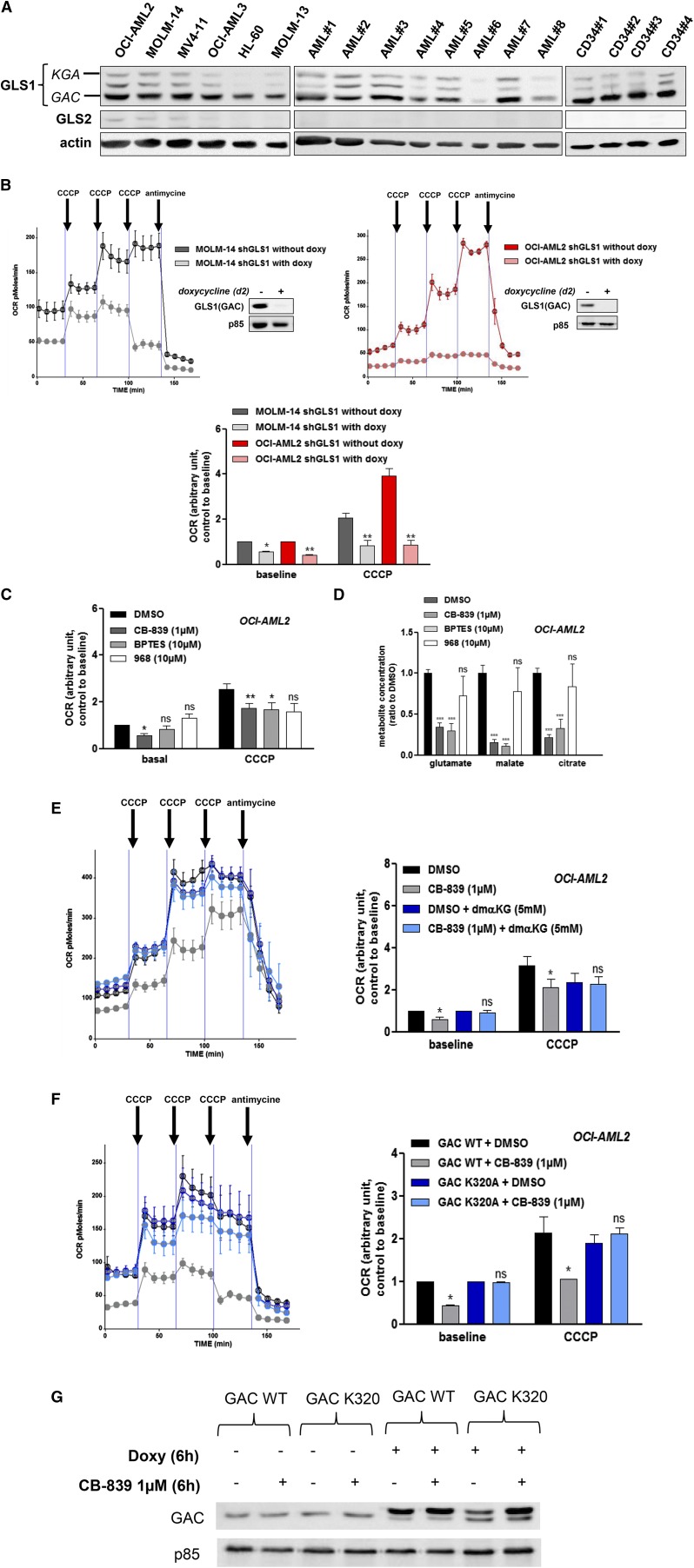
We measured the protein expression of KGA, GAC, and GLS2 in 26 primary AML samples, 11 AML cell lines, and CD34+ HPCs from 4 healthy donors. Across these samples, the GAC isoform of GLS1 was the most expressed in most AML cell lines and primary AML cells, whereas KGA was barely detectable and GLS2 was absent (Figure 2A; supplemental Figure 2A). GAC expression at the protein level was statistically increased compared with KGA in primary AML cells, in leukemic cell lines, and in normal CD34+ hematopoietic cells (supplemental Figure 2B). Similar results were observed at the mRNA level (data not shown). We used 2 Dox-inducible hairpins (shRNA#5 and shRNA#7) to target both GLS1 isoforms. The induction of GLS1 knockdown in MOLM-14 and OCI-AML2 cells reduced both the baseline OCR and CCCP-induced OCR (Figure 2B), suggesting a reduction in OXPHOS metabolism. Similar results were obtained in HL-60 cells, but not in OCI-AML3 cells (supplemental Figure 2C). We then tested the effects of 3 GSL1 inhibitors on the OCR. Compound CB-83924 and BPTES27 can inhibit both isoforms, whereas compound 968 is considered to be GAC specific.8 In OCI-AML2 cells, CB-839, but not BPTES or compound 968, could inhibit the baseline OCR (Figure 2C). After mitochondrial uncoupling by CCCP, both CB-839 and BPTES more robustly reduced OCR compared with compound 968. Similar results were observed in MOLM-14 cells (supplemental Figure 2D). In addition to OCR modulation in OCI-AML2 cells, CB-839 and BPTES, but not compound 968, reduced the intracellular concentrations of glutamate, malate, and citrate, suggesting that these compounds are useful tools to target glutaminolysis and the TCA cycle (Figure 2D). In OCI-AML2 cells, αKG could reverse CB-839–induced inhibition of OCR at both baseline and after decoupling, indicating that OCR suppression was related to CB-839–induced glutaminase inhibition in this setting (Figure 2E). Similar results were obtained in MOLM-14 cells (supplemental Figure 2E). OCI-AML2 cells were then transduced with Dox-inducible GACWT or GACK320A alleles (Figure 2F-G). The GACK320A allele encodes a fiber-prone super-active form of GAC that is insensitive to BPTES.21 Expression of the GACK320A mutant in OCI-AML2 cells promoted resistance to CB-839, as no changes were observed in the OCR (Figure 2F, right). Overall, these findings indicate that targeting glutaminolysis impacts mitochondrial respiration and the OCR.
Figure 2.
GAC protein is prominently expressed in AML and modulates the OCR. (A) Analysis of human leukemic cell lines. AML cells from 8 patients and normal CD34+ HPCs from 4 healthy donors were analyzed by western blotting using anti-GLS1, anti-GLS2, and anti-ACTIN antibodies. (B) MOLM-14 (left) and OCI-AML2 (right) cells were transfected with a lentiviral vector expressing a doxycycline-inducible GLS1 shRNA (#5) construct. Stably infected cell lines were established by puromycin selection. After 2 days of doxycycline exposure, the OCR was measured using a Seahorse XF96 extracellular flux analyzer under both basal conditions and after the addition of CCCP and antimycin, as indicated. The inhibition of GAC expression was controlled in western blots using anti-GAC antibody. Histograms show data that are representative of 3 independent experiments. (C) OCI-AML2 cells were cultured with or without CB-839 (1 µM), BPTES (10 µM), or compound 968 (10 µM) for 6 hours, and the OCR was measured using a Seahorse XF96 extracellular flux analyzer. Histograms show data that are representative of 3 independent experiments. (D) OCI-AML2 cells were cultured with or without CB-839 (1 µM), BPTES (10 µM), or compound 968 (10 µM) for 4 hours, and 5 × 106 cells were washed twice in cold PBS; the pellet was frozen and each indicted metabolite was measured. (E) OCI-AML2 cells were cultured with or without CB-839 (1 µM) and αKG (5 mM) for 6 h, and the OCR was measured. Histograms show data that are representative of 3 independent experiments. (F) OCI-AML2 cells were transfected with a lentiviral vector expressing a doxycycline-inducible V5-tagged GACWT or GACK320A construct. Stably infected cell lines were established by puromycin selection. After 6-hour doxycycline exposure with or without CB-839, the OCR was measured under both basal conditions and after the addition of CCCP and antimycin, as indicated. Histograms show data that are representative of 3 independent experiments (G) OCI-AML2 cells stably infected with GACWT or GACK320A were cultured for 6 hours with or without doxycycline or CB-839 (1 µM) and were analyzed by western blotting using anti-GAC and anti-ACTIN antibodies. *P < .05, **P < .01, ***P < .001.
Targeting glutaminase activity inhibits AML cell proliferation
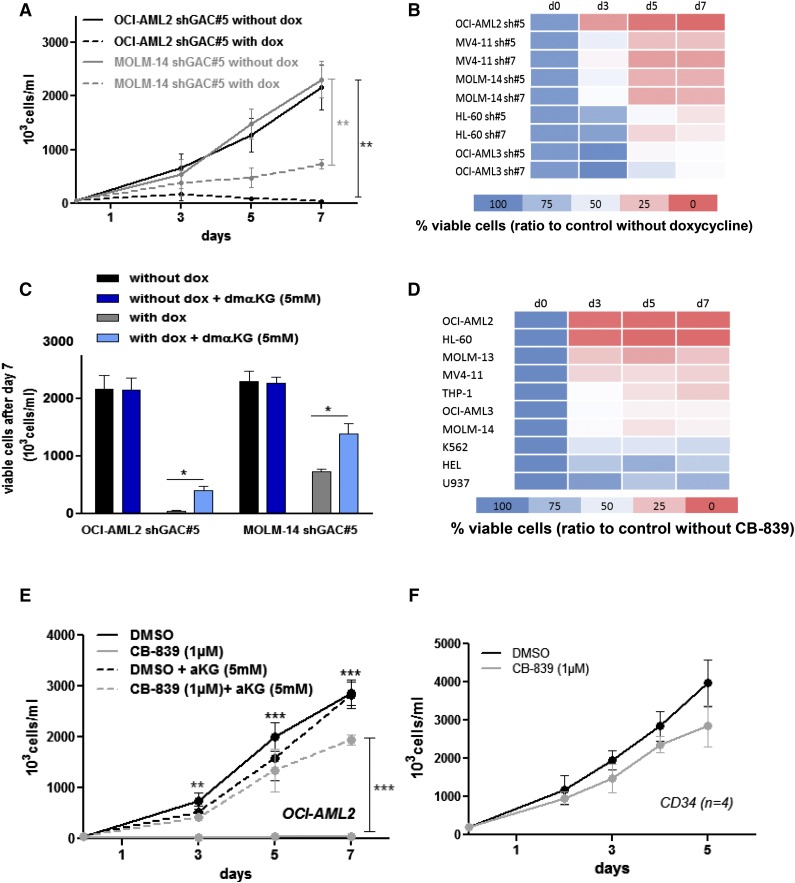
The expression of shGLS1#5 and shGLS1#7 were induced in several AML cell lines (OCI-AML2, MV4-11, MOLM-14, HL-60, and OCI-AML3) by doxycycline treatment (supplemental Figure 3A). On GLS1 knockdown, reduced cellular proliferation could be observed in all conditions, with a more dramatic effect occurring in OCI-AML2 cells (Figures 3A-B). Cell proliferation was partially restored by coincubation with αKG (Figure 3C). Among 10 distinct AML cell lines, compound CB-839 reduced cellular proliferation (Figure 3D), which could be blunted by coincubation with αKG in OCI-AML2, HL-60, MOLM-14, and MV4-11 cells (Figure 3E; supplemental Figure 3B). In agreement with the proliferation blockade findings, we observed the accumulation of p21cip1 and p27kip1 proteins in 2 cell lines treated with compound CB-839 (supplemental Figure 3C). By contrast, compound CB-839 did not reduce cellular proliferation across four different CD34-enriched HPCs samples (Figure 3F).
Figure 3.
Targeting glutaminase activity inhibits AML cell proliferation. (A) OCI-AML2 and MOLM-14 shGLS1#5 leukemic cells were seeded at 5 × 105 cells/mL with or without doxycycline; viable cells were counted manually following trypan blue staining on days 3, 5, and 7. Each experiment was performed independently ≥3 times. (B) OCI-AML2, MV4-11, MOLM-14, HL-60, and OCI-AML3 cells transfected with shGLS1#5 or #7 were seeded at 5 × 105 cells/mL with or without doxycycline; viable cells were counted following trypan blue staining on days 3, 5, and 7. Each experiment was performed independently ≥3 times. (C) OCI-AML2 and MOLM-14 shGLS1#5 leukemic cells were seeded at 5 × 105 cells/mL with or without doxycycline, and αKG (5 mM) and viable cells were counted following trypan blue staining on day 7. (D) A total of 10 cell lines were seeded at 5 × 105 cells/mL with or without CB-839 (1 µM); viable cells were counted following trypan blue staining on days 3, 5, and 7. (E) OCI-AML2 cells were seeded at 5 × 105 cells/mL with or without CB-839 (1 µM) and αKG (5 mM); viable cells were counted following trypan blue staining on days 3, 5, and 7. (F) CD34+ HPCs from 4 healthy donors were seeded at 5 × 105 cells/mL with and without CB-839 (1 µM); viable cells were counted following trypan blue staining on days 2, 3, 4, and 5. *P < .05, **P < .01, ***P < .001.
GLS1 controls AML survival by modulating the TCA cycle
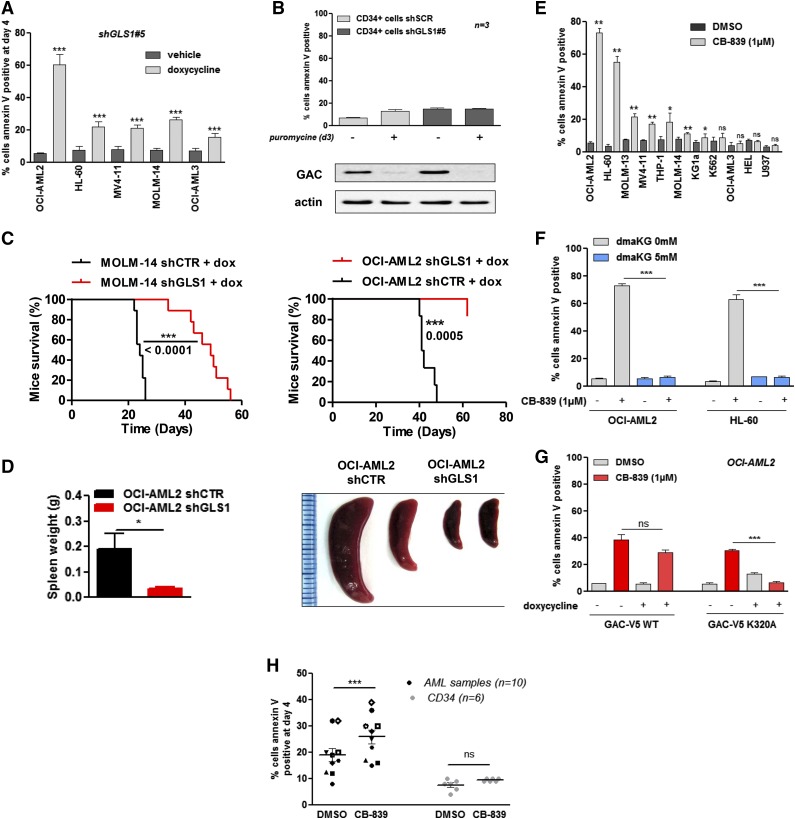
We observed apoptosis, as indicated by Annexin V staining, in 5 different AML cell lines on GLS1 knockdown, particularly in OCI-AML2 cells (Figure 4A). Apoptosis was reduced in cells coincubated with αKG (supplemental Figure 4A). In normal CD34+ HPCs, GLS1 knockdown did not result in significant apoptosis (Figure 4B). To test the in vivo antileukemic potential related to the inhibition of glutaminolysis, we generated a systemic xenotransplantation AML model in NSG mice injected with MOLM-14 or OCI-AML3 cell lines expressing either the shGLS1#5 or a control shRNA. Dox-induced GLS1 knockdown prevented AML growth in both cell lines, reduced splenomegaly development in OCI-AML2, and dramatically extended mouse survival (Figures 4C-D). The antileukemic activity that resulted from GAC inhibition by CB-839 was then tested in 11 AML cell lines; compound CB-839 induced apoptosis in 7 of these cell lines (Figure 4E). This proapoptotic activity could be fully reversed by coincubation with αKG in OCI-AML2 and HL-60 cells (Figure 4F). Similarly, ectopic expression of super-active GACK320A mutant protein preserved cell viability in CB-839–treated OCI-AML2 cells (Figure 4G). Compound CB-839 also induced apoptosis in primary AML samples but not in normal CD34+ HPCs (Figure 4H). These findings indicated that GLS1 inhibition targets leukemic, but not normal, hematopoietic cells.
Figure 4.
GLS1 controls AML survival by modulating the TCA cycle. (A) ShGLS1#5 was induced with doxycycline for 4 days in OCI-AML2, HL-60, MV4-11, MOLM-14, and OCI-AML3 leukemic cells, and apoptosis was evaluated based on Annexin-V binding. (B) CD34+ HPCs from 3 healthy donors were transfected with a lentiviral vector that expressed a noninducible GLS1 shRNA (#5), and transfected cells were selected with puromycin for 2 days; 3 days later, apoptosis was evaluated based on Annexin-V staining, and protein extracts were immunobloted with anti-GAC and anti-ACTIN antibodies. (C) MOLM-14 shGLS1 cells, MOLM-14 shCTR cells (9 mice each), and OCI-AML2 shGLS1 cells or OCI-AML2 shCTR cells (9 mice each) were intravenously injected into NSG mice (2 × 106 cells per mouse). Mice were treated with doxycycline by oral gavage. The Kaplan-Meier survival curves of mice treated with doxycycline are shown for both cell lines. (D) The spleens of the mice injected with the OCI-AML2 shGLS1 and OCI-AML2 shCTR cell lines were measured. (E) A total of 11 cell lines were cultured for 4 days with or without CB-839 (1 µM), and apoptosis was evaluated based on Annexin-V staining. (F) OCI-AML2 and HL-60 cells were cultured for 4 days with or without CB-839 (1 µM), and dmαKG (5 mM) and apoptosis was evaluated based on Annexin-V staining. (G) OCI-AML2 cells that were stably infected with GACWT or GACK320A were cultured for 2 days with or without doxycycline or CB-839 (1 µM). Apoptosis was evaluated based on Annexin-V staining. (H) AML samples from 10 patients and CD34+ HPCs from 6 healthy donors were cultured for 4 days with or without CB-839 (1 µM). Apoptosis was evaluated based on Annexin-V staining. *P < .05, **P < .01, ***P < .001.
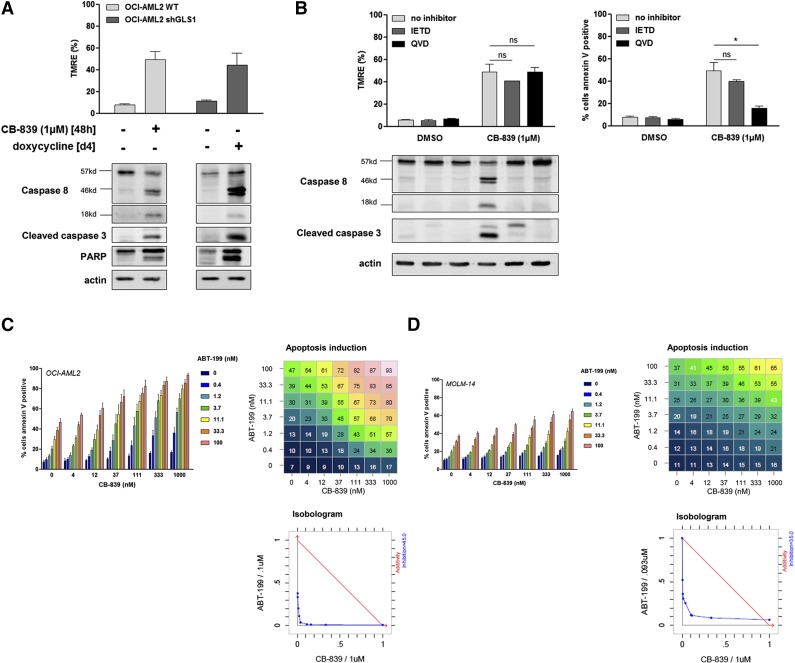
GLS1 inhibition induces mitochondrial apoptosis and sensitizes leukemic cells to priming with ABT-199
In OCI-AML2 cells, GLS1 inhibition was correlated with mitochondrial depolarization, as indicated by the reduced frequency of TMRE-positive cells in CB-839–treated and GLS1 knockdown cells (Figure 5A, upper). Accordingly, GLS1 blockade induced CASPASE-8, CASPASE-3, and PARP cleavage (Figure 5A, lower). To identify the proapoptotic pathway that was engaged downstream of GLS1 disruption, we used a specific CASPASE-8 inhibitor (IETD) and a pan-caspase inhibitor (QVD). The mitochondrial depolarization induced by compound CB-839 was not reversed by IETD or QVD, whereas CASPASE-8 cleavage could be abrogated by both inhibitors (Figure 5B). Compound CB-839–induced Annexin V staining was prevented by QVD, but not IETD, excluding a role for the CASPASE-8–dependent extrinsic pathway in CB-839–induced apoptosis (Figure 5B, right). Accordingly, we hypothesized that mitochondrial priming might potentiate the antileukemic effects that result from GLS1 inhibition. We cocultured AML cells with increasing doses of the BH3 mimetic ABT-199, which specifically inhibits BCL-2,28 along with CB-839. In OCI-AML2 and MOLM-14 cells, ABT-199 and CB-839 synergized to induce Annexin V staining (Figure 5C-D). The specificity of our observation was corroborated by the absence of BH3 mimetic priming effects on Annexin V staining in CB-839–resistant OCI-AML3 cells (supplemental Figure 5). Overall, these findings indicate that glutaminolysis inhibition relies on mitochondrial depolarization and intrinsic CASPASE-dependent apoptosis to produce antileukemic activity and supports the combination of GLS1 inhibitors and BH3 mimetic compounds as a promising potential drug combination in the clinical treatment of AML.
Figure 5.
GLS1 inhibition induces mitochondrial apoptosis and sensitizes leukemic cells to priming with ABT-199. (A) OCI-AML2 WT cells or cells transfected with shGLS1#5 were cultured with or without CB-839 (1 µM) or doxycycline as indicated. Apoptosis was evaluated by western blotting using anti-CASPASE-8, cleaved CASPASE-3, anti-PARP, and anti-ACTIN antibodies. (B) OCI-AML2 cells were cultured with or without CB-839 (1 µM) and with or without a CASPASE-8 inhibitor (IETD) or a pan-caspase inhibitor (QVD). Protein extracts were immunoblotted using anti-CASPASE-8, anti-cleaved CASPASE-3, and anti-ACTIN antibodies. Mitochondrial depolarization was evaluated using TMRE staining and apoptosis was quantified based on Annexin V staining. (C) OCI-AML2 cells were cultured for 1 day with or without the indicated doses of CB-839 and ABT-199; apoptosis was evaluated based on Annexin V staining. (D) MOLM-14 cells were cultured for 1 day with or without the indicated doses of CB-839 and ABT-199; apoptosis was evaluated based on Annexin-V staining. Histograms show data that are representative of 3 independent experiments. The response of the combination was compared with its single agents against the widely used Loewe model for drug-with-itself dose additivity using Chalice software26 and presented as an isobologram. *P < .05, **P < .01, ***P < .001.
Discussion
Targeting glutamine metabolism has recently emerged as a promising approach in cancer treatment, as it affects the energy generation, survival, and growth of cancer cells.29 The SLC1A5 plasma membrane transporter promotes intracellular glutamine accumulation, which in turn increases leucine uptake via the SLC7A5 amino acid transporter. This process is critical for activating the mTORC1 signaling pathway via the Ragulator-Rag complex that translocates mTORC1 to lysosomal membranes, which is the key event in amino acid signaling to mTORC1.30,31 Glutamine catabolism begins by converting glutamine to glutamate in reactions catalyzed by GA that either donate an amide nitrogen molecule to biosynthetic pathways or release it as ammonia. Glutamate, the product of a GA reaction, is the most important source of αKG, which is a major TCA intermediate and the substrate for dioxygenases, which include the prolyl hydroxylases, histone demethylases, and TET2 5-methylcytosine hydroxylase. The main function of αKG is to sustain OXPHOS through the TCA cycle, so it represents an important source of energy. However, glutamate also provides precursors for glutathione production, which contribute to maintaining the oxidative status of cells. It is also a source of amino groups for nonessential amino acids, such as alanine, aspartate, glycine, and serine. Finally, glutamine-derived nitrogen is a component of hexosamine for N-glycosylation reactions and can act to control nucleic acid synthesis.32
In AML, glutamine controls mTORC1 activity via the amino acid pathway, and glutamine depletion blocks protein synthesis.15 In addition to mTORC1, other metabolic pathways that are dependent on glutamine might account for the antileukemic activity that results from glutamine deprivation. In our present report, we show that AML cells are generally addicted to glutamine and dependent on mitochondrial OXPHOS for their survival. The critical role of glutamine as a limiting step in the TCA cycle, which is supported by the prosurvival activity of the TCA cycle intermediate αKG in glutamine-starved AML cells, prompted us to study the impact of glutaminolysis on AML cell proliferation and survival. In accordance with observations made in other types of cancer, we found that GLS1, particularly the GAC isoform, was the most frequently and strongly expressed GA isoform in AML cells.7 GLS1 expression levels were found to be within the same range across AML samples and also in comparison with normal HPCs, indicating that GLS1 overexpression is most likely not a leukemic feature, in contrast to other cancer models.33
Three GLS1 inhibitors are currently available: BPTES, compound 938, and compound CB-839. Among these compounds, CB-839 is currently being administered to humans in phase 1 clinical trials for solid tumor, lymphoid, and myeloid malignancies (NCT02071862, NCT02071888, and NCT02071927, respectively). Compound 968 was identified through a small molecule screening effort using RhoGTPase-transformed fibroblasts, and its activity has been reported to be directed against GAC but not KGA.8 In our present study, compound 968 had no effect on glutaminolysis, as it did not reduce the measured OCR or impact TCA metabolite intermediates in AML. Moreover, compound 968 induced mild apoptosis in AML cell lines, which was not rescued by αKG, strongly suggesting that these functional effects were related to off-target effects (data not shown). By contrast, both BPTES and CB-839 affected the TCA cycle and OCR. Strikingly, the proapoptotic activity of the clinical candidate molecule CB-839 was blunted by both αKG cotreatment and the expression of a GACK320A super-active mutant. These findings, which we obtained using a pharmacologic approach, were corroborated by the effects of GLS1 knockdown by 2 distinct hairpin sequences in AML cell lines. Based on these data, we conclude that specific GLS1 blockade, particularly using compound CB-839, efficiently eradicates AML cells in a preclinical setting.
In normal hematopoiesis, metabolism has recently emerged as being critically involved in survival, proliferation, and stem cell lineage specification. Normal murine HSCs exhibit high rates of glycolysis that are supported by the abundant expression of pyruvate dehydrogenase kinase, which suppresses mitochondrial glucose oxidation in a process that is essential to self-renewal capacities.34 It has also been shown that mice deficient in glycolysis enzymes, such as M2 pyruvate kinase or lactate dehydrogenase A, have hematopoietic defects with abnormal progenitor functions and, in the case of lactate dehydrogenase A knockdown, HSCs abnormalities with impaired long-term reconstitution capacities.35 Glutamine metabolism also regulates human HSC lineage specification, as it was demonstrated that the SLC1A5 glutamine transporter is required for erythroid specification by regulating de novo nucleotide biosynthesis.36 From the perspective of the therapeutic applications of GSL1 targeting in humans, we investigated the potential toxicity that results from GLS1 inhibition during normal hematopoiesis. Homozygous GLS1 deletion is lethal during the first postnatal day because of neurologic disorders.37 However, the impact of GLS1 knockout on blood formation has not yet been reported. In normal immature CD34+ hematopoietic progenitor cells, our data presented here show that GLS1 inhibition did not translate into reduced cellular proliferation and/or survival. Moreover, orally administered CB-839 did not modify blood cell count parameters in a mouse xenotransplantation model of breast cancer.24 The results of ongoing clinical trials will likely confirm the favorable therapeutic index of GLS1 targeting that we found in our preclinical analysis.
We further showed from our present analyses that GLS1 inhibition-induced apoptosis triggered caspase activation, which largely involved the intrinsic mitochondrial apoptotic pathway. Although the molecular mechanism leading from GLS1 disruption to mitochondrial membrane depolarization remains incompletely characterized, this pattern of caspase activation might suggest new therapeutic combinations. Indeed, BH3 mimetic compounds, such as ABT-199 or ABT-737, specifically act on BCL-2 and lead to subsequent cytochome C release and mitochondrial apoptosis via activate Bak/Bax dimer formation.38 In primary AML leukemic stem cells, high expression of BCL-2 mostly supports OXPHOS metabolism and correlates with a high sensitivity to BCL-2 inhibition in this cellular compartment.18 Our current findings regarding the synergic antileukemic activity of combined BCL-2 (using ABT-199) and GLS1 (using CB-839) inhibition open the possibility of testing this strategy in clinical trials.
In conclusion, AML cells have a unique sensitivity to glutaminolysis inhibition that results from the sustained suppression of the TCA cycle and OXPHOS activity. Our analysis of the molecular mechanisms of apoptosis downstream of GLS1 inhibition also provide insights into the molecular basis for the synergistic effects of a combination of GLS1 and BCL-2 inhibitors, which could represent a novel therapeutic strategy for AML.
Acknowledgments
The authors thank Dr Andre L. B. Ambrosio for providing pcDNA hGAC WT and K320A plasmids and Dr Virginie Penard-Lacronique for collaboration.
This work was supported by grants from the Institut National du Cancer (Projet Recherche Translationnelle TRANSLA13-087 to N.J.) and from the Ligue Nationale Contre le Cancer (Equipe Labellisée EL2014. N° Projet: R14077KK).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: N.J. performed experiments, analyzed data, and wrote the manuscript; A.M.R., C.L., G.M., R.B., E.S., L.W., J.D., L.P., M.A.H., P.S., L.J., and C.R. performed experiments; T.T.M., I.C.M., J.E.S., and C.R. performed in vivo experiments; M.L. helped design the molecular constructs; N.C. performed cytologic and flow cytometry analyses of primary AML samples; C.L. and P.M. wrote the manuscript; S.D. provided the CB-839 compound and analyzed data; J.T. analyzed data and wrote the manuscript; D.B. designed and supervised the research program, analyzed data, and wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Didier Bouscary, Service d’Hématologie, Hôpital Cochin, 27 rue du Faubourg Saint Jacques, AP-HP, Paris 75014, France; e-mail: didier.bouscary@cch.aphp.fr.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274(6):1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 3.DeBerardinis RJ, Mancuso A, Daikhin E, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104(49):19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr. 1995;15:133–159. doi: 10.1146/annurev.nu.15.070195.001025. [DOI] [PubMed] [Google Scholar]
- 5.Elgadi KM, Meguid RA, Qian M, Souba WW, Abcouwer SF. Cloning and analysis of unique human glutaminase isoforms generated by tissue-specific alternative splicing. Physiol Genomics. 1999;1(2):51–62. doi: 10.1152/physiolgenomics.1999.1.2.51. [DOI] [PubMed] [Google Scholar]
- 6.Gao P, Tchernyshyov I, Chang T-C, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458(7239):762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassago A, Ferreira APS, Ferreira IM, et al. Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc Natl Acad Sci USA. 2012;109(4):1092–1097. doi: 10.1073/pnas.1112495109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J-B, Erickson JW, Fuji R, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18(3):207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seltzer MJ, Bennett BD, Joshi AD, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70(22):8981–8987. doi: 10.1158/0008-5472.CAN-10-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aledo JC, Gómez-Fabre PM, Olalla L, Márquez J. Identification of two human glutaminase loci and tissue-specific expression of the two related genes. Mamm Genome. 2000;11(12):1107–1110. doi: 10.1007/s003350010190. [DOI] [PubMed] [Google Scholar]
- 11.de la Rosa V, Campos-Sandoval JA, Martín-Rufián M, et al. A novel glutaminase isoform in mammalian tissues. Neurochem Int. 2009;55(1-3):76–84. doi: 10.1016/j.neuint.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Martín-Rufián M, Tosina M, Campos-Sandoval JA, et al. Mammalian glutaminase Gls2 gene encodes two functional alternative transcripts by a surrogate promoter usage mechanism. PLoS One. 2012;7(6):e38380. doi: 10.1371/journal.pone.0038380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu W, Zhang C, Wu R, Sun Y, Levine A, Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci USA. 2010;107(16):7455–7460. doi: 10.1073/pnas.1001006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki S, Tanaka T, Poyurovsky MV, et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci USA. 2010;107(16):7461–7466. doi: 10.1073/pnas.1002459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willems L, Jacque N, Jacquel A, et al. Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood. 2013;122(20):3521–3532. doi: 10.1182/blood-2013-03-493163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skrtić M, Sriskanthadevan S, Jhas B, et al. Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell. 2011;20(5):674–688. doi: 10.1016/j.ccr.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scotland S, Saland E, Skuli N, et al. Mitochondrial energetic and AKT status mediate metabolic effects and apoptosis of metformin in human leukemic cells. Leukemia. 2013;27(11):2129–2138. doi: 10.1038/leu.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagadinou ED, Sach A, Callahan K, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12(3):329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiederschain D, Wee S, Chen L, et al. Single-vector inducible lentiviral RNAi system for oncology target validation. Cell Cycle. 2009;8(3):498–504. doi: 10.4161/cc.8.3.7701. [DOI] [PubMed] [Google Scholar]
- 20.Stewart SA, Dykxhoorn DM, Palliser D, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9(4):493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira APS, Cassago A, Gonçalves KA, et al. Active glutaminase C self-assembles into a supratetrameric oligomer that can be disrupted by an allosteric inhibitor. J Biol Chem. 2013;288(39):28009–28020. doi: 10.1074/jbc.M113.501346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meerbrey KL, Hu G, Kessler JD, et al. The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proc Natl Acad Sci USA. 2011;108(9):3665–3670. doi: 10.1073/pnas.1019736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varum S, Rodrigues AS, Moura MB, et al. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One. 2011;6(6):e20914. doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross MI, Demo SD, Dennison JB, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13(4):890–901. doi: 10.1158/1535-7163.MCT-13-0870. [DOI] [PubMed] [Google Scholar]
- 25.Saland E, Boutzen H, Castellano R, et al. A robust and rapid xenograft model to assess efficacy of chemotherapeutic agents for human acute myeloid leukemia. Blood Cancer J. 2015;5:e297. doi: 10.1038/bcj.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehár J, Krueger AS, Avery W, et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat Biotechnol. 2009;27(7):659–666. doi: 10.1038/nbt.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson MM, McBryant SJ, Tsukamoto T, et al. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES). Biochem J. 2007;406(3):407–414. doi: 10.1042/BJ20070039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan R, Hogdal LJ, Benito JM, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4(3):362–375. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123(9):3678–3684. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136(3):521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141(2):290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wellen KE, Lu C, Mancuso A, et al. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 2010;24(24):2784–2799. doi: 10.1101/gad.1985910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Heuvel APJ, Jing J, Wooster RF, Bachman KE. Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer Biol Ther. 2012;13(12):1185–1194. doi: 10.4161/cbt.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takubo K, Nagamatsu G, Kobayashi CI, et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013;12(1):49–61. doi: 10.1016/j.stem.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y-H, Israelsen WJ, Lee D, et al. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell. 2014;158(6):1309–1323. doi: 10.1016/j.cell.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oburoglu L, Tardito S, Fritz V, et al. Glucose and glutamine metabolism regulate human hematopoietic stem cell lineage specification. Cell Stem Cell. 2014;15(2):169–184. doi: 10.1016/j.stem.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Masson J, Darmon M, Conjard A, et al. Mice lacking brain/kidney phosphate-activated glutaminase have impaired glutamatergic synaptic transmission, altered breathing, disorganized goal-directed behavior and die shortly after birth. J Neurosci. 2006;26(17):4660–4671. doi: 10.1523/JNEUROSCI.4241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konopleva M, Watt J, Contractor R, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax). Cancer Res. 2008;68(9):3413–3420. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]