Key Points
The novel IFNα-2b, ropeginterferon alfa-2b, administered once every 2 weeks has low toxicity and induces high and sustained response rates in polycythemia vera patients.
Ropeginterferon alfa-2b induces significant partial and complete molecular response rates, as reflected by reduction of JAK2 allelic burden.
Abstract
In this prospective, open-label, multicenter phase 1/2 dose escalation study, we used a next-generation, mono-pegylated interferon (IFN) α-2b isoform, ropeginterferon alfa-2b. The unique feature of ropeginterferon alfa-2b is a longer elimination half-life, which allows administration every 2 weeks. We present data from 51 polycythemia vera patients. The main goal was to define the maximum tolerated dose and to assess safety and efficacy. A dose range of 50 to 540 µg was tested without the appearance of dose-limiting toxicities. All drug-related adverse events were known toxicities associated with IFN-α. The cumulative overall response rate was 90%, comprising complete response in 47% and partial response in 43% of patients; the best individual molecular response level was a complete response in 21% of patients and partial response in 47%. Notably, we did not observe any correlation between the dose level and the response rate or response duration, suggesting that already low levels of ropeginterferon alfa-2b are sufficient to induce significant hematologic and molecular responses. These data suggest promising efficacy and safety of ropeginterferon alfa-2b and support the development of the drug in a randomized phase 3 clinical trial. The study was disclosed at www.clinicaltrials.gov as #NCT01193699 before including the first patient.
Introduction
Polycythemia vera (PV) is a clonal myeloproliferative neoplasm (MPN) characterized by an increased red blood cell mass (hematocrit) as well as an increase in platelet and/or leukocyte counts. PV is associated with a risk of hemorrhage and thrombosis, and a long-term propensity to develop myelofibrosis and acute leukemia. Since 1985, observational, nonrandomized studies have provided convincing evidence that interferon α (IFN-α) treatment of patients with MPNs including PV can effectively normalize blood cell counts and prevent disease-related thromboembolic complications.1-4 Since the discovery of the gain-of-function mutation V617F of the JAK2 gene,5-8 which is detectable in >95% of PV patients, monitoring of JAK2 V617F allelic load emerged as a suitable tool to demonstrate the anticlonal activity of IFN-α therapy.9-12 This nonleukemogenic compound is therefore considered one of the most promising therapeutic tools for long-term treatment in PV, and is also used to treat other MPNs including essential thrombocythemia (ET) and early stages of primary myelofibrosis.13
The clinical use of IFN-α has mainly been limited because of poor tolerance in patients exposed to daily injections of the compound. To improve tolerability, pegylated (PEG) forms of IFN-α have been developed that can be administered once per week. Two PEG-IFN-α formulations are currently on the market in Europe and the United States; however, neither is approved for therapy of MPNs. Several phase 1 and 2 studies with PEG-IFN-α-2a and PEG-IFN-α-2b have been performed in patients with MPNs, some of which also included PV patients.14-21 All studies reported efficacy in hematologic response and/or JAK2 mutant burden upon weekly administration.9,12,14-21 However, there are no published data from comparative phase 3 clinical trials thus far, and no consensus on the optimum dose, dosing schedule, and duration of therapy has been reached. Particularly, there is no consensus on whether IFN-α treatment in PV should be initiated at low doses or more aggressively with higher doses to promote more rapid disease eradication, while accepting lower tolerability. An additional consideration for drug development is the optimal treatment duration, which is currently assumed to be lifelong. Indeed, drug tolerability and safety-related patient compliance are expected to be of the utmost importance for disease control but are also critically determined by the tolerability profile of a compound.13,22-24 Thus, a PEG interferon with a superior safety profile would represent a qualitatively novel therapeutic option for patients with PV.
Ropeginterferon alfa-2b is an innovative next-generation mono-pegylated IFN-α-2b. It is made from proline-IFN-α-2b (P1101) produced in Escherichia coli transformed with a plasmid-encoding human IFN-α fused with methionine and proline at the amino-terminus. The amino-terminal methionine of the translated product is cleaved by endogenous methionine aminopeptidase to produce the proline-IFN-α-2b polypeptide. The proline residue is then site-specifically pegylated to produce ropeginterferon alfa-2b (ie, AOP2014), which consists of only one isoform. Ropeginterferon alfa-2b has shown high tolerability and a longer elimination half-life compared with conventional PEG-IFN-α with fortnightly administration in healthy volunteers and in preclinical trials (unpublished results). It was assigned orphan status for the treatment of PV by the Food and Drug Administration and European Medicines Agency. We performed a first in-patient, multicenter phase 1/2 clinical study of ropeginterferon alfa-2b in PV to determine safety, efficacy, and tolerability and to define the optimal dosing schedule to be tested in a phase 3 clinical trial.
Methods
Study design
This phase 1/2 trial is a multicenter, open, dose-escalation study with 3 PV patients per cohort (total 25 patients) to determine maximum tolerated dose (MTD) (3 + 3 dose-escalation design). Briefly, 3 patients were treated with the lowest dose and observed for toxicity (see end points later) over 2 weeks. The dose was increased gradually for subsequent 3-patient cohorts in mutual agreement with the participating investigators depending on the safety signals observed in the previous cohort. After the MTD was determined, a cohort of 26 newly recruited patients was enrolled for the phase 2 part of the study to further explore efficacy and safety of ropeginterferon alfa-2b under primarily efficacy-based dosing rules. Long-term treatment is being performed (the study continues) using an intrapatient dose-escalation scheme to individual tolerable levels. The study was approved by the institutional review board of the Vienna Medical University and by the Austrian Drug Agency. lnformed consent was obtained from all patients in accordance with the Declaration of Helsinki. The study was disclosed at www.clinicaltrials.gov as #NCT01193699 before including the first patient.
Inclusion/exclusion criteria
Patients ≥18 years with confirmed diagnosis of PV according to either the World Health Organization criteria (2008) or the Polycythemia Vera Study Group criteria with mandatory JAK2 positivity. Newly diagnosed and pretreated and currently hydroxyurea (HU)-exposed PV patients from both high-risk and low-risk groups who had troubles in controlling hematocrit (Hct) with phlebotomies or because of other symptoms were declared eligible. Patients diagnosed with any other myeloproliferative disorder, previous treatment with IFN-α for PV, concurrent treatment with cytoreductive agents other than HU, and investigational agents of any type, and also any severe concomitant comorbidity, including autoimmune diseases, were excluded.
Treatment schedule and dosing
Ropeginterferon alfa-2b was provided in vials, each containing 0.18 mg of the investigational product in 1 mL saline solution, to be administered subcutaneously every 14 days. Dose levels of 50 µg, 100 µg, 150 µg, 225 µg, 300 µg, 360 µg, 450 µg, and 540 µg were explored. The starting dose in phase 1 was 50 µg, based on preclinical toxicology data. Intrapatient dose escalation was allowed throughout the entire study, but the dose administered to the patients could not exceed the highest dose under the exploration (before defining the MTD) or the MTD. After the MTD was defined, ropeginterferon alfa-2b dosing was adjusted based primarily on efficacy and long-term tolerability. The mean administered dose in the study was 263 µg (±104) every 14 days.
Patient evaluations
Visits were scheduled every 2 weeks (ie, 1 cycle) and involved documentation of adverse events, full scale hematology, blood biochemistry investigations, coagulation, and thyroid function tests and drug administration by the site staff. Serious adverse events (SAEs) were defined as: an undesirable sign, symptom, or medical condition that results in death; is life-threatening; requires inpatient hospitalization or prolongation of existing hospitalization; results in persistent or significant disability or incapacity; is a congenital anomaly or birth defect; or is medically significant (ie, jeopardizes the health of the patient and may require medical or surgical intervention to prevent one of the preceding outcomes). Autoimmunity tests,24 spleen sonography, blood tests to detect potential anti-IFN antibodies and JAK2 V617F mutation allelic burden assessment (detection limit 1%, see supplemental Methods, found on the Blood Web site) were performed every 8 weeks.
End points and statistical analysis
The end point for phase 1 was defining the MTD, which was the highest dose tested of ropeginterferon alfa-2b, at which no more than 1 of 6 patients experienced dose-limiting toxicity (DLT).25 The first assessment was done at week 10 after first drug application and then every 12 weeks. For phase 2, the end points of interest were hematologic and molecular response. For the hematologic response, complete response (CR) was defined using the modified European Leukemia Net criteria.26 All patients with available data up to the cutoff date of April 16, 2014 were included in the analysis (see supplemental Methods).
Results
The analysis was undertaken upon completion of the 1-year follow-up of the last patient enrolled in the study.
Patient characteristics
In total, 51 patients were treated with ropeginterferon alfa-2b in the study: 25 enrolled in the phase 1 study (eight 3-patient cohorts, one replacement because of an ischemic, non–drug-related event in the first cycle), and an additional 26 patients in the phase 2 dose extension period. Main baseline characteristics of the included patients are presented in Table 1. Median age at study entry was 56 years (range, 35-82), with 31 (61%) male participants. Thirty-one patients (61%) had splenomegaly defined as >12 cm by sonographic assessment, with a median spleen size of 13.1 cm (range, 8-22). Three months before study entry, 31 patients (61%) had undergone phlebotomies, with a median number of 2 phlebotomies in the 3 months before screening (ranging from 1-8). Seventeen patients (33%) were being treated with HU at the screening visit. Major cardiovascular events in the medical history included 6 cases of pulmonary embolism, 2 cerebrovascular accidents and 2 myocardial infarction cases, and a case of a portal vein thrombosis. The median follow-up duration reported in this study is 80 weeks (range, 4-174), and 38 (75%) patients were treated for 50 weeks or more, therefore being evaluable for response at week 50. Of 13 discontinued patients, 4 patients discontinued before the first assessment visit (week 10). The reasons for discontinuation were enrollment with violation of eligibility criteria (1 patient), administrative reasons (1 patient), adverse event (ie, sweating, fatigue, asthenia [1 patient]), and lack of efficacy (1 patient). Numbers of patients discontinued after the first assessment visit and reasons for discontinuation are as follows: 2 at week 18 (1 patient because of adverse event “deterioration of general condition” and 1 patient because of withdrawal of consent), 2 at week 24 (because of adverse event “depression” and “elevated thyroid antibody titers”), 3 at week 34 (2 patients because of adverse event “rheumatoid arthritis” and “depression” and 1 patient because of withdrawal of consent), 1 at week 42 (because of adverse event “ubiquitous hyperkeratosis” and “elevated antinuclear antibody titers”), and 1 at week 50 (because of adverse event “rheumatoid arthritis”), respectively.
Table 1.
Baseline characteristics
| Parameter | Value |
|---|---|
| Safety population, patients (all treated) | 51 |
| Age at study entry (y), median (min-max) | 56 (35-82) |
| Male, n (%) | 31 (61%) |
| Splenomegaly (length >12 cm on sonograph), patients (%) | 31 (61%) |
| Spleen length on sonograph, median in cm (min-max) | 13.1 (8.0-22.0) |
| Patients with phlebotomies in 3 mo before screening, n (%) | 31 (61%) |
| Number of phlebotomies in 3 mo before screening, median (range) | 2 (1-8) |
| PV history before entry (mo), median (Q1-Q3) | 17.0 (3.6-68.8) |
| Major cardiovascular events in the med history, patients (%) | 11 (22%) |
| HU pretreated, patients (%) | 17 (33%) |
| Hct, %, median (min-max) | 44.8 (36.9-53.8) |
| Platelets, G/L, median (min-max) | 429 (148-1016) |
| WBC, G/L, median (min-max) | 11.1 (4.7-30.9) |
| JAK2 V617F–positive | 100% |
| %V617F allelic burden, median (min-max) | 41% (2-100) |
Hct, hematocrit; WBC, white blood cells.
Phase 1: defining MTD
The phase 1 study was designed to define the MTD. Eight dose levels (from 50 µg up to 540 µg) administered every 2 weeks were explored in 25 PV patients over a 13-month period using a 3 + 3 dose-escalation design (see “Methods”). No DLT was observed, and the MTD was defined as the highest dose evaluated (ie, 540 µg).
Phase 2: Hematologic response to ropeginterferon alfa-2b
To evaluate optimal dosing and efficacy, 26 PV patients were recruited in addition to the 25 patients from phase 1. Again, ropeginterferon alfa-2b was administered once every 2 weeks, but dosing was based on efficacy rather than tolerability.
At the screening visit, 70% of patients needed phlebotomies, and 33% were being treated with HU, which was halted once ropeginterferon alfa-2b was administered. Administration of ropeginterferon alfa-2b stabilized Hct levels, and by 12 months, 28 (74%) of the 38 patients evaluated had Hct <45%, which is considered low enough to decrease the risk of thrombosis, and were phlebotomy free. Platelet and white blood cell counts decreased substantially after 10 weeks of treatment—at month 12 after the start of therapy, 34 (89%) patients had platelets <400 G/L and 35 (92%) patients had leukocytes <10 G/L. The values remained stabilized to the end of the observation period. Although patients had only mildly enlarged spleens at baseline, spleen size still tended to decrease over time (change from median at baseline 13.1 cm to 12.8 cm at month 12).
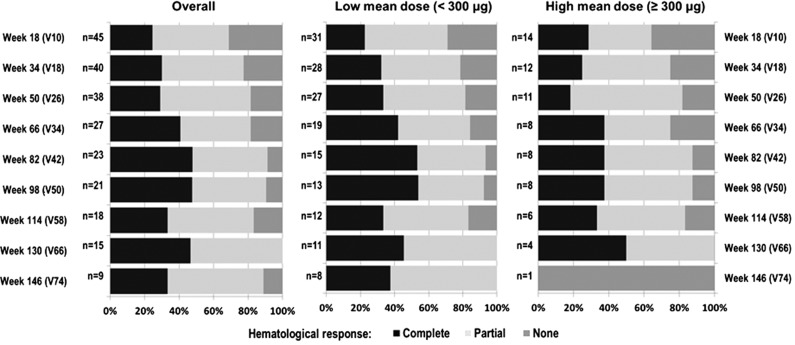
At week 10 (n = 47), the overall hematologic response was 75% (n = 35), with a CR rate of 26% (n = 12). At 1 year (n = 38), the overall response (OR) rate increased to 82% (n = 31), with a CR rate of 29% (n = 11) (Figure 1). The best individual response over the course of the evaluation period was 90% (ie, 46/51 enrolled patients: 47% CR, 43% partial response [PR]). One patient was nonresponding (NR), and 4 patients discontinued the study before the first assessment visit at week 10.
Figure 1.
Hematologic response over time in the study.
Phase 2: association of hematologic response and dose of ropeginterferon alfa-2b
Because of the wide dosing range (from 50 µg up to 540 µg), the relationship between dose and hematologic treatment response could also be analyzed. All treated patients were retrospectively divided into 2 groups based on their mean dose. In the group of patients who received low mean dose <300 µg (n = 37) vs high mean dose ≥300 µg (n = 14), 16 (43%) vs 8 (57%) patients achieved CR, and 16 (43%) vs 6 (43%) patients achieved PR as the best individual response over the course of the study. For the low-mean-dose group, CR steadily increased over the treatment duration. Response rates of patients in the higher dose group tended to be higher early after treatment initiation but did not show any further improvement (Figure 1). However, no significant association between dosing and response rates could be identified.
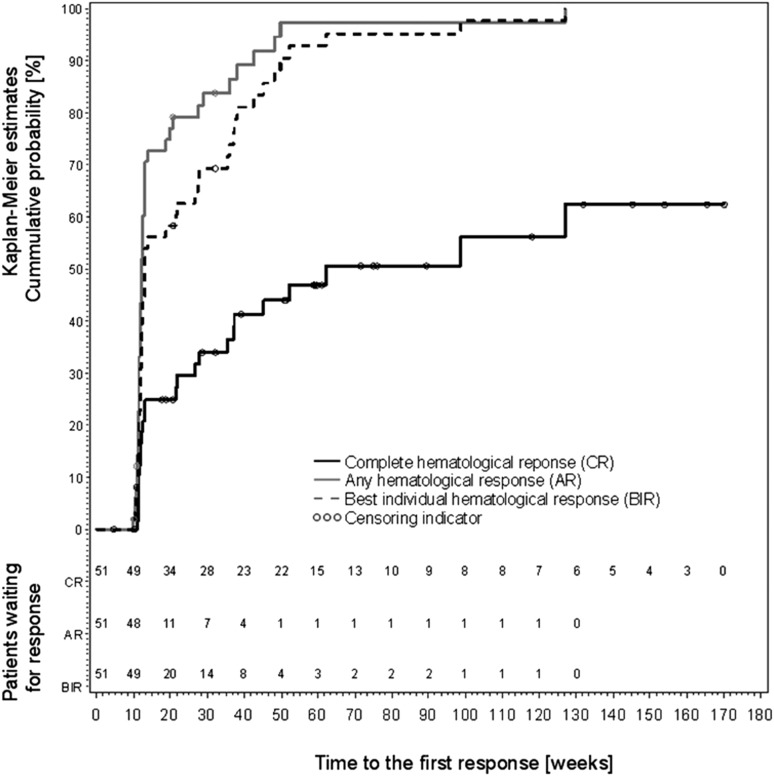
Most of the patients achieved their responses, in particular the best individual responses, before week 50. There was only one patient who responded after week 50, and only 3 patients achieved best individual response after week 50. Similarly, CRs were mainly achieved at 1 year, and only 6 PRs switched to CR in the later period of their study participation (Figure 2).
Figure 2.
Kaplan-Meier estimates of complete hematologic response, any response, and best individual hematologic response in the study (according to ELN 2009, see “Methods”).
Figure 1 shows efficacy data as proportions of CR, PR, and NR at individual assessment visits starting at week 18 of treatment. To evaluate kinetics of disease response regardless of maintenance in subsequent visits, Kaplan-Meier estimates of cumulative probability are presented in Figure 2. This figure displays the time and cumulative probability of the first response (CR, any response either CR or PR, best individual response). These data indicate that the first response is mostly achieved within 50 to 60 weeks after treatment start. At that time, cumulative probability is ∼45% (Figure 2) and the proportion of CR is between 25% and 40% (Figure 1), implying that there is some proportion of patients who do not continuously maintain CR. Figure 1 shows that proportion of NR patients decreased over time. Therefore, if CR was not maintained, the patients mostly changed their CR to PR. The median duration of maintaining the best individual response was 32 weeks (range, 8-64). In the group of patients with low vs high mean doses, the duration of response was 24 weeks (range, 4-64) and 36 weeks (range, 8-72), respectively. There was no significant association between the best treatment response and the mean dose (n = 51; P = .228), and we could not detect any correlation between the mean dose and the duration of best individual hematologic response (n = 46; r = 0.07; P = .645).
Phase 2: molecular response to ropeginterferon alfa-2b
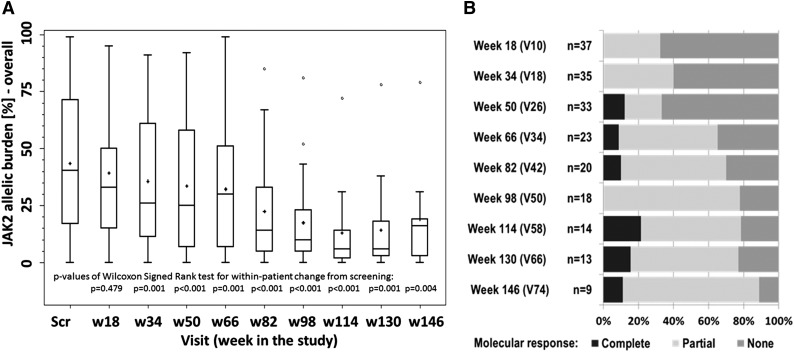
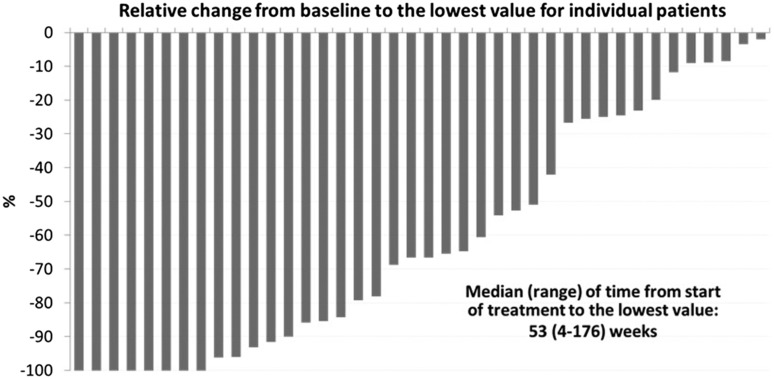
At study entry, the median JAK2 V617F allelic burden was 41% (range, 2%-100%), which was significantly reduced to 35% (range, 0%-95%) by week 18 of treatment (P = .479), and to 25% (range, 0%-92%) by week 50 (P < .001) (Figure 3A). A significant linear reduction of allelic burden over time could be shown, because each month allelic burden was reduced by 1.2% (95% confidence interval [CI], from −1.7 to −0.8; P < .001). Only 5 (10%) patients had an increase of their JAK2 allelic burden, which was <10% vs baseline, whereas in 24 (47%) patients, the decrease of allelic load was >20% (Figure 4).
Figure 3.
JAK2 molecular response in the study. (A) JAK2 allelic burden in the whole study population. (B) Molecular responses (rates) in patients with at least 20% JAK2 allelic burden at baseline.
Figure 4.
JAK2 allelic burden for each patient (baseline allelic burden ≥20%), comparing the best individual response with baseline.
The cumulative molecular response rate (mCR+mPR) at week 50 (n = 33) and at week 114 (n = 14) was 33% (12% CR, 21% PR) and 78% (21% CR, 57% PR), respectively (Figure 3B). The best individual molecular response was a CR in 21% of patients and a PR in 47%; of all 43 patients with baseline JAK2 V617F allelic burden of at least 20%, 10 patients (23%) did not respond and 4 patients withdrew their informed consent before the first study evaluation. Overall, 29 of 43 patients (67%) in this cohort achieved a molecular response. Duration of PV history before study entry was the most significant factor associated with slower allelic burden reduction under treatment.
Analysis of the 12-month data showed trends indicating that molecular responses were associated with the administered ropeginterferon alfa-2b dose as well as previous HU treatment. For mean dose increase of 100 µg, the odds ratio for improvement in molecular response was 1.8 (95% CI, 1.1-3.0; P = .028). Patients previously treated with HU had a lower probability of achieving better molecular response than treatment-naïve patients (OR = 0.4; 95% CI, 0.1-1.5; P = .167]).
Phase 2: association between hematologic and molecular response to ropeginterferon alfa-2b
A significant association between hematologic response and molecular response was found. Patients with complete hematologic response had a significantly higher likelihood of achieving a molecular response (OR = 2.8; 95% CI, 1.6-4.7; P < .001) compared with patients with a partial hematologic response. Similarly, patients with partial hematologic response were more likely to achieve molecular responses (OR = 1.5; 95% CI, 0.8-2.7; P = .216) than hematologic NR. Furthermore, the association between JAK2 V617F allelic burden and hematologic response (as a categorical variable) was also significant: complete hematological response was associated with 16% lower levels of JAK2 V617F allelic burden (95% CI, 5.5-25.5, P = .002) compared with NR.
Trends of positive correlation of higher JAK2 V617F allelic burden with increased spleen size at study entry (P = .100), higher platelet counts (P = .016), and increased leukocytes (P = .055) were observed. Moreover, bigger spleens (P = .033) and higher platelet counts (P = .002) were significantly independent predictors for better molecular responses during the study.
Disease-associated clinical complications
One transient ischemic attack in the first treatment cycle and a case of deep vein thrombosis (occurring also shortly after inclusion into the study) were observed. Apart from these events, there were no major, clinically durable cardiovascular events documented in the study.
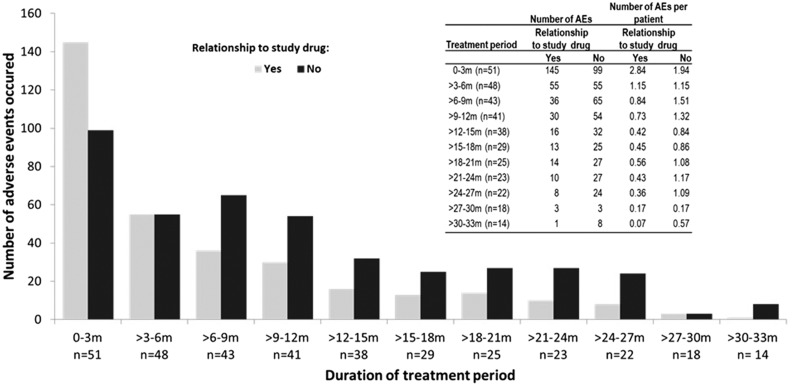
Adverse events
In total, 744 adverse events (defined according to the National Cancer Institute Common Toxicity Criteria AEv3.0) were noted, among which 330 were rated as drug-related. These events were reported in 45 of the 51 patients (88%). Ten patients (20%) permanently discontinued study drug treatment because of adverse events (AEs). The highest number of AEs was observed during the first 3 months of therapy and declined over time (Figure 5; this trend was also observed after adjusting per exposed patient). The following AEs, including disease-related and study drug–unrelated events, were observed in >20% of the patients: pruritus, arthralgia, fatigue, headache, diarrhea, influenzalike illness, and vertigo. In >10% of the patients, the following AEs were observed: nasopharyngitis and rhinitis, nausea, decreased appetite, pyrexia, myalgia, alopecia, chills, deterioration of general physical health, injection site reactions, leukopenia, thrombocytopenia, increase of γ-glutamyltransferase, pain in extremity, hyperhidrosis, and night sweats.
Figure 5.
Adverse events occurrence over time, shown separately for drug-related AEs (according to investigators) and adjusted for the number of patients exposed per period.
In 16 patients (31%), psychiatric AEs including acute stress disorder, aggression, apathy, depression, depressive symptoms, hallucination, insomnia, listlessness, altered mood, nervousness, sleep disorder, stress, and anxiety were observed. However, treatment had to be permanently discontinued because of psychiatric symptoms in only 2 patients (4%), and the majority of these symptoms (70%) were of mild intensity. In 77% of the patients, these symptoms completely resolved during the study. This observation is in line with the known safety product profile of the interferons and has also been observed by the group of Kiladjian et al20 (eg, at month six in 35 patients, 18 neuropsychiatric and asthenia events were observed). Clinically, we observed a relation of appearance of the psychiatric events with higher dose levels, but because of the small sample size, no statistical significance could be shown for this observation.
Autoimmune thyroiditis, the most frequent autoimmune side effect of IFN-α,24 was observed in 2 patients, and latent hyperthyroidism in 1 additional patient. Elevated antithyroid antibodies (anti-TG or anti-TGO) were documented in 4 additional patients; thyroid-stimulating hormone increase was reported once. Treatment had to be permanently discontinued in 2 patients (4%) with thyroid AEs. In some of these patients, preexisting antithyroid antibodies or history of past thyroiditis may have contributed to exacerbation of their conditions during the study. In 3 patients (one showing moderately elevated antinuclear antibody titer, one associated with skin rash), positive antinuclear antibodies were observed, which led to permanent discontinuation in 1 case (2%). Two cases of rheumatoid arthritis were observed in the study.
All of the drug-related AEs are known toxicities associated with IFN-α, and unless they led to discontinuation, were manageable with dose adjustment or additional supportive therapeutics.
There was no difference in mean dose for patients with vs patients without SAEs (252 µg vs 254 µg, respectively). Similarly, no correlation between mean dose and total number of AEs (r [Spearman] = –0.099; P = .489) or number of drug-related AEs (r [Spearman] = –0.081; P = .574) was seen.
Discussion
The present study shows that ropeginterferon alfa-2b is a safe, effective, and well-tolerated novel IFN-α formulation when administered every 2 weeks. No dose-limiting toxicities occurred within the dose range from 50 to 540 µg.
Ropeginterferon alfa-2b elicited similar response rates compared with other studies performed in PV patients receiving a weekly dosing schedule of PEG α-interferons. In these trials, complete hematologic response rates were 50% after a median treatment time of 24 months (weekly dosing),19 69% (including patients with ET) after a median treatment time of 24 months (weekly dosing),18 70% after a median treatment time of 21 months (weekly dosing),12 and 94.6% after a median treatment time of 12 months (biweekly dosing)20 compared with 47% after a median treatment time of 18.5 months in our study (biweekly dosing). Complete molecular response rates were reported in 6%12 and 24%20 compared with 21% in our study. Given the extended treatment durations that are required to achieve molecular responses, we hypothesize that ropeginterferon alfa-2b translates into a better tolerated and therefore more effective long-term therapy.
The data provide a safety corridor for the dose range to be tested in further studies using ropeginterferon alfa-2b in MPN. It allows for individual dosing regimens according to a personalized medicine approach in accordance with tolerability and efficacy. Previous HU treatment and duration of disease did not influence efficacy of ropeginterferon alfa-2b, measured as hematologic response during study. In contrast, the likelihood of achieving molecular response was lower in patients with a longer history of PV before study entry and in patients previously treated with HU. The chances of having molecular response increased in patients treated with higher doses of ropeginterferon alfa-2b. Duration of the observation period of 2 weeks was selected based on usual practices for such type of studies,14,17 without intention to interpret safety observations isolated from the further administration in the context of potentially long-term interferon use. After completion of phase 1, dose finding was based on the general safety assessment of the patient. In addition, the lack of DLT observation in the first MTD relevant cycle was predictive for the future mid- and long-term tolerability of the study drug ropeginterferon alfa-2b.
For the phase 2 efficacy study, the population included the patients from phase 1 whose initial dosage was determined by the toxicity-driven 3+3 dose-escalation design. However, during phase 2, all patients were dosed based on disease response and tolerability, combining both subgroups to be evaluated as one efficacy cohort. Because a substantial part of the patient cohort was treated with higher doses of ropeginterferon alfa-2b (toxicity-driven phase 1 part of the study, where dose increase had to be performed even in patients who responded, assuming good tolerability), the overall safety picture may not be fully representative for further efficacy-driven studies with ropeginterferon alfa-2b.
Analysis of the high- vs low-dose groups presented in the paper should be interpreted with caution; being a post hoc analysis, which is mainly based on the response-driven dose rules to maintain optimal response with acceptable toxicity, the data indicate the existence of a subgroup of well-tolerating patients that can be successfully treated with higher doses of ropeginterferon alfa-2b. The observed trend shows a slower rise of responses in low-dose–treated patients, yet slightly better maintenance of responses. The worsening of response seen at higher doses may also be caused by an attempt to increase the dose in patients whose disease failed to respond (and a subsequent inability to achieve a response by further dose increases).
The decrease of JAK2 allelic burden confirms the unique ability of α-interferons to work selectively on the malignant clone of the disease,12,20 while requiring it be balanced with medically significant drug-induced autoimmunity and psychiatric AEs.
In summary, ropeginterferon alfa-2b has been shown to be safe in this study in the administered dose range and exhibits profound efficacy, observed as normalization of blood parameters and reduction of JAK2 allelic burden. Because this enables a more convenient treatment schedule accompanied by low toxicity and high hematologic and molecular response rates, this study supports progression to phase 3 trials.
Acknowledgments
H. Pickersgill provided drafts and editorial assistance to the authors during preparation of this manuscript.
This study was supported by research funding from AOP Orphan to the authors’ institutions (except those of O.Z. and C.K.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.G. designed the study, performed the study, recruited patients, analyzed data, and wrote the paper; O.Z. designed the study, contributed analytical tools, analyzed data, and wrote the paper; R.K., V.B.-A., J.T., E.S., G.A.G., D.W., K.S., A.E., T.M., S.B., E.W., and R.G. performed the study, recruited patients, and contributed to the manuscript; and B.G., N.C.T., P.K., C.K., and M.S. contributed vital analytical tools, analyzed the data, and contributed to the manuscript.
Conflict-of-interest disclosure: O.Z. and C.K. are employees of the sponsor AOP Orphan Pharmaceuticals. H.G. acted as scientific advisor and is on the speakers honorary list of AOP Orphan Pharmaceuticals, Novartis, and Celgene.
Correspondence: Heinz Gisslinger, Department of Hematology and Blood Coagulation, Medical University of Vienna, Währinger Gürtel 18-20, 1090 Vienna, Austria; e-mail: heinz.gisslinger@meduniwien.ac.at.
References
- 1.Linkesch W, Gisslinger H, Ludwig H, Flener R, Sinzinger H. [Therapy with interferon (recombinant IFN-alpha-2C) in myeloproliferative diseases with severe thrombocytoses]. Acta Med Austriaca. 1985;12(5):123–127. [PubMed] [Google Scholar]
- 2.Gisslinger H, Ludwig H, Linkesch W, Chott A, Fritz E, Radaszkiewicz T. Long-term interferon therapy for thrombocytosis in myeloproliferative diseases. Lancet. 1989;1(8639):634–637. doi: 10.1016/s0140-6736(89)92142-9. [DOI] [PubMed] [Google Scholar]
- 3.Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer. 2006;107(3):451–458. doi: 10.1002/cncr.22026. [DOI] [PubMed] [Google Scholar]
- 4.Heis N, Rintelen C, Gisslinger B, Knöbl P, Lechner K, Gisslinger H. The effect of interferon alpha on myeloproliferation and vascular complications in polycythemia vera. Eur J Haematol. 1999;62(1):27–31. doi: 10.1111/j.1600-0609.1999.tb01110.x. [DOI] [PubMed] [Google Scholar]
- 5.Baxter EJ, Scott LM, Campbell PJ, et al. Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 6.James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 7.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 8.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Kiladjian JJ, Cassinat B, Turlure P, et al. High molecular response rate of polycythemia vera patients treated with pegylated interferon alpha-2a. Blood. 2006;108(6):2037–2040. doi: 10.1182/blood-2006-03-009860. [DOI] [PubMed] [Google Scholar]
- 10.Kiladjian JJ. Hematology Am Soc Hematol Educ Prog. 2012. The spectrum of JAK2-positive myeloproliferative neoplasms. pp. 561–566. [DOI] [PubMed] [Google Scholar]
- 11.Stein BL, Tiu RV. Biological rationale and clinical use of interferon in the classical BCR-ABL-negative myeloproliferative neoplasms. J Interferon Cytokine Res. 2013;33(4):145–153. doi: 10.1089/jir.2012.0120. [DOI] [PubMed] [Google Scholar]
- 12.Quintás-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009;27(32):5418–5424. doi: 10.1200/JCO.2009.23.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasselbalch HC. A new era for IFN-α in the treatment of Philadelphia-negative chronic myeloproliferative neoplasms. Expert Rev Hematol. 2011;4(6):637–655. doi: 10.1586/ehm.11.63. [DOI] [PubMed] [Google Scholar]
- 14.Talpaz M, O’Brien S, Rose E, et al. Phase 1 study of polyethylene glycol formulation of interferon alpha-2B (Schering 54031) in Philadelphia chromosome-positive chronic myelogenous leukemia. Blood. 2001;98(6):1708–1713. doi: 10.1182/blood.v98.6.1708. [DOI] [PubMed] [Google Scholar]
- 15.Alvarado Y, Cortes J, Verstovsek S, et al. Pilot study of pegylated interferon-alpha 2b in patients with essential thrombocythemia. Cancer Chemother Pharmacol. 2003;51(1):81–86. doi: 10.1007/s00280-002-0533-4. [DOI] [PubMed] [Google Scholar]
- 16.Langer C, Lengfelder E, Thiele J, et al. Pegylated interferon for the treatment of high risk essential thrombocythemia: results of a phase II study. Haematologica. 2005;90(10):1333–1338. [PubMed] [Google Scholar]
- 17.Talpaz M, Rakhit A, Rittweger K, et al. Phase I evaluation of a 40-kDa branched-chain long-acting pegylated IFN-alpha-2a with and without cytarabine in patients with chronic myelogenous leukemia. Clin Cancer Res. 2005;11(17):6247–6255. doi: 10.1158/1078-0432.CCR-05-0882. [DOI] [PubMed] [Google Scholar]
- 18.Samuelsson J, Hasselbalch H, Bruserud O, et al. Nordic Study Group for Myeloproliferative Disorders. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biologic effects, and impact on quality of life. Cancer. 2006;106(11):2397–2405. doi: 10.1002/cncr.21900. [DOI] [PubMed] [Google Scholar]
- 19.Jabbour E, Kantarjian H, Cortes J, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: final result of a phase 2 study. Cancer. 2007;110(9):2012–2018. doi: 10.1002/cncr.23018. [DOI] [PubMed] [Google Scholar]
- 20.Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112(8):3065–3072. doi: 10.1182/blood-2008-03-143537. [DOI] [PubMed] [Google Scholar]
- 21.Quintás-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon α-2a. Blood. 2013;122(6):893–901. doi: 10.1182/blood-2012-07-442012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiladjian JJ, Mesa RA, Hoffman R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood. 2011;117(18):4706–4715. doi: 10.1182/blood-2010-08-258772. [DOI] [PubMed] [Google Scholar]
- 23.Silver RT, Kiladjian JJ, Hasselbalch HC. Interferon and the treatment of polycythemia vera, essential thrombocythemia and myelofibrosis. Expert Rev Hematol. 2013;6(1):49–58. doi: 10.1586/ehm.12.69. [DOI] [PubMed] [Google Scholar]
- 24.Gisslinger H, Gilly B, Woloszczuk W, Mayr WR, Havelec L, Linkesch W, et al. Thyroid autoimmunity and hypothyroidism during long-term treatment with recombinant interferon-alpha. Clin Exp Immunol. 1992;90(3):363–367. doi: 10.1111/j.1365-2249.1992.tb05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crowley JC, Ankerst DP, editors. Handbook of statistics in clinical oncology. 2nd ed. Boca Raton: Chapman & Hall/CRC Taylor & Francis Group; 2006. [Google Scholar]
- 26.Barosi G, Birgegard G, Finazzi G, et al. Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood. 2009;113(20):4829–4833. doi: 10.1182/blood-2008-09-176818. [DOI] [PubMed] [Google Scholar]