Abstract
Introduction
Stage iii lung cancer is the most advanced stage of lung cancer for which radical (potentially curative) treatment is often discussed. Understanding the reasons for mortality and subsequent treatments in patients with stage iii non-small-cell lung cancer (nsclc) is important.
Methods
This retrospective cohort study extracted demographic, clinical, treatment, and outcomes data for patients with newly diagnosed stage iii nsclc diagnosed between 1 January 2008 and 31 December 2012 at a single institution.
Results
The study included 237 patients with stage iii nsclc, 130 of whom were not treated with radical or curative intent (55%). Median survival in the entire cohort was 14 months from diagnosis. For patients treated with radical-intent therapy, causes of death varied with the time period. The hazard rate for death was approximately 2.8 per 100 person–months of follow-up over the entire disease course and was highest between 6 months and 24 months. Over the entire time period, local causes accounted for 29% of deaths; systemic non–central nervous system metastases, for 25%; and brain metastases, for 14%. For patients treated with palliative intent, the overwhelming cause of death was local disease complications or progression (56% of deaths). Only 12% of patients in the palliative treatment group who progressed received subsequent chemotherapy; 23% of patients in the radical group who progressed received palliative chemotherapy. The most frequent subsequent treatment in both groups was radiation therapy.
Conclusions
The eventual life-ending event in stage iii nsclc varied for the patients who qualified for, and were treated with, radical or curative intent and for the patients who received palliative-intent therapy. Utilization of systemic chemotherapy in patients not fit for radical therapy is low.
Keywords: Chemotherapy, combined-modality therapy, chemoradiation, radiation, palliation, locally advanced disease
INTRODUCTION
In non-small-cell lung cancer (nsclc), stage iii disease represents the most advanced stage at which the possibility of cure is discussed, and it represents approximately 20%–25% of newly diagnosed cases1,2. Standard treatment options vary considerably throughout the world and include bi- or tri-modality therapy: surgery followed by chemotherapy, chemotherapy concurrent with radiation therapy, neoadjuvant concurrent chemotherapy and radiation therapy followed by surgery, and neoadjuvant chemotherapy alone followed by surgery2.
Since the early 2000s, clinical trials of strategies to improve outcomes in stage iii nsclc have failed to make a difference in overall survival. The new strategies have included the addition of surgery to bimodality therapy3,4, the addition of radiation to chemotherapy and surgery5, dose-escalation of radiation therapy6, the addition of prophylactic cranial radiation to standard treatment7, the addition of consolidation chemotherapy to chemoradiation8, and altered-fractionation radiotherapy with or without chemotherapy9.
Although all guidelines recognize bimodality therapy of some form as standard treatment for stage iii nsclc2,10,11, those guidelines are based on clinical trials that typically included patients with a relatively good performance status, minor weight loss, younger age, and fewer comorbidities than are seen in the general lung cancer patient population. The reasons for recommending palliative radiation therapy in stage iii nsclc patients with significant weight loss or borderline performance status include the potential for an increased risk of toxicity and a poorer prognosis overall.
To improve survival for all patients with stage iii nsclc, the current situation—including treatment patterns and actual causes of death for patients treated with both palliative and radical intent—has to be examined.
Causes of death in patients treated for stage iii nsclc include both pulmonary issues (progressive local cancer, lung toxicity from therapy, infectious complications and obstruction, and comorbid lung diseases) and nonpulmonary issues (including brain and non-brain distant metastases). Death can also occur from comorbid non-pulmonary conditions (often tobacco-related) and from cancer-associated paraneoplastic conditions such as pulmonary emboli and hypercalcemia. Trying to understand the ultimate cause of death and the risk over time for various causes of death could help to direct survival improvement efforts.
A review of patients diagnosed with stage iii nsclc and registered at an academic cancer centre in southeastern Ontario set out to answer two questions for patients initially treated with radical intent or palliative intent:
□ What are the causes of death and how do they vary during over time?
□ What are the subsequent treatments upon recurrence or progression?
METHODS
Cohort Definition
Patients were identified through the cancer centre database and included all patients with stage iii lung cancer diagnosed between 1 January 2008 and 31 December 2012. Patients were excluded if they had been treated predominantly elsewhere (that is, clinical data were not available) or if they had histologies other than nsclc.
Electronic charts were individually reviewed, and patients were excluded if, at diagnosis, they had stage iv lung cancer by the current American Joint Committee on Cancer staging system (7th edition)1 or if subsequent testing revealed that their disease was in fact metastatic disease from a non-lung primary. Patients were also excluded if they were initially classified as stage iii, but revealed by subsequent testing to be clearly stage i or ii.
Clinical and Treatment Characteristics
Diagnosis date, age, and sex were extracted from the clinical database. Histology, T stage, N stage, radiation treatment received, and chemotherapy treatment received were obtained from the cancer centre database and cross-checked with the clinical notes and medication administration record.
Date of death and last date known to be alive were extracted from the clinical database, supplemented by online obituary records and telephone contact with primary care. Survival was calculated from the date of pathologic confirmation to death or censoring.
Palliative radiation treatments included those given intentionally in the “low-dose” range, such as 20 Gy or less in 1 to 5 fractions, or in the “intermediate-dose” range, such as 30–39 Gy in 10 or more fractions. Radical-intent radiation regimens included those intended to deliver either 60 Gy or more, or 46 Gy or more in a neoadjuvant or adjuvant fashion combined with chemotherapy.
Data on weight loss and performance status were extracted from the consult notes made by the radiation oncologist, medical oncologist, respirologist, or thoracic surgeon. Data for weight loss were divided into less than 5%, 5%–10%, more than 10%, or unknown. If the absolute value of weight loss was recorded, the percentage was calculated directly. Eastern Cooperative Oncology Group performance status12 was also recorded. If the patient’s performance status was given as a range (2–3, for instance), the higher performance status (that is, 3) was recorded.
Smoking status was also reviewed from the medical record and was classified as never or light (<2 pack–year history), former (>2 pack-years, but quit more than 6 months earlier), and current or recent (>2 pack–years, and either still smoking or quit less than 6 months earlier).
Determination of Cause of Death
Probable cause of death was extracted from the medical records. For patients who died in hospital, discharge records and notes proximal to death were used. Patients were classified as “death from respiratory disease” if they died from a progressive local tumour, lung collapse, post-obstructive pneumonia, or respiratory failure without documentation of systemic metastatic disease. “Death from progressive brain metastases” occurred if the patient was diagnosed with brain metastases before death and had either absent or responding systemic disease. “Death from systemic progressive disease” occurred if metastatic disease was documented before the patient’s death. Initially, comorbid respiratory illness (patients with responsive primary disease, but death from respiratory failure) was recorded as a separate cause of death from local progression. However, it became clear that, although this cause was clearly a non-malignancy-related respiratory failure for a handful of patients, there was diagnostic uncertainty in most patients. Thus, respiratory failure deaths with and without disease progression had to be combined. “Unknown cause of death” was attributed to patients who died more than 30 days after last being seen at the cancer centre or hospital, to those for whom no new or progressive disease was documented, or to those for whom a cause could not be allocated.
For palliative patients, time periods were divided into less than 3 months, 3–9 months, 9–18 months, and more than 18 months from diagnosis. For radical patients, time periods were divided into less than 6 months, 6–12 months, 12–24 months, and more than 24 months from diagnosis.
Statistics are presented as proportions. The Kaplan– Meier method was used to estimate median survival. Hazard rates per 100 person–months of follow-up were calculated for each time period, and the proportions of deaths of each type are reported in each time period. Multivariate analysis of causes of death was not performed because the intention of the present analysis was not to determine the factors that predicted given outcomes, but to show the types of deaths in each group of patients. The significantly higher rate of deaths from local causes in patients treated with palliative-intent radiation could be a result of multiple factors, including a larger tumour, more weight loss, poorer performance status, and the radiation itself. Because of the high amount of (expected) collinearity between the factors of weight loss, tumour size, and performance status, associations with outcomes could be misleading and are not reported.
The statistical analysis was performed using the R software application (version 3.0.1: The R Foundation, Vienna, Austria). The study was approved by the research ethics board at Queen’s University, Kingston, Ontario.
RESULTS
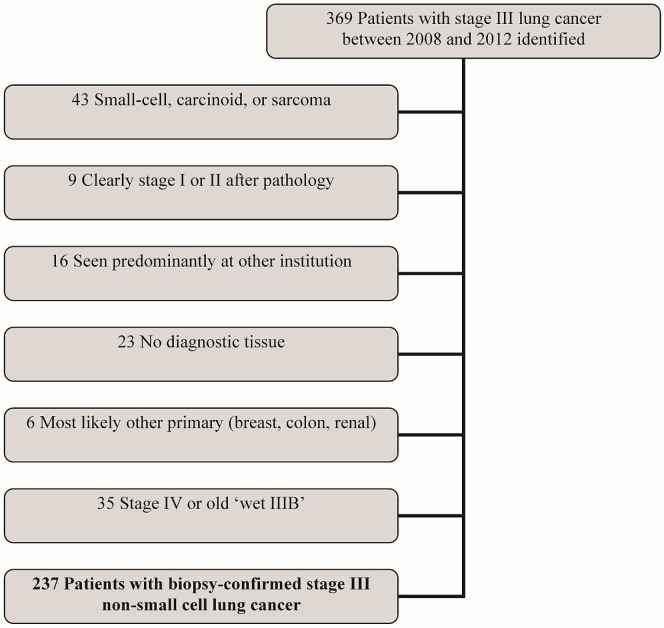
Of 369 patients with stage iii lung cancer extracted from the clinical database, 43 were excluded because of a non-nsclc diagnosis (carcinoid, small-cell, hemangiosarcoma); 9, because of downstaging; 16, because of treatment taking place mainly at an outside centre; 23, because of lack of tissue confirmation; 6, because of a non-lung primary; and 35, because of upstaging. Of the remaining 237 patients with biopsy-confirmed clinical or pathologic stage iii nsclc, 130 (55%) received palliative treatments, and 107 received radical-intent therapy (Figure 1).
FIGURE 1.
Selection of the study cohort.
Patient Characteristics
Table i presents the patient and treatment characteristics for the entire cohort. Median age was significantly higher for patients who received palliative therapy, as were the percentages of those who had a poor performance status, a T4 tumour, squamous cell histology, and weight loss.
TABLE I.
Characteristics of patients with stage III non-small-cell lung cancer, 2009–2013, at time of oncology assessment
| Characteristic | Patient group | p Value | ||
|---|---|---|---|---|
|
| ||||
| Overall | By treatment intent | |||
|
| ||||
| Palliative | Radical | |||
| Patients (n) | 237 | 130 | 107 | |
| Median age (years) | 68 | 72 | 64 | 0.001 |
| Sex [n (%)] | 0.06 | |||
| Women | 119 (50) | 70 (54) | 49 (46) | |
| Men | 118 (50) | 60 (46) | 58 (54) | |
| Smoking status (n) | 1.0 | |||
| Never | 7 | 4 | 3 | |
| Former | 111 | 61 | 50 | |
| Current | 116 | 62 | 54 | |
| Unknown | 3 | 3 | 0 | |
| Performance status (n) | 0.006 | |||
| 0–1 | 157 | 65 | 92 | |
| 2 | 42 | 33 | 9 | |
| 3–4 | 27 | 27 | 0 | |
| Unknown | 11 | 5 | 6 | |
| Weight loss (n) | 0.18 | |||
| <5% | 132 | 52 | 80 | |
| 5%–10% | 35 | 24 | 11 | |
| >10% | 59 | 45 | 14 | |
| Tumour histology (n) | 0.001 | |||
| Squamous | 103 | 72 | 31 | |
| Adenocarcinoma | 60 | 25 | 35 | |
| Not otherwise specified | 74 | 33 | 41 | |
| T Stage (n) | 0.001 | |||
| Tx | 11 | 10 | 1 | |
| T1,T2 | 66 | 25 | 41 | |
| T3 | 54 | 25 | 29 | |
| T4 | 106 | 70 | 36 | |
| N Stage (n) | 0.12 | |||
| Nx | 18 | 15 | 3 | |
| N0 | 26 | 13 | 13 | |
| N1 | 17 | 10 | 7 | |
| N2 | 149 | 75 | 74 | |
| N3 | 27 | 17 | 10 | |
Outcomes and Causes of Death for Radically-Treated Patients
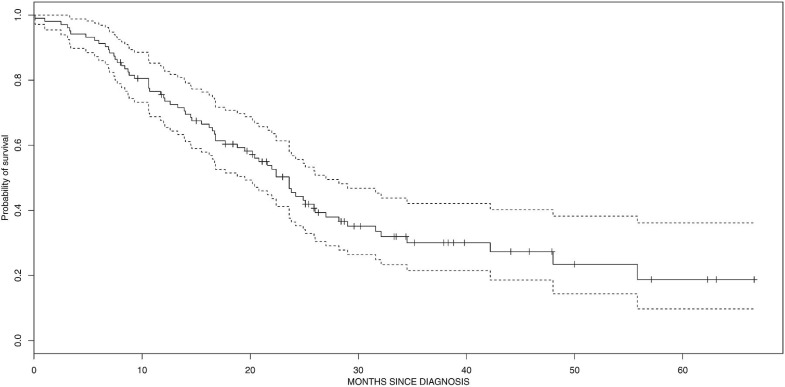
Median survival for the overall cohort was 14 months. It was 22.5 months for patients treated with radical-intent radiation (Figure 2).
FIGURE 2.
Kaplan–Meier probability of survival in radically-treated patients with stage III non-small-cell lung cancer.
Table ii shows causes of death by time period. The proportion of deaths attributable to systemic disease peaked between 12 and 24 months, with 46% of deaths being a result of non–central nervous system systemic disease, and 11%, a result of central nervous system metastases. Local issues accounted for a larger proportion of deaths in the 6- to 12-month and 24- to 60-month periods.
TABLE II.
Deaths from various causes in patients treated with curative intent
| Time from diagnosis | Deaths per 100 patient–months | Proportion of deaths (%) attributable to ... | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Local respiratory issuesa | Complications of treatment | CNS metastatic disease | Non-malignant cardiac cause | Non-CNS metastatic disease | Unknown | ||
| 0–6 Months | 1.3 | 15 | 38 | 23 | 0 | 0 | 23 |
| 6–12 Months | 3.8 | 37 | 0 | 16 | 11 | 5 | 32 |
| 12–24 Months | 3.7 | 19 | 0 | 11 | 46 | 8 | 16 |
| 24–60 Months | 2.5 | 44 | 0 | 8 | 20 | 4 | 20 |
| TOTAL | 2.8 | 29 | 4 | 14 | 25 | 7 | 22 |
Includes local progression and relapse.
CNS = central nervous system.
Outcomes and Causes of Death for Palliatively-Treated Patients
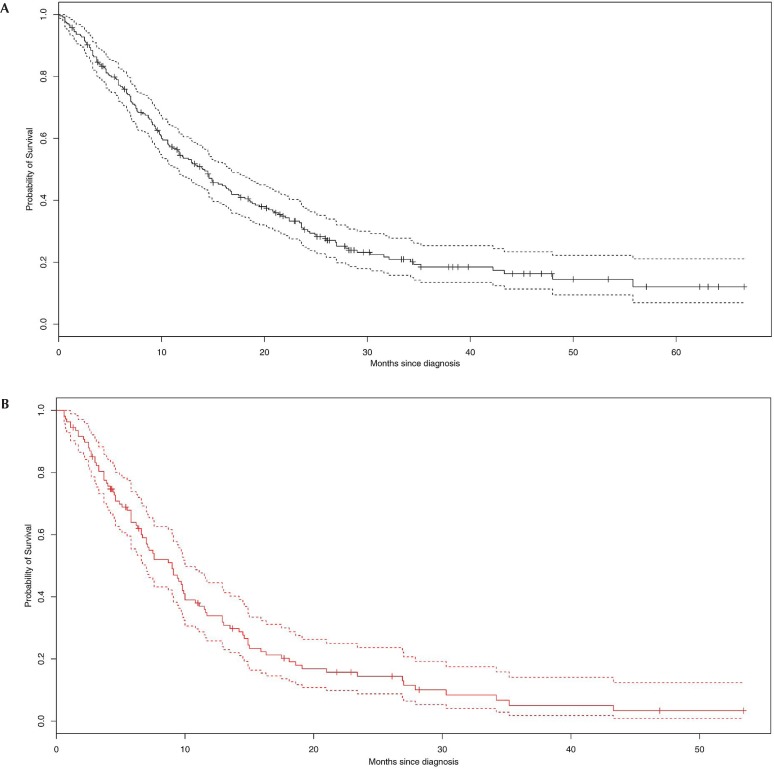
From the time of tissue diagnosis, median survival for patients treated with palliative-intent radiation was 9 months (Figure 3). The causes of death were overwhelmingly secondary to respiratory failure, and the deaths occurred in the early time periods(Table iii).
FIGURE 3.
Kaplan–Meier probabilities for survival (A) in all patients and (B) in palliatively-treated patients with stage III non-small-cell lung cancer.
TABLE III.
Deaths from various causes in patients treated with palliative intent
| Time from diagnosis | Deaths per 100 patient–months | Proportion of deaths (%) attributable to ... | |||
|---|---|---|---|---|---|
|
| |||||
| Local respiratory issuesa | CNS or non-CNS metastatic disease | Thrombosis or ischemia | Unknown | ||
| 0–3 Months | 7.6 | 83 | 8 | 0 | 8 |
| 3–9 Months | 10 | 59 | 28 | 13 | 0 |
| 9–18 Months | 11.4 | 47 | 38 | 12 | 4 |
| >18 Months | 9.1 | 35 | 40 | 10 | 15 |
| TOTAL | 9.5 | 56 | 29 | 9.5 | 5 |
Includes local progression and relapse.
CNS = central nervous system.
Subsequent Therapy in Radically-Treated Patients
Among the patients who received radical—potentially curative—therapy, 53 developed a recurrence during the observation period. Of those patients, 41 subsequently died, and 12 received subsequent palliative chemotherapy (9, a platinum doublet; 2, erlotinib; 1, docetaxel) in the first line after curative treatment. Two additional lines of therapy were given in 4 patients, including pemetrexed in 4, docetaxel in 1, and erlotinib in 1; and 2 patients received three additional lines of chemotherapy.
In total, 23% of the patients who received initial radical treatment and developed a recurrence received subsequent chemotherapy. Subsequent radiation therapy was administered in 23 patients who recurred after high-dose radical therapy (44% of those who recurred). Two or more subsequent radiation treatments were given in 7 patients. Cranial radiation was given in 16 patients; only 4 received subsequent lung radiation. Spine, femur, and other bony sites were also frequently irradiated. The likelihood of receiving subsequent radiation therapy was almost twice as high as the likelihood of receiving subsequent systemic therapy, largely because of central nervous system metastases and bony pain.
Subsequent Therapy in Palliatively-Treated Patients
Of the patients who were initially treated palliatively, 16 (12%) received palliative platinum doublet chemotherapy. Of those 16 patients, only 5 (4%) received more than 2 cycles. One patient received second-line docetaxel, and 7 patients received a tyrosine kinase inhibitor (1 in the first line).
Of the 110 patients who died, only 12 (11%) received doublet chemotherapy after their initial presentation. Numbers were too small to report predictors of systemic anticancer chemotherapy use.
Reirradiation to the ipsilateral lung occurred in 20% of patients who initially received low-dose radiation (n = 18); it was given in 5% of those who received intermediate-dose radiation (1 of 20). Of the 89 patients who received low-dose palliative radiation, 79 died, with 47 deaths being attributed to local disease or respiratory failure; 16 patients were retreated. Of the 24 patients initially treated with intermediate-dose palliative radiation, 18 died (6 apparently of local causes).
DISCUSSION
Main Findings
The main findings of the present study are that patterns of failure and causes of death in stage iii nsclc appear to vary substantially by time period. Local progression, obstruction, and respiratory failure contributed a great deal to morbidity and mortality in the patients treated with palliative-intent therapy to the lungs. Those local issues are significantly associated with early risk of death. The actual hazard rates for death from distant metastatic disease and brain metastases in the palliatively-treated group were similar to those in the radically-treated group, but the hazard rate for death from local complications and progression was much higher in the palliatively-treated group. In other words, far fewer patients in the palliatively-treated group than in the radically treated group died of metastatic brain and systemic cancer—largely because those in the palliatively-treated group had a much higher rate of death from local causes, presumably before death from distant disease could occur.
The lower contribution of distant metastasis to death (compared with historical series) and the favourable median survival of 22 months for radically-treated patients (compared with historical durations) could partly be a consequence of staging by combined positron-emission tomography and computed tomography, leading to stage migration.
In terms of therapies after initial treatment, radiation was reused significantly more often than systemic therapy was. The most common reasons for the difference were the use of radiation to treat brain or bone metastases in the radically-treated patients and to treat local progression or symptoms (pain, hemoptysis, shortness of breath) in the palliatively-treated patients. The frequent need for subsequent palliative radiotherapy highlights the need for multidisciplinary follow-up for this patient population.
Previous work has focused on sites of first recurrence for stage iii nsclc patients treated using various modalities. In 2014, Feng et al.13 published recurrence data for patients with N2 disease treated with surgery alone, without postoperative radiation. Of 250 patients, 173 relapsed, and the sites of first relapse were locoregional only in 29, locoregional and distant in 25, and distant in 119. This surgically treated population was younger in age, with a lower T stage and a higher percentage of adenocarcinoma.
Most recently, a detailed analysis of the International Adjuvant Lung Cancer Trial revealed that, in early lung cancer, roughly 20% of deaths resulted from non-lung-cancer causes, 15% from brain metastases, 3% from brain and local recurrence, 17% from local recurrence, 15% from local and distant non-brain recurrence, and 29% from distant non-brain metastases14. Although the patient population was different, the contribution of a number of different causes of death is evident.
In general, the causes of death in the present study are consistent with previous findings, although our study is the first to examine the patterns of progression and ultimate cause of death in patients deemed suitable for palliative treatment only. It is not surprising that patients who are not suitable for radical therapy often succumb to respiratory causes of death. The patients are “not suitable” often because of very large tumours, poor performance status, significant weight loss, and poor lung function. One frequently cited reason for a palliative approach to these patients is that they have a higher rate of death from metastatic disease. An equally plausible explanation for the poor outcomes in these patients receiving conventional therapy is not that they have subclinical metastatic disease causing their weight loss and poor performance status, but that they have aggressive local disease.
The Radiation Therapy Oncology Group 0214 trial7, which compared brain radiation with no brain radiation, showed how difficult it is to adequately power a trial to isolate one mode of death only. The trial was initially powered to show a reduction in the risk of death by 20% at 1 year and would have enrolled more than 1000 patients. In such a cohort, it would be impossible, given the competing hazards of death from other causes, to reduce the risk of death by 20% at any time, even if all brain metastases were prevented.
It is difficult to know how the high number of deaths from local causes (particularly among patients who received palliative low-dose radiation alone) can be improved. The recent meta-analysis by Fairchild and colleagues15 compared higher-dose with lower-dose radiation, showing an improvement with the higher dose at the expense of toxicity. The American Society for Radiation Oncology guidelines recently recommended higher-dose palliation in patients with a good performance status and the willingness to accept higher short-term toxicities16. The commentary accompanying the Fairchild analysis noted that changes in systemic therapy could change the risk–benefit analysis for short- compared with long-course palliation in such patients and questioned whether radiation as a single modality had run its course. Given the experience at our institution during 2008–2012, it would appear that, in practice, radiotherapy is much more likely to be the only palliative therapy delivered (only a small number of patients received systemic chemotherapy)17.
For patients treated with palliative intent, the high frequency of death from local progression suggests that significant gains might be possible with improvements in local control alone. Approaches could include optimizing local therapies and—possibly—using systemic therapies earlier in the course of treatment. It was difficult to elucidate the reasons that only 12% of patients received palliative doublet chemotherapy, but anecdotal reasons included patient refusal, patient comorbidity, and performance status. Although the numbers are quite small, it is also notable that fewer than half the patients who received doublet chemotherapy received more than 2 cycles. Determination of subsequent systemic treatment patterns and outcomes on a population level for palliatively irradiated patients is needed to validate our findings. Whether systemic therapy initiated earlier in the palliative care of these patients will help to delay local complications leading to death is unclear, but worthy of investigation. Given that the cause of death in palliatively irradiated stage iii patients appears overwhelming to be local disease or respiratory failure, the efficacy of early systemic interventions (such as immunotherapy) for local control should be evaluated.
Limitations of the present study include the number of patients with an unknown cause of death, which was significant in the sample, accounting for 22% of deaths in the radically-treated cohort and 5% of deaths in the palliatively-treated cohort, making firm conclusions difficult. Some reasons for the lack of knowledge about causes of death included patients being lost to follow-up and death after a “normal” checkup, which was a fairly common occurrence. A further limitation is the inability to retrospectively dissect local causes of death to determine iatrogenic causes (such as radiation pneumonitis or fibrosis), infection, and local progression. Even prospectively, such a determination is difficult18. Our inability to undertake a multivariate analysis because of the small sample size is a limitation, but does not affect the conclusions.
CONCLUSIONS
Patients with stage iii nsclc die of a variety of causes, with the differences between those treated with palliative intent and those treated with curative intent being significant. Optimizing treatment of local disease in palliative patients might improve survival. In contrast, for patients treated with curative-intent combination therapy, causes of death are distributed in such a fashion that tests of interventions that might improve outcomes for any single cause of death (brain metastasis, for instance) will require an extremely large sample to show statistically improved outcomes for survival as a composite. In contrast, clinical trials of multiple or single interventions expected to affect death from various causes—local, systemic, brain, and paraneoplastic—might be beneficial.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Groome PA, Bolejack V, Crowley JJ, et al. on behalf of the iaslc International Staging Committee The iaslc Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 2.Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. on behalf of the esmo Guidelines Working Group Early and locally advanced non-small-cell lung cancer (nsclc): esmo Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi89–98. doi: 10.1093/annonc/mdt241. [DOI] [PubMed] [Google Scholar]
- 3.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage iii non-small-cell lung cancer: a phase iii randomised controlled trial. Lancet. 2009;374:379–86. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Meerbeeck JP, Kramer GW, Van Schil PE, et al. on behalf of the European Organisation for Research and Treatment of Cancer–Lung Cancer Group Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage iiia-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99:442–50. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 5.Thomas M, Rübe C, Hoffknecht P, et al. on behalf of the German Lung Cancer Cooperative Group Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage iii non-small-cell lung cancer. Lancet Oncol. 2008;9:636–48. doi: 10.1016/S1470-2045(08)70156-6. [DOI] [PubMed] [Google Scholar]
- 6.Cox JD. Are the results of rtog 0617 mysterious? Int J Radiat Oncol Biol Phys. 2012;82:1042–4. doi: 10.1016/j.ijrobp.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Gore EM, Bae K, Wong SJ, et al. Phase iii comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of radiation therapy oncology group study rtog 0214. J Clin Oncol. 2011;29:272–8. doi: 10.1200/JCO.2010.29.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsujino K, Kurata T, Yamamoto S, et al. Is consolidation chemotherapy after concurrent chemo-radiotherapy beneficial for patients with locally advanced non-small-cell lung cancer? A pooled analysis of the literature. J Thorac Oncol. 2013;8:1181–9. doi: 10.1097/JTO.0b013e3182988348. [DOI] [PubMed] [Google Scholar]
- 9.Belani CP, Wang W, Johnson DH, et al. on behalf of the Eastern Cooperative Oncology Group Phase iii study of the Eastern Cooperative Oncology Group (ecog 2597): induction chemotherapy followed by either standard thoracic radiotherapy or hyperfractionated accelerated radiotherapy for patients with unresectable stage iiia and B non-small-cell lung cancer. J Clin Oncol. 2005;23:3760–7. doi: 10.1200/JCO.2005.09.108. [DOI] [PubMed] [Google Scholar]
- 10.Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage iii non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl):e314S–40S. doi: 10.1378/chest.12-2360. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger DS, Akerley W, Bepler G, et al. on behalf of the nccn Non-Small Cell Lung Cancer Panel Members Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8:740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 12.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Feng W, Fu XL, Cai XW, et al. Patterns of local-regional failure in completely resected stage iiia(N2) non-small cell lung cancer cases: implications for postoperative radiation therapy clinical target volume design. Int J Radiat Oncol Biol Phys. 2014;88:1100–7. doi: 10.1016/j.ijrobp.2013.12.048. [DOI] [PubMed] [Google Scholar]
- 14.Rotolo F, Dunant A, Le Chevalier T, Pignon JP, Arriagada R, on behalf of the ialt Collaborative Group Adjuvant cisplatin-based chemotherapy in non-small-cell lung cancer: new insights into the effect on failure type via a multistate approach. Ann Oncol. 2014;25:2162–6. doi: 10.1093/annonc/mdu442. [DOI] [PubMed] [Google Scholar]
- 15.Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26:4001–11. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: an American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1:60–71. doi: 10.1016/j.prro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner H., Jr Just enough palliation: radiation dose and outcome in patients with non-small-cell lung cancer. J Clin Oncol. 2008;26:3920–2. doi: 10.1200/JCO.2008.17.3674. [DOI] [PubMed] [Google Scholar]
- 18.Kocak Z, Evans ES, Zhou SM, et al. Challenges in defining radiation pneumonitis in patients with lung cancer. Int J Radiat Oncol Biol Phys. 2005;62:635–8. doi: 10.1016/j.ijrobp.2004.12.023. [DOI] [PubMed] [Google Scholar]