Abstract
Background
We documented changes in practice from 2009 to 2012 for cervical cancer brachytherapy in Canada.
Methods
Centres with gynecologic brachytherapy services were sent an e-mail questionnaire querying their 2012 practice. Responses are reported and compared with practice patterns identified in a similar survey for 2009.
Results
The response rate was 77% (24 of 31 centres). Almost all use high-dose-rate brachytherapy (92%); low-dose-rate brachytherapy has been completely phased out. Most continue to move patients from the site of applicator insertion to the radiation treatment simulation suite (75%) or to a diagnostic imaging department (29%), or both. In 2012, the imaging modalities used for dose specification were computed tomography [ct (75%)], magnetic resonance imaging [mri (38%)], plain radiography (21%), and cone-beam ct (8%). The number of institutions using mri guidance has markedly increased during the period of interest (9 vs. 1). Most respondents (58% vs. 14%) prescribed using guidelines from the Groupe Européen de Curiethérapie and the European Society for Therapeutic Radiology and Oncology, but they also used point A as a reference. Commonly used high-dose radiation regimens included 30 Gy in 5 fractions and 24 Gy in 3 fractions.
Conclusions
In Canada, image-guided brachytherapy for cervical cancer continues to evolve. Although ct-based imaging remains the most commonly used modality, many centres have adopted mri for at least 1 brachytherapy treatment. More centres are using fewer fractions and a slightly lower biologically effective dose, but are still achieving EQD2 (2-Gy equivalent) doses of 80–90 Gy in combination with external-beam radiation therapy.
Keywords: Cervical cancer, brachytherapy, radiation oncology
INTRODUCTION
The move to three-dimensional (3D) image-guided brachytherapy (bt) in Canada has been evolving since the early 2000s. The impetus to move away from the Manchester two-dimensional (2D) planning system was initiated by two international consensus working groups. In 2004, the Image-Guided Brachytherapy Working Group of the American Brachytherapy Society (abs) published their guidelines for image-based bt for cervical cancer for North America1.In 2005, at a joint consensus meeting, the abs and the Groupe Européen de Curiethérapie, with the European Society for Therapeutic Radiology and Oncology (gec-estro), decided to adopt the guidelines from gec-estro as their common template for implementation of 3D image-based planning2,3. However, adoption of the guidelines in Canada was slow until a major manufacturer of bt applicators and afterloaders announced discontinuation of service for low-dose-rate (ldr) afterloaders after 31 December 2009. As a result, many Canadian centres changed to a high-dose-rate (hdr) or pulsed-dose-rate (pdr) system4. By 2009, 78% of Canadian centres had phased out the ldr system. However, 50% of centres continued to use traditional 2D planning5.
To help centres considering a transition, the gec-estro group have now supplemented their recommendations with a document outlining technical considerations for 3D planning6, and the abs have updated their guidelines for volume-based planning of locally advanced carcinoma of the cervix7–9.
In Canada, 3D image-based bt for cervical cancer was the subject of a workshop at the Canadian Association of Radiation Oncology Annual Meeting in 2007. The workshop focused on the rationale for using imaging for 3D planning in cervical cancer bt and on 3D planning techniques. Previous Canadian patterns-of-practice surveys in 20095 and 201010 documented the change in practice to 3D-based planning, but transitions to 3D planning continue in many centres across Canada. Here, we report the use of, and future plans for, image-guided bt for cervical cancer and the bt dose and fractionation regimens commonly used by Canadian cancer centres in 2012.
METHODS
Canadian radiation oncology centres treating gynecologic malignancies were identified from the official Web site of the Canadian Association of Radiation Oncology (http://www.caro-acro.ca), from individual cancer centre directories, and by telephone queries to individual cancer centres. In December 2012, a 19-item questionnaire querying current practice in the use of imaging in bt planning and plans for transition to 3D image-guidance for bt in cervical cancer was created electronically using SurveyMonkey (Palo Alto, CA, U.S.A.) and sent to one representative radiation oncologist from each of the centres that treat gynecologic malignancies with bt. The questions focused on the individual’s practice of cervical cancer bt, including technology in use at their centre, dose and fractionation regimens, and plans for changes to 3D cervical cancer bt. Questions were specifically focused on the treatment of cervical cancer patients (intact cervix) using radical radiation therapy [external-beam radiation therapy (ebrt) and bt] with curative intent and did not pertain to patients treated in the postoperative setting. A reminder e-mail was sent to nonresponders in January 2013. Respondents could take part in the survey only once. Questionnaire responses were tabulated and analyzed by centre. Although respondents were not anonymized, results are reported without individual or site identifiers.
RESULTS
Centre Characteristics
Of 31 centres identified as treating gynecologic malignancies with bt in Canada, 24 replied, for a response rate of 77%. Of 10 Canadian provinces, 8 were represented in the responses. All respondents practiced in an academic medical centre or in a hospital-based practice with an academic affiliation.
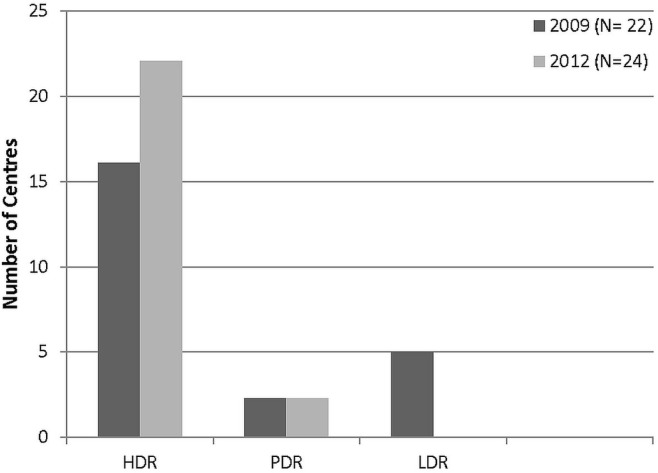
In 2012, respondents most commonly treated 10–20 patients (33%), followed by 20–30 patients (25%) and fewer than 10 patients (25%). Just 4 (17%) reported treating more than 30 patients in 2012. Figure 1 shows the bt modality used, by centre. Almost all used hdr bt (92% compared with 67% in 2009). Utilization of pdr was unchanged from 2009, and ldr bt had been phased out completely by the 5 centres that had been using it in 2009.
FIGURE 1.
Brachytherapy modalities in use. HDR = high dose rate; PDR = pulsed dose rate; LDR = low dose rate.
Imaging Modalities in Use
Imaging After Insertion
Some form of in-suite imaging was available in 42% of the surveyed cancer centres (10 of the 24). Nonetheless, most centres reported moving the patient out-of-suite for imaging, with 75% (18 of the 24) moving patients to the radiation therapy simulation suite and 29% (7 of the 22) transferring the patient to the diagnostic imaging department.
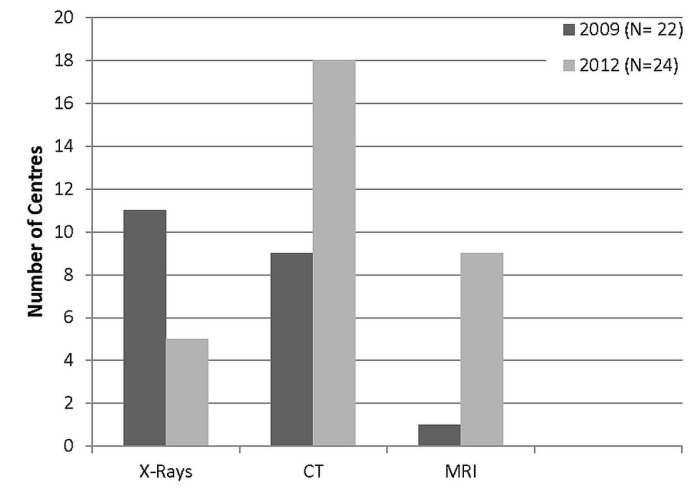
Figure 2 shows the imaging modalities used for bt planning. Use of plain radiography (orthogonal films) declined markedly, with fewer than half the 2012 respondent centres reporting use of that modality. A dramatic increase in 3D-based imaging for planning was observed: the use of computed tomography (ct) imaging almost doubled from 2009, and use of magnetic resonance imaging (mri) increased from a single centre in 2009 to 9 centres in 2012. Two centres (8%) reported using cone-beam ct.
FIGURE 2.
Imaging modalities in use for cervical brachytherapy planning. CT = computed tomography; MRI = magnetic resonance imaging.
Frequency of Post-Insertion Imaging
Of the centres using ct, 56% (10 of 18) acquired imaging with each insertion, including those using pdr, in which 1 insertion was performed. Two centres acquired a ct image only with the first insertion. When mri was used (9 centres), 8 of the centres obtained the image for only 1 insertion; ct planning was then used for the remaining fractions. Only 1 centre performed mri with each bt insertion. Of the 24 responding centres, 4 (17%) used 1 insertion to deliver more than one bt treatment.
Tumour and Normal-Tissue Contouring
In 2012, the uptake of volume-based definitions had increased compared with that in 2009. In 2012, 58% of centres (11 of 19) contoured the gross tumour volume at the time of the bt insertion (gtv bt). The high-risk clinical target volume (ctv) was delineated in 79% of centres (15 of 19); the intermediate-risk ctv was routinely contoured only in 21% (4 of 19). In 2009, of the respondents using ct for bt planning, 33% (5 of 15) contoured the gtv, 20% (3 of 15) contoured the high-risk ctv, none contoured the intermediate-risk ctv, and 44% (8 of 18) contoured none of those volumes. Per the earlier survey, contouring of the rectum and urinary bladder was performed by all respondents. In 2012, 95% of centres contoured the sigmoid colon as well (72% in 2009).
Dose Prescription and Reporting
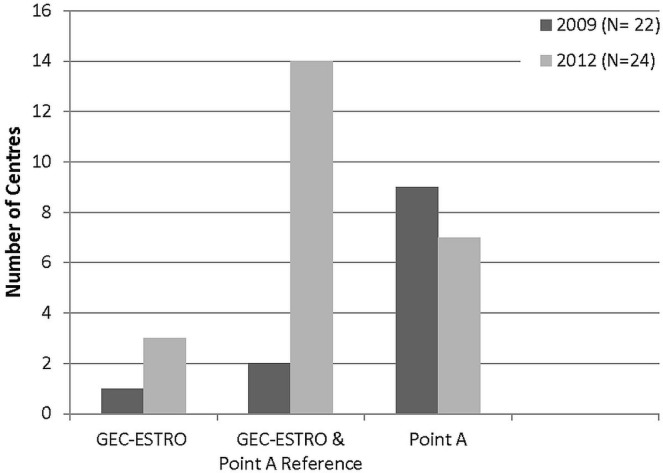
After publication of the gec-estro guidelines, a marked increase in centres using volume-based dose prescription (while still using point A as a reference) was observed [58% (14 of 24 centres) in 2012 vs. 9% (2 of 22 centres) in 2009]. Exclusive use of point A dose prescriptions declined to 29.2% (7 of 24 centres) in 2012 from 50% (9 of 18 centres) in the earlier survey. Three centres reported prescribing using the gec-estro guidelines exclusively (Figure 3).
FIGURE 3.
Dose prescription and reporting in use. GEC = Groupe Européen de Curiethérapie; ESTRO = European Society for Therapeutic Radiology and Oncology.
In 2012, the D90 and D100 were being more routinely reported for high-risk ctvs (54.2% in 2012, 13 of 24 centres, vs. 28% in 2009, 5 of 18 centres). Dose–volume histogram (dvh) parameters for the gtv and intermediate-risk ctv remained similar at 29.2% (7 of 24 centres) compared with 39% (7 of 18 centres) for the gtv and 12.5% (3 of 24 centres) compared with 11% (2 of 18 centres) for the intermediate-risk ctv. The 3 centres that used 3D planning did not define the D90 or D100 for any targets.
For organs at risk (oars), most respondents were reporting rectal, bladder, and sigmoid dose as the 3D dvh D2mL. For the same oars, the D1mL was reported in about 50% of centres (Table i). Nine centres continued to report the International Commission on Radiation Units and Measurements point dose for rectum and bladder, as before.
TABLE I.
Organs at risk reporting at the responding centres
| Organ | Dose to [% (n)] | ||||
|---|---|---|---|---|---|
|
| |||||
| 0.1 mL | 1 mL | 2 mL | 5 mL (wall) | 10 mL (wall) | |
| Rectum | 39 (7) | 56 (10) | 94 (17) | 6 (1) | 6 (1) |
| Bladder | 39 (7) | 56 (10) | 94 (17) | 6 (1) | 6 (1) |
| Sigmoid colon | 41 (7) | 53 (9) | 94 (16) | 6 (1) | 6 (1) |
All 24 Canadian centres prescribed 45 Gy in 25 fractions [equivalent dose in 2-Gy fractions (eqd2): 44.3 Gy] for ebrt of early-stage cervical cancer (stages ib and iia). Most also used that dose for advanced stages (iib, iii, iva), but some centres reported using 50.4 Gy in 28 fractions (eqd2: 49.6 Gy) for the whole pelvis. Parametrial boosts were also common.
In terms of bt dosing, 30 Gy in 5 fractions (eqd2: 40 Gy) was the most common regimen (6 centres), followed by 24 Gy in 3 fractions (eqd2: 36 Gy; 5 centres; Table ii). The bt doses were the same for both early- and advanced-stage disease.
TABLE II.
Brachytherapy dose and fractionation in use at the responding centres
| Dose | Fractions | EQD2 | Centres using (n) |
|---|---|---|---|
| 6 Gy | 5 | 40 Gy | 6 |
| 8 Gy | 3 | 36 Gy | 5 |
| 7 Gy | 4 | 39.7 Gy | 3 |
| 5.5 Gy | 5 | 35.5 Gy | 3 |
| 6.5 Gy | 4 | 35.8 Gy | 2 |
| 6 Gy | 3 | 24 Gy | 1 |
| 6 Gy | 4 | 32 Gy | 1 |
| 6.75 | 4 | 37.7 Gy | 1 |
| 35 Gya | — | 35 Gy | 2 |
Pulsed dose rate (60 cGy/pulse for 58 hourly pulses).
EQD2 = equivalent dose in 2-Gy fractions.
Plans to Transition to 3D Image-Based Planning
Only 1 of the 5 centres still using plain radiography at the time of the survey intended to continue with that type of planning. Preparations for transition to ct-based planning was under way in 3 centres, and 7 centres (currently using either ct or plain radiography planning) were preparing to switch to mri-based planning. Continuation of mri planning was expected at 8 centres, and 5 centres expected to continue with ct-based planning. Of the 10 centres intending a change in planning modality, 6 were expecting to transition within a 1- to 3-year window, 3 within 1 year, and 1 in more than 3 years. All centres that were undecided about transitioning or that were not intending to transition to 3D image-based bt planning cited budgetary reasons as a barrier. Technical reasons (43%) and lack of timely access to ct imaging or mri (14%) were also cited.
All 24 of the respondents strongly agreed that 3D image-based bt planning should be the standard of care for cervical cancer treatment in Canada.
DISCUSSION
The current survey updates the status of 3D image-based bt for cervical cancer in Canada and documents the changes in practice that have taken place since 2009. Since our first survey in 2006, a significant shift in bt practice for cervical cancer has occurred in Canada4,5,10. Instrumental changes—including a transition from ldr to hdr or pdr bt, uptake of 3D imaging-based treatment, and a move to 3D planning—constitute the new landscape of radiation treatment for patients with carcinoma of the cervix. Most centres (22 of 24) reported using hdr bt, 2 centres reported using pdr bt, and over 6 years, ldr bt had been completely phased out. This high uptake rate of hdr bt is comparable to bt practice both in the United States and internationally8,11,12. In an international survey by Viswanathan et al.13, 61 of 72 centres (85%) in Asia (Japan and Korea), Europe, Australia, New Zealand, and North America used hdr for treating carcinoma of the cervix.
Transition from 2D planning (orthogonal radiography) to 3D planning continues; in 2012, only 5 Canadian centres used orthogonal radiography for planning; most had adopted some form of 3D planning. Of centres responding to the current survey, 75% (18 of 24) reported using ct-based planning; in 2009, it had been 45%5. The use of mri for bt planning has also risen dramatically: In 2012, 9 centres were using mri for at least 1 bt insertion—in most cases, in conjunction with ct planning. Only 1 Canadian centre was exclusively using mri planning. Most centres incorporated mri planning by utilizing existing resources and infrastructure. Many were imaging after insertion by moving the patient to the ct simulator, or to the diagnostic imaging department, or both.
For centres using 3D imaging for planning, all respondents reported oar contouring for the rectum and bladder. The uptake of target volume contouring based on the guidelines of the gec-estro Working Group2,3 was a major change from 2009, when only a few centres were using volume-based planning. According to the guidelines for evaluation of complex dose heterogeneity, dvh parameters (that is, the D90 and D100, the minimum doses delivered to 90% and 100% of the volume) were to be reported for the gtv, the high-risk ctv, and the intermediate-risk ctv. The guidelines describe the V150 and V200 (the volumes enclosed by 150% and 200% of the prescribed dose) as essential for the overall assessment of high-dose volumes. Although the most important target volume concept in the gec-estro guidelines is the high-risk ctv, the challenge in defining that volume in the absence of mri was apparent in 2009. However, in 2012, with more centres having some access to mri, 79% were routinely reporting the high-risk ctv. Interestingly, some respondents reported contouring the gtv when using ct-based planning. The limitations of tumour delineation on ct have been well documented; however, use of that approach reflects the practice and limitations of centres that lack access to mri and could serve as a point for future dialog.
Adoption of the gec-estro guidelines for dose prescription is also gaining support. More than half the responding centres (n = 14) used volume-based dose prescription, but they were also still using point A as a reference, a marked increase from 2009. Exclusive use of point A dose prescriptions continued to decline as 2D planning was phased out; however, prescriptions that are exclusively volume-based were still used in a small number of centres. Both the abs and gec-estro recognize that many centres continue to record point A doses, but the goal should be to ensure adequate coverage of the high-risk ctv (that is, D90) to an eqd2 of 80–90 Gy2,3,7,9,13. In 2012, 23 Canadian centres reported prescribing eqd2 doses within that range, with variations in the bt and the ebrt doses being relatively uniform. Canadian centres did not vary their bt doses for early compared with advanced stages of cancer.
In terms of international practice, Viswanathan et al.13 reported that the most common hdr bt fractionation regimen was 6 Gy in 5 fractions (18%), which was also the most commonly reported regimen in the present survey. Other regimens used throughout the world include 6 Gy in 4 fractions (15%) and 7 Gy in 3 fractions (11%). Overall, the mean combined ebrt and bt eqd2 was 81 Gy. Analyzed by region, the mean combined eqd2 was 71.2 Gy in Asia, 81.18 Gy in Australia and New Zealand, 83.24 in Europe, and 81.66 Gy in North America11. The range of the combined eqd2 in the present survey was a mean of 81 Gy—that is, in line with international practice. In Canada, the ncic cxc.1/gog 0219 trial popularized the regimen of 6 Gy in 5 fractions, and that dose was also common in many centres in the United States. However, there has been concern about increased toxicity using that fractionation, given its combined eqd2 of 84.3 Gy. Forrest et al.14 reported increased toxicity in 122 patients treated with that regimen at the Odette Cancer Centre in Canada during 2006–2008. Grade 3 or 4 toxic effects were observed in 13 patients (11%), and the actuarial grade 3 or 4 toxic effect rate at 2 years was 14%, which is higher than the rate seen in other retrospective series14. Some Canadian centres have subsequently modified their dose and fractionation regimens to lower the combined eqd2 doses, as evidenced by the decline in the number of centres using 6 Gy in 5 fractions since the 2009 survey. A regimen commonly used in Canada in 2012 was 8 Gy in 3 fractions, which was shown by Souhami et al.15 to be comparable to regimens using a larger number of fractions and higher doses.
Our earlier survey identified the fact that nearly all Canadian centres lacked routine access to mri for bt planning for cervical cancer patients. That challenge is likely to be faced by many cancer centres worldwide. Viswanathan et al.13 reported that ct and mri are similar with respect to oar contouring and dvh analysis, but that mri is superior in terms of contouring the various gec-estro tumour definitions6. Other studies have shown that dvh analysis of 2D plans reveals suboptimal coverage of the ct-based cervix and a negative correlation between coverage and cervix size16. More Canadian centres have implemented some mri planning into their processes—albeit only for 1 insertion in most centres because of limited access to mri. Few centres in Canada have a dedicated mri machine in the radiation therapy department. Additional validation of the results of mri–guided bt in locally advanced cervical cancer is under way: the results of the embrace prospective multicentre observational trial are awaited.
Many centres using ct-based planning were intending to add mri to their planning—at least for 1 insertion. As in many countries, several barriers limit that transition, notably budgetary considerations. However, despite the constraints, support for both 3D-based imaging as the standard of care for cervical cancer bt and the development of national guidelines continues to be strong.
Several limitations to our study must be mentioned. This study is retrospective and not an audit of bt planning as it was actually delivered. The response rate, although very good, was not complete. Nonetheless, it is important to document image-guided bt practices for cervical cancer because participation by Canadian bt centres in national and international clinical trials continues to grow.
CONCLUSIONS
Image-guided bt for cervical cancer in Canada continues to evolve. Although ct-based imaging remains the modality most commonly used, many centres have adopted mri for at least 1 bt treatment. More centres are using fewer fractions and a slightly lower biologically effective dose, but are still achieving eqd2 doses of 80–90 Gy in combination with ebrt.
ACKNOWLEDGMENTS
We thank Dr. Boris Bahoric and the Canadian Brachytherapy Group for their support of our survey.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Nag S, Cardenes H, Chang S, et al. on behalf of the Image-Guided Brachytherapy Working Group Proposed guidelines for image-based intracavitary brachytherapy for cervical carcinoma: report from Image-Guided Brachytherapy Working Group. Int J Radiat Oncol Biol Phys. 2004;60:1160–72. doi: 10.1016/j.ijrobp.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 2.Haie-Meder C, Potter R, Van Limbergen E, et al. on behalf of the Gynaecological gec-estro Working Group Recommendations from Gynaecological (gyn) gec-estro Working Group (i): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on mri assessment of gtv and ctv. Radiother Oncol. 2005;74:235–45. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Potter R, Haie-Meder C, Van Limbergen E, et al. on behalf of the gecestro Working Group Recommendations from gynaecological (gyn) gec estro working group (ii): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy—3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Pearce A, Craighead P, Kay I, Traptow L, Doll C. Brachytherapy for carcinoma of the cervix: a Canadian survey of practice patterns in a changing era. Radiother Oncol. 2009;91:194–6. doi: 10.1016/j.radonc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Pavamani S, D’Souza DP, Portelance L, et al. Image-guided brachytherapy for cervical cancer: a Canadian Brachytherapy Group survey. Brachytherapy. 2011;10:345–51. doi: 10.1016/j.brachy.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Hellebust TP, Kirisits C, Berger D, et al. on behalf of the Gynaecological gec-estro Working Group Recommendations from Gynaecological (gyn) gec-estro Working Group: considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapy. Radiother Oncol. 2010;96:153–60. doi: 10.1016/j.radonc.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Viswanathan AN, Dimopoulos J, Kirisits C, Berger D, Pötter R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68:491–8. doi: 10.1016/j.ijrobp.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Viswanathan AN, Erickson BA. Three-dimensional imaging in gynecologic brachytherapy: a survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys. 2010;76:104–9. doi: 10.1016/j.ijrobp.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Lee LJ, Das IJ, Higgins SA, et al. on behalf of the American Brachytherapy Society American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part iii: low-dose-rate and pulsed-dose-rate brachy-therapy. Brachytherapy. 2012;11:53–7. doi: 10.1016/j.brachy.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Marchant KJ, Sadikov E. The evolving practice of intrauterine cervix brachytherapy in Canada: a medical physics perspective. Brachytherapy. 2013;12:324–30. doi: 10.1016/j.brachy.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Viswanathan AN, Creutzberg CL, Craighead P, et al. International brachytherapy practice patterns: a survey of the Gynecologic Cancer Intergroup (gcig) Int J Radiat Oncol Biol Phys. 2012;82:250–5. doi: 10.1016/j.ijrobp.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guedea F, Ventura M, Mazeron JJ, Torrecilla JL, Bilbao P, Borràs JM. Patterns of care for brachytherapy in Europe: facilities and resources in brachytherapy in the European area. Brachytherapy. 2008;7:223–30. doi: 10.1016/j.brachy.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Viswanathan AN, Thomadsen B, on behalf of the American Brachytherapy Society Cervical Cancer Recommendations Committee American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part i: general principles. Brachytherapy. 2012;11:33–46. doi: 10.1016/j.brachy.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Forrest JL, Ackerman I, Barbera L, et al. Patient outcome study of concurrent chemoradiation external beam radiotherapy and high-dose rate brachytherapy in locally advanced carcinoma of the cervix. Int J Gynecol Cancer. 2010;20:1074–8. doi: 10.1111/IGC.0b013e3181e6f321. [DOI] [PubMed] [Google Scholar]
- 15.Souhami L, Corns R, Duclos M, Portelance L, Bahoric B, Stanimir G. Long-term results of high-dose rate brachytherapy in cervix cancer using a small number of fractions. Gynecol Oncol. 2005;97:508–13. doi: 10.1016/j.ygyno.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Gao M, Albuquerque K, Chi A, Rusu I. 3D ct-based volumetric dose assessment of 2D plans using gec-estro guidelines for cervical cancer brachytherapy. Brachytherapy. 2010;9:55–60. doi: 10.1016/j.brachy.2009.05.004. [DOI] [PubMed] [Google Scholar]