Abstract
Background
Currently in Canada, several bone-targeted agents (btas) with varying characteristics are available for the prevention of skeletal-related events (sres) in patients with bone metastasis secondary to solid tumours. In the present study, we evaluated the preferences of physicians in Canada for the various attributes of the available btas.
Methods
Physicians treating patients with bone metastasis from solid tumours were invited to complete an online discrete-choice experiment. Respondents were asked to choose between pairs of hypothetical medications for virtual patients. Each hypothetical medication was described based on predefined key attributes: time until first sre, time until worsening of pain, medication-related annual risk of osteonecrosis of the jaw (onj), medication-related annual risk of renal impairment, and mode of administration. A random-parameters logit model was used to analyze the choices between hypothetical medications and thus infer physician preferences for medication attributes.
Results
Responses from the 200 physicians who completed the discrete-choice experiment suggested that months until first sre, risk of renal impairment, and months until worsening of pain were considered the most important attributes affecting choice of bta. The annual risk of onj was considered the least important attribute.
Conclusions
When making treatment decisions about the choice of bta for patients with bone metastasis from solid tumours, delaying sres and worsening of pain, and reducing the risk of renal impairment are primary considerations for physicians in Canada.
Keywords: Bone metastases, skeletal-related events, physician preferences, conjoint analysis, discrete-choice experiments, bone-targeted agents
INTRODUCTION
Historically, nearly half of the more than 140,000 new cases of cancer diagnosed annually in Canada metastasize to bone1. Approximately 70% of breast cancers, 80%–90% of prostate cancers, and 30%–40% of lung and other solid tumours can progress to metastatic bone disease2,3. Bone metastases often lead patients to experience bone complications—also known as skeletal-related events (sres)—that include pathologic fractures, spinal cord compression, and a need for surgery or radiation to bone4,5. In more than half these patients, bone complications can result in severe pain6, impaired mobility, increased morbidity and mortality, reduced health-related quality of life2,7,8, and increased health care costs9,10.
Before the approval of denosumab, treatment options for bone metastases from solid tumours in Canada were limited to bisphosphonates, with zoledronic acid and pamidronic acid being the ones most used. In 2011, denosumab was approved in Canada for reducing the risk of sres in patients with solid tumours and bone metastasis. The approval was based on demonstrated superiority, in three large randomized clinical trials that compared denosumab with zoledronic acid, for delaying first onset of sres11–14. However, efficacy might not be the only treatment characteristic considered during a physician’s decision-making process; a medication’s safety profile (that is, adverse events) and mode of administration can also play an important role. Descriptions of practice patterns at national and regional levels indicate variations in the use of bone-targeted agents (btas) that do not reflect current clinical evidence about treatments for bone metastases15 and support the idea that physicians incorporate their own views about the current clinical evidence—and other considerations—into their prescribing decisions.
Published guidelines (that is, those from the U.S. National Comprehensive Cancer Network, the American Society of Clinical Oncology, and the Canadian Urological Association) present various options for bone health in the setting of solid tumours. Recommendations in those guidelines, supported by pivotal clinical phase iii trials, are based on the primary outcome measure of reduction or delay in first or subsequent sres. As such, they fail to outline how to incorporate other important treatment characteristics into the decision-making process for individual patients.
The objectives of the present study were to evaluate physician preferences for attributes of btas used to delay sres in patients with bone metastasis from solid tumours, and to understand how physicians in Canada evaluate information about btas when making prescribing decisions. Although currently available btas are associated with risks of serious adverse events, determining physician perspectives on the acceptability of those risks for the potential benefits that their patients could receive is important. Results from the present study could shed light on the attributes that drive the decision to prescribe btas and on the levels of efficacy and treatment toxicity that physicians in Canada consider acceptable for such treatments.
METHODS
Study Population
To be eligible to complete the choice experiment, respondents had to be physicians involved in treating patients with bone metastasis from solid tumours in Canada. Members of online panels in Canada were invited by postal mail, fax, telephone, and e-mail to complete an online screener that would help a survey research company confirm that they met the study inclusion criteria. Only verified physicians were invited to complete the online discrete-choice experiment. All respondents provided informed consent.
Survey Instrument
An online discrete-choice instrument was developed for the study following good research practices16. Discrete-choice experiments, also known as choice-format conjoint analyses, are a common approach for assessing trade-off preferences for health interventions17–19. The method represents a rigorous way to collect evidence about preferences for aspects of medications by eliciting choices between hypothetical medication options with systematic differences in their attributes. In the present study, the hypothetical medication options were defined by their efficacy, safety, and administration requirements (“medication attributes”). Other potentially important aspects associated with treatment accessibility (such as drug and associated administration costs) were considered beyond the scope of the study because they were assumed to be influenced mostly by provincial funding formularies and clinical infrastructure rather than by variations in the features inherent to btas. For the study, each hypothetical medication could assume one of several attribute levels representing the degree to which the medication performed with respect to the associated attribute.
After a review of the prescribing information for the currently approved products, consultations with clinical experts, and in-person interviews with 15 oncology patients in the United States, 5 bta medication attributes were selected (Table i). Levels were defined for each attribute based on clinical evidence and prescribing information for the currently available btas.
TABLE I.
Medication attributes and levels for the choice questions
Time until first skeletal-related event
|
Time until a 2-point increase in pain on the BPI (“time until worsening of pain”)
|
Risk of osteonecrosis of the jaw each year
|
Risk of a 0.5 mg/dL increase in baseline creatinine each year (“risk of renal impairment each year”)
|
Mode of administration and frequency (“mode of administration”)
|
BPI = Brief Pain Inventory.
An overview of the medication attributes was provided to study respondents before they started the discrete-choice experiment. Descriptions of the attributes were taken from the prescribing information for the available btas20–23. In the case of time until first sre, physicians were told that this attribute referred to the time elapsed from the moment a patient started taking a bta to the moment that a need for radiation or surgery to bone was identified or that the patient experienced a pathologic fracture or spinal cord compression. No further information was provided about the specific events that a patient would experience after taking any of the hypothetical medications. The information thus provided was consistent with the available clinical trial evidence comparing the efficacy of btas.
Basic demographic data were collected from respondents, together with information about their experience treating patients with bone metastasis. The clarity and accuracy of the attribute definitions, background questions, and questions eliciting choices between the hypothetical medications were tested during semistructured in-person interviews with 8 physicians in the United States. During the interviews, the salience of the included medication attributes and the willingness of the physicians to make trade-offs between the medication attributes in the choice questions were also tested. The instrument with the choice experiments was finalized after the in-person interviews were completed; an updated instrument was programmed for online administration.
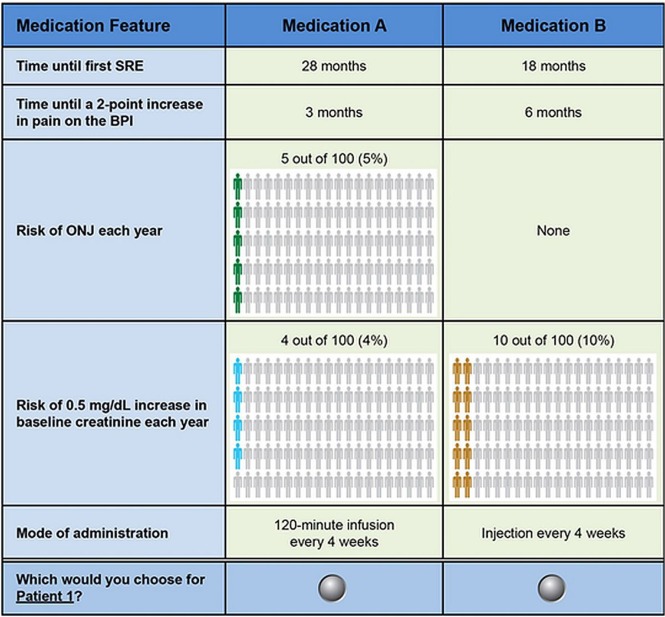
Each respondent was asked to answer choice questions involving pairs of hypothetical medications with different profiles (Figure 1) determined by an experimental design with known statistical properties. The experimental design defined the combinations of attribute levels associated with the medication profiles in the choice questions. The design generated 40 unbranded profile pairs and was developed using the SAS software application (version 9.3: SAS Institute, Cary, NC, U.S.A.), based on main-effects D-efficiency criteria24,25 consistent with good research practices26. Respondents were randomly assigned to 1 of 4 survey versions, each with 10 choice questions. To avoid order effects, the order of the choice questions within the survey versions was also randomized across respondents. Two profiles describing the characteristics of a typical breast cancer patient and a typical prostate cancer patient were provided (Table ii), and respondents were asked to evaluate the hypothetical medications for each of those patients.
FIGURE 1.
Example choice question: Patient 1—A 57-year-old woman who was diagnosed with breast cancer and developed bone metastases along with 2 cm mediastinal and supraclavicular adenopathy 3 years after her initial diagnosis. She initially received TC adjuvant chemotherapy. The tumour is ER/PR positive and HER2-negative. She was on an adjuvant aromatase inhibitor at the time of her relapse. Her recurrence was noted by examination identifying the supraclavicular adenopathy. On further questioning, she admits to increasing mid-back (thoracic area) pain, which she rates as a 4 on a scale of 0 to 10. The patient’s health is otherwise good (high performance status) with no history of kidney disease and no significant comorbidities. TC = docetaxel with cyclophosphamide; ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth factor receptor 2; SRE = skeletal-related event; BPI = Brief Pain Inventory; ONJ = osteonecrosis of the jaw.
TABLE II.
Patient profiles
| Profile 1 | A 57-year-old woman who was diagnosed with breast cancer and developed bone metastases along with 2-cm mediastinal and supraclavicular adenopathy 3 years after her initial diagnosis. She initially received TC adjuvant chemotherapy. The tumour is ER/PR positive and HER2 negative. She was on an adjuvant aromatase inhibitor at the time of her relapse. Her recurrence was noted by examination identifying the supraclavicular adenopathy. On further questioning she admits to increasing mid back (thoracic area) pain, which she rates as a 4 on a scale of 0 to 10. The patient’s health is otherwise good (high performance status) with no history of kidney disease and no significant comorbidities. |
| Profile 2 | A 71-year-old man who was initially diagnosed with Gleason 8–10 prostate cancer 3 years ago. He is now castration-resistant and has developed bone metastases. His PSA level is > 10. He is complaining of left hip pain when he walks and low back pain if he sits too long, which he rates as a 4 on a scale from 0 to 10. The patient’s health is otherwise good (high performance status) with no history of kidney disease and no significant comorbidities. |
TC = docetaxel/cyclophosphamide; ER/PR = estrogen receptor/progesterone receptor; HER2 = human epidermal growth receptor 2; PSA = prostate-specific antigen.
Analyses
A random-parameters logit (rpl) model developed using the nlogit software application (version 5.0: Econometric Software, Plainview, NY, U.S.A.) was used to quantify the trade-off preferences demonstrated by the physicians. An rpl regression model relates the likelihood of choosing a medication to its attributes and attribute levels26,27. Results from the rpl model are sometimes called “preference weights” and can be interpreted as parameters characterizing the relative preferences of respondents for medication attribute levels. The rpl model controls for preference heterogeneity across respondents by estimating a distribution of preferences for each preference parameter, estimating a mean preference parameter, and accounting for the panel nature of the dataset28,29.
Relative-importance weights of attributes were calculated as the difference between the mean preference weights for the most and least preferred levels of an attribute. Importance weights can be interpreted as the overall relative importance of the attributes for the outcome range presented in the choice questions27.
Predicted choice probabilities (pcps) were also calculated using the rpl model results. The pcps predict the percentage of respondents who would select a medication profile with specific attribute levels from a set of medication profiles, each with a given set of attribute levels. They provide a way to understand physician preferences for combinations of attribute levels that are similar to available btas. Our study estimated pcps for medications similar to currently available btas by using the preference weights for attribute levels in products with characteristics similar to those of denosumab, zoledronic acid, clodronate, and pamidronate (Table iii). It is important to note that, although the choice questions presented to physicians did not directly show profiles representing currently available medications, the pcps for currently available products can be calculated using the preference weights for the attribute levels in the study that were consistent with those products. The calculation of the pcps did not account for market conditions such as real-world availability of medication substitutes or differences in the information about btas and bta outcomes that physicians might take into account when making prescribing decisions.
TABLE III.
Product profiles
| Attribute | Characteristics similar to those of | |||
|---|---|---|---|---|
|
| ||||
| Denosumab20 | Zoledronic acid21 | Clodronate22 | Pamidronate23 | |
| Time until first SRE (months) | 27.7 | 19.5 | 15–20 (assumed 17.5) | 10.9 |
| Time until worsening of pain (months) | 5.9 | 5.6 | 3 | 0.03 to several (assumed 3) |
| Risk of ONJ each year (%) | 1.8 | 1.3 | Yes, but value not stated (assumed 1) | Yes, but value not stated (assumed 1) |
| Risk of renal impairment each year (%) | 0 | 9.3 | Yes, but value not stated (assumed 5) | 8.1 |
| Mode of administration | Injection every 4 weeks | 15-Minute infusion every 4 weeks | Daily oral tablet | 120-Minute infusion every 4 weeks |
SRE = skeletal-related event; ONJ = osteonecrosis of the jaw.
RESULTS
Physician Characteristics
Invitations to participate were extended to 3792 physicians in Canada currently treating patients with bone metastases, and 426 (11.2%) responded to the invitation. Of those respondents, 318 (74.6%) were eligible to participate. Of eligible respondents, 316 (99.4%) consented to participate, and 205 (64.5%) completed the survey by answering at least 1 choice question. In the choice questions, 5 participants always chose the same answer, Medication A or Medication B, suggesting a lack of attention to the medication profiles in the questions. Those respondents were excluded from the analysis. The final sample included 200 physicians.
Table iv reports the baseline characteristics of the final sample. Nearly 75% of the physicians had at least 10 years of experience since completing medical training, 57% reported practicing in an academic or teaching hospital, and approximately 58% were oncologists. More than one third of the group reported treating 11 or more patients with bone metastases from solid tumours each week.
TABLE IV.
Baseline characteristics of respondents
| Characteristic | Frequency [n (% of 200a)] |
|---|---|
| Age group | |
| 26–45 Years | 88 (44.0) |
| ≥46 Years | 112 (56.0) |
| Duration in practice since completion of medical training | |
| 1–6 Years | 30 (15) |
| 7–15 Years | 71 (35.5) |
| ≥16 Years | 99 (49.5) |
| Practice typeb | |
| General | 39 (19.5) |
| Academic or teaching hospital | 114 (57.0) |
| Community hospital | 56 (28.0) |
| Other | 7 (3.5) |
| Area of specialization | |
| Primary care | 7 (3.5) |
| Family medicine | 34 (17.0) |
| Oncology | 115 (57.5) |
| Other | 44 (22.0) |
| Patients with bone metastases from solid tumours treated each week (average) | |
| ≤10 | 127 (63.5) |
| >10 | 73 (36.5) |
Percentages exclude missing values.
Multiple options could be selected, if applicable.
Preference Weights
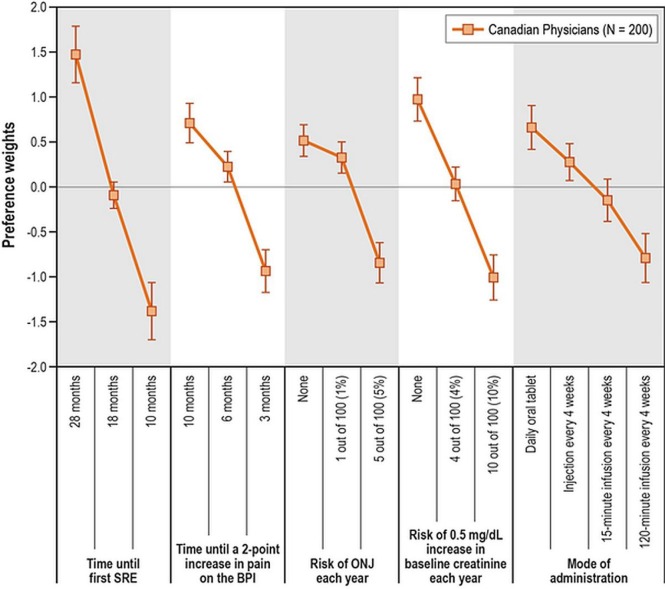
Figure 2 presents the estimated preference weights for all the attribute levels included in the study. Because the weights represent relative preferences, they cannot be interpreted in isolation, but only relative to other preference weights within each attribute. Attribute levels associated with higher weights are preferred over attribute levels with lower weights.
FIGURE 2.
Preference weights. The vertical bars surrounding each mean preference weight denote the 95% confidence interval (CI) for the point estimate; if the CIs for adjacent levels in a particular attribute do not overlap, the mean estimates for those levels are statistically different at the 5% level of significance. However, overlapping CIs do not necessarily imply a lack of statistical significance in the differences between preference weights within an attribute at the 5% level of significance. BPI = Brief Pain Inventory; ONJ = osteonecrosis of the jaw; SRE = skeletal-related event.
The vertical bars around each preference weight indicate the 95% confidence interval (ci) around the mean estimate. If the cis for any two levels in an attribute do not overlap, the mean estimates are statistically significantly different from each other at the 5% level of significance. However, overlapping cis do not necessarily imply that the estimated preferences at two levels within an attribute are not statistically significant at the 5% confidence level.
Preference weights for all the attribute levels were consistent with the expectation that better clinical outcomes are preferred to worse clinical outcomes. Although administration options were not defined solely based on the time required to administer the medication, more efficient administration options were preferred to options requiring a longer time for administration. Differences in the preference estimates were statistically significant (p < 0.05) for all levels of the attributes time until first sre, time until worsening of pain, risk of renal impairment each year, and mode of administration. The difference in the preference estimates for no risk and a 1% risk of onj was not statistically significant (p > 0.05); it did reach significance for an annual risk of 5%. Exploratory analyses did not reveal differences in preferences depending on physician specialty, type of practice, or experience (p > 0.05).
Relative Importance
The overall relative importance of attributes corresponded with the specific ranges of attribute levels presented in the choice questions27. In terms of relative importance for choice of a bta, physicians in Canada ranked the attributes as follows (in decreasing order):
□ Time until first sre
□ Annual risk of renal impairment
□ Time until worsening of pain
□ Mode of administration
□ Annual risk of onj
Predicted Choice Probabilities
Table v presents the predicted likelihood (pcp) that the surveyed physicians would choose each one of the drug profiles describing currently available medications, assuming that all the medication options were available to them. Based on the preference weights for the attributes and levels included in the choice questions, it was estimated that approximately 93% of physicians would prefer a medication with characteristics similar to denosumab over medications with characteristics similar to those of zoledronic acid, clodronate, or pamidronate. Differences between the likelihoods of choosing denosumab and of choosing other btas were statistically significant (p < 0.01).
TABLE V.
Predicted choice probabilities
| Characteristics similar to ... | Prediction | |
|---|---|---|
|
| ||
| Mean | 95% CI | |
| Denosumab | 93.4 | 88.4 to 96.2 |
| Zoledronic acid | 2.7 | 1.3 to 5.2 |
| Clodronate | 3.7 | 2.0 to 6.7 |
| Pamidronate | 0.2 | 0.1 to 0.5 |
CI = confidence interval.
DISCUSSION
Our findings suggest that physicians in Canada have well-defined preferences for attributes of btas and seem to consider mainly efficacy and safety when making prescribing decisions for patients with bone metastasis from solid tumours. In general, physicians who completed the survey seemed most concerned about delaying sres and reducing the risk of medication-related renal impairment. That result suggests that diminishing the risk of medication-related renal impairment was perceived by physicians in our sample to be more beneficial to patients than delaying pain using btas. It also suggests that the trade-offs between the efficacy and safety features of btas are not simplistic and vary depending on the type of benefit and risk evaluated.
In our study, the risk of onj was the attribute given the least importance by respondents, suggesting that the surveyed physicians were willing to accept increases in the level of that risk in exchange for improvements in any of the two treatment efficacy measures considered in the study. That result might be attributable to the inclusion of an onj risk range that was not salient to physicians relative to the other treatment attributes that they were asked to consider (despite its being a clinically relevant range). The result might also reflect an increased understanding on the part of the physicians about dental health management to reduce the medication-related risk of onj. Some recent publications include suggestions for new therapeutic approaches based on minimally invasive surgery, patient education, and proactive monitoring, thus showing that physicians in clinical practice accept the risk of onj and manage it during treatment with btas30–32.
The medication administration options for btas were a salient attribute for physicians. Although the options for medication administration were not defined solely on speed of administration, statistical significance across the various levels of that attribute suggests that a more efficient mode of bta administration is a desirable aspect of this attribute.
Overall, the findings in the study appear to be consistent with results from similar preference-elicitation studies among physicians in the United States33 and Europe34,35, for whom both efficacy and a reduced risk of renal impairment represented the primary goals in their decision-making process.
Preference results in the present study shed light on current discussions among physicians in Canada about btas and outcomes of btas. The fact that physicians had well-defined preferences for efficacy and safety outcomes is not surprising. Given that btas are supportive in nature, the results speak to an interest in controlling the advance of the disease, while managing the treatment-related risks that affect quality of life for patients. Also, physicians in Canada face challenges of limited resources and limiting geography; thus, the relative preference for more efficient and simplified administration could reflect how physicians in Canada manage those demands and restrictions.
Additionally, unlike current treatment guidelines, the preference results suggest that physicians have well-defined preferences for the attributes of currently available therapies and make their prescribing decisions based on several bta attributes. Among those attributes, physicians see a hierarchy of importance for clinical practice and supportive care that suggests a prioritization for preserving quality of life for patients with incurable malignancies. Based on physician desire to delay the symptoms of sres, manage the annual risk of renal impairment usually associated with the use of bisphosphonates, and prevent the worsening of pain, the pcp calculations show a physician preference for a hypothetical treatment profile whose attributes are similar to those of denosumab.
Several limitations of our study are worth noting. First, the decisions recorded in the discrete-choice experiment obviously do not have the same clinical and emotional consequences as real-world prescribing choices. Thus, physicians might, in reality, make different medication decisions based on prescribing aspects not explicitly captured in the study design. Also, the sampling process was not designed or weighted to ensure that the sample represented the population of Canadian physicians treating patients with bone metastases from solid tumours. Although we did not find statistically significant differences in the preferences of respondents with different specialties, types of practice, or levels of experience, it is possible that the lack of significance is attributable to a limited number of observations in any one of the subgroups defined for the comparisons. Without formal stratification of the sample at the time of data collection, the number of respondents in each subgroup is entirely determined by the natural variation of physician characteristics among those who agreed to participate in the study. Finally, we did not consider dosing options beyond those included in the prescribing information available for each bta. We recognize that varying intervals in the administration of therapies is an option for some patients. However, the literature concerning this topic is heterogeneous and evolving, and not part of the standard of care in Canadian centres.
Our study is the first to look at physician preferences for attributes of btas in Canada. Our results highlight that physicians in Canada are willing to accept trade-offs in the attributes of btas and to provide information about the trade-offs that they consider worthwhile for their patients. This information can help to characterize the medication decisions that physicians make for patients with metastatic bone disease, considering the Canadian health care context.
CONCLUSIONS
When making medication decisions about the choice of a bta for patients with bone metastasis secondary to solid tumours, the primary considerations for physicians in Canada are delaying the onset of sres, managing the risk of renal impairment, and preventing worsening of pain. Despite reacting to all the side effects included in the study, physicians were least concerned by the risk of onj and were willing to accept increases in the level of that risk for improvements in treatment efficacy. In addition to clinical considerations, physicians in Canada have well-defined preferences for practical medication considerations— namely, the medication’s mode of administration.
ACKNOWLEDGMENTS
Funding for this study was obtained from Amgen, Inc., Thousand Oaks, CA, U.S.A.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: ABH, JMG, and JP are employees of RTI Health Solutions, an independent scientific research organization. AFM was an employee of RTI Health Solutions at the time the study was conducted. The study that is the subject of this manuscript was conducted by RTI Health Solutions and was funded by Amgen, Thousand Oaks, CA, U.S.A. JA, YQ, MH, and FG are employees of Amgen. EC has received a teaching honorarium from Amgen and an educational grant for a handbook of bone metastases. NC has served as a member of an advisory board and has received honoraria from Amgen Canada.
REFERENCES
- 1.Kelly M, Lee M, Clarkson P, O’Brien PJ. Metastatic disease of the long bones: a review of the health care burden in a major trauma centre. Can J Surg. 2012;55:95–8. doi: 10.1503/cjs.049009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–9s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 3.Parker C, Nilsson S, Heinrich D, et al. on behalf of the alsympca investigators Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(suppl):1588–94. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1588::AID-CNCR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RE. Bisphosphonates: clinical experience. Oncologist. 2004;9(suppl 4):14–27. doi: 10.1634/theoncologist.9-90004-14. [DOI] [PubMed] [Google Scholar]
- 6.Hird A, Chow E, Zhang L, et al. Determining the incidence of pain flare following palliative radiotherapy for symptomatic bone metastases: results from three Canadian cancer centers. Int J Radiat Oncol Biol Phys. 2009;75:193–7. doi: 10.1016/j.ijrobp.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Chow E, Hoskin P, van der Linden Y, Bottomley A, Velikova G. Quality of life and symptom end points in palliative bone metastases trials. Clin Oncol (R Coll Radiol) 2006;18:67–9. doi: 10.1016/j.clon.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Langer C, Hirsh V. Skeletal morbidity in lung cancer patients with bone metastases: demonstrating the need for early diagnosis and treatment with bisphosphonates. Lung Cancer. 2010;67:4–11. doi: 10.1016/j.lungcan.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Delea T, Langer C, McKiernan J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology. 2004;67:390–6. doi: 10.1159/000082923. [DOI] [PubMed] [Google Scholar]
- 10.Lage MJ, Barber BL, Harrison DJ, Jun S. The cost of treating skeletal-related events in patients with prostate cancer. Am J Manag Care. 2008;14:317–22. [PubMed] [Google Scholar]
- 11.Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48:3082–92. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22:679–87. doi: 10.1007/s00520-013-2022-1. [DOI] [PubMed] [Google Scholar]
- 14.Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 15.Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75:1501–10. doi: 10.1016/j.ijrobp.2008.12.084. [DOI] [PubMed] [Google Scholar]
- 16.Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ispor good research practices for conjoint analysis task force. Value Health. 2011;14:403–13. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Bridges JF, Kinter ET, Kidane L, Heinzen RR, McCormick C. Things are looking up since we started listening to patients: trends in the application of conjoint analysis in health 1982–2007. Patient. 2008;1:273–82. doi: 10.2165/1312067-200801040-00009. [DOI] [PubMed] [Google Scholar]
- 18.Marshall D, Bridges JF, Hauber B, et al. Conjoint analysis applications in health—how are studies being designed and reported? An update on current practice in the published literature between 2005 and 2008. Patient. 2010;3:249–56. doi: 10.2165/11539650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21:145–72. doi: 10.1002/hec.1697. [DOI] [PubMed] [Google Scholar]
- 20.Amgen Inc . Xgeva (Denosumab). Full Prescribing Information. Thousand Oaks, CA: Amgen; 2013. [Google Scholar]
- 21.Novartis Pharmaceuticals Corporation . Zometa (Zoledronic Acid). Full Prescribing Information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2012. [Google Scholar]
- 22.Bayer Australia Limited . Product Information: Bonefos (Sodium Clodronate) Pymble, Australia: Bayer Australia Limited; 2013. [Google Scholar]
- 23.Novartis Pharmaceuticals Corporation . Aredia: Pamidronate Disodium for Injection. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2012. [Google Scholar]
- 24.Huber J, Zwerina K. The importance of utility balance and efficient choice designs. J Mark Res. 1996;33:307–17. doi: 10.2307/3152127. [DOI] [Google Scholar]
- 25.Kuhfeld W, Tobias F, Garratt M. Efficient experimental design with marketing research applications. J Mark Res. 1994;31:545–57. doi: 10.2307/3151882. [DOI] [Google Scholar]
- 26.Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ispor conjoint analysis experimental design good research practices task force. Value Health. 2013;16:3–13. doi: 10.1016/j.jval.2012.08.2223. [DOI] [PubMed] [Google Scholar]
- 27.Hauber AB, Arden NK, Mohamed AF, et al. A discrete-choice experiment of United Kingdom patients’ willingness to risk adverse events for improved function and pain control in osteoarthritis. Osteoarthritis Cartilage. 2013;21:289–97. doi: 10.1016/j.joca.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Train K. Discrete Choice Methods with Simulation. Cambridge, UK: Cambridge University Press; 2003. [DOI] [Google Scholar]
- 29.Train K, Sonnier G. Mixed logit with bounded distributions of correlated partworths. In: Scarpa R, Alberini A, editors. Applications of Simulation Methods in Environmental and Resource Economics. Dordrecht, Netherlands: Springer; 2005. pp. 117–34. [DOI] [Google Scholar]
- 30.Agrillo AF, Filiaci V, Ramieri E, et al. Bisphosphonate-related osteonecrosis of the jaw (bronj): 5 year experience in the treatment of 131 cases with ozone therapy. Eur Rev Med Pharmacol Sci. 2012;16:1741–7. [PubMed] [Google Scholar]
- 31.Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase iii trials in cancer patients with bone metastases. Ann Oncol. 2012;23:1341–7. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 32.Polascik TJ, Mouraviev V. Zoledronic acid in the management of metastatic bone disease. Ther Clin Risk Manag. 2008;4:261–8. doi: 10.2147/tcrm.s2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arellano J, Hauber AB, Mohamed AF, et al. Physicians’ preferences for bone metastases drug therapy in the United States. Value Health. 2015;18:78–83. doi: 10.1016/j.jval.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Qian Y, Hechmati G, Mohamed AF, et al. Physicians’ preferences for bone metastases treatments in France, Germany, and the United Kingdom [poster]. Presented at: ISPOR 16th Annual European Congress; Dublin, Ireland. 2–6 November 2013; [Available online at: https://www.rtihs.org/sites/default/files/Hauber_ISPOR%20EU_Nov2013.pdf; cited 17 July 2015] [Google Scholar]
- 35.Gonzalez JM, Gatta F, Arellano J, et al. Physicians’ preferences for bone metastases treatments in Turkey [abstract A570] Value Health. 2014;17 doi: 10.1016/j.jval.2014.08.1905. [DOI] [PubMed] [Google Scholar]