Abstract
Background
Metastatic colorectal cancer (mcrc) commonly affects elderly people, an understudied subset of patients. We analyzed the survival impact of the first and subsequent lines of chemotherapy in eligible non-trial patients 70 years of age and older with mcrc treated between 2004 and 2012.
Methods
This single-centre retrospective analysis estimated overall survival (os) and progression-free survival (pfs) using the Kaplan–Meier method. Multivariate analysis was used to adjust for age, sex, Eastern Cooperative Oncology Group performance status, score on the Charlson comorbidity index, dependency in activities of daily living, and exposure to 1 or more chemotherapy doublets, capecitabine alone, or best supportive care (bsc).
Results
Of 109 patients identified, 29 elected bsc, and 80 received chemotherapy. In multivariate analysis, age was not associated with os [hazard ratio (hr): 0.99; 95% confidence interval (ci): 0.92 to 1.05], but a performance status of 2 or higher was associated with a decreased likelihood of survival (hr: 3.12; 95% ci: 1.87 to 5.76), and exposure to 1 or more doublets was associated with improved survival (hr: 0.33; 95% ci: 0.17 to 0.66). In univariate analysis, a trend toward improved os was observed for first-line doublet chemotherapy compared with capecitabine (hr: 0.66; 95% ci: 0.41 to 1.07), and pfs was superior (hr: 0.46; 95% ci: 0.26 to 0.84). Compared with exposure to 1 doublet, exposure to the 3 potential cytotoxic chemotherapies was not associated with improved os (hr: 0.77; 95% ci: 0.41 to 1.43). The incidence of neutropenia with first-line folfiri was 40%; the incidences of bevacizumab-related arterial and venous thrombosis were both 8%.
Conclusions
Exposure to 1 or more doublet chemotherapies for mcrc was associated with better outcomes in non-trial patients 70 years of age and older. Elderly patients treated with palliative chemotherapy and bevacizumab should be monitored carefully for arterial and venous thrombotic events.
Keywords: Colorectal cancer, metastasis, elderly patients, bevacizumab, chemotherapy, oxaliplatin, irinotecan
BACKGROUND
Colorectal cancer is the fourth most prevalent cancer in North America. Nearly 50% of all cases are diagnosed in patients 70 years of age and older, and 20% of cases have metastasized by the time of the diagnosis1. Recent advances in the treatment of metastatic colorectal cancer (mcrc) have led to significant improvements in survival, increasing average survival to more than 24 months from 11–12 months2,3.
Cytotoxic 5-fluorouracil–capecitabine or doublets using oxaliplatin (folfox, xelox) or irinotecan (folfiri) in combination with bevacizumab, an inhibitor of vascular endothelial growth factor, are established as the standard of care in fit adults, but their benefits are difficult to extrapolate to unselected elderly patients for many reasons, including inadequate evidence to support the use of those regimens because of the exclusion of elderly patients from large clinical trials4. Heterogeneity of functional status in elderly patients, age-related alterations in drug metabolism, and a greater number of comorbidities can affect both the expected efficacy and the adverse effects of the regimens5,6.
Considerable efforts have recently been made to promote the enrolment of older patients in clinical trials. Pooled analyses that regroup the elderly patients from relevant trials7–10, as well as a limited number of focused trials11,12, have suggested that older patients can gain appreciable benefit from chemotherapy and targeted therapy, with acceptable safety. Compared with their younger counterparts, older patients eligible to receive chemotherapy are more likely to be initially offered capecitabine alone instead of a doublet regimen and are less likely to receive a doublet chemotherapy regimen13–15. Chemotherapy safety, tolerance, and efficacy are clinical concerns when older patients with mcrc who do not fit the stringent inclusion criteria of clinical trials are treated. Given that background, we conducted a retrospective study to analyze survival and toxicity data in patients 70 years of age and older treated for mcrc in our academic tertiary care centre.
METHODS
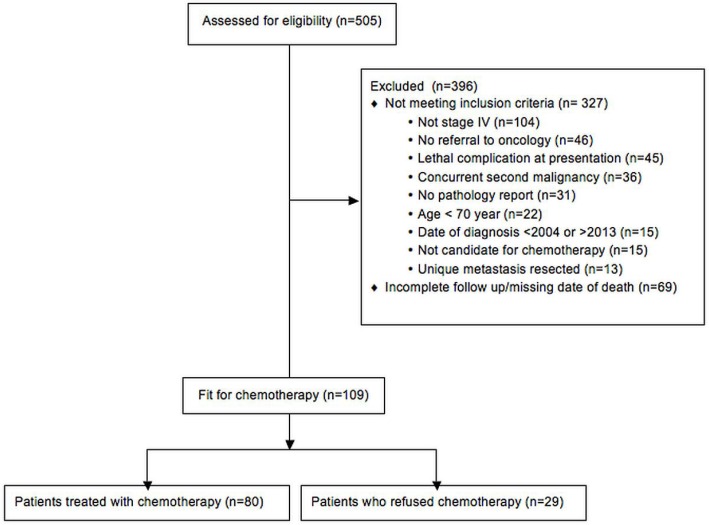
We retrospectively collected data for patients treated at the Centre hospitalier universitaire de Sherbrooke, Quebec, between January 2004 and December 2012. The inclusion criteria were histologically proven crc with radiologic metastasis, an age of 70 years or more at the time of diagnosis, oncology assessment at our centre, and candidacy for chemotherapy. To find charts for patients fulfilling the study criteria, the archive service at our institution used definitive mcrc-related key words to search admission summaries, radiology and pathology reports, and the oncology pharmacy’s drug delivery registry. Upon approval by our institutional ethics committee, the identified charts were manually reviewed, and those not meeting the study criteria were excluded (Figure 1). Patients who received no chemotherapy despite being deemed eligible by an oncologist were kept in the study.
FIGURE 1.
Flow diagram of patient selection for the study.
The patients were divided into two groups: best supportive care (bsc) and chemotherapy. The latter group was in turn subdivided in two age groups: 70–74 years and 75 years and older. The baseline characteristics extracted from the charts included primary tumour site, sites of metastasis, prior metastasectomy, prior adjuvant therapy, Eastern Cooperative Oncology Group (ecog) performance status, basic (adl) and instrumental (iadl) activities of daily living autonomy scales, and comorbidities. Autonomy was dichotomized into “autonomous” or “dependent,” indicating that the patient was dependent with respect to 1 or more adls or iadls. Comorbidities were scored according to the Charlson comorbidity index (cci)16. Additionally, prognostic biochemical determinants such as serum lactate dehydrogenase, carcinoembryonic antigen, and platelet count were collected. Chemotherapy toxicities were retrospectively graded using the U.S. National Cancer Institute’s Common Toxicity Criteria, version 2.0. The indexed treatments were capecitabine alone, doublets (folfox, xelox, folfiri), and bevacizumab.
Statistical Methods
The statistical analysis was performed using IBM SPSS Statistics (version 20.0: IBM, Armonk, NY, U.S.A.). Kaplan–Meier curves and log-rank tests were used to estimate median overall survival (os) and progression-free survival (pfs) with 95% confidence intervals (cis). Cox regression modelling was used to determine predictors of os and pfs; models included age, sex, cci score, ecog performance status, adl, doublet chemotherapy, capecitabine monotherapy, and bsc. Statistical significance was assumed at p ≤ 0.05.
RESULTS
Of the 109 patients who met the study criteria, 80 (73%) received chemotherapy, and 29 (27%) opted for bsc despite being deemed eligible for chemotherapy by an oncologist. Table i reports their baseline characteristics. In the chemotherapy group, 34 patients were 70–74 years of age, and 46 were 75 years of age or older. The age ranges were 70–87 years in the chemotherapy group and 70–94 years in the bsc group. Both groups contained 14 patients 80 years of age and older. The patients who selected bsc had higher ecog scores (p = 0.02).
TABLE I.
Baseline characteristics of the patients
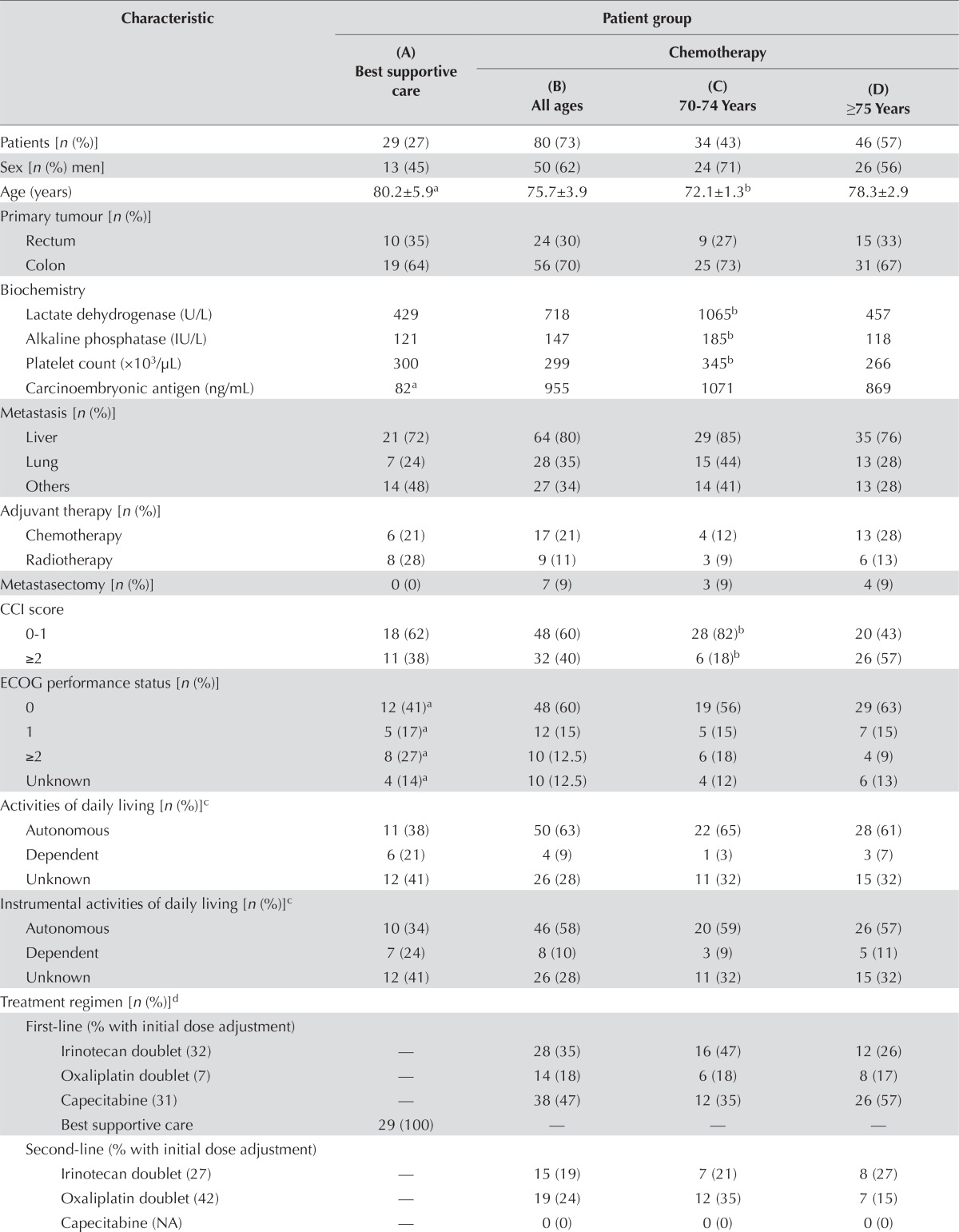
| Characteristic | Patient group | |||
|---|---|---|---|---|
|
| ||||
| (A) Best supportive care | Chemotherapy | |||
|
| ||||
| (B) All ages | (C) 70–74 Years | (D) ≥75 Years | ||
| Patients [n (%)] | 29 (27) | 80 (73) | 34 (43) | 46 (57) |
| Sex [n (%) men] | 13 (45) | 50 (62) | 24 (71) | 26 (56) |
| Age (years) | 80.2±5.9a | 75.7±3.9 | 72.1±1.3b | 78.3±2.9 |
| Primary tumour [n (%)] | ||||
| Rectum | 10 (35) | 24 (30) | 9 (27) | 15 (33) |
| Colon | 19 (64) | 56 (70) | 25 (73) | 31 (67) |
| Biochemistry | ||||
| Lactate dehydrogenase (U/L) | 429 | 718 | 1065b | 457 |
| Alkaline phosphatase (IU/L) | 121 | 147 | 185b | 118 |
| Platelet count (×103/μL) | 300 | 299 | 345b | 266 |
| Carcinoembryonic antigen (ng/mL) | 82a | 955 | 1071 | 869 |
| Metastasis [n (%)] | ||||
| Liver | 21 (72) | 64 (80) | 29 (85) | 35 (76) |
| Lung | 7 (24) | 28 (35) | 15 (44) | 13 (28) |
| Others | 14 (48) | 27 (34) | 14 (41) | 13 (28) |
| Adjuvant therapy [n (%)] | ||||
| Chemotherapy | 6 (21) | 17 (21) | 4 (12) | 13 (28) |
| Radiotherapy | 8 (28) | 9 (11) | 3 (9) | 6 (13) |
| Metastasectomy [n (%)] | 0 (0) | 7 (9) | 3 (9) | 4 (9) |
| CCI score | ||||
| 0–1 | 18 (62) | 48 (60) | 28 (82)b | 20 (43) |
| ≥2 | 11 (38) | 32 (40) | 6 (18)b | 26 (57) |
| ECOG performance status [n (%)] | ||||
| 0 | 12 (41)a | 48 (60) | 19 (56) | 29 (63) |
| 1 | 5 (17)a | 12 (15) | 5 (15) | 7 (15) |
| ≥2 | 8 (27)a | 10 (12.5) | 6 (18) | 4 (9) |
| Unknown | 4 (14)a | 10 (12.5) | 4 (12) | 6 (13) |
| Activities of daily living [n (%)]c | ||||
| Autonomous | 11 (38) | 50 (63) | 22 (65) | 28 (61) |
| Dependent | 6 (21) | 4 (9) | 1 (3) | 3 (7) |
| Unknown | 12 (41) | 26 (28) | 11 (32) | 15 (32) |
| Instrumental activities of daily living [n (%)]c | ||||
| Autonomous | 10 (34) | 46 (58) | 20 (59) | 26 (57) |
| Dependent | 7 (24) | 8 (10) | 3 (9) | 5 (11) |
| Unknown | 12 (41) | 26 (28) | 11 (32) | 15 (32) |
| Treatment regimen [n (%)]d | ||||
| First-line (% with initial dose adjustment) | ||||
| Irinotecan doublet (32) | — | 28 (35) | 16 (47) | 12 (26) |
| Oxaliplatin doublet (7) | — | 14 (18) | 6 (18) | 8 (17) |
| Capecitabine (31) | — | 38 (47) | 12 (35) | 26 (57) |
| Best supportive care | 29 (100) | — | — | — |
| Second-line (% with initial dose adjustment) | ||||
| Irinotecan doublet (27) | — | 15 (19) | 7 (21) | 8 (27) |
| Oxaliplatin doublet (42) | — | 19 (24) | 12 (35) | 7 (15) |
| Capecitabine (NA) | — | 0 (0) | 0 (0) | 0 (0) |
| Third-line (% with initial dose adjustment) | ||||
| Irinotecan doublet (NA) | — | 0 (0) | 0 (0) | 0 (0) |
| Oxaliplatin doublet (43) | — | 7 (9) | 2 (6) | 5 (11) |
| Capecitabine (75) | — | 4 (5) | 4 (12) | 0 (0) |
| Best supportive care | 29 (100) | 69 (86) | 28 (82) | 41 (89) |
| Exposure to all three drugs | — | 28 (35) | 18 (53) | 10 (22) |
| Bevacizumab | ||||
| First-line use | — | 33 (41) | 13 (38) | 20 (43) |
| Second-line use | — | 13 (16) | 8 (24) | 5 (11) |
Significant difference (p ≤ 0.05) compared with (B).
Significant difference (p ≤ 0.05) compared with (D).
Patient groups could not be compared because of missing data.
Statistical tests not applicable.
CCI = Charlson comorbidity index; ECOG = Eastern Cooperative Oncology Group; NA = not applicable.
Table ii presents the multivariate analysis of the 109 patients, which indicates that an ecog performance status of 2 or greater was associated with an increased risk of death [hazard ratio (hr): 3.12; 95% ci: 1.69 to 5.76]. Other baseline characteristics such as age, sex, and cci score were not associated with an increased risk of death. Unfortunately, too many adl and iadl data were missing to permit the functional status of the patients to be studied. A sensitivity analysis by multiple imputations was conducted, and the missing adls were placed in the multivariate model. The addition of those adls to the model did not change the significance of the other factors.
TABLE II.
Results of multivariate analysisa
| Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Age | 0.99 | 0.92 to 1.05 | 0.673 |
| Male sex | 0.93 | 0.58 to 1.51 | 0.775 |
| CCI score | 1.09 | 0.64 to 1.83 | 0.761 |
| ECOG performance status ≥2 | 3.12 | 1.87 to 5.76 | <0.001 |
| ADL dependencyb | 1.04 | 0.43 to 2.52 | 0.940 |
| ADL data missing | 2.03 | 1.20 to 3.41 | 0.008 |
| Doublet | 0.33 | 0.17 to 0.66 | 0.001 |
| Capecitabine | 0.57 | 0.28 to 1.18 | 0.130 |
A sensitivity analysis by multiple imputations was performed, and the factor of dependency for activities of daily living was added.
For activities of daily living, 35% of the data were missing.
HR = hazard ratio; CI = confidence interval; CCI = Charlson comorbidity index; ECOG = Eastern Cooperative Oncology Group; ADL = activities of daily living.
Advanced Age and Chemotherapy
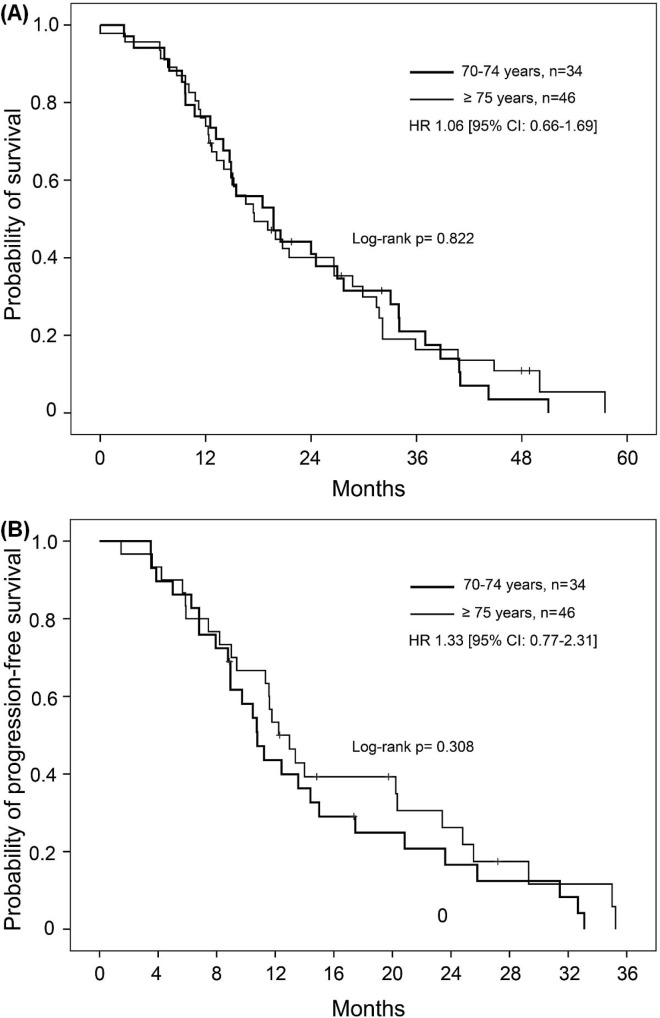
Median os was 19.7 months (95% ci: 12.6 to 26.7 months) for the 70–74 age group and 17.5 months (95% ci: 11.7 to 23.4 months) for the 75 and older group (log-rank p = 0.822). Univariate analysis revealed no significant difference in os between the age groups receiving chemotherapy [hr: 1.055; 95% ci: 0.66 to 1.67; Figure 2(A)]. No significant differences in the os and pfs rates were found between the age groups for any of the first-line chemotherapy subgroups, including capecitabine and all doublets. In the multivariate analysis, age was not a factor influencing the risk of death (hr: 0.97; % ci: 0.91 to 1.04; p = 0.43).
FIGURE 2.
Kaplan-Meier estimates for probability of (A) survival and (B) progression-free survival, by age group, for elderly patients who received chemotherapy. HR = hazard ratio; CI = confidence interval.
First-Line Chemotherapy
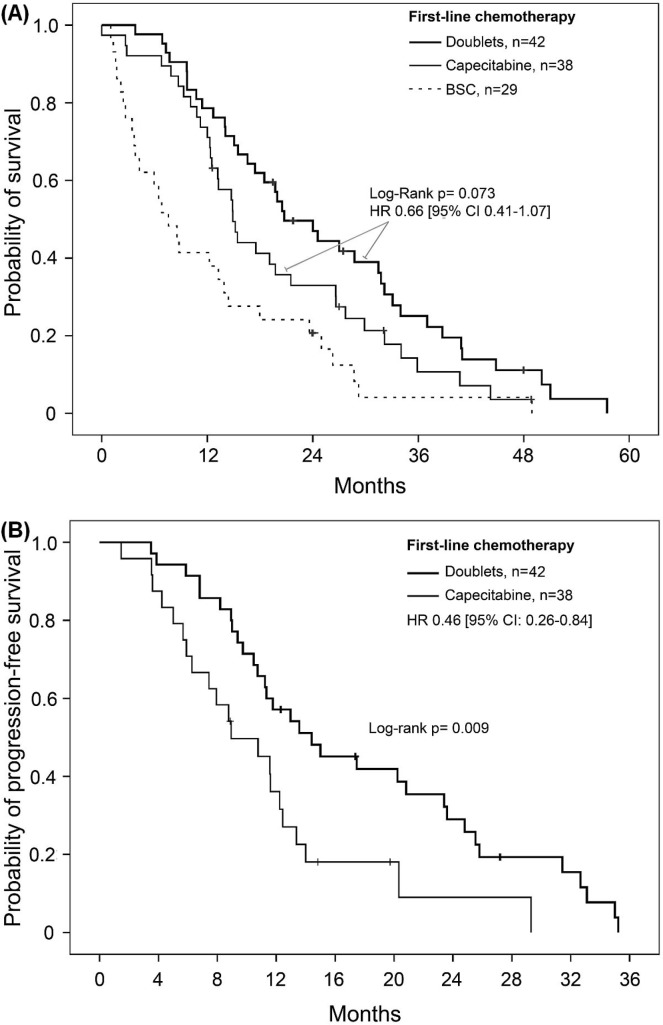
First-line oxaliplatin and irinotecan doublets resulted in similar os (p = 0.804) and pfs (p = 0.450) rates for all of the elderly patients who received chemotherapy and were therefore pooled. Figure 3 presents the Kaplan–Meier curves for the patients who received one of the doublets, capecitabine, or bsc as initial management. Compared with patients who received capecitabine, those who received a doublet as first-line chemotherapy showed a trend toward increased os (hr: 0.66; 95% ci: 0.41 to 1.07). Median survival rates in those groups were 20.7 months (95% ci: 14.9 to 26.6 months) and 14.9 months (95% ci: 12.3 to 17.4 months) respectively (log-rank p = 0.073). Capecitabine and doublet regimens were both superior to bsc, which was associated with a median os of 7.6 months [95% ci: 3.9 to 11.2 months; Figure 3(A)]. First-line pfs was longer with a doublet than with capecitabine [hr: 0.46; 95% ci: 0.26 to 0.84; p = 0.011; Figure 3(B)]. Compared with the 70–74 age group, the 75 and older age group contained a greater proportion of patients who received first-line capecitabine rather than a doublet (57% vs. 35%, p = 0.05). Nevertheless, pfs after any first-line chemotherapy was similar in the two age groups [hr: 1.33; 95% ci: 0.77 to 2.31; Figure 2(B)]. First-line bevacizumab was used in the same proportion in both groups. At the time of the study, no patients were given therapy targeting the epidermal growth factor receptor.
FIGURE 3.
(A) Kaplan–Meier estimate for probability of survival in elderly patients (≥70 years of age) who received a doublet and who received capecitabine as first-line chemotherapy. Survival was superior for patients who received a doublet or capecitabine alone compared with patients who selected no chemotherapy (log-rank p < 0.001 and p = 0.025 respectively). (B) Kaplan–Meier estimate of progression-free survival in elderly patients (≥70 years of age) treated with a doublet or capecitabine alone (log-rank p = 0.009). BSC = best supportive care; HR = hazard ratio; CI = confidence interval.
Treatment Intensity
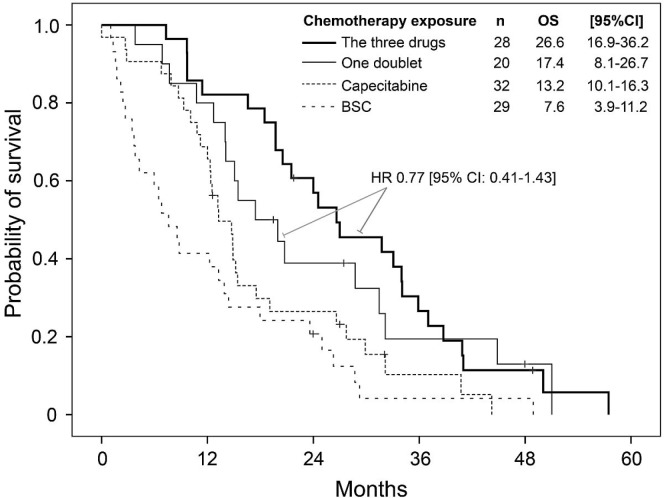
Patients 75 years of age and older were less likely than those 70–74 years of age to be exposed to all three chemotherapy backbones (5-fluorouracil–capecitabine, irinotecan, oxaliplatin: 22% versus 53%, p = 0.02), and a smaller proportion of the older group received second- and third-line chemotherapy (Table i). Exposure to two doublets compared with only one was not associated with a better os (hr: 0.77; 95% ci: 0.41 to 1.43; Figure 4). In the multivariate analysis, receipt of capecitabine as the only line of treatment was not statistically associated with longer survival (hr: 0.57; 95% ci: 0.28 to 1.12; p = 0.13); exposure to at least one doublet was associated with longer survival (hr: 0.36; 95% ci: 0.17 to 0.66). The 46 patients who received bevacizumab experienced a median survival of 24 months (95% ci: 16.4 to 31.6 months).
FIGURE 4.
Kaplan–Meier estimate for the probability of survival in elderly patients (≥70 years of age) exposed to all three major drugs (capecitabine, oxaliplatin, irinotecan), to just one doublet, or to capecitabine alone, or receiving only best supportive care (BSC). Exposure to the three drugs was superior to exposure to capecitabine alone (log-rank p = 0.004) and to BSC alone (log-rank p < 0.001), but not to exposure to just one doublet (log-rank p = 0.403). OS = overall survival; CI = confidence interval; HR = hazard ratio.
Toxicity
Table iii reports the grade 3 and 4 toxicities related to chemotherapy. Oxaliplatin doublets were associated with a significant rate of peripheral neuropathy (29%), and dose reductions were instituted in 71% of patients during their course of treatment. The irinotecan doublet led to a 40% rate of neutropenia, with 7% having febrile neutropenia, and 11% being admitted with an underlying infection. Of the patients on capecitabine, 13% developed hand–foot syndrome, and 11% had an infection requiring admission. Adverse effects of bevacizumab included 8% rates of both arterial (ate) and venous (vte) thrombotic events in our cohort of elderly patients. One patient (2.2%) experienced a visceral perforation while on bevacizumab (Table iv). Proteinuria, hypertension, and poor wound healing were inconsistently tracked in our retrospective review and were therefore not analyzed.
TABLE III.
Grade 3 or greater adverse effects of first-line chemotherapy
| Variable | Chemotherapy group [n (%)] | ||
|---|---|---|---|
|
| |||
| Irinotecan doublet | Oxaliplatin doublet | Capecitabine | |
| Patients | 28 | 14 | 38 |
| Dose reductiona | 10 (36) | 10 (71) | 5 (13) |
| Neutropenia | 10 (40) | 0 (0) | 0 (0) |
| Thrombocytopenia | 0 (0) | 0 (0) | 0 (0) |
| Anemia | 0 (0) | 0 (0) | 0 (0) |
| Hand–foot syndrome | 1 (4) | 2 (14) | 5 (13) |
| Peripheral neuropathy | 1 (4) | 4 (29) | 0 (0) |
| Diarrhea | 2 (7) | 0 (0) | 1 (3) |
| Febrile neutropenia | 2 (7) | 0 (0) | 0 (0) |
| Hospital admissionb | 3 (11) | 1 (7) | 4 (11) |
Includes all dose reductions that occurred after cycle 1 of chemotherapy.
Because of infectious complications.
TABLE IV.
Adverse effects with bevacizumab in elderly patients 70 or more years of age
| Line of treatment | Pts (n/N) | Effecta | ||
|---|---|---|---|---|
|
| ||||
| Arterial thrombosis | Venous thrombosis | Visceral perforation | ||
| First | 35/80 | 3 (9) | 3 (9) | 0 (0) |
| Second | 13/34 | 1 (8) | 1 (8) | 1 (8) |
| TOTAL | 46/80 | 4 (8.7) | 4 (8.7) | 1(2.2) |
Hypertension, proteinuria, and poor wound healing could not be collected retrospectively.
DISCUSSION
Treating elderly patients with mcrc can sometimes be challenging, given considerable patient heterogeneity, poor representation of this group in clinical trials, and the various available treatment strategies. Additionally, elderly patients not eligible for clinical trials might be more susceptible to drug toxicity or might receive less-intensive chemotherapies. The present retrospective study reports outcomes in elderly real-life non-trial patients (≥70 years of age) with mcrc treated with chemotherapy, and it explores the survival impact of the first and subsequent lines of treatment.
Compared with their counterparts who chose bsc, elderly patients who received chemotherapy experienced prolonged survival. In accord with the findings of the focus2 study of elderly and frail patients, the results of our multivariate analysis show that poor performance status was associated with worse outcomes, but that age and cci score were not11. In fact, number of comorbidities might be a better predictor of chemotherapy toxicity than of mortality17,18. Too many data on adl and iadl were missing to evaluate functional status in our patients.
Selection of the best first-line chemotherapy in elderly patients with mcrc has been poorly studied. In the focus2 trial, the addition of oxaliplatin to capecitabine failed to significantly improve pfs11. In comparison, in our study, doublets were associated with longer pfs and a trend toward better os that persisted beyond 2, 3, and 4 years (Figure 3). Given the lack of randomization, patients initially treated with capecitabine were likely to be more vulnerable or frail than the patients treated first with a doublet. Thus, the differences in functional and performance status could potentially account for the observed difference. Nevertheless, data from the U.S. Surveillance, Epidemiology, and End Results Medicare database suggest that choice of first-line of treatment could play an important role in older adults and could affect short-term and 5-year survival rates19.
The ideal intensity and number of lines of chemotherapy for this group of patients is difficult to define given their heterogeneity. In our study, multivariate analysis revealed that exposure to one doublet of chemotherapy (any line) was associated with better survival. However, and in contrast to results in younger trial-selected patients20, univariate analysis showed that exposure to 3, compared with just 2, active cytotoxic agents was not associated with a clear survival advantage. Similarly, patients 75 years of age and older experienced the same os as their counterparts 70–74 years of age despite receiving less-aggressive chemotherapy and fewer lines of treatment. The same observation was reported by Bakogeorgos et al.13, who described similar outcomes in non-trial patients 70 years of age and older (median: 76.6 years) and in younger patients (median: 57.4 years) with mcrc, despite less-intensive chemotherapy in the older group.
The retrospective nature of the data and the small number of patients limited interpretation of the results. However, it could be hypothesized that, although older and clinically fit patients clearly benefit from systemic therapy14,21, they might benefit not all from the same intensity and amount of chemotherapy. A prospective trial in elderly patients receiving comprehensive geriatric assessments would be necessary to make a more accurate determination. Among other factors, frailty and nutrition status could be particularly important in elderly patients with mcrc17.
Grade 3 and greater hematologic adverse effects of the 3 active standard chemotherapies were limited, but the irinotecan doublet was associated with 40% neutropenia and 7% febrile neutropenia, despite one third of the patients receiving dose-reduced chemotherapy. The rates of diarrhea, neuropathy, and hand–foot syndrome might have been underreported given the retrospective nature of the study.
Bevacizumab was associated with an 8% rate of ate and an additional 8% rate of vte. Although those rates are high, similar rates of thrombotic events have been reported in older adults10,22. In a pooled analysis, Cassidy et al.10 reported a 6.7% incidence of ate in adults 70 years of age and older with mcrc treated with bevacizumab. Likewise, those authors reported an incidence of vte of nearly 13%. Patients in the control groups who did not receive bevacizumab had incidences of ate and vte of 3% and 10% respectively. In the analysis, the proportion of bevacizumab-related ate increased with age. Other studies have proposed that bevacizumab-related thrombotic events are more common in colon cancer and in people with vascular comorbidities23,24, which emphasizes the need to carefully select elderly patients based on their comorbidities before adding bevacizumab to their chemotherapy. In our study, 29 of 46 patients who received bevacizumab had a cci score of 1 or higher, and it is therefore likely that some had one or more cardiovascular risk factors beyond advanced age. Of the 46 patients, one experienced a visceral perforation, for a rate comparable to that in the brite observational cohort study14.
Neutropenia associated with first-line folfiri was non-negligible, at an incidence of 40%, with a 7% rate of febrile neutropenia. Similar rates were reported in the bicc-c trial25, but the incidence of neutropenia remained high, given that up to 32% of the patients in that trial received initial dose-adjusted folfiri. Souglakos et al.26 reported a 20% incidence of grade 3 or greater neutropenia in patients 70 and more years of age treated with first-line folfiri.
The limitations of our study include its retrospective nature, and accordingly, the results should be carefully interpreted. The small number of patients, combined with their great heterogeneity, hindered our ability to adjust for all prognostic and predictive determinants in our analyses. Finally, an evaluation of the functional status of our patients, an important prognostic factor in elderly patients, could not be adequately performed given the amount of missing data.
CONCLUSIONS
Despite its limitations, our study emphasizes that elderly (70 and more years of age) non-trial patients with mcrc seem to derive benefit from a first-line doublet, with improvement in pfs compared with that achieved on capecitabine alone. Patients 75 and more years of age fared as well as those 70–74 years of age despite less-intense chemotherapy treatments. The high incidence of folfiri-related neutropenia and bevacizumab-related thrombotic events emphasizes the need to use adjusted-dose irinotecan in this population and to carefully select patients suitable for treatment with bevacizumab. Future prospective studies, with comprehensive systematic and geriatric assessment, are needed to evaluate the best treatment strategy in elderly patients and to better individualized the intensity and the type of chemotherapy delivered.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.United States Department of Health and Human Services. National Institutes of Health. National Cancer Institute . Home > Statistical Summaries > Cancer Stat Fact Sheets > Cancer of the Colon and Rectum [Web resource] Bethesda, MD: National Cancer Institute; Surveillance, Epidemiology, and End Results Program. n.d. [Available online at: http://seer.cancer.gov/statfacts/html/colorect.html; cited 1 October 2014] [Google Scholar]
- 2.Thirion P, Michiels S, Pignon JP, et al. on behalf of the Meta-Analysis Group in Cancer Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004;22:3766–75. doi: 10.1200/JCO.2004.03.104. [Erratum in: J Clin Oncol 2005;23:1337–8] [DOI] [PubMed] [Google Scholar]
- 3.Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the tree study. J Clin Oncol. 2008;26:3523–9. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 4.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–31. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 5.Droz JP, Aapro M, Balducci L. Overcoming challenges associated with chemotherapy treatment in the senior adult population. Crit Rev Oncol Hematol. 2008;68(suppl 1):S1–8. doi: 10.1016/j.critrevonc.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Denson AC, Mahipal A. Participation of the elderly population in clinical trials: barriers and solutions. Cancer Control. 2014;21:209–14. doi: 10.1177/107327481402100305. [DOI] [PubMed] [Google Scholar]
- 7.Folprecht G, Cunningham D, Ross P, et al. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials. Ann Oncol. 2004;15:1330–8. doi: 10.1093/annonc/mdh344. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–91. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 9.Folprecht G, Seymour MT, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol. 2008;26:1443–51. doi: 10.1200/JCO.2007.14.0509. [DOI] [PubMed] [Google Scholar]
- 10.Cassidy J, Saltz LB, Giantonio BJ, Kabbinavar FF, Hurwitz HI, Rohr UP. Effect of bevacizumab in older patients with metastatic colorectal cancer: pooled analysis of four randomized studies. J Cancer Res Clin Oncol. 2010;136:737–43. doi: 10.1007/s00432-009-0712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (mrc focus2): an open-label, randomised factorial trial. Lancet. 2011;377:1749–59. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozloff M, Yood MU, Berlin J, et al. on behalf of the investigators of the brite study Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: the brite observational cohort study. Oncologist. 2009;14:862–70. doi: 10.1634/theoncologist.2009-0071. [DOI] [PubMed] [Google Scholar]
- 13.Bakogeorgos M, Mountzios G, Kotsantis G, Economopoulou P, Fytrakis N, Kentepozidis N. Chemotherapy compliance, tolerance and efficacy in elderly and non-elderly patients with metastatic colorectal cancer: a single institution comparative study. J BUON. 2013;18:629–34. [PubMed] [Google Scholar]
- 14.Kozloff MF, Berlin J, Flynn PJ, et al. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: results from the brite observational cohort study. Oncology. 2010;78:329–39. doi: 10.1159/000320222. [DOI] [PubMed] [Google Scholar]
- 15.Foster JA, Salinas GD, Mansell D, Williamson JC, Casebeer LL. How does older age influence oncologists’ cancer management? Oncologist. 2010;15:584–92. doi: 10.1634/theoncologist.2009-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Aaldriks AA, van der Geest LG, Giltay EJ, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol. 2013;4:218–26. doi: 10.1016/j.jgo.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (crash) score. Cancer. 2012;118:3377–86. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 19.Hanna N, Onukwugha E, Bikov KA, Zheng Z, Seal BS, Mullins CD. Comparative analysis of second and subsequent chemotherapy lines on short- and long-term survival of elderly Medicare metastatic colon cancer patients [abstract 455] J Clin Oncol. 2013;31 [Available online at: http://meetinglibrary.asco.org/content/105754-133; cited 10 July 2015] [Google Scholar]
- 20.Grothey A, Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol. 2005;23:9441–2. doi: 10.1200/JCO.2005.04.4792. [DOI] [PubMed] [Google Scholar]
- 21.Ho C, Ng K, O’Reilly S, Gill S. Outcomes in elderly patients with advanced colorectal cancer treated with capecitabine: a population-based analysis. Clin Colorectal Cancer. 2005;5:279–82. doi: 10.3816/CCC.2005.n.040. [DOI] [PubMed] [Google Scholar]
- 22.Price TJ, Zannino D, Wilson K, et al. Bevacizumab is equally effective and no more toxic in elderly patients with advanced colorectal cancer: a subgroup analysis from the agitg max trial: an international randomised controlled trial of capecitabine, bevacizumab and mitomycin C. Ann Oncol. 2012;23:1531–6. doi: 10.1093/annonc/mdr488. [DOI] [PubMed] [Google Scholar]
- 23.Mohile SG, Hardt M, Tew W, et al. on behalf of the Cancer and Aging Research Group Toxicity of bevacizumab in combination with chemotherapy in older patients. Oncologist. 2013;18:408–14. doi: 10.1634/theoncologist.2012-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hershman DL, Wright JD, Lim E, Buono DL, Tsai WY, Neugut AI. Contraindicated use of bevacizumab and toxicity in elderly patients with cancer. J Clin Oncol. 2013;31:3592–9. doi: 10.1200/JCO.2012.48.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the bicc-c Study. J Clin Oncol. 2007;25:4779–86. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 26.Souglakos J, Pallis A, Kakolyris S, et al. Combination of irinotecan (cpt-11) plus 5-fluorouracil and leucovorin (folfiri regimen) as first line treatment for elderly patients with metastatic colorectal cancer: a phase ii trial. Oncology. 2005;69:384–90. doi: 10.1159/000089992. [DOI] [PubMed] [Google Scholar]