Abstract
Kaposi sarcoma (ks) is a vascular tumour caused by oncogenic human herpesvirus type 8; it often occurs with hiv-associated immunosuppression. Numerous cellular signalling pathways are involved in the pathogenesis of ks, among which receptor tyrosine kinases such as the c-Kit and platelet-derived growth factor receptors play an important role. Imatinib mesylate, a tyrosine kinase inhibitor, has resulted in partial regression of ks lesions in one third of treated patients, but its mechanism of action remains unclear.
Here, we report the case of a white man with recurrent ks despite well-suppressed hiv infection and multiple chemotherapies who received imatinib and showed a complete and sustained tumour response. To our knowledge, this report is the first showing the value of imatinib in the management of ks in the context of long-lasting hiv control with adequate quantitative CD4 recovery. Our case indicates that imatinib can be a treatment option for highly chemoresistant recurrent ks in patients on long-term antiretroviral therapy.
Keywords: hiv-1, Kaposi sarcoma, imatinib, autophagy
INTRODUCTION
Kaposi sarcoma (ks) is a multifocal angioproliferative tumour caused by oncogenic human herpesvirus type 8 (also known as ks-associated herpesvirus). It was the malignancy first identified to be associated with hiv infection at the onset of the aids epidemic.
Stabilization and regression of ks lesions, paralleling recovery of the CD4+ T-cell count, have been observed in almost all patients with hiv-associated ks (hiv-ks) because of viral load control after effective antiretroviral therapy (art). Cases of new-onset or unremitting ks in successfully treated hiv patients are rare, especially with a CD4+ T-cell count exceeding 500/μL. Managing such patients when their response to chemotherapeutic agents is limited can be very challenging.
Numerous cellular signalling pathways are involved in the pathogenesis of ks, including those involving receptor tyrosine kinases such as c-Kit and platelet-derived growth factor receptor (pdgfr)1,2. Imatinib mesylate (Gleevec: Novartis, Basel, Switzerland; hereinafter called imatinib), a tyrosine kinase inhibitor that was first approved as a treatment for chronic myeloid leukemia, was recently found to have effects on ks lesions3,4. Here, we report the case of a white man with recurrent ks despite well-controlled hiv infection who received imatinib and showed complete tumour response.
CASE DESCRIPTION
In July 2013, a 38-year-old white man was referred to the Chronic Viral Illness Service at McGill University Health Centre, Montreal, QC, with a 5-year history of persistent cutaneous nodular ks on the lower limbs, coupled with an 8-year history of hiv infection. The patient had, elsewhere in 2005, been diagnosed with hiv infection; he had continuously received art since then. He became aviremic—with a CD4+ T-cell count exceeding 500/μL—shortly after initiation of art. On presentation, he was on a regimen of tenofovir, emtricitabine, and raltegravir. His viral load was undetectable (<40 copies/mL), and his CD4+ T-cell count was 749/μL.
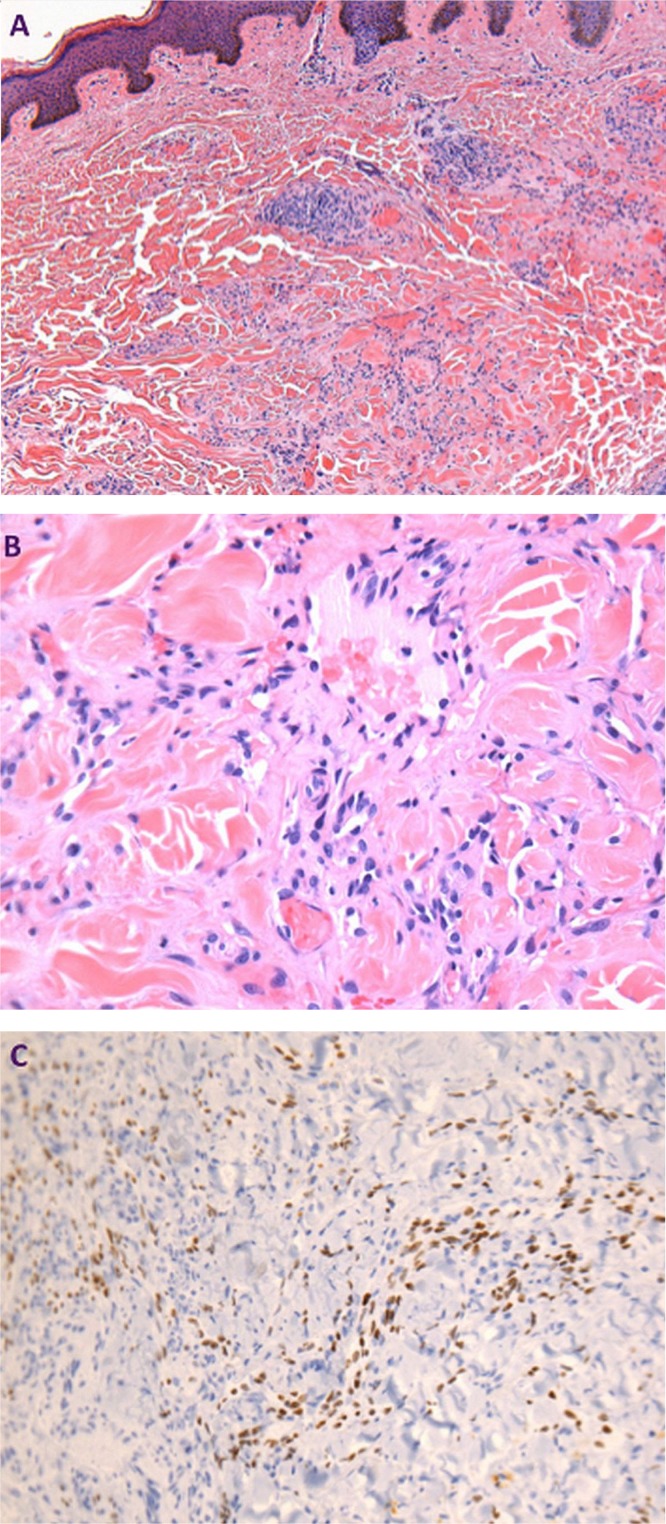
The patient developed cutaneous nodular ks on his lower limbs in 2008 while his hiv infection continued to be well controlled. Biopsy and pathology examination of the lesion on his right thigh revealed characteristic spindle cells and high vascularity, with positivity for the human herpesvirus type 8, determined to be ks (Figure 1). He had since been treated over a period of 5 years with multiple cycles of liposomal doxorubicin, paclitaxel, vinorelbine, and bortezomib, and had failed to sustain partial response for more than 6 months after chemotherapy.
FIGURE 1.
Pathology of the left thigh lesion. (A,B) Patch stage of Kaposi sarcoma, with capillary proliferation dissecting collagen. Hematoxylin and eosin stain, 100× and 400× magnification respectively. (C) Nuclear positivity of endothelial cells, highlighting vascular proliferation. HHV8 immunostain, 200× magnification.
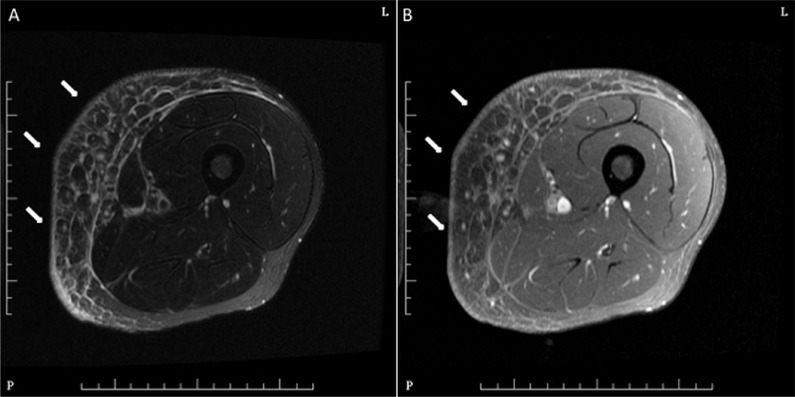
When referred to our centre in 2013, the patient had left lower limb ks lesions aggravated by prominent pain and swelling, and he had difficulty in moving. Physical examination revealed dark violet nodular lesions on both thighs, the left being more prominent and the biggest measuring 15×12 cm, with swelling and tenderness. Blood tests for immunoglobulin G antibodies to cytomegalovirus were positive, and tests for cytomegalovirus antigen, hepatitis B or C antigen, and syphilis were negative. Magnetic resonance imaging and ultrasonography of the lower limbs were confirmative of soft-tissue infiltration by ks (Figure 2).
FIGURE 2.
Magnetic resonance images of the left thigh. (A) Axial T2 weighted image and (B) contrast-enhanced T1 weighted image with fat suppression indicate mild skin thickening, subcutaneous edema, and thickening of the adjacent deep fascia (arrows), which are consistent with soft-tissue involvement of Kaposi sarcoma.
The patient received 8 cycles of paclitaxel with the mtor (mammalian target of rapamycin) inhibitor sirolimus as an immunomodulator for 1 year, which resulted only in transient and partial tumour control. Because of tumour progression, treatment options were discussed with the patient, including re-initiation of previously used chemotherapy, immunomodulators such as thalidomide, and imatinib. After consideration, the patient decided to receive imatinib. Oral imatinib 400 mg daily was therefore prescribed with liposomal doxorubicin beginning in September 2014.
The patient was closely followed in clinic, and major regression of the lesions started to take place 1 month after imatinib initiation—something not previously observed. At the beginning of imatinib therapy, the patient reported mild swelling of the eyelids and lower limbs and extreme fatigue and malaise, which were treated with furosemide and nonpharmacologic measures. The symptoms gradually improved after the first 2 months. Chemotherapy was stopped after 2 cycles, and the symptoms continued to improve on imatinib monotherapy. At his last visit (after 9 months of treatment), all ks lesions had completely resolved for the first time since the ks diagnosis.
DISCUSSION
HIV-KS in the Context of Virus-Controlled HIV Infection
Patients with ks typically present with a CD4+ T-cell count below 350/μL in absence of art. However, new-onset or unremitting ks has been reported in hiv-infected patients with an undetectable viral load and restored CD4+ T-cell count. In 1999, the first 2 cases of hiv-ks in the setting of viral suppression were reported by Chan et al.5, followed by several case series of hiv-ks with CD4+ T-cell counts exceeding 300/μL and an undetectable hiv viral load6,7. Still, ks persistence despite effective art remains rare.
In addition, our patient was unique because of the onset of ks with a CD4+ T-cell count exceeding 600/μL combined with 3 years of hiv viral suppression. However, unlike the indolent course of ks usually reported in controlled hiv infection, the ks lesions in our patient were progressive and refractory to multiple chemotherapies despite a well-restored CD4+ T-cell count. Those observations raise questions about the interplay between human herpesvirus type 8, hiv, and the development of ks, especially in the context of effective art.
Factors that have been postulated to play a role in the development or persistence of ks in such patients include older patient age, duration of hiv infection, and type of art used6,8. More recently, Unemori et al. observed that increased frequencies of immunosenescence phenotypes (CD57+ and CD28−) and lower frequencies of naïve T cells (CD27+, CD28+, CD45RA+) are associated with the presence of ks in art-treated patients, indicating a role for “immune aging” in relation to an increasing vulnerability to ks9. Nevertheless, more investigation in this population is required to elucidate the underlying mechanisms. Furthermore, whether an approach other than chemotherapy in addition to art will better target such ks lesions remains unknown. In that sense, our case suggests a treatment option for recurrent and refractory ks in patients treated with long-term art.
Imatinib As a Pathogenesis-Based Therapy
A reduced incidence and remarkable regression of ks have been reported in hiv-infected patients treated with art or with chemotherapy. However, none of the ks treatments to date are curative. The development of ks is currently well recognized to be an inflammation-driven angiogenic and oncogenic process in which a number of cytokines and growth factors with autocrine and paracrine growth effects create a favourable microenvironment for ks formation10. Targeted therapies based on antiangiogenic agents and cytokine signalling pathway inhibitors have therefore been regarded as a priority in treatment development.
Activation of the receptor tyrosine kinases such as pdgfr and c-Kit receptor has been proposed to play a role in mediating the growth of hiv-ks1,2,11. As a multikinase inhibitor, imatinib has shown activity against pdgfr and c-Kit in treating gastrointestinal stromal tumours with activating mutations in pdgfr and c-Kit12,13. The response of hiv-ks to imatinib has also been investigated (detailed in Table i). In 2005, a pilot study by Koon et al. evaluated the clinical and histologic effects of imatinib on 10 patients with progressive cutaneous hiv-ks despite chemotherapy or art, or both3. After 4 weeks of oral imatinib (300 mg twice daily), 5 of 10 participants experienced a partial response. In addition, biopsies of lesions in the responders demonstrated histologic regression, correlated with inhibition of pdgfr and its downstream effector, extracellular receptor kinase. However, a phase ii study by the same group, in which 30 patients received imatinib 400 mg daily for an intended 52 weeks, failed to identify a link between clinical outcomes and the alteration in pdgfr or c-Kit downstream effectors, despite an observed partial response in one third of participants4. Those results raise the question of the operating mechanisms underlying imatinib’s efficacy.
TABLE I.
Summary of clinical studies of imatinib mesylate in Kaposi sarcoma (KS)
| Reference | Pts (n) | Study details | Drug regimen | Clinical outcome | Safety |
|---|---|---|---|---|---|
| Koon et al., 20053 | 10 | Patients with progressive cutaneous HIV-KS were eligible. All received ART for a mean duration of 37.5 weeks; 7 had received prior single-agent chemotherapy for KS. |
Oral imatinib 300 mg twice daily for 4 weeks | Partial clinical response (by tumour measurements) was observed in 5 of the 10 patients. Histologic regression was demonstrated in 4 of 6 patients, and inhibition of PDGFR was observed in 4 of 4 patients. |
Gastrointestinal (drug withdrawn in 5 patients; dose reduction applied in the rest) |
| Koon et al., 20144 | 30 | Patients with HIV-KS were eligible. ART had been received by 77% (23 of 30), and prior therapy for KS, by 60%. |
Oral imatinib 400 mg daily for up to 52 weeks, with dose escalation up to 600 mg daily at the 3rd month, if stable | Partial response was observed in 10 of 30 patients, and stable disease in 6 (20%). No significant decrease in ERK phosphorylation was observed in post-treatment biopsies. No mutation in PDGFR or c-Kit receptors was identified. |
Grade 3 or 4 imatinib-related adverse events were reported in 8 patients; 5 of those patients terminated the therapy. |
Pts = patients; ART = antiretroviral therapy; PDGFR = platelet-derived growth factor receptor; ERK = extracellular receptor kinase.
A study by Ertmer et al.14 showed that treatment with imatinib could lead to a dose-dependent activation of cellular autophagy, which was likely induced by inhibition of c-Abl rather than by inhibition of pdgfr or c-Kit receptor. Autophagy is a cellular catabolic mechanism involved mainly in the recycling and turnover of cytoplasmic constituents. Regarded as a double-edged sword in tumour progression, autophagy can promote cell adaption and survival in some circumstances and induce autophagic cell death and growth arrest in tumours in others. Imatinib is known for its effect of inducing tumour cell apoptosis by inhibition of oncogenic tyrosine kinase signalling and subsequent enhanced CD8 cytotoxic function and decreased regulatory T-cell inhibition, as demonstrated in the case of gastrointestinal stromal tumours15. However, imatinib might also have the capacity to shift the balance between apoptosis and autophagy in ks tumour cells. Basciani et al.16 provided evidence that imatinib results in increased autophagy in multidrug-resistant ks cells, indicating that autophagy might represent an additional mechanism of tumour regression in ks.
Given that resistance or unresponsiveness to chemotherapeutic agents results predominantly from defects in the apoptotic signalling pathway, agents such as imatinib that can induce autophagy could be important in managing recurrent ks such as that experienced by our patient. In addition, previous studies have indicated that autophagic activity is inversely correlated with virus production in hiv infection, and pharmacologically induced autophagy in infected CD4+ T cells could lead to a significant decline in viral replication17,18. In that sense, whether imatinib might generate a synergistic effect in terms of viral control and reservoir eradication in hiv-ks patients would be of special interest.
SUMMARY
We report a rare case of highly chemoresistant ks in well-controlled hiv infection, which was successfully treated using imatinib. Imatinib can be a treatment option for multiresistant hiv-ks, and its operating mechanism needs more investigation.
ACKNOWLEDGMENTS
We thank the patient for his consent to publish this case report. We acknowledge Jacquie Sas and Jim Pankovich for coordinating the research with the support of Canadian Institutes of Health Research (cihr), cihr Canadian hiv Trials Network (ctn). We thank Angie Massicotte for coordination and assistance during the writing of this case report.
WC is supported by the 2014 cihr ctn International Postdoctoral Fellowship. BR is supported by Fondation Philanthropia and the Catherine and Stuart Townsend Hematology Fellowship Award. JPR is holder of the Louis Lowenstein Chair in Hematology and Oncology, McGill University.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Sturzl M, Roth WK, Brockmeyer NH, Zietz C, Speiser B, Hofschneider PH. Expression of platelet-derived growth factor and its receptor in aids-related Kaposi sarcoma in vivo suggests paracrine and autocrine mechanisms of tumor maintenance. Proc Natl Acad Sci U S A. 1992;89:7046–50. doi: 10.1073/pnas.89.15.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moses AV, Jarvis MA, Raggo C, et al. Kaposi’s sarcoma–associated herpesvirus-induced upregulation of the c-Kit proto-oncogene, as identified by gene expression profiling, is essential for the transformation of endothelial cells. J Virol. 2002;76:8383–99. doi: 10.1128/JVI.76.16.8383-8399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koon HB, Bubley GJ, Pantanowitz L, et al. Imatinib-induced regression of aids-related Kaposi’s sarcoma. J Clin Oncol. 2005;23:982–9. doi: 10.1200/JCO.2005.06.079. [DOI] [PubMed] [Google Scholar]
- 4.Koon HB, Krown SE, Lee JY, et al. Phase ii trial of imatinib in aids-associated Kaposi’s sarcoma: aids Malignancy Consortium Protocol 042. J Clin Oncol. 2014;32:402–8. doi: 10.1200/JCO.2012.48.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J, Kravcik S, Angel JB. Development of Kaposi’s sarcoma despite sustained suppression of hiv plasma viremia. J Acquir Immune Defic Syndr. 1999;22:209–10. doi: 10.1097/00126334-199910010-00017. [DOI] [PubMed] [Google Scholar]
- 6.Maurer T, Ponte M, Leslie K. hiv-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. N Engl J Med. 2007;357:1352–3. doi: 10.1056/NEJMc070508. [DOI] [PubMed] [Google Scholar]
- 7.Krown SE, Lee JY, Dittmer DP, on behalf of the aids Malignancy Consortium More on hiv-associated Kaposi’s sarcoma. N Engl J Med. 2008;358:535–6. doi: 10.1056/NEJMc072994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leitch H, Trudeau M, Routy JP. Effect of protease inhibitor-based highly active antiretroviral therapy on survival in hiv-associated advanced Kaposi’s sarcoma patients treated with chemotherapy. HIV Clin Trials. 2003;4:107–14. doi: 10.1310/VQXJ-41X6-GJA2-H6AG. [DOI] [PubMed] [Google Scholar]
- 9.Unemori P, Leslie KS, Hunt PW, et al. Immunosenescence is associated with presence of Kaposi’s sarcoma in antiretroviral treated hiv infection. AIDS. 2013;27:1735–42. doi: 10.1097/QAD.0b013e3283601144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707–19. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betsholtz C. Insight into the physiological functions of pdgf through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–28. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 13.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 14.Ertmer A, Huber V, Gilch S, et al. The anticancer drug imatinib induces cellular autophagy. Leukemia. 2007;21:936–42. doi: 10.1038/sj.leu.2404606. [DOI] [PubMed] [Google Scholar]
- 15.Balachandran VP, Cavnar MJ, Zeng S, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of ido. Nat Med. 2011;17:1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basciani S, Vona R, Matarrese P, et al. Imatinib interferes with survival of multi drug resistant Kaposi’s sarcoma cells. FEBS Lett. 2007;581:5897–903. doi: 10.1016/j.febslet.2007.11.069. [DOI] [PubMed] [Google Scholar]
- 17.Sagnier S, Daussy CF, Borel S, et al. Autophagy restricts hiv-1 infection by selectively degrading Tat in CD4+ T lymphocytes. J Virol. 2015;89:615–25. doi: 10.1128/JVI.02174-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandell MA, Kimura T, Jain A, Johansen T, Deretic V. trim proteins regulate autophagy: trim5 is a selective autophagy receptor mediating hiv-1 restriction. Autophagy. 2014;10:2387–8. doi: 10.4161/15548627.2014.984278. [DOI] [PMC free article] [PubMed] [Google Scholar]