Summary
Purpose
Few studies have found differences in rates of epilepsy by race or ethnicity although previous reports indicate strong links between epilepsy and socioeconomic indicators. We investigated social and demographic factors as they relate to prevalence and incidence of epilepsy in Washington, DC, a culturally diverse area.
Methods
Probability-based sampling was used to select 20,000 DC households that were mailed an epilepsy screening survey. Demographic and epilepsy data were obtained on all household members. Screened individuals with a history of epilepsy or seizure disorder were sent a case survey asking more detailed questions about seizures and treatment which were used to verify case status using the standard case definition. Survey data were weighted to match characteristics of DC residents. Lifetime and active prevalence and incidence of epilepsy were estimated using weighted data and appropriate survey procedures in SAS.
Key Findings
Overall survey response rate was 36.6%. 208 cases of epilepsy were identified during screening and 14% with a case survey were considered false positive. Using the verified dataset, lifetime prevalence was 1.53% overall; 0.77% in Whites, 2.13% in Blacks, 3.4% in those with less than a high school diploma, and 2.27% in those with household income less than $30,000. Overall prevalence of active epilepsy was 0.79% and followed similar subgroup comparisons as lifetime prevalence. Age-adjusted lifetime and active epilepsy from multivariate analyses demonstrated significantly higher rates for Blacks compared to Whites and for those not completing high school compared to those that attended graduate school. The overall incidence of epilepsy was 71 per 100,000 persons. Adults with active epilepsy were significantly less likely to live alone than those without epilepsy (36.0% versus 46.1%). Residents of DC for less than four years had the lowest prevalence and incidence of all subgroups indicating a possible healthy mover effect.
Significance
Our study is the first to provide region-specific estimates and profiles of the epilepsy population in DC which can help inform policy makers and healthcare providers on where to better target resources to improve the health and outcomes of people with epilepsy and their families.
Keywords: epilepsy, incidence, prevalence, race, education
Introduction
General surveys measuring self-reported epilepsy occurrence among U.S. adult populations have yielded estimates of lifetime prevalence ranging from 1.2 to 2.9%, and estimates of active or point prevalence ranging from 0.8 to 1.6% (Konda et al., 2009; Kobau et al., 2008; Kobau et al., 2006; Ottman et al., 2011). These variations may be due to differences in study population demographics and survey methodology. From population-based studies in developed countries, which include additional methods to confirm clinical diagnoses of epilepsy, estimates of epilepsy prevalence across all ages range from 0.4% to 0.9% and in children range from 0.4% to 0.5% (Hirtz et al., 2007). The median estimate of age-adjusted epilepsy incidence among such studies is 48 per 100,000 (Hirtz et al., 2007).
In most population-based surveys, prevalence has been estimated using a single screening question that asks about the history of a diagnosis of epilepsy or a seizure disorder. However, a study that involved additional self-reported information about seizures and treatment found that 18.5% of reports from a single screening question were false positive (Kelvin et al., 2007). When medical records were used as the gold standard for a diagnosis of epilepsy compared to a self-report, the false positive rate from a single screening question rose to 23.8% (Ottman et al., 2010).
With few exceptions, U.S. studies have found no significant differences in rates of epilepsy by race or ethnicity (Burneo et al., 2009; Ottman et al., 2011; Kelvin et al., 2007; Kobau et al., 2006; Kobau et al., 2008; Haerer et al., 1986). However, there are previous reports of strong links between epilepsy and lower educational attainment and income (Elliott et al., 2009; Kobau et al., 2007; Elliott et al., 2008; Konda et al., 2009; Kobau et al., 2006; Ottman et al., 2011; Ferguson et al., 2008, Geerts et al., 2011, Sillanpaa, 2004). While education is highly correlated with income and expected to produce analogous results, it also involves cognitive aspects that can impact recognition of and self-care for epilepsy, as well as the ability to live independently.
The Washington DC Health Study (DCHS) was initiated to estimate the incidence and prevalence of epilepsy among underrepresented groups to help guide policy makers and health care organizations in understanding potential disparities in access to care. The District of Columbia (DC) was chosen as the study site because of its rich cultural, racial and socioeconomic diversity. According to 2009 census data, DC includes a high proportion of non-Hispanic Blacks (52.7%) compared to the national estimate of 12.1%. Although the median household income for DC is higher than the country as a whole, the number of families living below poverty level is also higher than the national average (14.6% versus 10.5%). In addition, compared to national averages, DC has more adult residents that live alone (46.6% versus 27.4%) and are highly educated (28.0% versus 10.3%). We sought to investigate these factors and other demographic indicators as they relate to the prevalence and incidence of epilepsy.
METHODS
Sampling Frame
Address Based Sampling (ABS) was used to reach a representative sample of 20,000 households in DC. This method has gained recent popularity because of evolving problems associated with telephone-based samples, eroding rates of response to single methods of contact, and improvements in the databases of household addresses available to researchers. Specifically, the Computerized Delivery Sequence File (CDSF) of the US Postal Service, the most complete address database available in the US providing near perfect coverage, was used as the frame to select a representative sample of household addresses. In order to identify enough minority households with a member with epilepsy, a stratified sampling design was used whereby DC households in certain Census Block Groups (CBG) were over-sampled according to the allocation summarized in Table 1.
Table 1.
Distribution of all households in DC and in the sample by percent Blacks and median household income from 2006 Census
| Percent Blacks in CBG |
Median Household Income for Census Block Group (CBG) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Less than $30,000 | $30,000– $39,999 | $40,000– $49,999 | More than $49,999 |
Total | ||||||
| DC | Sample | DC | Sample | DC | Sample | DC | Sample | DC | Sample | |
| > 95% | 15.2% | 18.0% | 6.9% | 8.1% | 1.2% | 1.4% | 1.2% | 8.7% | 24.5% | 36.2% |
| 70 – 94% | 8.8% | 5.2% | 7.7% | 4.5% | 4.4% | 2.6% | 3.9% | 2.3% | 24.8% | 14.6% |
| 10 – 69% | 4.5% | 2.6% | 6.3% | 3.7% | 3.8% | 2.3% | 8.8% | 5.2% | 23.4% | 13.8% |
| < 10% | 0.5% | 3.7% | 1.6% | 1.9% | 3.8% | 4.5% | 21.4% | 25.3% | 27.3% | 35.4% |
| Total | 29.0% | 29.5% | 22.5% | 18.2% | 13.2% | 10.8% | 35.3% | 41.5% | 100.0% | 100.0% |
Survey Design and Administration
There were three phases of data collection for the DCHS, involving four data collection instruments. In Phase I a one-page bi-lingual screening survey asked five basic demographic and three epilepsy screening questions for all household members. Demographic data included age, gender, race/ethnicity, education, and length of residency in DC. Answer choices for race/ethnicity included White, Black, Hispanic, Asian, and Other. The epilepsy screening questions were derived from the Behavioral Risk Factor Surveillance System’s (BRFSS) epilepsy module (Kobau et al., 2008) and included “Ever diagnosed with epilepsy or a seizure disorder?”, “Currently taking any medication to control seizures?”, and “What year was the first seizure?”. Limited space on the survey did not allow for a question about the date of the most recent seizure.
In Phase II, a case survey was mailed to each household that had identified a prevalent case of epilepsy from the first screening question in the Phase I survey. The case survey included detailed questions about seizures and treatment, co-morbid conditions, quality of life, and social factors such as marital status, school and employment. Parents were asked to complete the case surveys on behalf of children. Also in Phase II was a control survey which was mailed to households that completed a screening survey and reported no prevalent cases. The control survey was identical to the case survey without the questions about seizures and treatment.
The Phase III survey was developed after preliminary analyses of the case survey suggested that the number of prevalent cases of epilepsy from the screening survey was overestimated due to self-reporting of febrile, provoked, and isolated unprovoked seizures. This supplemental survey was sent to all cases and included two pages of questions about the causes of the seizures.
The surveys and letters of invitation, which included a statement of informed consent, were approved by the institutional review board for protection of human subjects at RTI International, Research Triangle Park, NC.
Multi-Mode Data Collection
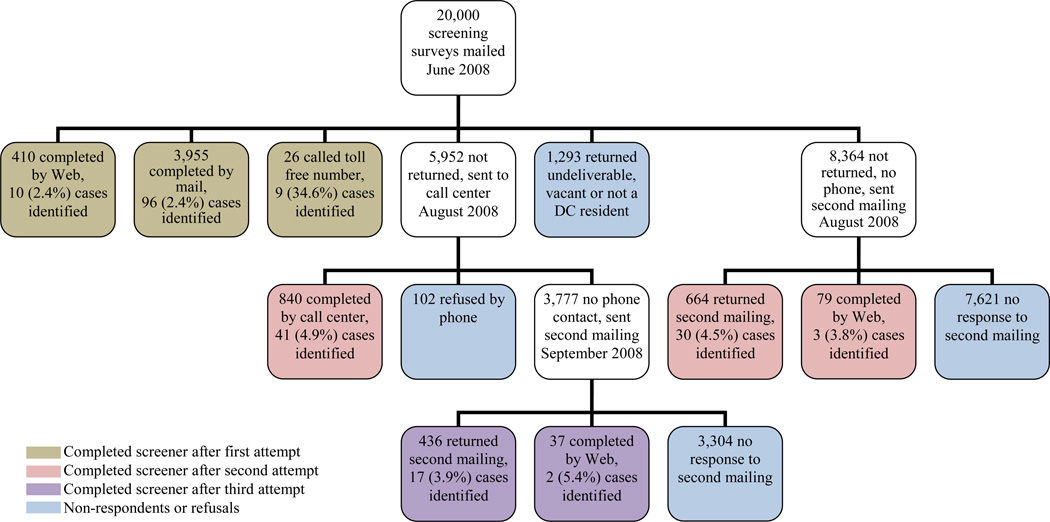
In order to increase response rates, data were collected using several modes of administration: mail, telephone, and internet. In June 2008, sample households were initially mailed a postcard announcing the upcoming screening survey mailing and the study sponsor and local supporters. One week later, households were mailed the Phase I screening survey and asked to complete and return it using the postage-paid envelope enclosed. All mailings were addressed to the name associated with the household telephone number, when available, followed by “or current resident”. A $1 bill was included in the mailing to encourage response. The survey was re-sent to all non-responding households without a corresponding telephone number approximately 6 weeks after the initial screening survey was mailed. At this point, an incentive of $5 was provided for each completed survey. Non-responding households with a corresponding telephone number, including those for which the screener was returned as undeliverable, were called by a trained interviewer beginning seven weeks after the initial survey was mailed. If multiple attempts to reach these households by telephone were unsuccessful, all non-responding households were re-mailed the screening survey. Figure 1 presents the data collection scheme for the screening survey.
Figure 1.
Data Collection Flow and Response Rates
Throughout Phase I data collection, any household identified as having an individual with epilepsy or a seizure disorder was sent the Phase II case survey to be completed by, or on behalf of, the person with seizures. An incentive of $40 was provided for each completed case survey. At least one additional attempt was made to contact by mail or telephone each non-respondent to the Phase II case survey. Additionally, online versions of Phase I and Phase II surveys were available for completion through the study website. Moreover, households and cases were invited to call a toll-free number if they preferred to complete the surveys by telephone. Data collection for both the screening and case surveys ended in January 2009.
In August 2009, the Phase II control survey was administered as a mail-only survey, with one mail follow-up of non-respondents six weeks later. The Phase III supplemental survey was mailed only once in February 2011 to the initial prevalent cases identified from the screening survey, regardless of whether a case survey was already completed. Communication with respondents was through household address and not name, so there was no way of verifying whether the household members that completed the initial screening survey were the same as those that completed the control and supplemental surveys. A change in residents for the control survey was not a concern. Effects on the Phase III supplemental survey are discussed in the results section.
Case Definition and Validation
In order to compare the findings from the DCHS with other population-based estimates of epilepsy, such as the BRFSS (http://www.cdc.gov/brfss), the results from the Phase I screening survey were summarized without influence from the Phase II and III case and supplemental surveys. For the Phase I analyses of screened cases, a lifetime prevalent case of epilepsy was defined as a positive response to the first screening question about a diagnosis of epilepsy or a seizure disorder. For the Phase II/III analyses (the verified cases), an epileptologist (WDG) reviewed all case and supplemental surveys and assigned a final status of positive or negative for each of the lifetime prevalent cases from the screening survey. Epilepsy was confirmed in cases with evidence of two or more unprovoked seizures, consistent with the International League Against Epilepsy (ILAE) case definition (Commission on Epidemiology and Prognosis of the ILAE, 1993). In addition, missing or obvious discrepant data on the Phase I screening survey were replaced with data from the Phase II case survey, when available. All survey weights were then adjusted to reflect the fact that a subset for which no validation data were available could emerge as false positive cases.
An active case of epilepsy was defined as a lifetime prevalent case currently taking a medication to control seizures, according to the screening survey. This definition differs slightly from that used in the BRFSS and Healthstyles surveys where active epilepsy also included a seizure within the previous three months in individuals not taking an anti-epileptic drug (AED) (Kobau et al 2006, Kobau et al. 2008). Annual incidence of epilepsy was calculated from the mean annual number of lifetime prevalent cases with a first seizure during the three-year period 2005–2007 based on the screening survey.
Imputation, Weighting and Statistical Analysis
Virtually all survey data are weighted before they can be used to produce reliable estimates of population parameters. For this study survey weights were calculated in several steps. In the first step, design weights were constructed to reflect different selection probabilities used to oversample households in certain block groups based on household income and percent Black race. These weights were adjusted to account for unknown eligibility and differential non-response, while the resulting weights were then post-stratified to reported counts of DC households indexed by characteristics such as household size. In addition to these household-level weights, a second set of person-level weights were calculated by reflecting the number of household members and post-stratification of the resulting weights to match weighted distributions of DC residents with respect to gender, race, and age from the 2009 Current Population Survey (March Supplement, http://www.census.gov/cps/). Depending on the level at which survey estimates were produced (household or person) the corresponding set of weights were applied.
Missing values for all variables were first imputed using a sequential hot-deck technique (Kalton & Kasprzakand, 1986). Specifically, missing values were replaced in a random fashion so that their corresponding observed distributions would be retained for the combined data. This process was implemented by first imputing the variable with the smallest number of missing values (gender) followed by the imputation of other variables, such as age, race, and education.
Subsequently, all estimations and analyses were carried out using weighted data and appropriate survey procedures in SAS to reflect the resulting weights and design features employed to select sample households (http://support.sas.com/rnd/app/da/new/dasurvey.html). Such procedures, including SurveyMeans and SurveyLogistic rely on Taylor Linearization technique to approximate variances of weighted survey estimates.
RESULTS
Response Rates
There were 6,447 households, representing 12,894 individuals (10,753 adults, 2,141 children), that responded to the DCHS screening survey for an overall unadjusted response rate of 32.2%. After adjustment for returned vacancies and undeliverable mail, the response rate increased to 36.6%. The mode of response to the completed surveys included 78.4% by mail, 13.4% by telephone, and 8.2% by web (Figure 1).
The demographic composition of the individuals in the study is presented in Table 2. There were more females (55.6%) than males (44.4%) and more Blacks (48.3%) than Whites (40.2%). Almost 54% of the study population was between the ages of 18 and 54 and 12.5% had recently moved into the DC city limits. There were 208 cases of epilepsy (174 adults, 34 children) reported by the screening survey in 201 households. Over half of the cases (115, 55%) were identified from responses to the first mailing, but the proportion of households with cases among the responders was higher for the subsequent mailings (Figure 1).
Table 2.
Prevalence of Lifetime and Active Epilepsy
| N1 | Lifetime Epilepsy Percent (95% CI) |
Active Epilepsy Percent (95% CI) |
|||
|---|---|---|---|---|---|
| Screened Cases | Verified Cases | Screened Cases | Verified Cases | ||
| All Cases | 12,894 | 1.78 (1.52– 2.05) | 1.53 (1.28– 1.77) | 0.86 (0.67– 1.05) | 0.79 (0.62– 0.97) |
| Gender | |||||
| Male | 5,726 | 1.90 (1.50– 2.31) | 1.62 (1.26– 1.99) | 0.95 (0.67– 1.24) | 0.86 (0.59– 1.13) |
| Female | 7,168 | 1.68 (1.34– 2.02) | 1.44 (1.13– 1.74) | 0.78 (0.54– 1.01) | 0.73 (0.51– 0.95) |
| Children/Adults | |||||
| 0–17 years | 2,163 | 1.76 (1.13– 2.40) | 1.29 (0.76– 1.81) | 0.68 (0.26– 1.09) | 0.61 (0.24– 0.98) |
| More than 17 years | 10,731 | 1.79 (1.50– 2.07) | 1.58 (1.32– 1.85) | 0.90 (0.70– 1.11) | 0.84 (0.65– 1.03) |
| Age Group | |||||
| 0–3 years | 519 | 1.87 (0.49– 3.25) | 1.42 (0.28– 2.55) | 0.91 (0.04– 1.78) | 0.80 (0.04– 1.57) |
| 4–17 years | 1,644 | 1.73 (1.01– 2.45) | 1.25 (0.66– 1.84) | 0.60 (0.13– 1.07) | 0.55 (0.12– 0.97) |
| 18–54 years | 6,901 | 1.71 (1.37– 2.06) | 1.53 (1.21– 1.85) | 0.76 (0.53– 0.99) | 0.71 (0.49– 0.93) |
| 55–69 years | 2,420 | 2.10 (1.43– 2.77) | 1.85 (1.24– 2.46) | 1.32 (0.79– 1.85) | 1.25 (0.74– 1.75) |
| More than 69 years | 1,410 | 1.69 (0.94– 2.43) | 1.43 (0.76– 2.10) | 1.16 (0.53– 1.79) | 0.95 (0.38– 1.51) |
| Race/Ethnicity | |||||
| White | 5,178 | 0.95 (0.67– 1.23) | 0.77 (0.52– 1.03) | 0.36 (0.19– 0.53) | 0.33 (0.17– 0.49) |
| Black | 6,224 | 2.44 (2.01– 2.87) | 2.13 (1.73– 2.53) | 1.22 (0.92– 1.53) | 1.13 (0.84– 1.42) |
| Hispanic | 786 | 1.27 (0.37– 2.17) | 0.95 (0.22– 1.68) | 0.69 (0.03– 1.35) | 0.61 (0.03– 1.19) |
| Other | 706 | 1.06 (0.11– 2.02) | 0.97 (0.10– 1.83) | 0.54 (0.00– 1.15) | 0.48 (0.00– 1.03) |
| Household Income2 | |||||
| Less than $30,000 | 3,160 | 2.68 (2.07– 3.28) | 2.27 (1.72– 2.83) | 1.25 (0.82– 1.68) | 1.12 (0.73– 1.51) |
| $30,000 – $49,999 | 4,031 | 1.42 (1.02– 1.83) | 1.26 (0.89– 1.63) | 0.65 (0.39– 0.91) | 0.63 (0.38– 0.88) |
| More than $49,999 | 5,703 | 1.28 (0.95– 1.61) | 1.06 (0.77– 1.35) | 0.70 (0.44– 0.95) | 0.64 (0.41– 0.88) |
| Highest Education3 | |||||
| Less than high school diploma | 619 | 4.22 (2.49– 5.96) | 3.40 (1.87– 4.92) | 2.80 (1.42– 4.19) | 2.46 (1.19– 3.72) |
| High school graduate | 1,552 | 2.59 (1.72– 3.45) | 2.43 (1.60– 3.25) | 1.36 (0.75– 1.98) | 1.31 (0.72– 1.91) |
| College | 3,810 | 1.92 (1.41– 2.42) | 1.68 (1.21– 2.15) | 0.87 (0.54– 1.21) | 0.80 (0.48– 1.11) |
| Graduate/professional school | 3,725 | 0.77 (0.48– 1.06) | 0.69 (0.41– 0.96) | 0.39 (0.18– 0.60) | 0.38 (0.17– 0.58) |
| Residence in DC3 | |||||
| 0–3 years | 1,614 | 0.92 (0.42– 1.42) | 0.65 (0.22– 1.08) | 0.34 (0.04– 0.64) | 0.32 (0.04– 0.61) |
| More than 3 years | 8,092 | 2.01 (1.65– 2.36) | 1.80 (1.47– 2.13) | 1.07 (0.81– 1.32) | 0.99 (0.75– 1.23) |
Number of unweighted observations
Median household income from Census blocks. Results were similar for adults over 24 years.
Adults over 24 years of age
Respondents to the Phase II case survey included 122 (58.7%) of the individuals identified as epilepsy cases on the screening survey (105 adults, 17 children). Those that returned a case survey compared to those that did not were more likely to be White (30.9% versus 11.8%), female (54.5% versus 47.6%), have post high school education (59.7% versus 37.8%), have lived in DC for three years or less (75.6% versus 54.8%), and be taking an AED (53.7% versus 45.9%). Sixty-three (30.3%) of 208 Phase III surveys were returned. Phase II control surveys mailed to 4,994 adults and 1,244 adult-child pairs were completed for 35.9% children and 35.5% adults. Findings from the case and control surveys will be the subject of a separate report.
Case validation
For the analyses of verified cases, 141 (67.8%) of the 208 cases from the Phase I screening survey had at least one Phase II or Phase III survey for review of their epilepsy status. However, 16 of 63 (25.4%) Phase III surveys reported no evidence of seizures in a household member and 15 of these 16 reported no recollection of previously being in the study. These 16 surveys were excluded from further consideration of epilepsy status because of the likelihood that the household residents had changed since the screening survey was completed three years prior. This left 133 of 208 (63.9%) screened cases with at least one Phase II or III survey for review. Of these 133, 112 (84.2%) were given a final case status of positive and 21 (14 adults and 7 children) were re-assigned as a non-case. Reasons for the reassignment included febrile seizures only (28.6%), provoked only (42.9%), single unprovoked (14.3%), and a single seizure of unknown cause (14.3%).
Three people (14.3%) classified as a non-case, including two older than 80 years of age, reported currently taking an AED on the screening survey. In comparison, 58 of the 112 verified cases (51.8%) reported currently taking an AED on the screening survey.
Prevalence of Lifetime and Active Epilepsy
The weighted lifetime prevalence of self-reported epilepsy in DC was 1.78% [95% CI 1.52–2.05] for the screened cases (Table 2). After restricting the analysis to verified cases, the prevalence of lifetime epilepsy declined to 1.53% [1.28–1.77]. With this adjustment, lifetime prevalence was highest in those 55–69 years of age (1.85% [1.24–2.46]) compared to the other age groups (range 1.25–1.53%) although none of the differences were significant. There was also no appreciable difference in prevalence by gender. In contrast, Blacks had nearly a three-fold higher prevalence (2.13% [1.73–2.53]) than Whites (0.77% [0.52–1.03]) and over twice the prevalence of those of Hispanic origin (0.95% [0.22–1.68]). Lifetime prevalence of epilepsy was inversely associated with the highest level of education in adults and ranged from 3.40% [1.87–4.92] for those with less than a high school education to 0.69% [0.41–0.96] for those that attended graduate school. Although the categories for annual household income spanned just $20,000 from highest to lowest, income was also inversely associated with lifetime prevalence of epilepsy in adults. The prevalence was significantly higher for those with income less than $30,000 (2.27% [1.72–2.83]) compared to those with income of $30,000-$49,000 (1.26% [0.89–1.63]) and greater than $49,000 (1.06% [0.77–1.35]). Length of residency in DC was also significantly associated with epilepsy prevalence. Adults that recently relocated into the city had a lifetime prevalence of epilepsy that was almost a third (0.65% [0.22–1.08]) of that found in residents living in DC for more than three years (1.80% [1.47–2.13]).
Prevalence of active epilepsy followed the same trends among subgroups as lifetime prevalence and overall was 0.86% [0.67–1.05] for screened cases and 0.79% [0.62–0.97] for verified cases (Table 2). In verified analyses, active epilepsy was highest in Blacks (1.13% [0.84–1.42]) compared to Whites (0.33% [0.17–0.49], individuals age 55–69 years of age (1.25% [0.74–1.75]) compared to other ages (range 0.55–0.95%), those with annual household incomes less than $30,000 (1.12% [0.73–1.51]) compared to greater income levels, those with less than a high school diploma (2.46% [1.19–3.72]) compared to high school graduates (1.31% [0.72–1.91]) or attendees of college (0.80% [0.48–1.11]) or graduate school (0.38% [0.17–0.58]). Adults living in DC for more than 3 years had a prevalence of active epilepsy that was three times greater (0.99% [0.75–1.23]) than those that had more recently moved into the city (0.32% [0.04–0.61]).
Cohabitation was another factor related to lifetime (p<.001) and active (p=.05) epilepsy status. Of adults with a history of epilepsy from weighted data, 31.6% lived alone and 68.4% shared a dwelling. In comparison, 46.7% of the non-epilepsy households were single occupancy. Adults with active epilepsy were also less likely to live alone than those without epilepsy (36.0% versus 46.1%).
Results for age-adjusted lifetime and active epilepsy from multivariate analyses using the verified cases dataset are presented in Table 3. Independent of age, education and income, Blacks had a lifetime prevalence rate (PR) that was 1.74 higher [1.08–2.82] than Whites and an active epilepsy PR that was 2.05 higher [1.05–3.98] than Whites. Those of Other race were at lower risk compared to Whites (PR 0.75 [0.33–1.69] for lifetime prevalence and PR 0.64 [0.20–2.00] for active prevalence), however these rates were not significantly different from 1.0. Education was also independently associated with epilepsy prevalence in adults after adjusting for age, income and race/ethnicity. Compared to those that attended graduate school, adults with less than a high school diploma had a significantly higher PR of lifetime epilepsy (3.92 [1.93 – 7.96]) and of active epilepsy (4.61 [1.84–11.55]). PRs for lifetime and active epilepsy for other levels of education compared to graduate school included 2.33 [1.24–4.36] and 2.04 [0.89–4.69, respectively, for high school diploma and 2.01 [1.23–3.28] and 1.64 [0.80–3.36], respectively, for college.
Table 3.
Age-adjusted Predictors of Lifetime and Active Epilepsy in Multivariate Analyses1
| Lifetime Epilepsy | Active Epilepsy | |
|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | |
| Race | ||
| Black | 1.74 (1.08 – 2.82) | 2.05 (1.05 – 3.98) |
| Other2 | 0.75 (0.33 – 1.69) | 0.64 (0.20 – 2.00) |
| White | 1.0 | 1.0 |
| Highest Education | ||
| Less than high school diploma | 3.92 (1.93 – 7.96) | 4.61 (1.84 – 11.55) |
| High school graduate | 2.33 (1.24 – 4.36) | 2.04 (0.89 – 4.69) |
| College | 2.01 (1.23 – 3.28) | 1.64 (0.80 – 3.36) |
| Graduate school | 1.0 | 1.0 |
| Annual Household Income | ||
| Less than $30,000 | 1.39 (0.94 – 2.05) | 1.33 (0.80 – 2.18) |
| $30,000 of more | 1.0 | 1.0 |
Adults over 24 years of age using the verified dataset
Other race = other than non-Hispanic Black and non-Hispanic White
Incidence of Epilepsy
There were 23 cases of epilepsy (15 adults, 8 children) with a year of first seizure between 2005 and 2007 that were weighted and included in the incidence estimates. Overall, the annual incidence per 100,000 persons was 75 [41–110] and was similar for males and females. Incidence for all children was more than twice that for adults (140 compared to 60). The incidence rate by age group was highest in children less than four years of age (430 [52–807]) and then declined to 49–50 per 100,000 until 55 years of age. The incidence reached a second peak between 55–69 years (115 [18–211]) and then fell sharply to 19 [0–57] in those 70 years of age and older. The incidence rate for Blacks was 109 [49–169] which was more than three times that for Whites (34 [6–62]). Unlike results for epilepsy prevalence, the incidence was higher among those of Other non-Hispanic race (80 [0–235]) compared to Hispanics (26 [0–76]) although the weighted numbers of new cases in these subgroups were small (18 and 13, respectively).
DISCUSSION
This is the first study to our knowledge that has demonstrated a significant association between epilepsy, race, and socioeconomic indicators in multivariate analysis. Blacks had a PR for lifetime epilepsy that was 1.74 times higher than that of Whites after adjusting for age, education and income, and a corresponding PR for active epilepsy that was twice that for Whites. Adults with less than a high school education had a PR for lifetime and active epilepsy that was 4 times higher than those with an advanced degree, independent of age, race, ethnicity, and income.
The higher risk of epilepsy in Blacks may be related to environmental factors, quality of health care, cultural knowledge and beliefs, or other confounding factors that are related to both self-reported epilepsy and race. People of Hispanic origin prefer the word “attacks” to epilepsy or seizures (Sirven et al.,2005). There may be other racial or ethnic preferences that influence self-identification with the clinical term epilepsy among minorities and immigrants and how they respond to a survey about epilepsy. Sorting out potential barriers to accurate survey responses in these populations requires further study.
Similar to most cross-sectional studies (Banerjee & Hauser, 2008), we did not see a steady increase in lifetime prevalence with age, with a peak at 55–69 years. The subsequent decline in prevalence in the elderly in our study may have been the result of unrecognized epileptic seizures, reduced survival among people with epilepsy, an incomplete medical history of childhood onset seizures passed from the parents, or recall bias, particularly of a distant history of epilepsy in childhood.
We demonstrated an over-reporting of lifetime prevalence of epilepsy among residents of DC by 16% (1.78 compared to 1.53) after including additional screening questions about seizures and treatment. This finding is similar to the 18% over-estimation of epilepsy from another population-based study with limited screening (Kelvin et al. 2007). For active epilepsy, the over-reporting declined to 9% which was not surprising given that AED use defined the condition and was relatively rare in the false positive cases.
Epilepsy was more commonly over-reported in children, the elderly, Hispanics, under-educated adults, and adults that were new residents to DC in our study. During the validation process, febrile seizures were easy to identify but provoked seizures were sometimes harder to distinguish from remote symptomatic seizures based on the limited number of questions and the responses that were provided. Nine of 21 (42.8%) false positive cases had provoked seizures while 42 of 101 (41.6%) verified cases had remote symptomatic seizures. Trauma accounted for 26% of the epilepsy in adults in DC compared to less than 9% reported in a meta-analysis of over thirty studies (Banerjee & Hauser, 2008). Classifying acute and remote symptomatic seizures as epilepsy or not in a population-based study may be challenging without support from accompanying medical records or targeted interview data.
The overall incidence of epilepsy (71 per 100,000) was higher than that found in other US populations but similar or lower than that reported in some African countries (WHO, 2004). Within racial subgroups, incidence was highest among Blacks. Similar to other studies, we found peaks in incidence of epilepsy in the youngest age group (0–3 years of age) and in the older middle age group (55–69 years of age). The incidence was very low (19 per 100,000) in elderly DC residents which may be related to unrecognized epileptic seizures or survival bias. The life expectancy of 74 years for DC residents is the lowest of all 50 States.
The lower prevalence and incidence of epilepsy in new residents of DC probably represents a “healthy mover” effect where individuals without chronic health problems are more likely to relocate to start a new job or attend higher education and be less concerned about maintaining consistency in medical providers than those with chronic disease. The Hispanic population in DC has nearly doubled in the past decade and this ability to immigrate may account for the lower rates of epilepsy in the Hispanic population in our study.
In one respect, it was reassuring to find that adults with active epilepsy were significantly less likely to live alone than the general adult population in DC and that at least one other household member may be available to provide assistance in the event of a serious seizure. On the other hand, sharing a dwelling may also indicate that some adults with epilepsy in DC are more likely to need assistance with their daily living activities than those without epilepsy. Lower neurocognitive functioning and lower educational achievement are well documented outcomes of childhood onset epilepsy, even in those without remote symptomatic causes (Shinnar & Pellock, 2002, Sillanpaa, 1990, Fastenau et al., 2008; Geerts et al., 2011, Berg et al., 2011), and these factors can also influence independent living. Two prospective studies found that adults with childhood onset epilepsy were more likely to live with a family member than a life partner or independently (Sillanpaa, 1990; Geerts et al., 2011). Further research is needed to address the full impact of epilepsy on caregivers and on the quality of care received by dependent adults with epilepsy. This research should extend to families of children with epilepsy to identify areas for early intervention in impaired family functioning (Duffy, 2011).
There are several limitations to our study that should be noted, the primary one being the low response rate. An address-based sampling methodology was employed to give all households in DC an equal chance of selection by administering the survey using several modes of data collection, as well as incentives, to increase response rates. In spite of this, the rate of response to our survey was lower than desired (37%) but not different from that found in other population-based surveys. Biener et al. (2004) and Curtin et al. (2005) note that the rate of response to general public surveys has been on a steady decline. More recent investigations by Fahimi et al. (2008) reveal that response rates to well-founded government surveys, such as the BRFSS, follow this trend as well. We took steps to reduce as much of the potential non-response bias as possible by further adjusting the non-response-adjusted weights to match the demographic composition of the DC households.
A low response rate may have resulted in non-random bias regarding the epilepsy status of household members that returned the screening survey compared to those that did not. Unlike the BRFSS, sample households were aware of the nature of our study after the first contact was made. Households that had a member with a history of epilepsy may have been more likely (or less likely due to stigma) to complete the survey than households without a history of epilepsy.
Our definition of active epilepsy differed from the BRFSS in that we were not able to include occurrence of a seizure in the previous three months in those not taking an AED, which has been estimated to be 7% of active cases (Kobau et al., 2008). However, in our analysis of verified cases, this difference in definition would tend to be off-set by exclusion of false positive cases that were taking an AED which we found to be 14%.
Another limitation of the study includes the proxy reporting of information about most of the household members. The data collected for all individuals living in a household was presumably provided by one person who completed the screening survey. This person may not have been familiar with the complete health history of other household members. This limitation would cause an underestimate of the lifetime prevalence of epilepsy, particularly of resolved childhood onset.
Nonetheless, our results are well within the range of prevalence estimates reported by others. Furthermore, our study is the first to provide region-specific estimates and profiles of the epilepsy population in DC which can help inform policy makers and healthcare providers on where to better target resources to improve the health and outcomes of people with epilepsy and their families. Subsequently, we have initiated a large clinical cohort study of DC children with epilepsy and their families to address pediatric and parental co-morbidities and the effects of family functioning on access to quality medical care in a vulnerable population.
Table 4.
Incidence of Epilepsy
| N1 | Screened Cases Per 100,000 (95% CI) |
Verified Cases 100,000 (95% CI) |
|
|---|---|---|---|
| Overall | 12,741 | 75 (41–110) | 71 (39–103) |
| Gender | |||
| Male | 5,649 | 74 (23–124) | 69 (21–116) |
| Female | 7,092 | 77 (30–124) | 73 (29–116) |
| Children/Adults | |||
| 0–17 years | 2,142 | 140 (32–248) | 135 (29–240) |
| >17 years | 10,599 | 60 (28–92) | 55 (26–84) |
| Age Group | |||
| 0–3 years | 517 | 430 (52–807) | 408 (43–773) |
| 4–17 years | 1,625 | 49 (0–127) | 49 (0–127) |
| 18–54 years | 6,821 | 50 (14–86) | 46 (13–78) |
| 55–69 years | 2,386 | 115 (18–211) | 105 (17–194) |
| More than 69 years | 1,392 | 19 (0–57) | 19 (0–57) |
| Race/Ethnicity | |||
| White | 5,138 | 34 (6–62) | 33 (6–60) |
| Black | 6,123 | 109 (49–169) | 102 (46–158) |
| Hispanic | 779 | 26 (0–76) | 22 (0–65) |
| Other | 701 | 80 (0–235) | 68 (0–201) |
| Highest Education2 | |||
| Less than high school diploma | 601 | 76 (0–226) | 76 (0–226) |
| High school graduate/equivalency | 1,525 | 89 (0–189) | 84 (0–179) |
| College | 3,756 | 89 (23–154) | 78 (21–134) |
| Graduate or professional degree | 3,700 | 21 (0–51) | 21 (0–51) |
| Residence in DC2 | |||
| 0–3 years | 1,600 | 28 (0–82) | 28 (0–82) |
| More than 3 years | 7,982 | 71 (31–112) | 65 (28–102) |
Number of unweighted observations without epilepsy prior to 2005
Adults over 24 years of age
Acknowledgements
This study was made possible through the Centers for Disease Control and Prevention (CDC) and the Association for Prevention Teaching and Research (APTR) Cooperative Agreement, No. 5U50CD300860, Project TS-1389.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Disclosure of Conflicts of Interest
None of the authors has any conflict of interest to disclose.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Banerjee PN, Hauser WA. Incidence and prevalence. In: Engel J Jr, Pedley TA, editors. Epilepsy: a comprehensive textbook. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 45–56. [Google Scholar]
- Berg AT, Hesdorffer DC, Zelko FA. Special education participation in children with epilepsy: what does it reflect? Epilepsy Behav. 2011;22(2):336–341. doi: 10.1016/j.yebeh.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, Garrett CA, Gilpin EA, Roman AM, Currivan DB. Consequences of declining survey response rates for smoking prevalence estimates. Am J Prev Med. 2004;27(3):254–257. doi: 10.1016/j.amepre.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Burneo JG, Jette N, Theodore W, Begley C, Parko K, Thurman DJ, Wiebe S Task Force on Disparities in Epilepsy Care; North American Commission of the International League Against Epilepsy. Disparities in epilepsy: Report of a systematic review by the North American Commission of the International League Against Epilepsy. Epilepsia. 2009;50(10):2285–2295. doi: 10.1111/j.1528-1167.2009.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission on Epidemiology and Prognosis of the International League Against Epilepsy. Guidelines for epidemiologic studies on epilepsy. Epilepsia. 1993;34:592–596. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Curtin R, Presser S, Singer E. Changes in telephone survey nonresponse over the past quarter century. Public Opinion Quarterly. 2005;69:87–98. [Google Scholar]
- Duffy LV. Parental coping and childhood epilepsy: the need for future research. J Neurosci Nurs. 2011;43(1):29–35. [PubMed] [Google Scholar]
- Elliott JO, Moore JL, Lu B. Health status and behavioral risk factors among persons with epilepsy in Ohio based on the 2006 Behavioral Risk Factor Surveillance System. Epilepsy Behav. 2008;12(3):434–444. doi: 10.1016/j.yebeh.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Elliott JO, Charyton C, Lu B, Moore JL. Serious psychological distress and health outcomes for persons with epilepsy in poverty. Seizure. 2009;18(5):332–338. doi: 10.1016/j.seizure.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Fahimi M, Link MW, Schwartz D, Levy P, Mokdad A. Tracking Chronic Disease and Risk Behavior Prevalence as Survey Participation Declines: Statistics from the Behavioral Risk Factor Surveillance System and Other National Surveys. Preventing Chronic Disease. 2008;5(3) [PMC free article] [PubMed] [Google Scholar]
- Fastenau PS, Jianzhao S, Dunn DW, Austin JK. Academic underachievement among children with epilepsy: proportion exceeding psychometric criteria for learning disability and associated risk factors. J Learn Disabil. 2008;41(3):195–207. doi: 10.1177/0022219408317548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson PL, Chiprich J, Smith G, Dong B, Wannamaker BB, Kobau R, Thurman DJ, Selassie AW. Prevalence of self-reported epilepsy, health care access, and health behaviors among adults in South Carolina. Epilepsy Behav. 2008;13(3):529–534. doi: 10.1016/j.yebeh.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Geerts A, Brouwer O, van Donselaar C, Stroink H, Peters B, Peeters E, Arts WF. Health perception and socioeconomic status following childhood-onset epilepsy: The Dutch study of epilepsy in childhood. Epilepsia. 2011 Oct 17; doi: 10.1111/j.1528-1167.2011.03294.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Haerer AF, Anderson DW, Schoenberg BS. Prevalence and clinical features of epilepsy in a biracial United States population. Epilepsia. 1986;27:66–75. doi: 10.1111/j.1528-1157.1986.tb03503.x. [DOI] [PubMed] [Google Scholar]
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68(5):326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- Kalton G, Kasprzak D. The treatment of missing survey data. Survey Methodology. 1986;12:1–16. [Google Scholar]
- Kelvin EA, Hesdorffer DC, Bagiella E, Andrews H, Pedley TA, Shih TT, Leary L, Thurman DJ, Hauser WA. Prevalence of self-reported epilepsy in a multiracial and multiethnic community in New York City. Epilepsy Res. 2007;77(2–3):141–150. doi: 10.1016/j.eplepsyres.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Kobau R, Gilliam F, Thurman DJ. Prevalence of self-reported epilepsy or seizure disorder and its associations with self-reported depression and anxiety: Results from the 2004 HealthStyles Survey. Epilepsia. 2006;47(11):1915–1921. doi: 10.1111/j.1528-1167.2006.00612.x. [DOI] [PubMed] [Google Scholar]
- Kobau R, Zahran H, Grant D, Thurman DJ, Price PH, Zack MM. Prevalence of active epilepsy and health-related quality of life among adults with self-reported epilepsy in California: California Health Interview Survey, 2003. Epilepsia. 2007;48(10):1904–1913. doi: 10.1111/j.1528-1167.2007.01161.x. [DOI] [PubMed] [Google Scholar]
- Kobau R, Zahran H, Thurman DJ, Zack MM, Henry TR, Schachter SC, Price PH Centers for Disease Control and Prevention (CDC) Epilepsy surveillance among adults--19 States, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveill Summ. 2008;57(6):1–20. [PubMed] [Google Scholar]
- Konda K, Ablah E, Konda KS, Liow K. Health behaviors and conditions of persons with epilepsy: A bivariate analysis of 2006 BRFSS data. Epilepsy Behav. 2009;16(1):120–127. doi: 10.1016/j.yebeh.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Ottman R, Barker-Cummings C, Leibson CL, Vasoli VM, Hauser WA, Buchhalter JR. Validation of a brief screening instrument for the ascertainment of epilepsy. Epilepsia. 2010;51:191–197. doi: 10.1111/j.1528-1167.2009.02274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottman R, Lipton RB, Ettinger AB, Cramer JA, Reed ML, Morrison A, Wan GJ. Comorbidities of epilepsy: results from the Epilepsy Comorbidities and Health (EPIC) survey. Epilepsia. 2011;52(2):308–315. doi: 10.1111/j.1528-1167.2010.02927.x. [DOI] [PubMed] [Google Scholar]
- Shinnar S, Pellock JM. Update on the epidemiology and prognosis of pediatric epilepsy. J Child Neurol. 2002;17(Suppl 1):S4–S17. doi: 10.1177/08830738020170010201. [DOI] [PubMed] [Google Scholar]
- Sillanpaa M. Children with epilepsy as adults: outcome after 30 years of follow-up. Acta Paediatr Scand Suppl. 1990;368:1–78. [PubMed] [Google Scholar]
- Sillanpaa M. Learning disability: occurrence and long-term consequences in childhood-onset epilepsy. Epilepsy Behav. 2004;5:937–944. doi: 10.1016/j.yebeh.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Sirven JI, Lopez RA, Vazquez B, Van Haverbeke P. Que es la epilepsia? Attitudes and knowledge of epilepsy by Spanish-speaking adults in the United States. Epilepsy Behav. 2005;7:259–265. doi: 10.1016/j.yebeh.2005.04.015. [DOI] [PubMed] [Google Scholar]
- US Census Bureau. 2009 American Community Survey 1-Year Estimate, Summary Tables S0201 and B03002. [Accessed September 6, 2011]; http://factfinder.census.gov.
- World Health Organization. Epilepsy in the WHO African Region: Bridging the Gap. WHO-ARF/MNH/04.1. 2004