Abstract
Beekeepers who use honey bees (Apis mellifera L.) for crop pollination services, or have colonies making honey on or in close proximity to agricultural crops, are concerned about the reductions of colony foragers and ultimate weakening of their colonies. Pesticide exposure is a potential factor in the loss of foragers. During 2009–2010, we assessed changes in the field force populations of 9–10 colonies at one location per crop on each of the eight crops by counting departing foragers leaving colonies at regular intervals during the respective crop blooming periods. The number of frames of adult bees was counted before and after bloom period. For pesticide analysis, we collected dead and dying bees near the hives, returning foragers, crop flowers, trapped pollen, and corn-flowers associated with the cotton crop. The number of departing foragers changed over time in all crops except almonds; general patterns in foraging activity included declines (cotton), noticeable peaks and declines (alfalfa, blueberries, cotton, corn, and pumpkins), and increases (apples and cantaloupes). The number of adult bee frames increased or remained stable in all crops except alfalfa and cotton. A total of 53 different pesticide residues were identified in samples collected across eight crops. Hazard quotients (HQ) were calculated for the combined residues for all crop-associated samples and separately for samples of dead and dying bees. A decrease in the number of departing foragers in cotton was one of the most substantial crop-associated impacts and presented the highest pesticide risk estimated by a summed pesticide residue HQ.
Keywords: honey bee, pesticide, pollination, toxicity, multiresidue analysis
Honey bees are a key component of global food security. Of the 100 crops that provide 90% of the world’s food, 71 are bee pollinated, and honey bees (Apis mellifera L.) are the managed pollinator conscripted to provide the necessary pollination services for most of these crops (United Nations Food and Agriculture Organization 2005). In the United States alone, honey bee pollination is valued at US$20 billion (Calderone 2012). Over the past decade, there has been a sharp increase in the number of honey bee colony losses in the United States, often exceeding 30% per year (Lee et al. 2015). Beekeepers renting their colonies for pollination, or making honey on or in close proximity to agricultural crops, are concerned about pesticide exposure and its potential negative impacts on their colonies. This includes sublethal impacts that may affect forager performance and are more difficult to diagnose.
This research was undertaken as a result of an interactive workshop Protecting Honey Bees from Pesticides at the 2009 American Beekeeping Federation Conference. Ten commercial beekeepers were present in addition to two regulators and the Penn State Extension Specialist. All of the participating beekeepers rent bees for pollination of various crops and all reported experiencing declines in their colony populations while working certain crops. This phenomenon has been reported anecdotally by many beekeepers for several years but to date has not been documented.
Honey bee exposure to pesticides in contaminated wax and pollen as a result of in-hive miticides for Varroa mite control and agrochemicals used for agricultural pest control, especially in bee-pollinated crops, is now known to be prevalent (Frazier et al. 2008, Mullin et al. 2010, Chauzat et al. 2011). An acute toxic result of pesticide exposure is typically characterized by piles of dead bees outside of hives; however, evidence of sublethal effects of pesticides, as well as associated adjuvants, has been mounting in the literature. These impacts include, but are not limited to, reduced longevity, reduced immune function, impacts on learning, and impaired orientation, foraging, and motor coordination (Thompson 2003, Decourtye et al. 2004, Desneux et al. 2007, Ciarlo et al. 2012, Oruc et al. 2012, Garrido et al. 2013).
Based on the concerns of beekeepers using their colonies for commercial pollination of agriculture crops, the present research was undertaken to address two questions: 1) Are honey bee field force populations (assessed as the number of departing foragers) decreasing while bees are foraging on certain crops? and 2) Are sufficient levels of pesticides detected in bees and the surrounding environment potentially contributing to changes in departing foragers? In order to address these questions, we assessed field force populations of colonies present during pollination or in association with eight different crops and analyzed samples of live and dead or dying bees, crop flowers, and bee-collected pollen for pesticide residues.
Materials and Methods
Field populations (measured as the number of departing foragers) during pollination and colony adult populations pre- and postpollination were measured in association with eight different crops at one location per crop in Pennsylvania, California, and Maine. Samples for pesticide analysis were concurrently collected from the colonies (dead bees, returning foragers, and trapped pollen), target crop (flowers), and in the case of cotton, plants (corn flowers) growing close to the crop. In Pennsylvania, nine colonies were assessed in each of the following settings: apples (Malus domestica Borkh.) located outside of Biglerville, in Adams County; pumpkins (Cucurbita pepo L.) located outside of Benton, Columbia County; and colonies surrounded by corn (Zea mays L.), located at the home apiary of the participating Pennsylvania beekeeper near West Milton, Union County (Table 1). The commercial apple orchard consisted of 20 acres of apples with some stone fruit, Christmas trees, and woodland within the foraging ranges of the colonies. The pumpkin planting consisted of >500 acres of pumpkins with some acreage of soybeans within the foraging ranges of the colonies. In the location where corn was assessed, soybeans, hay, and woodland were within the foraging range of the colonies. In California, 10 colonies were assessed in each of the following settings: alfalfa (Medicago sativa L.) for seed production, located near Tranquility, Fresno County; cantaloupe (Cucumis melo L.), near Los Banos, Merced County; almond (Prunus dulcis (Mill.) D. A. Webb), near Hughson, Stanislaus County; and colonies making honey on cotton (Gossypium hirsutum L.), near Tranquility, Fresno County (Table 1). The alfalfa planting consisted of 60 acres. In addition, 50 acres of cotton and 100 acres of tomatoes were within the foraging range of these colonies. Bees in almonds also had access to some natural forage including mustard, radish, black locust, Eucalyptus, and vetch. In addition, five acres of cherries and 20 acres of peaches were within the foraging range of the colonies. The 30-acre cantaloupe field had alfalfa hay, cotton, other melon fields, and processing tomatoes within flight range of the colonies. The cotton planting covered 180 acres, but colonies were within a mile of alfalfa and lettuce seed, cotton, and corn. In Maine, nine colonies pollinating low-bush blueberries (Vaccinum angustifolium Aiton) were located on Passamaquoddy tribal lands in Washington County (Table 1). The approximate 100 acres of blueberries was contiguous with other blueberry fields. At a radius of 4 km around the hive drop, as a measure of foraging distance (50 square kilometers), the composition of the landscape was roughly 35% wild blueberry, 5% wetland (mostly fern), and 60% spruce-fir forest. There were no other residences or agricultural sites in the area.
Table 1.
Geographic locations where field force assessments were conducted
| Crop | Location of initial assessment | Location of crop assessment |
|---|---|---|
| Alfalfa | Hughson, CA | Tranquility, CA |
| (Medicago sativa) | 37° 33′12.84″ N | 36° 37′45.86″ N |
| 120° 50′31.80″ W | 120° 15′51.57″ W | |
| Almonds | Hughson, CA | Hughson, CA |
| (Prunus dulcis) | 37° 33′53.26″ N | 37° 33′53.26″ N |
| 120° 51′39.26″ W | 120° 51′39.26″ W | |
| Apples | Penn State University Park, PA | Orchard |
| (Malus domestica) | 40° 49′18.6″ N | Biglerville, Adams Co., PA |
| 77° 51′34″ W | 39° 97′30.74″ N | |
| 77° 31′71.13″ W | ||
| Blueberry | Passamaquoddy tribal land, ME | Passamaquoddy tribal land, ME |
| (Vaccinum angustifolium) | 44° 45′48.57″ N | 44° 45′48.57″ N |
| 67° 43′36.20″ W | 67° 43′36.20″ W | |
| Corn | West Milton, PA | West Milton, PA |
| (Zea mays) | 41° 01′15.6″ N | 41° 01′15.6″ N |
| 76° 53′21.4″ W | 76° 53′21.4″ W | |
| Cotton | Soledad, CA | Tranquility, CA |
| (Gossypium hirsutum) | 36° 24′02.92″ N | 36° 39′42.07″ N |
| 121° 14′17.71″ W | 120° 17′25.91″ W | |
| Cantaloupe | Los Banos, CA | Los Banos, CA |
| (Cucumis melo) | 37° 04′50.06″ N | 37° 04′39.54″ N |
| 120° 44′57.37″ W | 120° 44′57.37″ W | |
| Pumpkin | West Milton, PA | Benton, Columbia Co., PA |
| (Cucurbita pepo) | 41° 01′15.6″ N | 41° 22′59.7″ N |
| 76° 53′21.4″ W | 76° 40′96″ W |
In some cases, initial assessments could not be done prior to moving bees onto the crop. In this case, the initial assessment was done the second day after the bees were moved onto the crop.
Colony Assessments
All colonies in the study were confirmed to be queen-right at the beginning and end of the assessment period. The number of frames of adult bees was counted at the beginning and end of the assessment period for colonies pollinating alfalfa, almond, apple, cantaloupe, pumpkin, and those in association with cotton and cornfields. These assessments were made early in the morning. Each frame was removed and the percent of the frame covered by adult bees was estimated for both sides of each frame. Measurements of all frames with adults were summed for each colony and then averaged for each crop.
Field-Force Determination
Nine to ten (depending on the crop, see above) queen-right colonies, in association with each of the eight different crops, were randomly identified for assessment. All colonies were owned and operated by commercial beekeepers except those assessed during apple pollination. These were established from 3-pound packages 1 mo prior to being moved to a commercial apple orchard and were owned by Pennsylvania State University. Field force populations were measured by making three 3-min counts of the number of worker bees leaving the colony and averaged for each of the nine colonies. These assessments were performed from five to eight times per crop, depending on the length of bloom, which ranged from 11 d in apples to 53 d in alfalfa (Fig. 1). The initial count was made before or 2 d after the bees were moved on to the crop. The time of day and air temperature were recorded. Assessments were made between 10 a.m. and 3 p.m., and temperatures ranged from a low of 11°C to a high of 41°C.
Fig. 1.
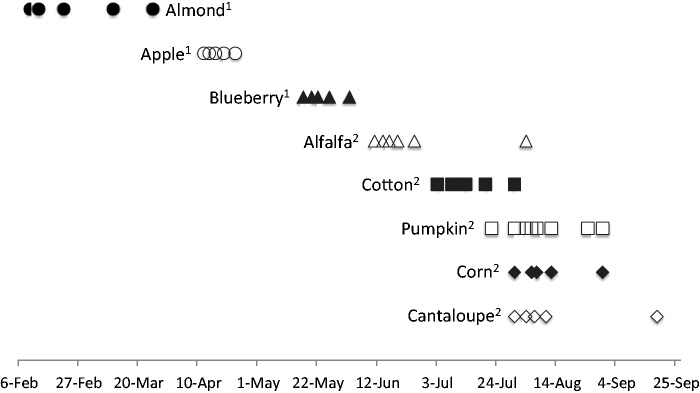
Timeline showing the dates and length of time field force populations were assessed and pesticide samples were collected for each crop type. Monitoring and sampling coincided with the bloom period of each crop. 1Field work conducted in 2010. 2 Field work conducted in 2009.
Pesticide Residue Analysis and Hazard Quotient
In order to assess pesticide exposure, 31 samples of crop flowers, trapped pollen, dead and dying bees, returning foragers, and in some cases blooms from bee-attractive plants in close proximity to the target crop, were collected and submitted for pesticide residue analysis (Table 2). All samples were collected on ice and stored in a freezer until the end of the study when they were shipped to Pennsylvania State University. Once received, samples were stored in a standard −15°C freezer until 3-g portions were weighed into 50-ml plastic centrifuge tubes and sent at ice temperature to the USDA-AMS National Science Laboratory in Gastonia NC for multipesticide residue analysis. Samples were extracted and analyzed for 171 pesticides and associated degradates at the part per billion (ppb) level as described in Mullin et al. (2010).
Table 2.
Samples taken from each of the crops used to assess for honey bee field force
| Sample type | Alfalfa | Almond | Apple | Blueberry | Corn | Cotton | Cantaloupe | Pumpkin |
|---|---|---|---|---|---|---|---|---|
| Crop flowers | X | Xa | X | X | X | X | X | |
| Trapped pollen | X | Xa | X | X | X | X | ||
| Dead and dying bees before exposure to crop | X | |||||||
| Returning foragers before exposure to crop | X | |||||||
| Dead and dying bees initial exposure to crop | X | |||||||
| Returning foragers initial exposure to crop | X | |||||||
| Dead and dying bees during exposure to crop | X | X | X | X | X | X | X | X |
| Returning foragers during exposure to crop | X | X | X | X | X | |||
| Additional sample | Corn tassels |
“X” indicates samples that were sent for pesticide analysis for each crop.
a Sampled on the day of or day after pesticide spray.
Because samples collected for pesticide analysis varied in number and composition depending on the crop [alfalfa (n = 8), almond (4), apple (9), blueberry (3), corn (3), cotton (4), cantaloupe (2), and pumpkin (3)], pesticide residue values in ppb ( = µg/kg) for each detection across all sample types per crop were averaged for use in calculation of a hazard quotient (Stoner and Eitzer 2013). The total pesticide hazard quotient (HQ) for a crop is the summation of each average pesticide residue in ppb divided by the respective honey bee LD50 (µg/bee). LD50 values (Table 3) represent averaged 24–72-h adult acute toxicities available from the US EPA Ecotox Database (http://cfpub.epa.gov/ecotox/, accessed 6 July 2015), the University of Hertfordshire Pesticide Properties DataBase (PPDB, http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm, accessed 6 July 2015), and some additional primary literature (e.g., Atkins et al. 1981, Atkins and Kellum 1986, Graham 1992). Crop Hazard Quotient = ∑ (each residue in ppb ÷ respective honey bee LD50 in ppb), and a value of 10,000 equals one LD50 equivalent assuming an average adult bee weight of 100 mg, equal weight equivalent of exposure to the crop, and that pesticides interact additively. A HQ was similarly calculated for average dead and dying bee pesticide residues to explore their independent role in colony assessments.
Table 3.
Summary of residues detected in crop-associated samples (residues are averaged across all samples collected in each crop), their adult bee LD50s, and crops where the highest detections were identified
| Pesticide | Classa | Ave. LD50 (µg/bee)b | Toxicity rank | Crop with highest residue | Ave. crop residue (ppb) | Crop with 2nd highest residue | Ave. crop residue (ppb) |
|---|---|---|---|---|---|---|---|
| Acephate | S OP | 0.513 | High | Cotton | 306.0 | ||
| Acetamiprid | S NEO | 9.9 | Moderate | Apple | 60.6 | Blueberry Cotton | 4.0 |
| Azinphos-methyl | OP | 0.179 | High | Apple | 2,680.0 | ||
| Azoxystrobin | S F | 112 | Nontoxic | Cotton | 3.5 | ||
| Bifenthrin | PYR | 0.0412 | High | Cotton | 21.8 | Pumpkin | 4.2 |
| Boscalid | S F | 155 | Nontoxic | Almond | 16,649.1 | Cotton | 36.1 |
| Captan | F | 135 | Nontoxic | Blueberry | 1,310.0 | Apple | 51.5 |
| Carbaryl | PS CAR | 0.442 | High | Apple | 70.6 | Blueberry | 3.0 |
| Chlorothalonil | F | 111 | Nontoxic | Pumpkin | 1,131.6 | Corn | 513.8 |
| Chlorpyrifos | OP | 0.0762 | High | Almond | 69.9 | Cotton | 46.2 |
| Chlorpyrifos-methyl | OP | 0.246 | High | Almond | 209.0 | Apple | 43.5 |
| Clothianidin | S NEO | 0.0184 | High | Cotton | 1.0 | ||
| Coumaphos | MIT OP | 5.93 | Moderate | Pumpkin | 18.6 | 3.7 | |
| Cyfluthrin | PYR | 0.0279 | High | Cotton | 48.5 | Cantaloupe | 2.8 |
| Cyhalothrin | PYR | 0.183 | High | Cotton | 630.0 | Alfalfa | 12.9 |
| Cypermethrin | PYR | 0.188 | High | Cotton | 347.1 | Alfalfa | 8.8 |
| Cyprodinil | S F | 332 | Nontoxic | Apple | 2,825.0 | Almond | 53.2 |
| Dicofol | OC | 18.6 | Moderate | Cotton | 2.5 | ||
| Dieldrin | OC | 0.227 | High | Pumpkin | 24.3 | ||
| Difenoconazole | S F | 126 | Nontoxic | Apple | 914.0 | ||
| DMPF (Amitraz degradate) | MIT | 75 | Low | Corn | 2,773.0 | Cantaloupe | 702.0 |
| Endosulfan I | OC | 7.05 | Moderate | Pumpkin | 239.2 | Almond | 6.7 |
| Endosulfan II | OC | 7.05 | Moderate | Pumpkin | 154.3 | Alfalfa | 3.1 |
| Endosulfan sulfate | OC | 21.8 | Moderate | Pumpkin | 51.6 | Alfalfa | 1.9 |
| Esfenvalerate | PYR | 0.162 | High | Cotton | 7,240.0 | Alfalfa | 26.3 |
| Fenbuconazole | S F | 149 | Nontoxic | Apple | 4.3 | ||
| Fenhexamid | F | 159 | Nontoxic | Corn | 77.1 | Pumpkin | 56.2 |
| Fenpyroximate | MIT | 248 | Nontoxic | Cotton | 131.0 | ||
| Flonicamid | S I | 71.2 | Low | Cotton | 862.6 | Alfalfa | 42.4 |
| Fluvalinate-tau | PYR | 4.32 | Moderate | Alfalfa | 45.2 | Pumpkin | 37.3 |
| Hexythiazox | MIT | 156 | Nontoxic | Cotton | 70.9 | ||
| Imidacloprid | S NEO | 0.0398 | High | Apple | 15.9 | ||
| Indoxacarb | I | 147 | Nontoxic | Cotton | 80.3 | ||
| Iprodione | F | 91.7 | Low | Almond | 1,191.3 | ||
| Malathion | OP | 0.232 | High | Cotton | 5,550.0 | ||
| Metalaxyl | S F | 113 | Nontoxic | Pumpkin | 2.1 | ||
| Methamidophos (acephate degradate) | S OP | 0.498 | High | Cotton | 269.0 | Alfalfa | 20.7 |
| Methidathion | OP | 0.201 | High | Cotton | 94.3 | ||
| Myclobutanil | S F | 161 | Nontoxic | Pumpkin | 258.5 | Cotton | 57.4 |
| Oxamyl | S CAR | 0.259 | High | Cotton | 292.0 | ||
| Oxyfluorfen | H | 100 | Nontoxic | Almond | 29.2 | Alfalfa | 11.6 |
| Pendimethalin | PS H | 74.9 | Low | Almond | 119.9 | Alfalfa | 11.7 |
| Permethrin | PYR | 0.115 | High | Pumpkin | 275.0 | ||
| Phosmet | OP | 0.803 | High | Blueberry | 738.4 | ||
| Propiconazole | S F | 67.5 | Low | Blueberry | 442.0 | ||
| Pyraclostrobin | F | 86.6 | Low | Almond | 10,458.0 | Pumpkin | 267.0 |
| Pyrimethanil | PS F | 100 | Nontoxic | Almond | 41.3 | ||
| Spiromesifen | PS I | 200 | Nontoxic | Cotton | 7,000.0 | ||
| Thiacloprid | S NEO | 25.2 | Moderate | Apple | 85.0 | ||
| Thiamethoxam | S NEO | 0.0196 | High | Alfalfa | 12.7 | ||
| THPI (Captan degradate) | F | 135 | Nontoxic | Blueberry | 201.0 | ||
| Trifloxystrobin | PS F | 175 | Nontoxic | Apple | 12.6 | ||
| Trifluralin | H | 68.5 | Low | Almond | 7.5 | Alfalfa | 1.7 |
a CAR, carbamate; F, fungicide; H, herbicide; I, insecticide (other); MIT, miticide; NEO, neonicotinoid; OC, organochlorine; OP, organophosphate; PS, partially systemic; PYR, pyrethroid; S, systemic.
b Sources: US EPA Ecotox Database http://cfpub.epa.gov/ecotox/, accessed 6 July 2015; University of Hertfordshire (2013); The Pesticide Properties DataBase (PPDB) http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm, accessed 6 July 2015; and some additional primary literature.
Statistical Analyses
To test the hypothesis that field force population size changes over time, we compared mean field force measures at each time point for each of the eight crop types, individually. Because the same colonies were used to estimate field force at each time point, we used a one-way repeated measures ANOVA in SPSS v. 22 (IBM Corp. 2013) and tested for the effect of time. For significant results, we included a post hoc Tukey HSD test to identify which time points were significantly different from one another. Similarly, we used a one-way repeated measures ANOVA to determine if the number of adult bee frames changed from pre- to post-crop exposure for each crop type.
Because our field force assessments took place over multiple dates, we recognized that temperature may have potentially impacted bee activity patterns. Using experiment-wide data, we performed a regression analysis with temperature as a predictor and field force population as a response, and found no relationship between the two factors (F1,43 = 0.69, P = 0.794; R2 = 0.002).
Results
A total of 53 pesticide residues were identified in samples collected across eight crops (Table 3). Fungicide residues were detected frequently and often were found at higher levels than insecticides. In-hive miticides, except for amitraz residues, were typically found at low levels, where the highest levels of fluvalinate and coumaphos were 103 and 18.6 ppb for alfalfa and pumpkin colonies, respectively. N-(2,4-Dimethylphenyl) formamide (DMPF), a metabolite of amitraz, was found in trapped pollen, dead and dying bees, and returning foragers with the highest level being 5,160 ppb in dead and dying bees in corn. Two non-bee toxic herbicides, pendimethalin and oxyfluorfen, were detected at low levels in some samples. Average crop HQ levels ranged from 30 (corn) to 14,333 (cotton). A field force and colony assessment relative to pesticide exposure for each crop is detailed below (and summarized in Tables 4 and 5).
Table 4.
Summary of the number of pesticides detected in collected materials associated with all crops
| Crop | Flowers | Trapped pollen | Live returning foragers | Dead and dying foragers |
|---|---|---|---|---|
| Alfalfa | 3/1 | 5/1 | 4/1 (samples first & during comb.) | 14/4 (samples first & during comb.) |
| Almonds | 8/2 (day of spray) | 15/2 (day after spray) | 3/0 | 9/1 |
| Apples | 13/2 (2 samples comb.) | 13/4 | 2/1 | 2/1 |
| Blueberries | 2/1 | 4/2 | N/A | 4/1 |
| Cantaloupes | N/A | N/A | 4/2 | 2/2 |
| Cotton | 4/0 (corn tassels: 21/8) | N/A | 0/0 | 11/6 |
| Corn | 3/0 | 3/0 | N/A | 2/0 |
| Pumpkin | 8/1 (anthers only) | 11/1 | N/A | 14/4 |
Indicated are the total number of pesticides detected relative to the number pesticides considered toxic to honey bees based on their published LD50s.
Table 5.
Quick reference summary of results for colony assessment over time
| Crop | Field force size |
No. adult frames |
||
|---|---|---|---|---|
| Sig. change? | Direction? | Sig. change? | Direction? | |
| Alfalfa | Yes | Variable; increase–decrease | Yes | Decrease |
| Almonds | No | N/A | Yes | Increase |
| Apples | Yes | Increase | Yes | Increase |
| Blueberries | Yes | Variable; increase–decrease | – | – |
| Cantaloupes | Yes | Increase | No | N/A |
| Corn | Yes | Decrease | Yes | Increase |
| Cotton | Yes | Decrease | Yes | Decrease |
| Pumpkins | Yes | Variable; decrease–increase–decrease | Yes | Increase |
Alfalfa
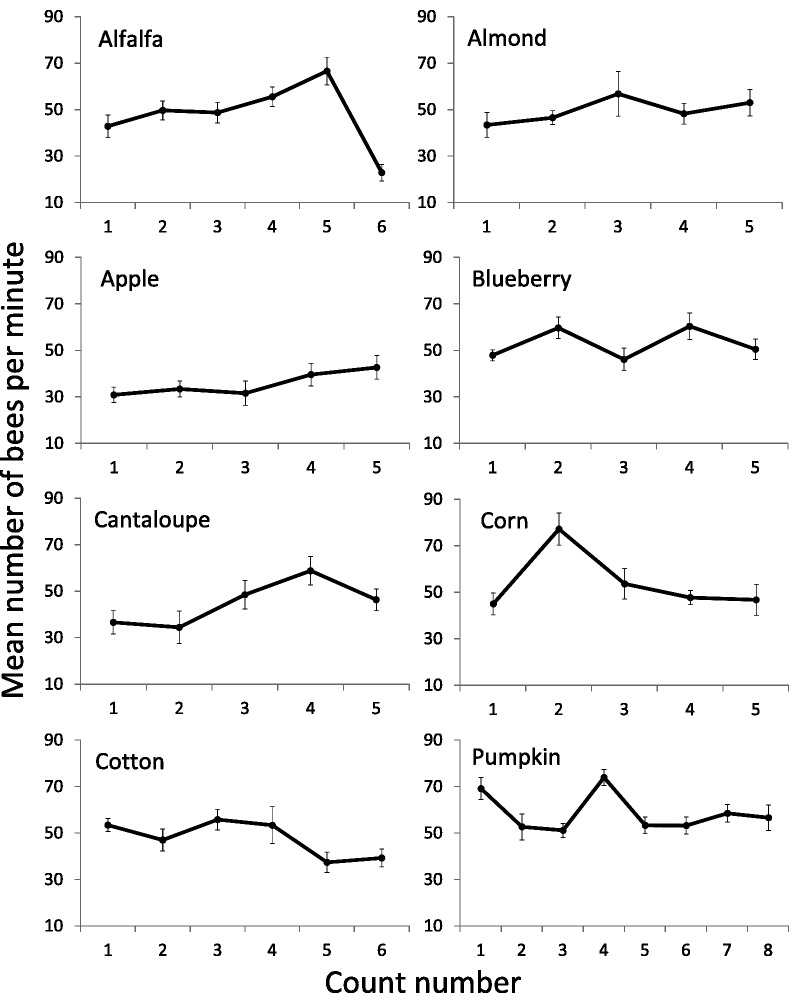
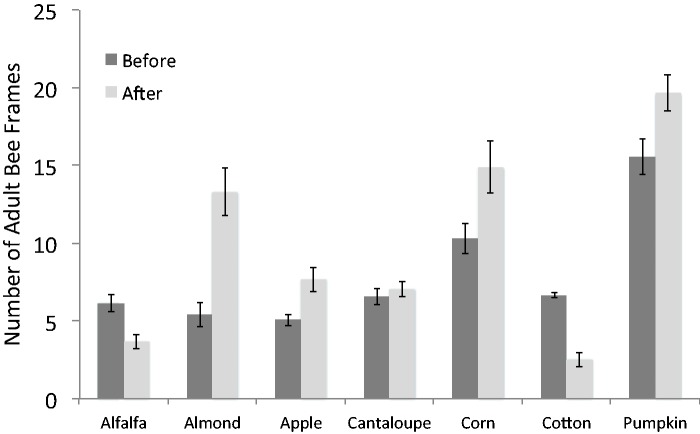
Field force populations in colonies pollinating alfalfa ranged from 22.8 to 66.5 bees per minute (Fig. 2). There was a significant change in field force over time (F2.27, 20.39 = 15.66, P < 0.001). Mauchly’s test found that the assumption of sphericity had been violated, χ2(14), P = 0.007; therefore, degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity (ε = 0.45). In general, field force populations steadily increased and then dramatically decreased at the final count. A means comparison test revealed that Count 5 was higher than Counts 2 (P = 0.006), 3 (P = 0.003), 4 (P = 0.033), and 6 (P = 0.003). Additionally, Count 6 was lower than Counts 2 (P = 0.015), 3 (P = 0.030), 4 (P = 0.007), and 5 (P = 0.003). The average number of adult bee frames per colony decreased from 6.14 to 3.66 (F1,9 = 18.74, P = 0.002; Fig. 3).
Fig. 2.
Changes in foraging force population size over time for colonies adjacent to eight different crop types.
Fig. 3.
Number of adult bee frames (mean ± SE) before and after the pollination period.
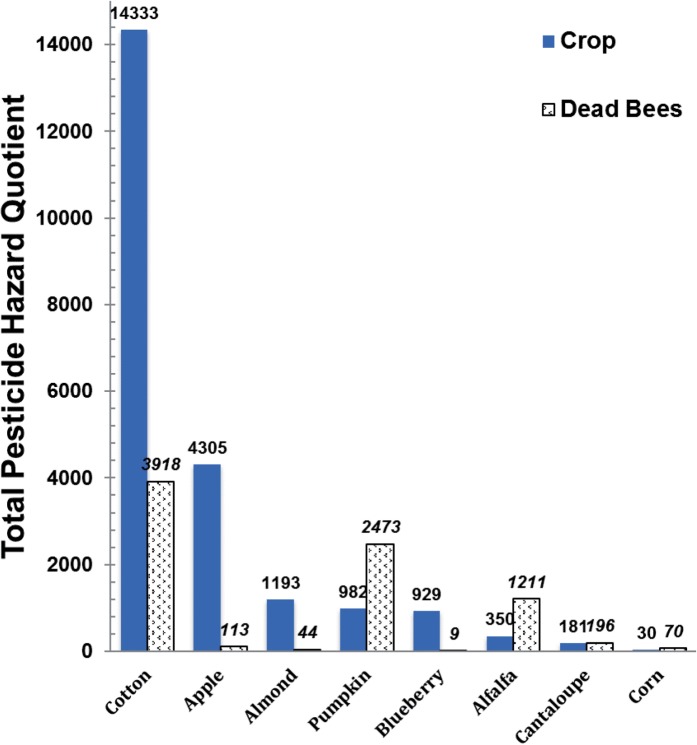
A total of 18 pesticide residues were found in eight samples collected in association with the alfalfa seed crop. The samples included alfalfa flowers, trapped pollen, dead and dying bees, and returning foragers. Each of the bee samples were collected at two distinct time periods: before colonies were moved on to the crop (pre-crop), and the first day of residency on the crop. Two additional samples, one of dead and dying bees and one of returning foragers, were composed of bees collected and combined over the course of the observation period. Alfalfa flowers had only two pesticides detected > 10 ppb, methamidophos (30.6 ppb) and pendimethalin (11.7 ppb). Trapped pollen (five residues total) had none of those found in flowers. Only cyhalothrin (11.7 ppb), flonicamid (22.6 ppb), and fluvalinate (16.9 ppb) were found at levels > 10 ppb. The number of detections in dead and dying bees increased from four pesticides pre-crop assessment, to seven in the initial sample on the crop, to 10 in the combination sample of bees collected over the course of the observation period. Also the pre-crop and initial crop exposure pesticides levels were relatively low compared to those in the combined sample. In the combined sample the insecticides thiamethoxam (12.7 ppb), cyhalothrin (14.0 ppb), esfenvalerate (59.5 ppb), and flonicamid (62.1 ppb), and miticides fluvalinate (103 ppb), and DMPF (273 ppb, amitraz degradate) were present at higher levels. The returning foragers had fewer pesticide detections than the dead and dying bee samples overall. Returning foragers prior to crop residency had only two pesticide detections, as did the initial sample, and both had relatively low levels of pesticide residues compared to the combined sample. The combination sample of returning foragers over the course of the observation period had three residues detected: DMPF (50.2 ppb), boscalid (23.5 ppb), and fluvalinate (87.4 ppb). Fluvalinate was found in all samples except the alfalfa flower sample, and was the highest detection in three of the six bee samples. The average alfalfa HQ of 350 (Fig. 4) represents a pesticide exposure risk of less than 0.035 × LD50 for adult bees, which is low, but the HQ for dead and dying bees (Fig. 4) of 1,211 (0.12 × LD50) may pose a significant sublethal risk to honey bees.
Fig. 4.
Total pesticide hazard quotient relative to crop or for only dead and dying bees foraging in crop. Crop values represent an average across all sample types (Table 2) collected for each crop.
Almonds
Field force populations near almonds ranged from 43.3 to 56.7 bees per minute (Fig. 2). There was no significant change in field force over time (F1.78, 16.04 = 1.13, P = 0.34). Mauchly’s test indicated that the assumption of sphericity had been violated, χ2(9), P = 0.045; therefore, degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity (ε = 0.45). Despite no change in field force populations over time, the average number of adult bee frames per colony increased significantly from 5.39 to 13.34 (F1,9 = 37.49, P < 0.001; Fig. 3).
A total of 15 pesticides were found in the four samples (almond flowers, trapped pollen, dead and dying bees, and returning foragers) collected in association with the almond crop. Unlike the other crops sampled, the almond flowers sample was collected the day a spray event occurred, and the trapped pollen was collected the day after spraying. There were eight pesticide residues detected in flowers including boscalid (75,500 ppb), chlorothalonil (22.1 ppb), esfenvalerate (18 ppb), iprodione (270 ppb), pendimethalin (17.7 ppb), and pyraclostrobin (27,700 ppb). In trapped pollen 15 pesticide residues were detected consisting of boscalid (7,270 ppb), chlorothalonil (30.5 ppb), chlorpyrifos and its metabolite (413 ppb), cyprodinil (53.2 ppb), esfenvalerate (15.7 ppb), iprodione (3,260 ppb), pendimethalin (222 ppb), pyraclostrobin (3,480 ppb), and pyrimethanil (41.3 ppb). Nine pesticides were detected in the dead and dying bee sample including boscalid (347 ppb), chlorothalonil (13.7 ppb), fluvalinate (10 ppb), iprodione (344 ppb), oxyfluorfen (48.6 ppb), and pyraclostrobin (194 ppb). Only three residues were found in the returning forager sample—boscalid (92.3 ppb), iprodione (891 ppb), and trace amounts of pyrimethanil. Boscalid, iprodione, and pyrimethanil were found at some level in all four samples, boscalid and iprodione among the highest levels of any detection within the sample. Chlorothalonil, chlorpyrifos, and pyraclostrobin were found in all but the returning forager sample, with pyraclostrobin being detected among the highest levels of any detection. The average almond HQ of 1,193 (Fig. 4) represents a notable pesticide exposure risk of 0.12 × LD50 for adult bees, while the HQ for dead and dying bees (Fig. 4) of 44 is of negligible risk.
Apples
Field force populations near apples ranged from 30.9 to 42.7 bees per minute (Fig. 2). There was a significant change in field force over time (F4, 36 = 4.16, P = 0.007). In general, field force populations increased as values for Count 4 (P = 0.026) and Count 5 (P = 0.023) were higher than Count 3. The average number of adult bee frames per colony also increased from 4.94 to 7.67 (F1,8 = 28.00, P = 0.001; Fig. 3).
There were 19 pesticide detections in nine samples collected in association with the apple crop. Residues found in the flower anthers and nectaries included: acetamiprid (3,820 and 12,390 ppb), chlorothalonil (20.4 and 33.4 ppb), chlorpyrifos and its methyl analog (59.15 and 92.6 ppb), cyprodinil (18.6 and 454 ppb), and thiacloprid (55 and 114.8 ppb), respectively. In addition trifloxystrobin was found in both samples, but only in nectaries (17.5 ppb) at > 10 ppb. Trapped pollen contained 13 residues at levels of, acetamiprid (60.6 ppb), azinphos methyl (2,680 ppb), captan (51.5 ppb), carbaryl (70.6 ppb), chlorothalonil (44.2 ppb), chlorpyrifos (70.3 ppb), cyhalothrin total (12.8 ppb), cyprodinil (2,825 ppb), difenoconazole (914 ppb), and imidacloprid (15.9 ppb); all others were < 10 ppb. Dead and dying bees contained two residues but only chlorothalonil (30.9 ppb) was above > 10 ppb. The returning foragers sample contained two, both < 10 ppb. Chlorpyrifos was the only pesticide found in all samples, and chlorothalonil was found in all samples except the returning forager sample. The average apple HQ of 4,305 (Fig. 4) represents an appreciable pesticide exposure risk of 0.43 × LD50 for adult bees, while the HQ for dead and dying bees (Fig. 4) of 113 is of negligible risk.
Blueberries
Field force populations near blueberries ranged from 46.1 to 60.4 bees per minute (Fig. 2). There was a significant change in field force over time (F4,32 = 3.10, P = 0.029). Field force populations increased and decreased over the course of the bloom period and peaked at Count 2 and Count 4.
There were eight pesticide detections in the three samples collected in association with the blueberry crop. The blueberry flower sample had only two detections—phosmet (60.4 ppb) and propiconazole (442 ppb). The trapped pollen sample contained four detections including captan and its metabolite THPI (1,511 ppb) and phosmet (2,150 ppb); all others were <10 ppb. The dead and dying bees sample contained four pesticides but only DMPF (197 ppb) and boscalid (31.3 ppb) were >10 ppb. Phosmet was the only pesticide detected in all three samples. The average blueberry HQ of 929 (Fig. 4) represents a low pesticide exposure risk of 0.09 × LD50 for adult bees, while the HQ for dead and dying bees (Fig. 4) of 9 is of negligible risk.
Cantaloupes
Field force populations near cantaloupes ranged from 34.4 to 58.8 bees per minute (Fig. 2). There was a significant change in field force over time (F4, 36 = 4.12, P = 0.007). In general, field force populations increased over time as the lowest values were Counts 1 and 2, and the highest values were Counts 3, 4, and 5. The difference in field force between Count 2 and Count 4 was marginally significant (P = 0.06). However, this increase in frequency may be artificial. Suspected pesticide exposure at the time the colonies were placed in the field resulted in an initial low count (Mussen 2009; E. Mussen personal communication). The average number of adult bee frames per colony near cantaloupes experienced no significant change—6.58 to 7.04 (F1,9 = 1.12, P = 0.319; Fig. 3).
There were four pesticide detections in the two samples (dead and dying bees and returning foragers) collected in association with the cantaloupe crop. The dead and dying bee sample had residues of DMPF at 702 ppb, which was the highest detection in this crop; all other residues were present at < 10 ppb. The returning foragers had two pesticide detections, which were also found in the dead and dying bees, both < 10 ppb. The average cantaloupe HQ of 181 (Fig. 4) and HQ for dead and dying bees of 196 (Fig. 4) both represent a very low pesticide exposure risk of 0.02 × LD50 for adult bees.
Corn
Field force populations near corn ranged from 45.0 to 77.2 bees per minute (Fig. 2). There was a significant change in field force over time (F4, 32 = 9.60, P < 0.001). Field force values peaked at Count 2 and then declined. Count 2 was significantly higher than Count 1 (P = 0.010), 3 (P < 0.001), 4 (P = 0.007), and 5 (P = 0.040). The average number of adult bee frames per colony near corn increased significantly from 10.3 to 14.9 (F1,8 = 10.66, P = 0.011; Fig. 3).
A total of four pesticide residues were found in three samples (corn tassels, trapped pollen, and dead and dying bees) collected in association with the corn crop. Corn tassels contained residues of chlorothalonil (1,020 ppb), fenhexamid (77.1 ppb), and fluvalinate (9.3 ppb). Trapped pollen had residues of DMPF (386 ppb), chlorothalonil (7.5 ppb), and fluvalinate (20.9 ppb). The dead and dying bee sample had residues of DMPF at 5,160 ppb and fluvalinate at 3.4 ppb. Only fluvalinate was detected in all three samples. The average corn HQ of 30.5 (Fig. 4) and HQ for dead and dying bees of 70 (Fig. 4) both represent a negligible pesticide exposure risk of less than 0.01 × LD50 for adult bees.
Cotton
Field force populations near cotton ranged from 37.5 to 55.9 bees per minute (Fig. 2). There was a significant change in field force over time (F5, 45 = 4.60, P = 0.002). In general, field force populations declined as values were lowest at Time 5 and 6. Count 5 was significantly lower than Count 3 (P = 0.001) and marginally lower than Count 1 (P = 0.051). The average number of adult bee frames per colony near cotton decreased from 6.65 to 2.51 (F1,9 = 68.23, P < 0.001; Fig. 3).
A total of 29 pesticide residues were found in four samples (cotton flowers, corn tassels from corn growing in close proximity to the cotton, returning foragers, and dead and dying bees) associated with the cotton crop. The cotton flower sample had residues of fenpyroximate at 131 ppb, the highest detection in the sample, while chlorothalonil (14.6 ppb), boscalid (36.1 ppb), and flonicamid (trace) were also present. However, the corn tassels had the most pesticide detections (21) of any sample with esfenvalerate present at the highest level (7,240 ppb) in this sample. It is unlikely that this number of pesticides was used on corn, but as corn is a tall plant in the landscape, it is likely “catching” pesticide drift from crop applications in the area (E. Mussen, personal communication). It is unknown if bees were collecting corn pollen in this location. The dead and dying bees had a total of 11 pesticide residues while returning foragers had none. Pesticide residues in dead and dying bees > 10 ppb included acephate and its toxic degradate methamidophos (575 ppb), chlorpyrifos (88.4 ppb), fenhexamid (40.5 ppb), flonicamid (65.2 ppb), methidathion (94.3 ppb), and oxamyl (292 ppb). The average cotton HQ of 14,333 (Fig. 4) represents a high pesticide exposure risk of 1.4 × LD50 for adult bees, and the HQ for dead and dying bees of 3,918 (Fig. 4) or 0.39 × LD50 both indicate that pesticides are a major toxic risk for the bees in cotton.
Pumpkins
Field force populations near pumpkins ranged from 51.3 to 74.0 bees per minute (Fig. 2). There was a significant change in field force over time (F7, 56 = 4.17, P = 0.001). The field force population peaked in the middle of the bloom period and then declined. Count 4 was significantly higher than Count 2 (P = 0.014), 3 (P = 0.001), and 5 (P = 0.008). The average number of adult bee frames per colony near pumpkin increased from 15.6 to 19.7 (F1,8 = 9.59, P = 0.015; Fig. 3).
There were a total of 19 pesticide residues in the three samples (pumpkin anthers only, trapped pollen, and dead and dying bees) collected in association with the pumpkin crop. Pumpkin flower anthers had eight residues detected and contained combined endosulfan residues (296.8 ppb) and dieldrin (24.3 ppb); all others were < 10 ppb. In the trapped pollen sample 11 pesticide residues were detected, including DMPF (60.4 ppb), chlorothalonil (1,100 ppb), coumaphos (18.6 ppb), endosulfan (1,012 ppb), fluvalinate (60.4 ppb), myclobutanil (128 ppb), and pyraclostrobin (267 ppb). The dead and dying bee sample had 14 pesticide residues detected including boscalid (26.7 ppb), chlorothalonil (2,290 ppb), endosulfan (26.5 ppb), fenhexamid (56.2 ppb), fluvalinate (49.2 ppb), myclobutanil (389 ppb), and permethrin (275 ppb). Chlorothalonil, endosulfan, and fluvalinate were detected in all three samples. Chlorpyrifos, cyhalothrin, and myclobutanil were detected in both the trapped pollen and the dead and dying bee sample. Metalaxyl was the only pesticide detected in both the anthers and dead and dying bee samples. The average pumpkin HQ of 982 (Fig. 4) represents a low pesticide exposure risk of 0.10 × LD50 for adult bees, while the HQ for dead and dying bees (Fig. 4) of 2,473 or 0.25 × LD50 is the second highest noted, and suggests a substantial pesticide toxicity risk was present.
Discussion
Honey bee colonies pollinating, or in association with, the crops in this study were clearly exposed to a diverse array of pesticides. Furthermore, we see that they were typically being exposed to residues beyond the target crop, even in large agricultural monocultures; cotton flowers had four residues while dead and dying bees had 11, and alfalfa flowers had only three residues while dead and dying foragers had 14.
Field force populations changed significantly over time in nearly all crops, with changes being highly variable depending on the crop and the time of year. A decrease in field force in cotton was one of the most substantial crop-associated impacts noted for foraging honey bees in this study. A corn tassel sample adjacent to cotton also presented the highest pesticide risk estimated by a summed pesticide residue HQ for any crop-associated or dead and dying bee sample measured, and provided the highest HQ of 78,161 or 7.8 × bee LD50. Clearly, pesticides may have posed a hazard to bees foraging at this cotton site. Other significant changes in field force may also be explained by the notable amounts of pesticide residues found. Nevertheless, pesticide residues cannot explain the later decline of returning foragers at our corn site.
The hazard quotient provides a convenient estimate of pesticide exposure risk to bee-toxic pesticides. It only estimates the toxicity of the active ingredients without other formulation ingredients or spray tank adjuvants used in actual field applications. Assumptions used for estimating risk via the HQ are that bees are exposed to a body weight equivalent of the residues in a crop sample, i.e., 100 mg, over one or a couple days, thus an acute exposure. This would mean that a value of 10,000 is equivalent to 1 × LD50 for the honey bee. The US EPA is now using 0.4 × LD50 exposure as its “level of concern” threshold (United States Environmental Protection Agency [US EPA] 2014), while the European Food Safety Authority (EFSA, 2013) uses a more conservative 0.1 × LD50 value (EFSA 2013). Using the latter safety factor with a threshold of HQ = 1,000, we have calculated HQ values of concern for bees pollinating cotton>apple>pumpkin>alfalfa > almond but not in blueberry, cantaloupe, or corn. Using this standard, we estimate that 43% of our crop samples and 38% of our dead and dying bee samples contain total pesticide residues hazardous to the bees. These values can be further refined to food residue inputs by expressing the HQ relative to an estimated daily honey bee consumption rate for pollen and nectar (Stoner and Eitzer 2013). This results in a constant multiplying factor that does not change the predicted risk trend, but better reflects the absolute hazard for the honey bee on a daily basis.
Activities that workers engage in depend on their ages and on colony needs (Winston 1987). The ability of honey bees to respond to their colony’s needs, such as through the recruitment of young workers, is well documented (Sagili and Pankiw 2007, Fewell and Winston 1996). If there is a loss or decline in foragers, younger hive bees are known to accelerate their behavioral development and forage precociously to compensate (Huang and Robinson 1996, Robinson et al. 1994). This dynamic and adaptive nature of colonies may explain the roller-coaster patterns seen in foraging populations of colonies on blueberries and pumpkins (Fig. 2) and could be a response to pesticide exposure, although other factors could also cause this phenomenon such as sudden changes in the detection of exploitable pollen and nectar. The pattern was less pronounced in blueberries, where the crop HQ represents a low pesticide exposure risk and the HQ for dead and dying bees is of negligible risk, compared to pumpkins where the pattern is more pronounced and the average pumpkin crop HQ represents a low pesticide exposure risk while the HQ for dead and dying bees suggests a substantial pesticide toxicity risk.
The most dramatic drop in field force population was seen in colonies pollinating alfalfa, the only crop where an assessment was made 2 mo after the initial assessment. After a steady increase, foraging activity was reduced from a high of 60 bees per minute at Count 5 to less than 20 per minute at Count 6. Also the adult bee population had a corresponding reduction from an average of six frames to less then four. While the average alfalfa crop HQ represents a low risk, the HQ for dead and dying bees suggests significant sublethal effects that became evident only after several brood cycles (Zhu 2013).
Apples and almonds are early spring crops and colonies used in the pollination of these crops would be expected to increase in size rapidly as was seen in the number of frames of adult bees in both cases. Field forces of colonies in apples steadily increased as colony populations nearly doubled; however, in almonds field force counts remained nearly constant and only slightly increased while colony populations nearly tripled in size. In this case the HQ for the almond crop represented a notable pesticide exposure risk while the HQ for dead and dying bees did not.
Honey bee colonies, especially those used for crop pollination, are without question being exposed to a diverse array of agrochemicals, especially fungicides and some bee-toxic pesticides as seen here and in other published studies (Mullin et al. 2010, Orantes-Bermejo et al. 2010, Chauzat et al. 2011, Krupke et al. 2012). Honey bee colonies likely have some colony-level capabilities for dealing with chemical exposure that are not fully understood, but it is also likely that pesticide effects are being experienced with variable time delays after the exposure period. Brood is particularly vulnerable to impacts from pesticide toxicity (Atkins and Kellum 1986, Nation et al. 1986, Davis et al. 1988, Zhu et al. 2014). The impacts of in-hive chemicals continue to be a concern given that the amitraz metabolite, DMPF, was found at 5,160 ppb in dead and dying bees near corn.
Honey bees require diverse sources of pollen (Maurizio 1950, Brodschneider and Crailsheim 2010) and need large stores of honey to overwinter. To achieve this, foraging radius can range from a few hundred meters to significant forging at 3,700 meters and up to 10,000 meters (Gary et al. 1972, Visscher and Seeley 1982). In another study that looked at bees pollinating certain crops, Pettis et al. (2013) found in some cases, little to no pollen from the crop being pollinated in trapped pollen samples. The behavior to forage over large acreage and collect a diverse array of plant pollens could work to their advantage; pesticide-contaminated pollen could be diluted by the pollen from other noncontaminated sources. Conversely, it could also be a disadvantage; bees may collect contaminated pollen from flowering crops and weeds beyond the target crop that have pesticide residues due to treatment or drift, as seems to be the case here. Within the agriculture community there is much interest in establishing flowering refugia near bee-pollinated crops to provide improved nutrition and help mitigate exposure to, and impacts of, pesticides for both honey bees and native pollinators. However, making these areas large and diverse enough and adequately protecting them from pesticide drift, especially in areas with diverse agriculture, will be challenging.
Lastly it is worth noting that pest control and thus pesticide application is a moving target. Newly introduced pests and pathogens, for example, citrus greening disease (Candidatus Liberibacter spp.) transmitted by the Asian citrus psyllid (Diaphorina citri), weather patterns, and economics, can all influence the need for, the type, and the timing of pesticide applications, thus making the protection of both managed and wild pollinators an ongoing challenge.
Acknowledgments
We would like to thank the beekeepers who helped develop this proposal and allowed us to use their bees for the project: Dave Hackenberg, Dave Mendez, Tory Bunch, Gene Brandi, Bob Miller, and Orin Johnson. We are especially thankful to PSU technicians Lauren Rusert and Sara Ziegler, UMaine technician George Cooper, and Wanyi Zhu who helped collect data and conducted an initial analysis of the data. Without their help, this project would not have been possible. Funding for this research was provided by a grant from the National Honey Board.
References Cited
- Atkins E. L., Kellum D. 1986. Comparative morphogenic and toxicity studies on the effect of pesticides on honeybee brood. J. Apicult. Res. 25: 242–255. [Google Scholar]
- Atkins E. L., Kellum D., Atkins K. W. 1981. Reducing pesticide hazards to honey bees: Mortality prediction and integrated management strategies. Univ. Calif. Div. Agric. Sci. Leafl. 2883. [Google Scholar]
- Brodschneider R., Crailsheim K. 2010. Nutrition and health in honey bees. Apidologie 41: 278–294. [Google Scholar]
- Calderone N. W. 2012. Insect pollinated crops, insect pollinators and US agriculture: Trend analysis of aggregate data for the period 1992–2009. PLoS ONE 7: e37235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauzat M. P., Martel A. C., Cougoule N., Porta P., Lachaize J., Zeggane S., Aubert M., Carpentier P., Faucon J. P. 2011. An assessment of honeybee colony matrices, Apis mellifera (Hymenoptera Apidae) to monitor pesticide presence in continental France. Environ. Toxicol. Chem. 30: 103–111. [DOI] [PubMed] [Google Scholar]
- Ciarlo T. J., Mullin C. A., Frazier J. L., Schmehl D. R. 2012. Learning impairment in honey bees caused by agricultural spray adjuvants. PLoS ONE 7: e40848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. R., Solomon K. R., Shuel R. W. 1988. Laboratory studies of honeybee larval growth and development as affected by systemic insecticides at adult-sublethal levels. J. Apicult. Res. 27: 146–161. [Google Scholar]
- Decourtye A., Devillers J., Cluzeau S., Charreton M., Pham-Delegue M. H. 2004. Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol. Environ. Saf. 57: 410–419. [DOI] [PubMed] [Google Scholar]
- Desneux N., Decourtye A., Delpuech J. M. 2007. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52: 81–106. [DOI] [PubMed] [Google Scholar]
- (EFSA) European Food Safety Authority. 2013. EFSA guidance document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 11: 3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell J. H., Winston M. L. 1996. Regulation of nectar collection in relation to honey storage levels by honey bees, Apis mellifera. Behav. Ecol. 7: 286–291. [Google Scholar]
- Frazier M., Mullin C., Frazier J., Ashcraft S. 2008. What have pesticides got to do with it? Am. Bee J. 148: 521–523. [Google Scholar]
- Garrido P. M., Antunez K., Martin M., Porrini M. P., Zunino P., Eguaras M. J. 2013. Immune-related gene expression in nurse honey bees (Apis mellifera) exposed to synthetic acaricides. J. Insect Physiol. 59: 113–119. [DOI] [PubMed] [Google Scholar]
- Gary N. E., Witherell P. C., Marston J. M. 1972. Foraging range and distribution of honey bees used for carrot and onion pollination. Environ. Entomol. 1: 71–78 [Google Scholar]
- Graham J. M. 1992. The hive and the honey bee. Dadant & Sons, Hamilton, IL. [Google Scholar]
- Huang Z. Y., Robinson G. E. 1996. Regulation of honey bee division of labor by colony age demography. Behav. Ecol. Sociobiol. 39: 147–158. [Google Scholar]
- IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. IBM Corp, Armonk, NY. [Google Scholar]
- Krupke C. H., Hunt G. J., Eitzer B. D., Andino G., Given K. 2012. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PloS ONE 7: e29268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. V., Steinhauer N., Rennich K., Wilson M. E., Tarpy D. R., Caron D. M., Rose R., Delaplane K. S., Baylis K., Lengerich E. J., et al. 2015. A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie 46: 292–305. [Google Scholar]
- Maurizio A. 1950. The influence of pollen feeding and brood rearing on the length of life and physiological condition of the honey bee - preliminary report. Bee World 31: 9–12. [Google Scholar]
- Mullin C. A., Frazier M., Frazier J. L., Ashcraft S., Simonds R., van Engelsdorp D., Pettis J. S. 2010. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PloS ONE 5: e9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation J. L., Robinson F. A., Yu S. J., Bolten A. B. 1986. Influence upon honeybees of chronic exposure to very low-levels of selected insecticides in their diet. J. Apicult. Res. 25: 170–177. [Google Scholar]
- Orantes-Bermejo F. J., Pajuelo A. G., Megias M. M., Fernandez-Pinar C. T. 2010. Pesticide residues in beeswax and beebread samples collected from honey bee colonies (Apis mellifera L.) in Spain. Possible implications for bee losses. J. Apicult. Res. 49: 243–250. [Google Scholar]
- Oruc H. H., Hranitz J. M., Sorucu A., Duell M., Cakmak I., Aydin L., Orman A. 2012. Determination of acute oral toxicity of flumethrin in honey bees. J. Econ. Entomol. 105: 1890–1894. [DOI] [PubMed] [Google Scholar]
- Pettis J. S., Lichtenberg E. M., Andree M., Stitzinger J., Rose R., van Engelsdorp D. 2013. Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS ONE 8: e70182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G. E., Page R. E., Huang Z. Y. 1994. Temporal polyethism in social insects is a developmental process. Anim. Behav. 48: 467–469. [Google Scholar]
- Sagili R., Pankiw T. 2007. Effects of protein-constrained brood food on honey bee (Apis mellifera L.) pollen foraging and colony growth. Behav. Ecol. Sociobiol. 61: 1471–1478. [Google Scholar]
- Stoner K. A., Eitzer B. D. 2013. Using a hazard quotient to evaluate pesticide residues detected in pollen trapped from honey bees (Apis mellifera) in Connecticut. PLoS ONE 8: e77550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. M. 2003. Behavioural effects of pesticides in bees - Their potential for use in risk assessment. Ecotoxicology 12: 317–330. [DOI] [PubMed] [Google Scholar]
- United Nations Food and Agriculture Organization. 2005. Protecting the pollinators. FAO spotlight. (http://www.fao.org/ag/magazine/0512sp1.htm) (accessed 6 July 2015). [Google Scholar]
- (US EPA) United States Environmental Protection Agency. 2014. Guidance for assessing pesticide risks to bees, p. 59. (http://www2.epa.gov/pollinator-protection/pollinator-risk-assessment-guidance) (accessed 6 July 2015). [Google Scholar]
- Visscher K. P., Seeley T. D. 1982. Foraging strategy of honeybee colonies in a temperate deciduous forest. Ecology 63: 1790–1801. [Google Scholar]
- Winston M. L. 1987. The biology of the honey bee. Harvard University Press, Cambridge, MA. [Google Scholar]
- Zhu W. Y. 2013. Assessing impacts of pesticides and other stressors on honey bee colony health: Experimental and modeling approaches. Ph.D. dissertation; The Pennsylvania State University; University Park, PA. [Google Scholar]
- Zhu W. Y., Schmehl D. R., Mullin C. A., Frazier J. L. 2014. Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS ONE 9: e77547. [DOI] [PMC free article] [PubMed] [Google Scholar]