Abstract
Objective
This study is a placebo-controlled comparison of the response to alfuzosin treatment for lower urinary tract symptoms (LUTS) in patients with and without metabolic syndrome (MetS).
Material and methods
A total of 80 men with LUTS were included in the study. Patients had a maximum flow rate of <15 mL/sec, prostate volume of >20 mL, and International Prostate Symptom Score (IPSS) of >8. All eligible men (n=68) for evaluation were initially divided into two groups as MetS (n=34) and non-MetS (n=34) groups. Patients were further randomized to receive alfuzosin (10 mg/day) or placebo (n=17/group; a total of four groups). The outcome was measured at 12th week according to the changes from baseline in IPSS, quality of life (QoL) scores, maximum flow rate (Qmax), and postmictional residue.
Results
Alfuzosin significantly improved LUTS in men with and without MetS compared with patients receiving placebo (p<0.05). Mean IPSS scores in treatment groups decreased significantly, whereas patients receiving placebo had no statistically significant difference (p>0.05). Similarly, alfuzosin treatment resulted in a significant increase in Qmax in patients with LUTS/benign prostatic enlargement when compared with patients in placebo group (p<0.05). Mean QoL scores measured by IPSS-QoL and QoL questionnaires also improved significantly in patients receiving alfuzosin for 3 months regardless of the presence of MetS (p<0.05).
Conclusion
Our results revealed that the presence of MetS in patients with LUTS did not impair the response to alfuzosin treatment.
Keywords: Alfuzosin, lower urinary tract symptoms treatment, metabolic syndrome
Introduction
Metabolic syndrome (MetS) has been considered to have an important role in the development or progression of lower urinary tract symptoms (LUTS) and/or benign prostatic enlargement (BPE).[1, 2] The incidence of LUTS was reported to be significantly higher in patients with MetS, which increases the evidence of a relationship between the presence of MetS and LUTS.[1–3] The pathophysiology of LUTS is not limited to benign prostatic hyperplasia (BPH) and it is rather multifactorial. Chronic diseases, such as diabetes, cardiac pathologies or lifestyle changes, and components of MetS, such as insulin resistance (IR), obesity, and hyperlipidemia, may lead to the development or progression of LUTS.[4–6]
A possible link between LUTS and MetS may be accompanied by IR.[7] Hyperinsulinemia, the core pathophysiology of MetS, was previously shown to be associated with increased annual growth of prostate volume and increased smooth muscle tone.[7, 8] Increased sympathetic tone caused by increased serum insulin levels in the presence of MetS has been reported to contribute to the development of LUTS in patients with BPE or BPH.[9] Similarly, insulin-like growth factor (IGF) and chronic inflammation induced by MetS were shown to stimulate the growth of prostatic stromal and epithelial cells.[10] Hammersten et al.[7] examined a total of 280 men with and without hyperinsulinemia and found the median annual prostatic growth rate to be significantly higher in patients with increased insulin levels. The Third National Health and Nutrition Examination Survey conducted in men >60 years revealed that the odds of having LUTS increased significantly in men with three or more components of MetS.[2] Similarly, in a community-based healthy survey, a trend in the increasing prevalence of MetS with increasing American Urological Association Symptom Index (AUA-SI) was observed.[11]
Currently, the standard pharmacological treatment for men with LUTS is alpha (α)-adrenergic receptor blockers.[12] Indirect and limited direct comparisons between different α-blockers revealed that all α-blockers have similar efficacy in therapeutic doses.[13] However, there is limited data regarding the efficacy of α-blocker use in the treatment of patients with concomitant LUTS and MetS. In this double-blind, randomized, and placebo-controlled study, we aimed to evaluate the response to alfuzosin treatment for LUTS in patients with and without MetS.
Material and methods
Patient enrollment and study groups
The study has been reviewed by the local ethics committee for human subjects after detailed examination and is addressed by the approval number of 07/07–2.4.2011. Between May 2011 and May 2012, a total of 80 men with or without MetS having severe LUTS presented to Urology and/or Endocrinology outpatient clinics were enrolled into the study. All patients were informed about the study protocol, and written consents were obtained. Frail elderly patients and patients with the following criteria were excluded from the study: neurogenic lower urinary tract dysfunction, previous lower urinary tract surgery, active urinary tract infection, increased prostate-specific antigen (PSA) levels (without documented pathology in biopsy), history of lower urinary tract malignancy, urethral stricture, history of previous medication for LUTS, or hormonal treatment,. Uroflowmetric study (MMS Flowmaster; Earth City, MO, USA) and prostate volume measurement (Esaote Biomedica AU3 Partner Advanced Ultrasonography, Genoa, Italy) were performed. All of the enrolled patients had a maximum flow rate of <15 mL/s, prostate volume of >20 mL, and International Prostate Symptom Score (IPSS) of ≥8. Patients with PSA levels of >4 ng/dL underwent biopsy and were enrolled after a negative histopathological examination.
Of the 80 patients with LUTS, 68 men were eligible to participate in the study and were randomly assigned to four equal groups. All men were first randomized according to the presence of MetS and then according to the administration of either alfuzosin 10 mg/day (Generica, İstanbul, Turkey) or placebo for 3 months. All patients were assigned a number at the beginning of the study, and each number was randomly assigned to one of the two treatment options using computed generated by GraphPad software (GraphPad Software Inc., La Jolla, CA). The study medications and placebo provided by the same company (Generica, Istanbul, Turkey) were identical in appearance to preserve blinding. The study design and patient allocation was presented in Figure 1.
Figure 1.
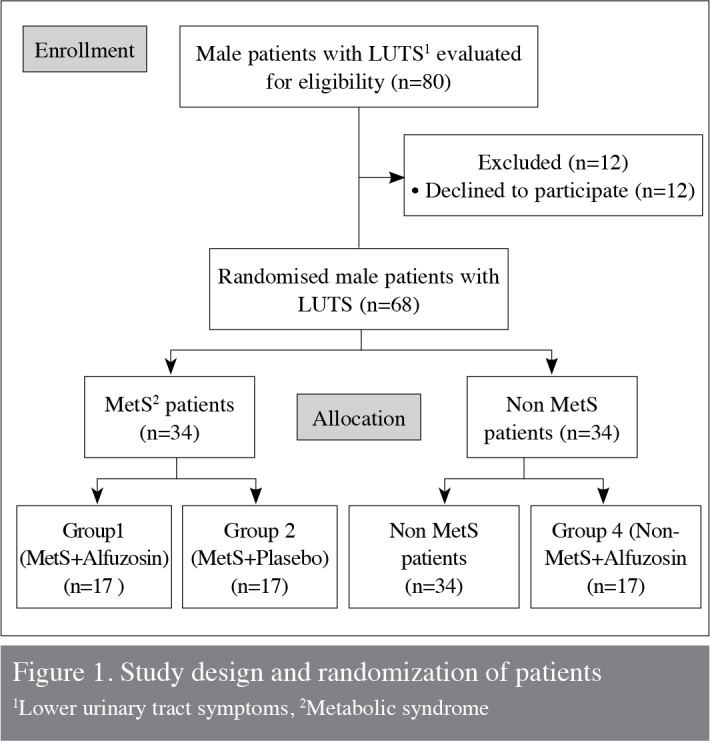
Study design and randomization of patients
1Lower urinary tract symptoms, 2Metabolic syndrome
Assessment of LUTS and diagnosis of MetS
Detailed physical and neurological examinations and laboratory assessment were performed for all patients; inspection and palpation of the organs belonging to the system including the digital rectal examination of the prostate was performed. Sensations and reflexes in the urogenital area have been tested. The anal sphincter and pelvic floor functions must be extensively tested. The symptoms and their effects on quality of life (QoL) were evaluated by IPSS and disease-specific QoL question score from IPSS, respectively, as described previously. IPSS was used to assess the degree of severity of LUTS. Each symptom is graded from 0 (not at all) to 5 (almost always), and the scores of each individual symptom were added to reach the total score of 35. Urinary flow rate and post-void residual urine measurements (Esaote Biomedica AU3 Partner Advanced Ultrasonography, Genoa, Italy) were performed in all patients both prior to and at the end of the study (3 months).
Blood pressure, body weight, body height, waist/hip circumferences, and body mass index (BMI) were measured in all men. Biochemical analyses including glucose and lipid pro- files and PSA levels were measured using spectrophotometric (Siemens Advia 2400; Healthcare Dgn., Tarrytown, NY, USA) and chemiluminescence methods (Siemens Advia Centaur XP, Healthcare Dgn., Tarrytown, NY, USA), respectively.
Diagnosis of MetS was made according to the most recent consensus report of the National Cholesterol Education Program’s Third Adult Treatment Panel (NCEP ATP III).[2] The diagnostic criteria for MetS had to satisfy three or more of the NCEP-ATP III criteria, which are as follows: 1) hypertension (blood pressure of ≥130/85 mmHg); 2) hyperglycemia (fasting blood glucose level of ≥110 mg/dL); 3) abdominal obesity (waist circumference of >102 cm); 4) hypertriglyceridemia (serum triglyceride (TG) levels of ≥150 mg/dL); and 5) reduced levels of high-density lipoprotein (HDL) cholesterol (<40 mg/dL).
The primary outcome measures examined in our study were the percentage of changes in IPPS and maximum flow rates. Secondary measures were QoL scores and change in postmictional residue (PVR). All patients were evaluated at 4th and 8th week for any side-effects and compared at the end of 12th week.
Statistical analysis
All data were presented as mean±standard deviation. Statistical analyses were performed using the Statistical Package of the Social Sciences (SPSS) 10 software (Chicago, IL, USA). Comparisons of parameters between the groups at baseline and after 3 months of treatment were performed using Kruskal Wallis and Mann Whitney U-tests, and the efficacy of treatment was assessed by the Wilcoxon test within the groups. A p value of <0.05 was considered to be statistically significant.
Results
The demographic data and laboratory findings of men in the study groups were shown in Table 1. Mean age of the patients was 64.18±7.61 (50–84). There was no significant difference between groups with respect to age, prostate volume, and PSA (p>0.05). Similarly, baseline IPSS, mean QoL scores, maximum flow rate (Qmax), and PVR measurements showed no significant difference among each group (p>0.05). However, men with MetS in groups 1 and 2 had significantly higher serum levels of TG, HDL, and HbA1c compared with groups 3 and 4 (p<0.05). Patients with MetS in groups 1 and 2 also had significantly higher BMI, waist circumference, body weight, and blood pressure measurements than those of the patients in group 3 and 4 (p<0.05) (Table 1).
Table 1.
Patient demographics and laboratory findings of each group
| MetS+alfuzosin (Group 1) | MetS+placebo (Group 2) | Alfuzosin (Group 3) | Placebo (Group 4) | |
|---|---|---|---|---|
| Age | 63.94±6.05 | 66.88±7.45 | 65.67±9.68 | 60.18±5.21 |
| T PSA (ng/mL) | 0.9±2.37 | 1.9±1.10 | 1.67±1.25 | 1.07±1.23 |
| HDL (mg/dL) | 46.23±12.87a,b | 41.88±6.20a,b | 49.27±16.45 | 50.00±9.42 |
| BMI (kg/m2) | 31.20±3.55a,b | 30.01±4.26a,b | 25.94±3.04 | 27.04±3.26 |
| Waist circumference (cm) | 110±5.09a,b,c | 103±8.46a,b | 90±6.45 | 90±11.17 |
| Body weight (kg) | 90±10.40a,b | 86±10.50a,b | 77±10.20 | 78±10.06 |
| HbA1c | 9.6±2.14a,b | 8±2.40a,b | 5.4±1.00 | 4.9±1.20 |
| IPSS | 18.24±6.39 | 17.82±5.92 | 17.67±5.92 | 18.18±6.26 |
| Qmax (mL/sn) | 12.19±2.13 | 12.51±2.59 | 10.59±2.63 | 13.26±1.71 |
| PVR (mL) | 54.59±26.64 | 43.71±29.24 | 43.78±30.55 | 45.88±32.76 |
Data are presented as mean±SD.
Statistically significant difference compared with placebo group (p<0.05)
Statistically significant difference compared with alfuzosine group
Statistically significant difference compared with MetS+placebo group
PSA: prostate-specific antigen; MetS: metabolic syndrome; HDL: high density lipoprotein; BDI: body mass index; IPSS: international prostate symptom score; Qmax: maximum flow rate; PVR: post-voiding residue
After 3 months of 10-mg alfuzosin treatment, LUTS in patients with and without MetS showed significant improvement compared with patients receiving placebo (groups 2 and 4; p<0.05). Mean IPSS scores in groups 1 and 3 increased significantly compared with pre-treatment levels, whereas patients receiving placebo had no statistically significant difference in the IPSS scores (p<0.05) (Table 2). The improvement of mean IPSS in the group of patients with MetS was similar to the improvement observed in the group of patients without MetS. The percentage change in primary outcome measures (mean IPSS and Qmax scores) in all groups were −37%, −9.8%, −7.8%, and −19%, respectively (Table 3).
Table 2.
Assessment of primary and secondary outcome measures before (BT) and after (AT) treatment in each group
| MetS+alfuzosin (Group 1) | MetS+placebo (Group 2) | Alfuzosin (Group 3) | Placebo (Group 4) | |
|---|---|---|---|---|
| IPSS BT | 18.24±6.39 | 17.82±5.92 | 17.67±9.92 | 18.18±6.26 |
| IPSS AT | 11.53±8.35a,c,d | 16.06±6.99b | 12.50±6.36a,d | 14.18±7.89 |
| IPSS-QoL BT | 3.47±0.71 | 3.59±0.62 | 3.44±0.62 | 3.59±0.62 |
| IPSS-QoL AT | 2.65±0.79d | 3.18±0.95 | 2.72±0.83d | 3.29±0.95 |
| BPH-QoL | ||||
| BT | 16.53±11.54 | 18.88±6.72b | 12.78±6.65 | 15.71±10.91 |
| BPH-QoL AT | 9.65±12.26c,d | 16.94±7.62b | 7.06±6.37c,d | 12.18±10.82 |
| Qmax (mL/s) BT | 12.19±2.13 | 12.51±2.59b | 10.59±2.63a | 13.26±1.71 |
| Qmax (mL/s) AT | 16.25±5.48c,d | 12.12±3.32 | 13.32±3.33d | 12.96±3.24 |
| PVR (mL) BT | 54.59±26.64 | 43.71±29.24 | 43.78±30.55 | 45.88±32.76 |
| PVR (mL) AT | 57.65±35.33 | 41.18±20.80 | 47.11±30.99 | 42.76±27.97 |
Data are presented as mean±SD
Statistically significant difference compared to placebo group
Statistically significant difference compared to alfuzosine group
Statistically significant difference compared to MetS+placebo group
Statistically significant difference compared to before treatment scores; p<0.05
MetS: metabolic syndrome; IPSS: international prostate symptom score; IPSS-QoL: international prostate symptom score-quality of life; BPH-QoL: benign prostate hypertrophy-quality of life; Qmax: maximum flow rate; PVR: post-voiding residue
Table 3.
Percent changes (Δ%) of primary outcome measures after 12 weeks of treatment among each group
| MetS+alfuzosin group 1 | MetS+placebo group 2 | Alfuzosin group 3 | Placebo group 4 | |
|---|---|---|---|---|
| Percent change of IPSS (Δ%) | −37.77%a,b,c | −9.8% | −27.89%a,c | −19.58% |
| Percent change of Qmax (Δ%) | +32.82%a,c | +2.25% | +30.10%a,c | +2.67% |
Data are presented as mean±SD
Statistically significant difference compared to placebo group
Statistically significant difference compared to alfuzosine group
Statistically significant difference compared to MetS+placebo group; p<0.05
MetS: metabolic syndrome; IPSS: international prostate symptom score; Qmax: maximum flow rate
The most significant improvement was observed in patients with MetS who received alfuzosin treatment (p<0.05). Post-void residual urine measurements before and after treatment were not significantly different between the groups (p<0.05) (Table 2).
Mean QoL scores measured by IPSS-QoL and BPH-QoL questionnaires also improved significantly in patients receiving alfuzosin for 3 months, regardless of the presence or absence of MetS (p<0.05). However, mean QoL scores of patients did not show any difference in patients receiving placebo compared with pre-treatment scores (p>0.05).
Discussion
The prevalence of MetS varies in different parts of the world. The prevalence of MetS was reported as 21.7%, 36.3%, and 17.9% in China, Jordan, and Greenland, respectively.[14–16] Athyros et al.[17] reviewed its prevalence in Greece and found it in 23.6% of the population. Our study was conducted in Turkey, which is considered as minor Asia or Anatolia. Kozan et al.[18] reported the prevalance of MetS in Turkey as 33.9%, whereas in another study conducted by Ozsahin et al.[19] it was detected as 33.4%. Several studies have suggested an increased association between LUTS and the presence of MetS in men.[3] NCEP-ATP III showed the relationship between the markers of MetS and LUTS defined as having three of four urinary symptoms (such as nocturia, incomplete voiding, weak stream, and hesitancy).[2] In a community-based survey analysing 1899 men, increased odds of MetS were observed even with mild symptoms, primarily for incomplete emptying, intermittency, and nocturia.[11] The association of weight gain, increased BMI, and LUTS supports the major role of insulin-mediated effect on LUTS development and increased levels of tissue growth factors for prostate enlargement.[20] Another hypothesis for the increased detection of LUTS in men with MetS is atherosclerosis of pelvic vessels and subsequent chronic pelvic ischemia of the bladder and prostate.[21] Alternatively, the inflammatory molecules, such as C-reactive proteins, or impaired endothelium-derived nitric oxide pathway may be the mediators of intraprostatic inflammation, thereby contributing to LUTS development.[22, 23] However, the most important pathophysiological event for the development of LUTS in men with BPH was reported to be IR and compensatory hyperinsulinemia, which induces autonomic hyperactivity.[24] Subsequent unbalanced loss of autonomic neurons was suggested to induce an oversupply of sympathetic tone over parasympathetic tone, resulting in increased bladder neck obstruction.[25]
Lower urinary tract symptoms secondary to BPH are primarily treated by α-adrenergic blockers, which aim to lower the sympathetic tone and increase urinary flow rate. By inhibiting smooth muscle α-adrenergic receptors, the drugs relax prostatic and bladder neck smooth muscles and partially improve LUTS by relieving bladder outlet obstruction.[26] Although α-blockers have a proven role for the symptomatic treatment of LUTS, there is little evidence about their efficacy for the treatment of LUTS in men with MetS. Gökkaya et al.[27] prospectively evaluated the effects of IR on the outcomes of doxazosin treatment for LUTS in 64 patients. Doxazosin treatment significantly lowered the mean IPSS levels and increased Qmax in men without IR, whereas this treatment did not affect the mean IPSS and Qmax. Authors concluded that IR impaired the response to doxazosin treatment for LUTS caused by BPH. However, the similar pre-and post-treatment values of IPSS and Qmax in patients with improved and non-improved IR in that study suggested that the disappearance of IR after doxazosin had no impact on the outcomes of BPH therapy. They commented that this result was related to their patients’ high baseline homeostasis model assessment (HOMA) scores, shorter duration of treatment, and differences in patient profile. In addition to these limitations, the study also had no placebo arm. In a recent study evaluating the responsiveness to α1-blocker treatment in men with concomitant LUTS and MetS, Lee et al.[28] examined the efficacy of 4-mg doxazosin GITS administered once daily in 109 patients. After 12 weeks of drug treatment, the responders were defined as those having a decrease in the total IPSS by >4 points from baseline. Sixty-six percent of the patients responded to α1-blocker treatment, and multivariate analysis revealed that MetS was significantly higher in the non-responder group. Similarly, IPSS improvements from baseline in patients with MetS significantly decreased as the number of MetS components increased. Thus, the authors concluded that MetS may lead to a different drug response than that expected and α-blocker treatment with the aim of interrupting sympathetic adrenergic activity may not produce the desired treatment efficacy. Despite these findings, authors acknowledged that because of the lack of molecular investigations, their study did not provide further evidence about the possible mechanisms on how MetS influenced the responsiveness to a1-blocker therapy in men with BPH/LUTS.[28]
Our randomized, placebo-controlled study revealed that alfuzosin was similarly effective, regardless of the presence or absence of MetS in men with BPH. In a recent study examining the association between MetS, hyperinsulinemia, and LUTS, Eom et al.[29] found that voiding symptoms were decreased in men with MetS compared with men without MetS. However, LUTS had a strong, positive, and significant association when they were present at a severe level of metabolic derangement (HbA1c level of ≥8%). We measured serum HbA1c levels of ≥8% in patients with MetS; however, we randomized the patients with similar IPSS scores into groups to prevent a potential bias of selection. On the contrary, Roehrborn et al.[26] found a close association of MetS components with LUTS in older men, whereas Joseph et al.[30] detected that hypertension or diabetes mellitus were at a risk of moderate-to-severe LUTS. However, in a recent survey, a significant positive association was not found between MetS or IR and LUTS in men or women.[31] Similarly, Park et al.[32] showed no significant differences in voiding symptoms between MetS and non-MetS patients. Age, duration of the components of MetS, HbA1c level, and long-term hyperglycemia were all reported to have either favorable or unfavorable effects on LUTS.[29] The glomerular filtration and insulin are commonly increased in early diabetes, and these early compensatory mechanisms may favorably affect LUTS; however, with time the decreased glomerular filtration and advanced diabetes may worsen different components of LUTS.[2, 3] Thus, considering the controversial data regarding relationship between LUTS and MetS, it cannot be concluded that MetS may or may not worsen LUTS everytime. Similarly, it cannot always be stated that there will be poor responsiveness to α1-blocker therapy in men with LUTS and concomitant MetS. To our knowledge, our study is the first to provide a similar response to alfuzosin in men with BPH and MetS compared with the response obtained in men without MetS.
In the Korean Longitidunal Study on Health and Aging, there was no significant change in the IPSS between the metabolic and non-metabolic groups. We also detected no significant difference between the groups in the pretreatment period with respect to IPSS and QoL scores. After 12 weeks of the treatment period, IPSS significantly decreased whereas QoL improved in groups 1 and 3 compared with placebo groups. According to the AUA guidelines, α-blockers provide four- to six-point decrease in AUA symptoms score.[33] In our study, we detected a 6.7 (−37.7%) and 5.1 (−27.8%) IPSS improvement in Group 1 and Group 3, respectively. The highest change in the percentage of IPSS and IPSS-QoL score was recorded in men with MetS patients receiving alfuzosin (groups 1 and 3; Figure 2). This improvement may be explained by the favorable effects of MetS on LUTS, which has been previously described in some studies. In a recent study conducted on 707 men with and without MetS, it was shown that men with MetS had significantly lower IPSS and better Qmax levels.[34] Similarly, in another large series examining 33481 patients, it was reported that MetS and accompanying hyperinsulinemia could have a positive effect on voiding symptoms, particularly in the early compensatory stage.[29]
Figure 2.
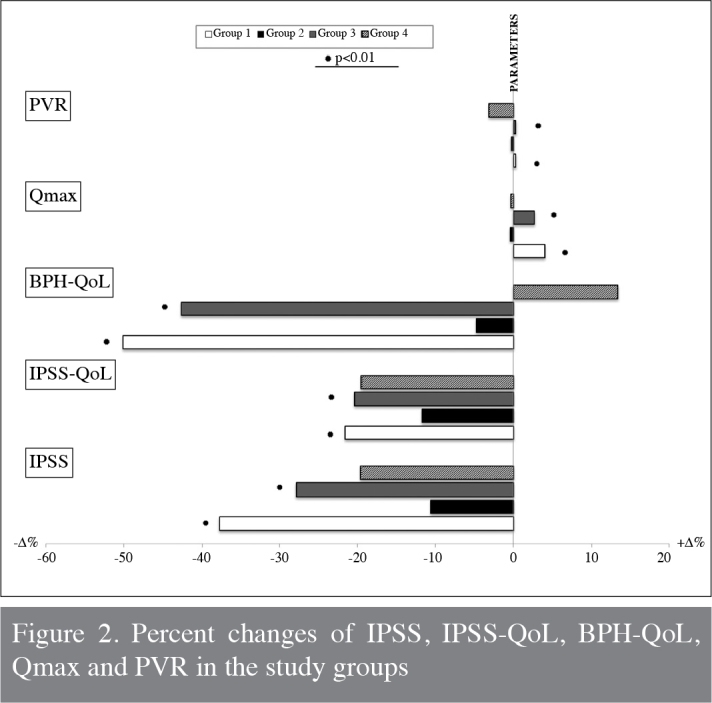
Percent changes of IPSS, IPSS-QoL, BPH-QoL, Qmax and PVR in the study groups
The present study is one of the limited clinical examples to the relationship between MetS and responsiveness of α-blocker treatment in men with BPH. However, we have to acknowledge some of the limitations of our study. First, we could only enroll a relatively small group of patients. The subject population came from a single institution, but this group had well-defined symptoms evaluated by self-administered and validated questionnaires. Sample size was intended to be prospectively determined. Because we examined a specific group of patients, a total of 20 patients with MetS and LUTS demanding treatment could be recruited to receive alfuzosin. Similarly, other groups were maintained with similar numbers. Secondly, we did not assess the degree of response to alfuzosin therapy with regard to the duration of MetS nor to the level of HbA1c. Men with MetS in our study group had mean HbA1c levels of ≥8.8±2.27, which may introduce a selection bias or a potential for a response bias.
In conclusion, our data suggested that the presence of MetS in men with BPH did not impair the response to alfuzosin treatment, and patients’ symptoms and QoL parameters showed similar improvements. However, our results need to be confirmed with further studies.
Footnotes
Ethics Committee Approval: Ethics committee approval was obtained.
Informed consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - R.O, S.A.; Design - R.O., S.A.; Supervision - R.O., T.O.; Funding - R.O., S.A.; Materials - N.İ.; Data Collection and/or Processing - S.A.; Analysis and/or Interpretation - T.O., R.O.; Literature Review - T.O.; Writer - R.O.; Critical Review - T.O., R.O.; Other - S.İ.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Kasturi S, Russell S, McVary KT. Metabolic syndrome and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Curr Urol Rep. 2006;7:288–92. doi: 10.1007/s11934-996-0008-y. http://dx.doi.org/10.1007/s11934-996-0008-y. [DOI] [PubMed] [Google Scholar]
- 2.Rohrmann S, Smit E, Giovannucci E, Platz EA. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III) Int J Obes (Lond) 2005;29:310–6. doi: 10.1038/sj.ijo.0802881. http://dx.doi.org/10.1038/sj.ijo.0802881. [DOI] [PubMed] [Google Scholar]
- 3.Moul S, McVary KT. Lower urinary tract symptoms, obesity and the metabolic syndrome. Curr Opin Urol. 2010;20:7–12. doi: 10.1097/MOU.0b013e3283336f3f. http://dx.doi.org/10.1097/MOU.0b013e3283336f3f. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald MP, Link CL, Litman HJ, Travison TG, McKinlay JB. Beyond the lower urinary tract: the association of urologic and sexual symptomps with common illnesses. Eur Urol. 2007;52:407–15. doi: 10.1016/j.eururo.2007.03.014. http://dx.doi.org/10.1016/j.eururo.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joseph MA, Harlow SD, Wei JT, Sarma AV, Dunn RL, Taylor JM, et al. Risk factors for lower urinary tract symptoms in a population based sample of African-American men. Am J Epidemiol. 2003;157:906–7. doi: 10.1093/aje/kwg051. http://dx.doi.org/10.1093/aje/kwg051. [DOI] [PubMed] [Google Scholar]
- 6.Michel MC, Mehlburger L, Schumacher H, Bressel HU, Goepel M. Effect of diabetes on lower urinary tract symptoms in patients with benign prostatic hyperplasia. J Urol. 2000;163:1725–9. http://dx.doi.org/10.1097/00005392-200006000-00021. [PubMed] [Google Scholar]
- 7.Hammarsten J, Hogstedt B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur Urol. 2001;39:151–8. doi: 10.1159/000052430. http://dx.doi.org/10.1159/000052430. [DOI] [PubMed] [Google Scholar]
- 8.Hammarsten J, Damber JE, Karlsson M, Knutson T, Ljunggren O, Ohlsson C, et al. Insulin and free oestradiol are independent risk factors for benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2009;12:160–5. doi: 10.1038/pcan.2008.50. http://dx.doi.org/10.1038/pcan.2008.50. [DOI] [PubMed] [Google Scholar]
- 9.Vikram A, Jena G, Ramarao P. Insulin-resistance and benign prostatic hyperplasia: the connection. Eur J Pharmacol. 2010;641:75–81. doi: 10.1016/j.ejphar.2010.05.042. http://dx.doi.org/10.1016/j.ejphar.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 10.Peehl DM, Cohen P, Rosenfeld RG. The role of insulin-like growth factors in prostate biology. J Androl. 1996;17:2–4. [PubMed] [Google Scholar]
- 11.Kupelian V, McVary KT, Kaplan SA, Hall SA, Link CL, Aiyer LP, et al. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston Area Community Health Survey. J Urol. 2009;182:616–24. doi: 10.1016/j.juro.2009.04.025. http://dx.doi.org/10.1016/j.juro.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung MS, Lee SH, Park KK, Yoo SJ, Chung BH. Comparative rapid onset of efficacy between doxazosin gastrointestinal therapeutic system and tamsulosin in patients with lower urinary tract symptoms from benign prostatic hyperplasia: a multicentre, prospective, randomised study. Int J Clin Pract. 2011;65:1193–9. doi: 10.1111/j.1742-1241.2011.02759.x. http://dx.doi.org/10.1111/j.1742-1241.2011.02759.x. [DOI] [PubMed] [Google Scholar]
- 13.Djavan B, Chapple C, Milani S, Marberger M. State of the art on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology. 2004;64:1081–8. doi: 10.1016/j.urology.2004.07.031. http://dx.doi.org/10.1016/j.urology.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Yu R, Yau F, Ho SC, Woo J. Associations of cardiorespiratory fitness, physical activity, and obesity with metabolic-syndrome in Hong Kong Chinese midlife women. BMC Public Health. 2013;13:614. doi: 10.1186/1471-2458-13-614. http://dx.doi.org/10.1186/1471-2458-13-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khader Y, Bateiha A, El-Khateeb M, Al-Shaikh A, Ajlouni K. High prevalence of the metabolic syndrome among Northern Jordanians. J Diabetes Complications. 2007;21:214–9. doi: 10.1016/j.jdiacomp.2005.11.003. http://dx.doi.org/10.1016/j.jdiacomp.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Jørgensen ME, Bjerregaard P, Gyntelberg F, Borch-Johnsen K. Greenland Population Study. Prevalence of the metabolic syndrome among the Inuit in Greenland. A comparison between two proposed definitions. Diabet Med. 2004;21:1237–42. doi: 10.1111/j.1464-5491.2004.01294.x. http://dx.doi.org/10.1111/j.1464-5491.2004.01294.x. [DOI] [PubMed] [Google Scholar]
- 17.Athyros VG, Bouloukos VI, Pehlivanidis AN, Papageorgiou AA, Dionysopoulou SG, Symeonidis AN, et al. The prevalence of the metabolic syndrome in Greece: the MetS-Greece Multicentre Study. Diabetes Obes Metab. 2005;7:397–405. doi: 10.1111/j.1463-1326.2004.00409.x. http://dx.doi.org/10.1111/j.1463-1326.2004.00409.x. [DOI] [PubMed] [Google Scholar]
- 18.Kozan O, Oguz A, Abaci A, Erol C, Ongen Z, Temizhan A, et al. Prevalence of the metabolic syndrome among Turkish adults. Eur J Clin Nutr. 2007;61:548–53. doi: 10.1038/sj.ejcn.1602554. [DOI] [PubMed] [Google Scholar]
- 19.Ozsahin AK, Gokcel A, Sezgin N, Akbaba M, Guvener N, Ozisik L, et al. Prevalence of the metabolic syndrome in a Turkish adult population. Diabetes Nutr Metab. 2004;17:230–4. [PubMed] [Google Scholar]
- 20.Homer J, Jones A, Seville D, Essien J, Milstein B, Murphy D CDC. National Health Interview Survey: Adults with Diabetes, 2002. Centers for Disease Control And Prevention, National Center for Health Statistics; 2004a. [Google Scholar]
- 21.Shenfeld OZ, Meir KS, Yutkin V, Gofrit ON, Landau EH, Pode D. Do atherosclerosis and chronic bladder ischemia really play a role in detrusor dysfunction of old age? Urology. 2005;65:181–4. doi: 10.1016/j.urology.2004.08.055. http://dx.doi.org/10.1016/j.urology.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 22.Ehren I, Iversen H, Jansson O, Adolfsson J, Wiklund NP. Localization of nitric oxide synthase activity in the human lower urinary tract and its correlation with neuroeffector responses. Urology. 1994;44:683–7. doi: 10.1016/s0090-4295(94)80206-8. http://dx.doi.org/10.1016/S0090-4295(94)80206-8. [DOI] [PubMed] [Google Scholar]
- 23.Teoh H, Verma S. C-reactive protein, metabolic syndrome, and end organ damage. Metabolism. 2007;56:1620–2. doi: 10.1016/j.metabol.2007.07.002. http://dx.doi.org/10.1016/j.metabol.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Golomb E, Rosenzweig N, Eilam R, Abramovici A. Spontaneous hyperplasia of the ventral lobe of the prostate in aging genetically hypertensive rats. J Androl. 2000;21:58–64. [PubMed] [Google Scholar]
- 25.Cellek S, Rodrigo J, Lobos E, Fernández P, Serrano J, Moncada S, et al. Selective nitrergic neurodegeneration in diabetes mellitus-a nitric oxide-dependent phenomenon. Br J Pharmacol. 1999;128:1804–12. doi: 10.1038/sj.bjp.0702981. http://dx.doi.org/10.1038/sj.bjp.0702981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roehrborn CG, Rosen RC. Medical therapy options for aging men with benign prostatic hyperplasia: focus on alfuzosin 10 mg once daily. Clin Interv Aging. 2008;3:511–24. doi: 10.2147/cia.s3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gokkaya CS, Oztekin CV, Ozden CO, Ozturk EO, Aktas BK, Memis A. Insulin resistance impairs response to doxazosin therapy in men with benign prostatic hyperplasia. Turk J Med Sci. 2013;43:1–5. [Google Scholar]
- 28.Lee YC, Liu CC, Juan YS, Wu WJ, Li WM, Yeh HC, et al. The impact of metabolic syndrome on the responsiveness to a1-blocker in men with BPH⁄LUTS. Int J Clin Pract. 2013;67:356–62. doi: 10.1111/ijcp.12086. http://dx.doi.org/10.1111/ijcp.12086. [DOI] [PubMed] [Google Scholar]
- 29.Eom CS, Park JH, Cho BL. Metabolic syndrome and accompanying hyperinsulinemia have favorable effects on lower urinary tract symptoms in a generally healthy screened population. J Urol. 2011;186:175–9. doi: 10.1016/j.juro.2011.03.025. http://dx.doi.org/10.1016/j.juro.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Joseph MA, Harlow SD, Wei JT, Sarma AV, Dunn RL, Taylor JM, et al. Risk factors for lower urinary tract symptoms in a population based sample of African-American men. Am J Epidemiol. 2003;157:906–7. doi: 10.1093/aje/kwg051. http://dx.doi.org/10.1093/aje/kwg051. [DOI] [PubMed] [Google Scholar]
- 31.Temml C, Obermayr R, Marszalek M, Rauchenwald M, Madersbacher S, Ponholzer A. Are lower urinary tract symptoms influenced by metabolic syndrome? Urology. 2009;73:544–8. doi: 10.1016/j.urology.2008.10.027. http://dx.doi.org/10.1016/j.urology.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 32.Park HK, Lee HW, Lee KS, Byun SS, Jeong SJ, Hong SK, et al. Relationship between lower urinary tract symptoms and metabolic syndrome in a community-based elderly population. Urology. 2008;72:556–60. doi: 10.1016/j.urology.2008.03.043. http://dx.doi.org/10.1016/j.urology.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 33.AUA practice guidelines committee. AUA guidelines on management of benign prostatic hyperplasia: Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530–47. doi: 10.1097/01.ju.0000078083.38675.79. http://dx.doi.org/10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 34.Yang TK, Hsieh JT, Chen SC, Chang HC, Yang HJ, Huang KH. Metabolic syndrome associated with reduced lower urinary tract symptoms in middle-aged men receiving health checkup. Urology. 2012;80:1093–7. doi: 10.1016/j.urology.2012.08.002. http://dx.doi.org/10.1016/j.urology.2012.08.002. [DOI] [PubMed] [Google Scholar]


