Abstract
This report describes a Spanish water dog with an ammonium urate urethrolith which was diagnosed with primary portal vein hypoplasia and was found to be homozygous for the mutated SLC2A9 gene. This is the first Spanish water dog described with the SLC2A9 mutation and the first case of concurrent portal vascular abnormalities and SLC2A9 mutation.
Résumé
Hypoplasie de la veine porte primaire et mutation SLC2A9 associée à l’urolithiase d’urate chez un Chien d’eau espagnol. Ce rapport décrit un cas d’uréthrolithes d’urate d’ammonium chez un Chien d’eau espagnol pour lequel un diagnostic d’hypoplasie de la veine porte primaire a été posé et qui a été déclaré comme étant homozygote pour la mutation du gène SLC2A9. C’est le premier Chien d’eau espagnol dont la mutation SLC2A9 est décrite et le premier cas d’anomalies portes vasculaires concomitantes et la mutation SLC2A9.
(Traduit par Isabelle Vallières)
Urate urolithiasis occurs when the urine is supersaturated with uric acid (hyperuricosuria). In dogs this is reported secondary to either metabolic defects or hepatic dysfunction (1,2). Metabolic hyperuricosuria has been documented in several breeds including dalmatians, black Russian terriers, and bulldogs (1,3). The condition is due to a defect in the uric acid transporter in both the kidney and liver, altering the ability to metabolize uric acid. Mutation of the SLC2A9 gene has been identified as the cause of the defect in the transporter and the resultant predisposition for urate urolithiasis in these breeds (1,3).
Dogs with primary hepatic disease, most commonly congenital portal vasculature anomalies, can have reduced conversion of uric acid to allantoin and ammonia to urea (4). This can lead to hyperuricemia and hyperammonemia resulting in hyperuricuria and hyperammonuria and development of urate urolithiasis (4).
The case described in this report represents a novel presentation of both primary portal vein hypoplasia (PPVH) and hyperuricosuria mutation in a dog causing urate urolithiasis and is the first Spanish water dog to be diagnosed with SLC2A9 mutation.
Case description
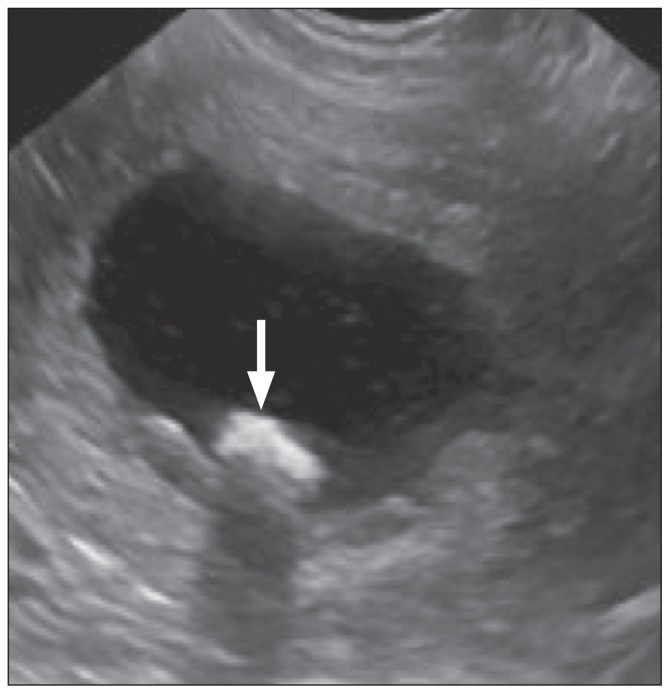
A 19-month-old male neutered Spanish water dog was presented to the University of Glasgow Small Animal Hospital with a 2-week history of dysuria, stranguria, and hematuria. On physical examination the dog had a markedly distended urinary bladder and was painful on caudal abdominal palpation. Through the initial consultation, the dog was noted to be dribbling a constant stream of urine. The remainder of the physical examination was unremarkable and vital parameters were within normal limits. Complete blood cell count did not reveal any abnormalities. Serum biochemical abnormalities included hypoalbuminemia and increased alkaline phosphatase (ALP) (Table 1). All other parameters, including electrolytes, serum ammonia levels, and pre- and post-prandial bile acids were within normal limits (Table 1). An abdominal ultrasound examination revealed a distended bladder, with echogenic sediment and suspected cystoliths (Figure 1). Bilateral pyelectasia was noted, most likely secondary to partial or complete obstruction of the lower urinary tract. The ureters were not distended. There was subjective mild microhepatica with normal parenchyma.
Table 1.
Results of significant biochemical tests
| Results | |||
|---|---|---|---|
|
|
|||
| Analyte | Day 1 | Day 28 | Reference range |
| Total protein | 57 | 62 | 50 to 78 g/L |
| Albumin | 27 | 30 | 29 to 36 g/L |
| Globulin | 30 | 32 | 28 to 42 g/L |
| Glucose | 4.7 | 3.4 | 3.3 to 5.5 mmol/L |
| Sodium | 147.1 | 143.4 | 135 to 159 mmol/L |
| Potassium | 5.1 | 5.6 | 3.4 to 5.8 mmol/L |
| Chloride | 113.6 | 109.4 | 9 to 115 mmol/L |
| Calcium | 2.49 | 2.64 | 2.34 to 3 mmol/L |
| Phosphate | 1.58 | 1.59 | 1.29 to 2.9 mmol/L |
| Urea | 7.7 | 7.7 | 2.5 to 8.5 mmol/L |
| Creatinine | 115 | 118 | 45 to 155 μmol/L |
| Cholesterol | 6.46 | 6.32 | 2.0 to 7.0 mmol/L |
| Triglyceride | 0.54 | 0.52 | < 0.6 mmol/L |
| Total bilirubin | 1 | 1 | < 10 μmol/L |
| ALT | 20 | 19 | < 90 U/L |
| AST | 24 | 24 | < 40 U/L |
| ALP | 247 | 213 | < 230 U/L |
| GGT | 0 | 3 | < 20 U/L |
| Bile acids (fasting) | 3.2 | 3 | < 10 μmol/L |
| Bile acids (post-prandial) | 3.6 | N/A | < 15 μmol/L |
| Ammonia | 22 | 18 | < 94 μmol/L |
ALT — alanine aminotransferase; AST — aspartase aminotransferase; ALP — alkaline phosphatase; GGT — gamma-glutamyl transferase; N/A — Not available.
Figure 1.
Saggital ultrasonographic view of the urinary bladder with a dependent space-occupying hyperechoic object, with distal acoustic shadowing (white arrow), consistent with a cystolith.
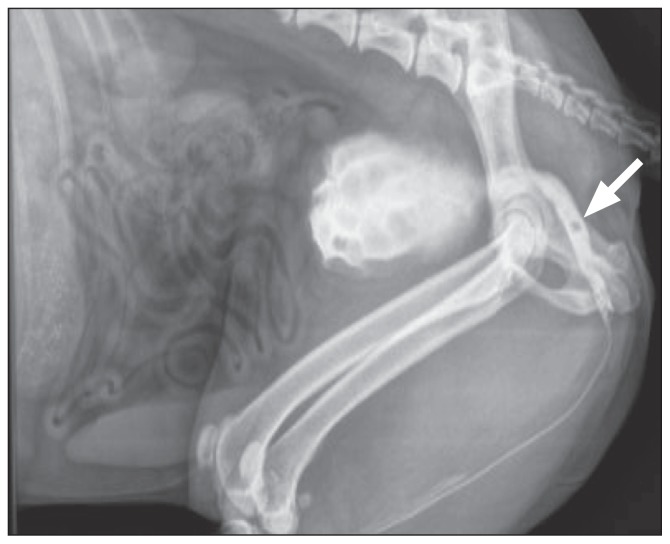
Assessment for congenital or acquired portosystemic shunts was performed; however, no evidence of an anomalous vessel(s) was detected. Plain abdominal radiographs revealed no evidence of radiopaque uroliths. Mild microhepatica was also detected. The dog was sedated with medetomidine (Domitor 1 mg/mL; Elanco Animal Health, Basingstoke, Hampshire, UK), 0.01 mg/kg body weight (BW), IV, and butorphanol (Torbugesic 10 mg/mL; Zoetis, London, UK) 0.2 mg/kg BW, IV. The distal urethra was catheterized (Mila 10 Fr × 90 cm Foley catheter; Mila international USA, Erlanger, Kentucky, USA) and retrograde positive contrast urethrography showed a small polygonal filling defect (5.2 mm × 5.7 mm) in the mid pelvic urethra, which was most consistent with a urolith (Figure 2). The bladder neck and proximal urethra were dilated, consistent with partial urethral obstruction. There were several small radiolucent areas at the level of the end of the urinary catheter, consistent with small gas bubbles, although small urethroliths could not be excluded (Figure 2). The urethrolith was retropulsed into the bladder. Repeat contrast radiographs confirmed relocation and the previous radiolucent areas seen at the tip of the catheter were no longer visible. The catheter was left indwelling to prevent relocation of the urolith back into the urethra prior to surgery. Analysis of urine taken via cystocentesis revealed a specific gravity of 1.030, and a pH of 5.0. Sediment analysis of the urine revealed erythrocytes and ammonium urate crystalluria. Aerobically cultured urine was negative for bacterial growth (Table 2).
Figure 2.
Radiograph of right lateral abdomen — retrograde positive contrast urethrogram. A polygonal filling defect is seen within the lumen of the pelvic urethra (white arrow) consistent with a urethrolith.
Table 2.
Results of urinalysis
| Results | |||
|---|---|---|---|
|
|
|||
| Test | Day 1 | Day 28 | Day 343 |
| Color | Yellow | Yellow | Yellow |
| Appearance | Clear | Clear | Clear |
| Specific gravity | 1.030 | 1.046 | 1.048 |
| pH | 5.0 | 8.0 | 6.5 |
| Protein | + | Trace | Trace |
| Protein to creatinine ratioa | 0.77 | 0.03 | 0.08 |
| Glucose | Negative | Negative | Negative |
| Ketones | Negative | Negative | Negative |
| Bilirubin | Negative | Negative | Negative |
| Blood/hemoglobin | ++ | Negative | Negative |
| Red blood cellsb | 50 to 100 | < 5 | < 5 |
| White blood cellsb | < 5 | < 5 | < 5 |
| Epithelial cells | None seen | None seen | None seen |
| Casts | None seen | None seen | None seen |
| Crystals | Moderate ammonium urate crystals | None seen | None seen |
| Urine culture | No bacterial growth | N/A | N/A |
Reference range < 0.5.
Reference range < 5 per high power field.
N/A — Not available.
The dog was hospitalized, with the indwelling urinary catheter in place. Given the abdominal pain, buprenorphine (Vetergesic 0.3 mg/mL; Sogeval, Sheriff Hutton, York, UK), 0.02 mg/kg BW, IV, q6h, was administered. Intravenous fluid therapy (0.9% NaCl; Vetivex 1, 9 mg/mL, Dechra Veterinary Products, Shrewsbury, Shropshire, UK), 2 mL/kg BW per hour was administered and urine production was monitored (2 to 2.97 mL/kg BW per hour). Based on the radiolucency of the stones and the urinalysis sediment examination, it was suspected that the uroliths were likely composed of ammonium urate. The patient was anesthetised the following day and a dual phase CT angiogram of the abdomen was performed with the aim of assessing the portal vasculature, primarily for a single congenital portosystemic shunt. No obvious decrease in the size of the portal vein or abnormal extrahepatic or intrahepatic vessels were detected and there was no evidence of hepatic arteriovenous malformations. A mild degree of microhepatica was detected. Following this a mid-line ceilotomy was performed. No anomalous vessels or abnormalities consistent with acquired portosystemic shunting were detected. A hepatic biopsy from the left medial liver lobe was obtained using a guillotine technique with 3 metric Polydioxanone (PDS®; Ethicon, Norderstedt, Germany). This was submitted for histopathological assessment. The upper and lower urinary tracts were grossly normal on inspection. Three cystoliths were removed through a stab incision on the ventral aspect of the bladder and were submitted for quantitative analysis. The dog received buprenorphine (Vetergesic), 0.02 mg/kg BW, IV, q8h after surgery and was observed urinating normally 6 h later. Intravenous fluid therapy (NaCl 0.9%, Vetivex), 2 mL/kg BW per hour was continued for a further 16 h. Ultrasound examination following a normal urination confirmed that the bladder had emptied. The dog was discharged the following day on meloxicam (Metacam 1.5 mg/mL, Boehringer Ingelheim, Bracknell, Berkshire, UK), 0.1 mg/kg BW, PO, q24h, for 5 d.
The uroliths were submitted to the Minnesota Urolith Center, University of Minnesota, USA. Quantitative analysis (polarizing light microscopy and/or infrared spectroscopy) indicated that the urolith was comprised of ammonium urate (100%). Histopathological evaluation of the liver revealed that approximately 25% of the portal triads had no discernible portal vein, and some were expanded by immature vascular structures which extended into the parenchyma. Larger portal triads were surrounded by mildly increased fibrous connective tissue. Scattered Kupffer cells and clusters of macrophages with intracytoplasmic hemosiderin accumulation were also noted. In the absence of detectable intra- or extra-hepatic portosystemic shunting or hepatic arteriovenous malformations on CT angiography and at surgical exploration, these findings were consistent with PPVH (previously termed microvascular dysplasia). Given these findings, the dog was managed with a commercially available hepatic diet (Canine hepatic; Royal Canin, Castle Cairy, Somerset, UK), ampicillin (Ampicare 250 mg capsule; Animalcare, Nether Poppleton, York, UK), 20 mg/kg BW, PO, q8h, and lactulose (Lactulose oral solution 3.3 g/5 mL; Resolution Chemicals, Stevenage, Hertfordshire, UK), 0.25 mL/kg BW, PO, q8h. An ethylenediamine tetraacetic acid (EDTA) blood sample was submitted to Genetic Services, Animal Health Trust, Newmarket, Suffolk, UK, to determine whether the dog had the SLC2A9 gene mutation.
Follow-up consultation 28 d post-surgery revealed complete resolution of the dysuria, stranguria, and hematuria. Physical examination was unremarkable. Serum biochemistry, electrolytes, ammonia, and fasting bile acids were within normal limits. Analysis of a urine sample taken via free catch revealed a specific gravity of 1.046 and a pH of 8. Sediment analysis of the urine was unremarkable, as was repeat ultrasonography of the urinary tract. The results of the genetic test were available at this stage and indicated that the dog was homozygous for the mutated SLC2A9 gene.
The dog’s diet was changed to a commercial restricted protein and low purine diet (Urinary U/C low purine, Royal Canin, UK). Ampicillin and lactulose were discontinued. Future follow-up was performed by the referring veterinarian. Repeat urinalysis performed 343 d after initial presentation was unremarkable. No signs of urinary tract disease had developed at the time of writing (420-day follow-up period).
Discussion
To the authors’ knowledge this is the first reported case of a Spanish water dog diagnosed with the SLC2A9 mutation. It is also the first reported case of a dog that was homozygous for the SLC2A9 gene mutation and was diagnosed concurrently with PPVH.
Primary portal vein hypoplasia is characterized by small intrahepatic portal vessels and portal endothelial hyperplasia resulting in abnormal communication between the portal and systemic circulation (5). This condition is a diagnosis of exclusion, as dogs with macroscopic portosystemic shunts can have concurrent microscopic hepatic abnormalities, which cannot be differentiated from PPVH on the basis of histopathological analysis of the liver alone (6).
It has been long recognized that dogs with portosystemic shunts, PPVH, and other hepatic conditions are at high risk of ammonium urate urolithiasis and crystalluria, as they can develop hyperuricemia and hyperammonemia (4,7). Dogs with portal vascular abnormalities have a higher incidence of urate urolithiasis than patients with other hepatic conditions (8). The hyperuricemia results from incomplete conversion of purine to allantoin, as this requires the hepatic enzyme uricase (urate oxidase), which is stored in hepatic peroxisomes (4,7). The hyperammonemia is a consequence of inefficient conversion of ammonia to urea, which normally occurs in the mitochondria of hepatocytes (4,9). Hyperuricuria and hyperammonuria result and if the saturation of urine ammonia and uric acid exceeds the solubility product of ammonium urate, then uroliths will precipitate (4). Up to 50% of dogs with portal vascular anomalies can develop ammonium biurate crystalluria and between 20% to 30% can develop urate uroliths (9–11). Dogs with PPVH can have similar rates of urogenital signs, including urate crystalluria, to dogs with congenital portosystemic shunts, with 29.41% of dogs with PPVH found to have crystalluria compared with 32% of dogs with a single congenital portosystemic shunt (5). However in a group of cairn terriers diagnosed with PPVH, none were clinically affected and there was no evidence of urate urolithiasis or crystalluria, suggesting the condition is variable between individuals and possibly among breeds (12). In general, dogs with PPVH frequently had less severe clinical signs and better long-term prognosis compared to dogs with a congenital portosystemic shunt (6,13).
The mild hypoalbuminemia at presentation may have been secondary to decreased hepatic synthesis or inflammation associated with the urinary tract obstruction (14,15). The marginal increase in ALP detected at presentation may be attributed to the PPVH; however, although ALP is sensitive for hepato-bilary disease, it is highly non-specific (16,17). As the case described had normal bile acids (pre- and post-prandial), and normal fasting serum ammonia, we suspect the PPVH may have been subclinical. Alternatively, the bile acid stimulation test may not have been representative as several factors can influence the post-prandial serum bile acids (18). Given the normal pre- and post-prandial bile acids, normal fasting ammonia and minimal biochemical and hematological abnormalities, the authors elected to investigate other causes of urate urolithiasis. Whilst awaiting the results of genetic testing, the dog was treated with dietary modification (hepatic diet), ampicillin, and lactulose due to the PPVH. Medical management is the only treatment available for clinical primary PPVH with the mainstay of treatment for dogs with this condition being dietary modification (19).
The metabolic hyperuricosuria mutation was originally characterized in the dalmatian dog and was determined to be caused by a missense mutation of the SLC2A9 gene (1). The mutation is recessive; therefore, dogs must be homozygous for the mutation to develop the condition (1). In affected patients the mutation leads to inefficient transport of uric acid in the liver and renal proximal tubules (1,20,21). All purebred dalmatians are homozygous for the mutated gene; however, only 19.6% of 179 individuals assessed were clinically affected by urate uroliths (1,22). Additionally, most of those affected were male, most likely due to anatomical reasons (22). Other factors such as lower concentrations of Tamm Horsfall protein and concentration of uric acid in the urine may also play a role in the variation in development of urolithiasis (2,23). The SLC2A9 mutation has also been correlated with urate urolithiasis in other dog breeds including the black Russian terrier, bulldog, weimaraner, large munsterlander, and the Parson Russell terrier (24,25). Similarly, not all black Russian terriers or bulldogs that were homozygous for the SLC2A9 mutation developed clinical signs related to urate urolithasis (25). Unlike the Dalmatian, however, not all individuals within these breeds had the mutation and of those with the mutation, the majority were heterozygous (25). Although clinically unaffected, these carriers have the potential to transmit to their offspring (24). To the authors’ knowledge, the Spanish water dog had not previously been tested for the mutation. The dog presented herein was found to be homozygous for the mutation; however, the frequency of the mutation in the breed is unknown. Further studies into the allele frequency in the Spanish water dog could avoid inadvertent breeding of affected individuals.
In dogs diagnosed with urate urolithiasis secondary to SLC2A9 homozygous mutation the mainstay of preventing further formation after surgical removal of the uroliths, is to minimize the urinary concentration of ammonia and uric acid. This is achieved by feeding a low-protein diet. Allopurinol, a synthetic isomer of hypoxanthine that inhibits the action of xanthine oxidase resulting in decreased uric acid production can be used as adjunctive therapy, but is generally used in cases in which medical dissolution is preferred over surgical removal (26). Prophylactic treatment with allopurinol is not routinely recommended as it carries a risk of xanthine urolith formation (2). Added to this it should be used with caution in patients with hepatic dysfunction (27). We therefore elected to use only dietary modification in this case.
Dogs with cancer, renal disease, and hyperadrenocorticism have been reported to have increased uric acid to creatinine ratios compared with healthy dogs (28). There was no evidence of these conditions in the dog in this case; however, these diseases should be considered in dogs which present with urate urolithiasis or crystalluria of unknown origin.
In this case it is possible that the 2 diagnosed conditions together lead to the urate uroliths. The authors elected to proceed with a commercial low purine diet as opposed to a hepatic diet. As discussed, dogs with congenital hepatic vascular anomalies and dogs which are homozygous for the SLC2A9 mutation are at a similar risk of developing urate uroliths (1,9–11,22). However in this case, it was felt that given the normal bile acids and serum ammonia, the degree of contribution to the urate uoliths by PPVH was less than the SLC2A9 mutation. The low purine diet is protein restricted as well as specifically purine restricted. If the PPVH was also contributing to the urate uroliths, this diet is not contraindicated and in fact may also aid in the reduction of hyperuricaemia given the purine restriction. We elected not to continue with ampicillin and lactulose for the same reason; however, we have recommended ongoing monitoring of clinical signs, hematology, biochemistry, serum bile acids, ammonia, and abdominal ultrasound as it is possible that in the future the dog will require more specific treatment for the PPVH.
This case highlights the importance of examining all possible differentials for the development of urate urolithiasis as, although there was a documented PPVH, the dog had normal bile acids and serum ammonia, leading the authors to pursue other diagnoses. This is the first case of a Spanish water dog documented to have the SLC2A9 mutation and therefore this should be considered a differential in this breed for urinary tract signs and/or urolithiasis. Further investigation into the prevalence within this breed should also be considered. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Bannasch D, Safra N, Young A, Karmi N, Schaible RS, Ling GV. Mutations in the SLC2A9 gene cause hyperuricosuria and hyperuricemia in the dog. PLoS Genet. 2008;4:1–8. doi: 10.1371/journal.pgen.1000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams LG, Syme HM. Canine ureteral and lower urinary tract diseases. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 7th ed. St. Louis, Missouri: Elsevier Saunders; 2010. pp. 2086–2115. [Google Scholar]
- 3.Bende B, Nemeth T. High prevalence of urate urolithiasis in the Russian black terrier. Vet Rec. 2004;155:239–240. doi: 10.1136/vr.155.8.239. [DOI] [PubMed] [Google Scholar]
- 4.Bartges JW, Cornelius LM, Osborne CA. Ammonium urate uroliths in dogs with portosystemic shunts. In: Bonagura JD, editor. Kirks Current Veterinary Therapy XIII. Philadelphia, Pennsylvania: Saunders WB; 2000. pp. 872–874. [Google Scholar]
- 5.Allen L, Stobie D, Mauldin N, Baer KE. Clinicopathologic features of dogs with hepatic microvascular dysplasia with and without portosystemic shunts: 42 cases (1991–1996) J Am Vet Med Assoc. 1999;214:218–220. [PubMed] [Google Scholar]
- 6.Christiansen JS, Hottinger HA, Allen L, Phillips L, Aronson LR. Hepatic microvascular dysplasia in dogs: A retrospective study of 24 cases (1987–1995) J Am Anim Hosp Assoc. 2000;36:385–389. doi: 10.5326/15473317-36-5-385. [DOI] [PubMed] [Google Scholar]
- 7.Bannasch D, Henthorn PS. Changing paradigms in diagnosis of inherited defects associated with urolithiasis. Vet Clin North Am Small Anim Pract. 2009;39:111–125. doi: 10.1016/j.cvsm.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marretta SM, Pask AJ, Greene RW, Liu S. Urinary calculi associated with portosystemic shunts in six dogs. J Am Vet Med Assoc. 1981;178:133–137. [PubMed] [Google Scholar]
- 9.Webster CRL. History, clinical signs and physical findings in hepatobillary disease. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 7th ed. St. Louis, Missouri: Elsevier Saunders; 2010. pp. 1612–1625. [Google Scholar]
- 10.Johnson CA, Armstrong PJ, Hauptman JG. Congenital portosystemic shunts in dogs: 46 cases (1979–1986) J Am Vet Med Assoc. 1987;191:1478–1483. [PubMed] [Google Scholar]
- 11.Winkler JT, Bohling MW, Tillson DM, Wright JC, Ballagas AJ. Portosystemic shunts: Diagnosis, prognosis and treatment of 64 cases (1993–2001) J Am Anim Hosp Assoc. 2003;39:169–185. doi: 10.5326/0390169. [DOI] [PubMed] [Google Scholar]
- 12.Schermerhorn T, Center SA, Dykes NL, et al. Characterization of hepatoportal microvascular dysplasia in a kindred of Cairn terriers. J Vet Intern Med. 1996;10:219–230. doi: 10.1111/j.1939-1676.1996.tb02053.x. [DOI] [PubMed] [Google Scholar]
- 13.Berent AC, Weisse C. Hepatic vascular anomalies. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 7th ed. St. Louis, Missouri: Elsevier Saunders; 2010. pp. 1649–1672. [Google Scholar]
- 14.Adam FH, German AJ, McConnell JF, et al. Clinical and cliniopathologic abnormalities in young dogs with acquired and congential portosystemic shunts: 93 cases (2003–2008) J Am Vet Med Assoc. 2012;241:760–765. doi: 10.2460/javma.241.6.760. [DOI] [PubMed] [Google Scholar]
- 15.Ceron JJ, Eckersall PD, Martýnez-Subiela S. Acute phase proteins in dogs and cats: Current knowledge and future perspectives. Vet Clin Pathol. 2005;34:85–99. doi: 10.1111/j.1939-165x.2005.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 16.Willard MD, Twedt DC. Gastrointestinal, pancreatic, hepatic disorders. In: Willard MD, Tvedten H, Turnwald GH, editors. Small Animal Clinical Diagnosis by Laboratory Methods. 3rd ed. Philadelphia, Pennsylvania: Saunders; 1999. [Google Scholar]
- 17.Gray AT, Twedt DC. Evaluation of elevated serum alkaline phosphatase in the dog. In: Bonagura JD, Twedt DC, editors. Kirk’s Current Veterinary Therapy XIV. St. Louis, Missouri: Elsevier Saunders; 2009. pp. 549–553. [Google Scholar]
- 18.Bridger N, Glanemann B, Neiger R. Comparison of postprandial and ceruletide serum bile acid stimulation in dogs. J Vet Intern Med. 2008;22:873–878. doi: 10.1111/j.1939-1676.2008.0114.x. [DOI] [PubMed] [Google Scholar]
- 19.Christiansen JS, Hottinger HA, Allen L, Phillips L, Aronson LR. Hepatic microvascular dysplasia in dogs: A retrospective study of 24 cases 1987–1995. J Am Anim Hosp Assoc. 2000;36:385–389. doi: 10.5326/15473317-36-5-385. [DOI] [PubMed] [Google Scholar]
- 20.Giesecke D, Tiemeyer W. Defect of uric acid uptake in Dalmatian dog liver. Experientia. 1984;40:1415–1416. doi: 10.1007/BF01951919. [DOI] [PubMed] [Google Scholar]
- 21.Simkin PA. The Dalmatian defect. A hepatic endocrinopathy of urate transport. Arthritis Rheum. 2005;52:2257–2262. doi: 10.1002/art.21241. [DOI] [PubMed] [Google Scholar]
- 22.Bannasch DL, Ling GV, Bea J, Famula TR. Inheritance of urinary calculi in the Dalmatian. J Vet Intern Med. 2004;18:483–487. doi: 10.1892/0891-6640(2004)18<483:ioucit>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Carvalhi M, Lulich JP, Osborne CA, Nakagawa Y. Role of urinary inhibitors of crystallization in uric acid nephrolithiasis: Dalmatian dog model. Urology. 2003;62:566–570. doi: 10.1016/s0090-4295(03)00406-0. [DOI] [PubMed] [Google Scholar]
- 24.Karmi N, Brown EA, Hughes SS, et al. Estimated frequency of the canine hyperuricosuria mutation in different dog breeds. J Vet Intern Med. 2010;24:1337–1342. doi: 10.1111/j.1939-1676.2010.0631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karmi N, Safra N, Young A, Bannasch D. Validation of a urine test and characterization of the putative genetic mutation for hyperuricosuria in Bulldogs and Black Russian Terriers. Am J Vet Res. 2010;71:909–914. doi: 10.2460/ajvr.71.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling GV, Case LC, Nelson H, Harrold DR, Johnson DL, Vulliet PR. Pharmacokinetics of allopurinol in Dalmatian dogs. J Vet Pharmacol Ther. 1997;20:134–138. doi: 10.1046/j.1365-2885.1997.00817.x. [DOI] [PubMed] [Google Scholar]
- 27.Plumb DC. Plumb’s Veterinary Drug Handbook. 7th ed. Stockholm, Wisconsin: Pharma Vet Inc; 2011. pp. 40–43. [Google Scholar]
- 28.Rivara CM, Johnson CR, Lulicj JP, Osborne CA, Murtaugh M. The effect of disease on the urinary purine metabolite concentrations in dogs. Vet Rec. 2013;173:219. doi: 10.1136/vr.101237. [DOI] [PubMed] [Google Scholar]