Abstract
Background
We tested the hypothesis that developmental effects of repeated neonatal exposure to sevoflurane in rats are exacerbated by stressful experiences received later in life.
Methods
Sprague-Dawley male rats received sequential exposures to 3% sevoflurane for two h on postnatal days (P) six, seven, and eight. After weaning at P21, rats were housed either in pairs in an enriched environment (EE) or singly in an enrichment-deprived environment (an adverse environment, AE). The hippocampal concentrations of brain-derived neurotrophic factor (BDNF), and synaptic markers were assessed at P8 and P53. The dentate gyrus neural progenitor proliferation was evaluated at P11 and P53 after administration of bromodeoyuridine (BrdU) at P8 to P10 and at P22 to P27, respectively. Neurobehavioural evaluations were performed at P49 to P53.
Results
Repeated sevoflurane exposure acutely reduced concentrations of BDNF, synaptic markers and neural progenitor proliferation. The sevoflurane group housed in the AE conditions (sevoflurane+AE) had decreased concentrations of BDNF and synaptic markers, and survival of new granule cells and impaired cognitive function compared with the control+AE, control+EE, and sevoflurane+EE groups. The neurobehavioural parameters in the sevoflurane+EE and control+EE groups were similar.
Conclusions
Neurocognitive abnormalities induced by repeated neonatal exposure to sevoflurane can be aggravated by stressful conditions such as social isolation and enrichment deprivation.
Keywords: brain-derived neurotrophic factor, cognition, environment, rats, sevoflurane, social isolation
Editor's key points.
Repeated anaesthesia in the neonatal period is linked to cognitive and behavioural deficits later in childhood
Effects of early anaesthesia may be compounded by environmental factors
This study assessed the effect of an enriched or deprived environment on neurobehavioural effects of early anaesthesia in rat pups
Social isolation and lack of an enriched environment worsened the anaesthesia effects
The majority of human retrospective epidemiological evaluations of children who underwent surgical procedures with general anaesthesia at an early postnatal age, report impairments in cognition and behavior [for a review, see Sanders and colleagues1]. Although human studies performed thus far do not provide a definitive answer to the question of whether neonatal anaesthesia itself affects brain development, similar findings in healthy animals exposed to anaesthesia in the absence of surgery strongly support this possibility. Furthermore, findings in affected children indicate that the duration of anaesthesia and repeated exposures to anesthetics may be among the aggravating factors.2–4 The latter is in agreement with the results of well-controlled laboratory studies.5,6 Such parallels between the findings of human and animal studies suggest that understanding the underlying mechanisms of the developmental effects of neonatal anaesthesia in animal models may facilitate an understanding of the developmental effects of neonatal exposure to anaesthesia in humans. Unfortunately, molecular targets and cellular pathways involved in the mediation of neonatal anaesthesia-induced abnormalities are incompletely understood, even in animal models.
It was recently reported by several laboratories that the adverse developmental effects of neonatal anaesthesia in rodents may be alleviated, not only by pharmacological interventions before exposure to anaesthesia, but also by post-weaning housing of the exposed animals in an enriched environment.6–8 This brings us to the possibility that the functional consequences of the exposure of neonates to general anaesthetics may result from a combination of the acute effects of anaesthetics at the time of anaesthesia and the effects of ‘post-anaesthesia’ environmental factors, such as a healthy and fulfilled life or disease, hunger, pain, maternal deprivation, etc. Consequently, the effects of such environmental factors may be alleviating or exacerbating. In other words, two subjects exposed to the same anaesthesia protocol may have different long-term neurobehavioural outcomes based on post-anaesthesia life experiences. We tested this hypothesis by comparing the developmental effects of repeated exposures to sevoflurane in rats that were housed either in an enriched environment or in social isolation, deprived of an enriched environment.
Methods
Animals
The present study was approved by the Ethics Committee of Jinling Hospital, Nanjing University, China, and was performed in accordance with the relevant aspects of ARRIVE guidelines and the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (Bethesda, MD, USA). Pregnant Sprague-Dawley rats were purchased from the Animal Center of Jinling Hospital, Nanjing, China, and were housed individually in standard conditions with a 12-h light/dark cycle (light from 07:00–19:00) at 24±1°C and ad libitum access to food and water.
Anaesthesia
The P6 to P8 male rat pups were randomly assigned to the sevoflurane (SEV) or control (CON, not exposed to sevoflurane) groups. Rats in the SEV group received 3% sevoflurane in O2/N2 (fraction of inspired oxygen 50%, or 50%) for two h daily on three consecutive days, in a thermostated chamber set to 37±1°C. The total gas flow was two Litre min−1. The rats breathed spontaneously, and concentrations of anaesthetic and oxygen were measured continuously using a calibrated Datex side stream analyser that sampled from the interior of the chamber. After anaesthesia, the rat pups were allowed to recover and were returned to the mothers on gaining the righting reflex. Rat pups in the non-exposed group were separated from the dams for the same duration of time in identical conditions (37 ± 1°C), 50%), except for exposure to the anaesthetic. Arterial blood for gas analysis was obtained from separate groups of sevoflurane-exposed (n=5) and non-exposed (n=5) rat pups by thoracotomy and orthoptic puncture of the left ventricle at the end of the last sevoflurane exposure (i.e. third exposure), while the animals were still anaesthetized. The maternally separated non-exposed pups received pentobarbital (50 mg kg−1, intraperitoneally, i.p.) before the arterial blood sampling at the end of the third maternal separation. Upon completion of the bleeding procedures and after all other procedures that required euthanasia, the animals received pentobarbital (100 mg kg−1, i.p.) followed by decapitation. An adequate depth of anaesthesia before decapitation was ensured by lack of response to a nociceptive stimulus. The mean (SD) blood gas values in the non-exposed and exposed animals were: pH: 7.36 (0.039) and 7.30 (0.041); : 45.8 (3.4) and 40.2 (3.1) mm Hg; PO2: 163.4 (7.3) and 154.2 (5.7) mm Hg. These findings are in agreement with the results of previous studies showing that anaesthesia with 3% sevoflurane for two h does not significantly change blood gas values.6
Experimental design of the study
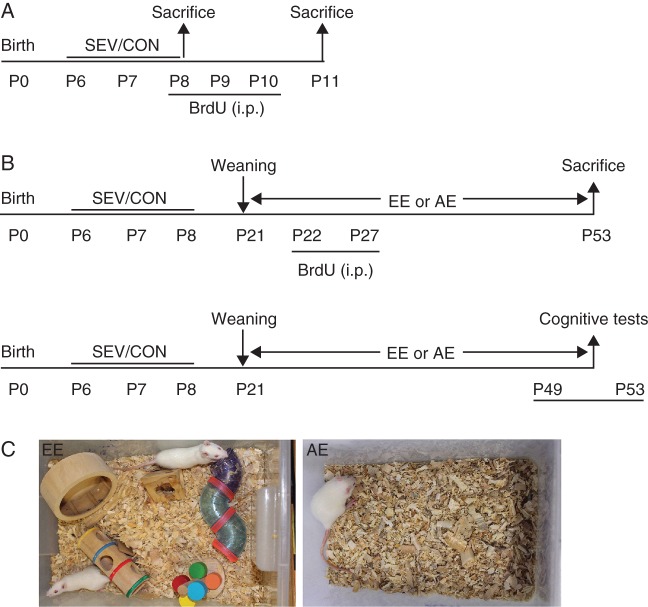
The experimental design of the study is illustrated in Fig. 1. In the first set of experiments (Fig. 1a) the acute effects of sevoflurane were studied by assessing apoptotic neuronal profiles, concentrations of brain derived neurotrophic factor (BDNF) and synaptic markers in the hippocampus of the sevoflurane-exposed and control rats six h after the last sevoflurane exposure (n=6) or last maternal separation only (n=6), respectively. To determine the effect of sevoflurane on neural progenitor proliferation in the hippocampus, the sevoflurane-exposed (n=6) and control (n=6) rat pups received the thymidine analog, bromodeoxyuridine (BrdU, 75 mg kg−1, i.p.), over three consecutive days from P8 to P10. The BrdU+ cells were determined at P11 using immunohistochemistry.
Fig 1.
Experimental design of the study.
In the second set of experiments (Fig. 1b), the effects of the adverse and enriched environments (AE and EE, respectively) on sevoflurane-induced changes in brain biochemical markers and neurocognitive function were studied. To create the EE, large Plexiglas cages (55×40×30 cm) were equipped with a series of novel objects representing different types of stimuli: a voluntary running wheel for physical exercise, environmental complexity for social interaction, and environmental novelty (Fig. 1c). Every three days the objects were cleaned, disinfected, and rearranged to ensure novelty. Plexiglas cages (32×22×17 cm) lacking any enrichment objects represented the AE (Fig. 1c). The rat pups were weaned at P21 and housed one per cage in a cage deprived of enrichment objects (AE), or two per large cage filled with enrichment objects (EE). The rats were divided into four treatment groups: sevoflurane-exposed rats housed in the AE (SEV+AE); sevoflurane-exposed rats housed in the EE (SEV+EE); control rats housed in the AE (CON+AE); and control rats housed in the EE (CON+EE). A subset of animals (n=6) from each treatment group received BrdU (75 mg kg−1, i.p.) for six consecutive days from P22 to P27. At P53, the survival of BrdU+ cells, the concentrations of BDNF and synaptic markers in the hippocampus were evaluated (n=6 per measurement per treatment group). Separately, the neurocognitive function tests were performed at P49 to P53 (n=10 per treatment group). Upon completion of these tests, the rats were not used in any other experiments and were killed as described above.
Behavioural tests
Behavioural tests were performed successively in the open-field test, social interaction test and fear conditioning tests at P49 to P53 (n=10 per treatment group). The movement of each rat was automatically monitored and analysed using a computer-operated video tracking system. The apparatus was thoroughly cleaned with 75% alcohol before each trial. All apparatus used in the tests were from the Shanghai Softmaze Information Technology Co., Ltd., China.
Open-field test
At P49, the rats were placed in the centre of the open-field chamber (100×10040 cm) with two-feet-high opaque walls made of grey acrylic. The rat activities were assessed by measuring the total distance travelled during a 10-min session as previously described by Satomoto and colleagues.9
Social interaction test
At P50, the rats were studied in the modified social interaction test, as previously described.10 In brief, the cage was 70 cm long and 30 cm wide, with three communicating compartments. The test rat was first placed in the middle chamber and allowed to explore for 10 min. The doorways into the two side chambers were obstructed by plastic boxes during the habituation phase. After the habituation period, an unfamiliar, age-matched (P50) rat (a ‘stranger’ rat) that had no prior contact with the subject rat was placed in one of the side chambers. Five rats, weighted about 220–270 g on P50, were used as strangers to evaluate later-life social interactions in different treatment groups. Between social pairings, one of the five stranger rats was placed in turns in one of the side chambers for each treatment group. The location of the stranger rat in the left vs right empty side chamber was systematically alternated between trials. The stranger rat was enclosed in a small, round wire cage with a radius of 10.5 cm, which allowed nose contact between the bars but prevented fighting. The doors to the side chambers were then unblocked and the subject was allowed to explore the entire social test box during a 10-min session. Measures were taken of the time spent in the stranger rat chamber and the sociability index (the ratio of duration in the stranger-rat side to duration in the empty side). The chambers of the social apparatus were thoroughly cleaned with 75% alcohol before each trial.
Fear conditioning test
Separate groups of the P51 to P53 rats were evaluated in the fear conditioning test. The test was performed in a black plastic chamber equipped with a stainless steel grid floor as previously described.11 The conditioning (acquisition) trial for fear conditioning consisted of a two min exploration period followed by three conditioned stimulus (CS)–unconditioned stimulus (US) pairings (CS, 70 dB white noise, 20-s duration; US, 1.0 mA footshock intensity, two-s duration; US was delivered during the last two s of the CS presentation) separated by one min each. A contextual test was performed in the conditioning chamber for four min without any stimulation 48 h after the conditioning trial. A cued test (for the same set of rats) was performed by presentation of the cue (70 dB white noise, three min duration) in an alternative context with distinct visual and tactile cues; the cued test was conducted after the contextual test was finished. The rate of the rat's freezing response was used to measure the fear memory. The level of nonspecific freezing provoked by the new context was controlled for two min before the presentation of the cue in that new context.
Brain tissue harvest and protein level quantification
Separate groups of rats that were not used in the behavioural tests were used in different biochemistry studies at P8, P11 and P53. In brief, sodium pentobarbital (50 mg kg−1, i.p.) was administered followed by thoracotomy and transcardial perfusion with phosphate-buffered saline (pH 7.35) for western blot studies and 4% phosphate-buffered paraformaldehyde for immunohistochemistry studies. For the western blot analysis the hippocampus tissues were harvested and homogenized on ice using immunoprecipitation buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, and 0.5% Nonidet P-40) plus protease inhibitors (1 μg mL−1 aprotinin, 1 μg mL−1 leupeptin, and 1 μg mL−1 pepstatin A) as described in previous studies.12 The lysates were collected, centrifuged at 10 000 g for 10 min, and quantified for total protein with a bicinchoninic acid protein assay kit (Pierce Technology Co., Iselin, NJ).
Western blot
Western blot analysis was performed as previously described.13 Apoptosis, BDNF and synaptic proteins were evaluated in the hippocampal tissue. BDNF antibody (1:1000; sc-546, Santa Cruz Biotechnology, Inc.), PSD-95 antibody (1:1000; ab18258, Abcam, Cambridge, UK) and synaptophysin antibody (1:1000; ab8049, Abcam) were used to detect BDNF (15 kDa), PSD-95 (95 kDa), and synaptophysin (34 kDa). Antibody anti-β-actin (1:10 000; Sigma, St. Louis, MO) was used to detect β-actin (44 kDa). The membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2000; sc-2004, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or goat anti-mouse IgG (1:2000; sc-2005, Santa Cruz Biotechnology, Inc.). The Image J software (NIH Image, Bethesda, MD) was used for semiquantification of the bands. Expression of β-actin, determined with β-actin antibody, was used to control for loading differences in the total protein amount, and presented semiquantitive data are normalized to β-actin levels.
Immunohistochemistry and cell counting
Sodium pentobarbital (50 mg kg−1 i.p.) was administered before transcardial perfusion with saline, followed by 4% paraformaldehyde. Brains were removed and postfixed overnight in the same solution. For progenitor proliferation, coronal free-floating sections were obtained.
Briefly, brains were immersed in 30% sucrose overnight until they sank before they were cut on a freezing microtome. Coronal brain sections were taken at a thickness of 40 µm through the rostro-caudal extent of the hippocampus. Every sixth section throughout the hippocampus was processed for BrdU staining. BrdU immunostaining was done as described previously.14 DNA was first denatured by incubating tissue sections in 2N HCl for 30 min at 37°C followed by a 10 min wash in 0.1 M borate solution, pH 8.5. Diaminobenzidine peroxidase immunohistochemistry for BrdU was performed. Primary antibody dilutions used were 1:400 for BrdU (mouse monoclonal; Boehringer Mannheim). An overnight incubation in primary antibody at room temperature was used for immunostaining and images were captured at 200× magnification.
An experimenter blinded to the treatment groups counted the BrdU+ cells in the subgranular zones and granule cell layers. The positive cells were counted on sets of every sixth section (six sections per rat) through the rostral-caudal extent of the hippocampal DG. The first section of each set from each rat was randomly selected from the first to sixth section of DG. BrdU+ cells were counted by multiplying the number six (the section interval) to generate a stereological estimate of total cell number without fractionating section thickness.
Statistical analysis
Values are reported as mean (SD). SPSS (version 17.0) was used for statistical analysis. Distribution of data was analysed using the Kolmogorov-Smirnov test. Student's t-test was used for single comparisons and multiple comparisons among groups were analysed using analysis of variance (ANOVA) followed by post hoc Bonferroni test. A P value less than 0.05 was considered statistically significant.
Results
Exposure of the P6 to P8 rats to sevoflurane decreased the concentrations of BDNF in the neonatal brain hippocampus at P8 (P=0.011; Fig. 2a and b). At P53, the sevoflurane-exposed rats housed in the AE had lower concentrations of BDNF (F3,23=18.099, P=0.014; Fig. 2c and d) compared with non-exposed rats housed in the AE. The sevoflurane-exposed rats housed in the EE had similar concentrations of BDNF as non-exposed rats housed in the EE (Fig. 2c and d). Rats previously not exposed to sevoflurane and housed in the EE compared with non-exposed rats housed in the AE had higher concentrations of BDNF (F3,23=18.099, P=0.049; Fig. 2c and d). Similarly, higher concentrations of BDNF (F3,23=18.099, P<0.001; Fig. 2c and d) were detected in the sevoflurane-exposed rats housed in the EE compared with the SEV+AE group. Two-way ANOVA showed that there was an interaction between the EE and sevoflurane (F1,23=8.432, P=0.009), and there were statistically significant main effects of the EE (F1,23=51.856, P<0.001) and sevoflurane (F1,23=4.132, P=0.041) based on the concentration of BDNF.
Fig 2.
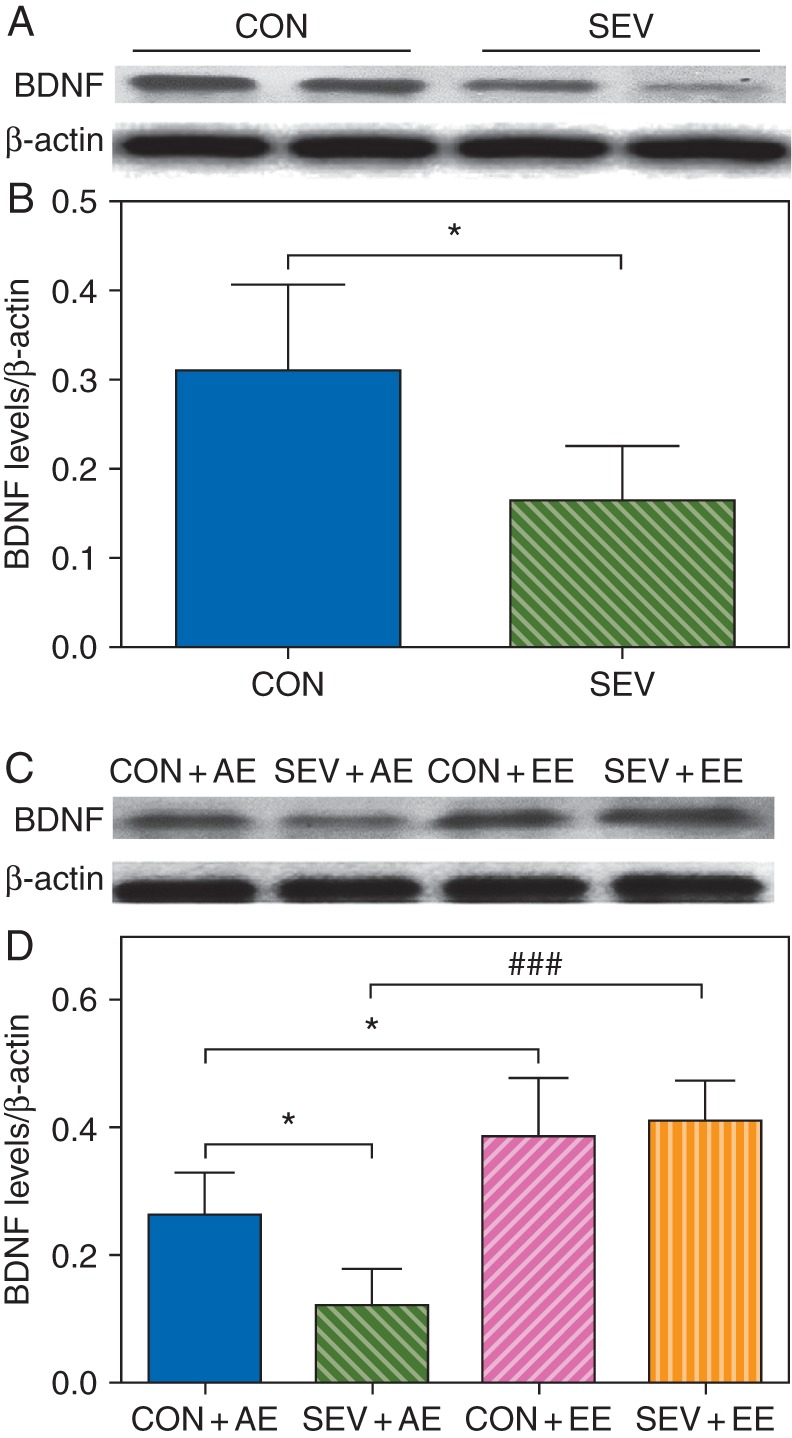
Sevoflurane-exposed rats showed a decline in the concentrations of BDNF in the neonatal brain hippocampus and demonstrated an environment-dependent decline in the concentrations of BDNF after growing up. (a and b) Exposure of the P6 to P8 rats to sevoflurane decrease the concentrations of BDNF in the neonatal brain hippocampus at P8. (*P<0.05 vs CON group, n=6). (c and d) Sevoflurane-exposed rats demonstrated an environment-dependent decline in the concentrations of BDNF in the hippocampus after growing up at P53. (*P<0.05 vs CON+AE group, ###P<0.001 vs SEV+AE group, n=6). (a and c) Representative western blot images of BDNF and β-actin in the hippocampus of different groups of rats to illustrate band intensities. (b and d). Histograms showing results of the densitometric analysis of western blot images of the concentrations of BDNF.
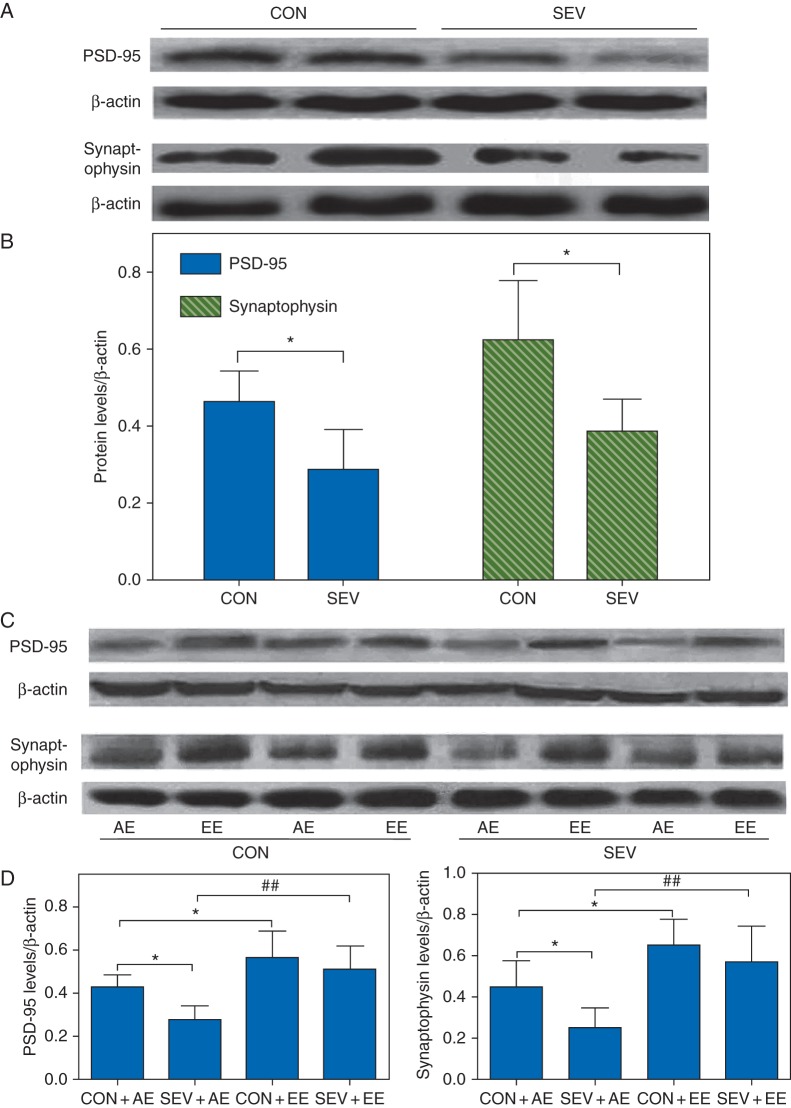
Exposure of the P6 to P8 rats to sevoflurane decreased the concentrations of PSD-95 (P=0.027) and synaptophysin (P=0.033) in the neonatal brain hippocampus at P8 (Fig. 3a and b). At P53, the sevoflurane-exposed rats housed in the AE had lower concentrations of PSD-95 (F3,23=8.065, P=0.021; Fig. 3c and d) and synaptophysin (F3,23=11.038, P=0.015; Fig. 3c and d) compared with non-exposed rats housed in the AE. The sevoflurane-exposed rats housed in the EE had similar concentrations of PSD-95 and synaptophysin to those of non-exposed rats housed in the EE (Fig. 3c and d). Rats previously not exposed to sevoflurane and housed in the EE compared with non-exposed rats housed in the AE had higher concentrations of PSD-95 (F3,23=8.065, P=0.024; Fig. 3c and d) and synaptophysin (F3,23=11.038, P=0.026; Fig. 3c and d). Similarly, higher concentrations of PSD-95 (F3,23=8.065, P=0.007; Fig. 3c and d) and synaptophysin (F3,23=11.038, P=0.002; Fig. 3c and d) were detected in the sevoflurane-exposed rats housed in the EE compared with the SEV+AE group. Two-way ANOVA showed that there was no interaction between the EE and sevoflurane, but there were statistically significant main effects of the EE (F1,23=19.199, P<0.001) and sevoflurane (F1,23=4.534, P=0.039) based on the concentration of PSD-95; Also there was a statistically significant main effect of the EE (F1,23=21.746, P<0.001) and sevoflurane (F1,23=4.289, P=0.046), based on the concentration of synaptophysin.
Fig 3.
Sevoflurane-exposed rats showed lower concentrations of synaptic markers in the neonatal brain hippocampus and demonstrated an environment-dependent decline in the concentrations of synaptic markers after growing up. (a and b) Exposure of the P6 to P8 rats to sevoflurane decrease the concentrations of PSD-95 and synaptophysin in the neonatal brain hippocampus at P8. (*P<0.05 vs CON group, n=6). (c and d) Sevoflurane-exposed rats demonstrated an environment-dependent decline in the concentrations of synaptic markers in the hippocampus after growing up at P53. (*P<0.05 vs CON+AE group, ##P<0.01 vs SEV+AE group, n=6). (a and c) Representative western blot images of PSD-95, synaptophysin and β-actin in the hippocampus of different groups of rats to illustrate band intensities. (b and d). Histograms showing results of the densitometric analysis of western blot images of the concentrations of PSD-95 and synaptophysin.
Compared with the control group, the number of the BrdU+ cells in the dentate gyrus was significantly reduced at P11 (P=0.049; Fig. 4a and b) in the sevoflurane-exposed rats injected with BrdU for three consecutive days from P8 to P10. For determining the long-term survival of new granule cells, BrdU was injected for six consecutive days from P22 to P27. At P53, the sevoflurane-exposed rats and housed in the EE and non-exposed rats housed in the same conditions did not differ in terms of the number of the BrdU+ cells (Fig. 4c and d). The P53 sevoflurane-exposed rats housed in the AE, on the other hand, when compared with non-exposed rats housed in the AE, had reduced BrdU+ cells (F3,23=4.948, P=0.035; Fig. 4c and d). There was significant increase in the number of BrdU+ cells in the CON+EE group compared with CON+AE group (F3,23=4.948, P=0.015). The SEV+EE group had a higher number of the BrdU+ cells than the SEV+AE group (F3,23=4.948, P=0.003). Two-way ANOVA showed that there were statistically significant main effects of the EE (F1,23=8.797, P=0.006) and sevoflurane (F1,23=4.325, P=0.048), based on numbers of the BrdU+ cells, but there was not an interaction between the EE and sevoflurane (F1,23=1.370, P=0.252).
Fig 4.
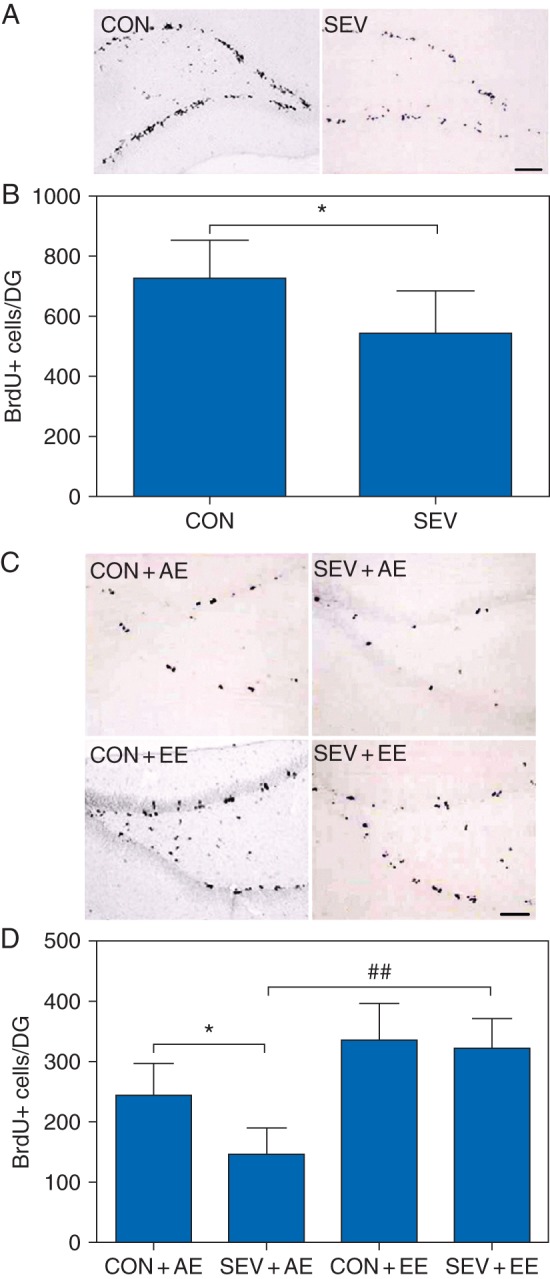
Sevoflurane-exposed rats showed a decline in the neural progenitor proliferation in the neonatal dentate gyrus and demonstrated an environment-dependent decline in the long-term survival of new granule cells after growing up. (a and b) The number of the BrdU+ cells in the dentate gyrus was significantly reduced at P11 in the sevoflurane-exposed rats injected with BrdU for three consecutive days from P8 to P10 compared with the control group. (*P<0.05 vs CON group, n=6). (c and d) Sevoflurane-exposed rats demonstrated an environment-dependent decline in the long-term survival of new granule cells after growing up. (*P<0.05 vs CON+AE group, ##P<0.01 vs SEV+AE group, n=6). (a and c) Representative images of BrdU+ cells in the dentate gyrus of different groups of rats (original magnification 200×, scale bar 50 µm). (b and d) Histogram showing results of the number of the BrdU+ cells.
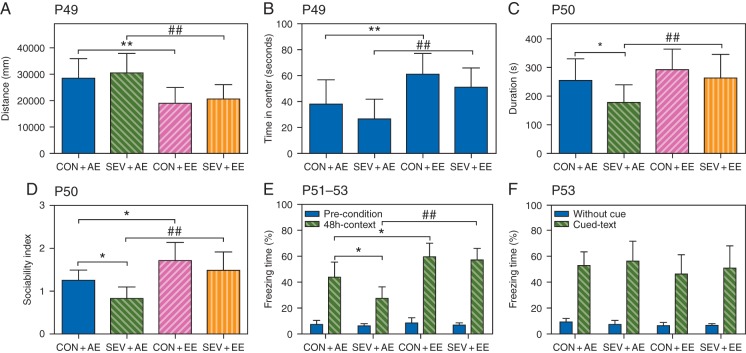
The P49-P53 rats previously exposed to sevoflurane and housed in the AE exhibited neurobehavioural abnormalities when compared with those housed in the EE. The sevoflurane-exposed rats housed in the AE exhibited unaltered total distance travelled (F3,39=5.201, P=0.824; Fig. 5a) and no significant decline in the time spent in the centre (F3,39=1.276, P=0.124; Fig. 5b), but reduced social interaction duration (F3,39=4.358, P=0.029; Fig. 5c) and sociability index (F3,39=7.737, P=0.050; Fig. 5d) compared with the CON+AE group. The sevoflurane-exposed rats housed in the AE exhibited reduced freezing time in contextual fear conditioning (F3,39=11.877, P=0.011; Fig. 5e), but unaltered freezing time in the hippocampal-independent cued test compared with the CON+AE group (Fig. 5f). The P49 rats in the control and sevoflurane-exposed groups housed in the EE exhibited significantly lower locomotion activity and more time spent in the centre compared with those housed in the AE (Fig. 5a and b). The sevoflurane group housed in the EE showed significant improvement in social interaction duration (F3,39=4.358, P=0.015; Fig. 5c), the sociability index (F3,39=7.737, P=0.005; Fig. 5d) and freezing time in contextual fear conditioning (F3,39=11.877, P=0.002; Fig. 5e) compared with the SEV+AE group. Two-way ANOVA showed that there was no interaction between the EE and sevoflurane, but there were statistically significant main effects of the EE and sevoflurane based on social interaction duration and sociability index (social interaction duration: EE, F1,39=7.070, P=0.011; sevoflurane, F1,39=5.100, P=0.029; interaction, F1,39=0.905, P=0.347; social-interaction index: EE, F1,39=17.436, P<0.001; sevoflurane, F1,39=5.517, P=0.024; interaction, F1,39=0.257, P=0.615; Fig. 5c and d). There was no significant difference in social interaction duration but there was a significant difference in the sociability index between the CON+AE and CON+EE groups (F3,39=7.737, P=0.013; Fig. 5d).
Fig 5.
The P49-P53 rats previously exposed to sevoflurane and housed in the adverse environment (AE) exhibited impaired behaviour in comparison with those housed in the enriched environment (EE). (a–f). Histograms showing the results of the neurobehavioral tests in the P49-P53 rats housed in the AE conditions that were either not exposed to sevoflurane (CON+AE), exposed to sevoflurane (SEV+AE) or those housed in the EE that were either not exposed to sevoflurane (CON+EE) or exposed to sevoflurane (SEV+EE). (**P<0.01, *P<0.05 vs CON+AE; ##P<0.01, #P<0.05 vs SEV+AE, n=10). See text for details.
Two-way ANOVA showed that there was no interaction between the EE and sevoflurane (F1,39=2.670, P=0.111; Fig. 5e), but there were statistically significant main effects of the EE (F1,39=28.083, P<0.001) and sevoflurane (F1,39=5.216, P=0.028) based on freezing time in hippocampal-dependent contextual fear conditioning. There was a significant difference in freezing time in contextual fear conditioning between the CON+AE and CON+EE groups (F3,39=7.070, P=0.013; Fig. 5d). The freezing time in hippocampal-independent cued fear conditioning was not significantly different among all groups (Fig. 5f).
Discussion
The main finding of this study is that the developmental outcome of repeated neonatal exposure of rats to sevoflurane may be determined by environmental factors presented at a relatively distant life period after exposure to the anaesthetic (in rodents after weaning). Specifically, at ∼P50, the sevoflurane-exposed rats housed in EE or AE conditions since weaning were either indistinguishable from the non-exposed controls or exhibited significant neurobehavioural abnormalities, respectively. It suggested that the brain development of neonatal anaesthesia-initiated deficiencies might be a gradually expanding pathophysiological process that can be either exacerbated or alleviated by a stressful or enriched (non-stressful) environment throughout life, respectively. Our findings allow speculation that subjects exposed to the same anaesthesia regimen during the early life period may be affected to a different extent, depending on their subsequent life experiences.
The adverse effects of repeated neonatal exposure to sevoflurane were detected at cellular and behavioural levels. Multiple sevoflurane exposures of neonatal rats reduced the neonatal neural progenitor proliferation and the long-term survival of new granule cells in rats housed in the AE from weaning day. It is known that new neurones are generated in the dentate gyrus throughout adulthood and may play an important role in cognitive functions, and alterations in this process may contribute to various brain diseases.14 Our results suggest that repeated neonatal exposure to sevoflurane results in impairment in neural progenitor proliferation. The survival of new granule cells may be further decreased by adverse environmental factors presented later in life. We did not find any significant difference in the survival number of BrdU+ cells between the CON+EE and the SEV+EE groups at P53, but we found that control rats, after being housed in the EE for one month, showed a higher survival of new granule cells in the GCL compared with the CON+AE group. These results support the idea that the EE exerts a survival-promoting effect on the proliferating neuronal precursor cells.15
Similar to the survival-promoting effect of EE in new granule cells, our results showed that repeated neonatal exposure to sevoflurane-induced reduction in synaptic markers, may be reversed by enriched environmental factors presented later in life. We did not find any significant difference in the concentrations of synaptic markers in the hippocampus between the CON+EE and the SEV+EE groups at P53, but we found that sevoflurane-exposed rats, after being housed in the AE for one month, showed a lower concentrations of synaptic markers in the hippocampus compared with the CON+AE group. These results support previous reports that EE induces neural changes in the cerebral cortex and hippocampus16 and raise the possibility that the neurobehavioural developmental effects of repeated neonatal exposure to sevoflurane can be aggravated by adverse environmental factors.
Similar to the survival of new granule cells and the changes in concentrations of synaptic markers in the hippocampus, the P49 to P53 rats that were neonatally exposed to sevoflurane and subsequently housed in the AE conditions, but not those housed in the EE conditions, exhibited abnormal behaviour. The sevoflurane-exposed rats housed in the AE conditions exhibited reduced freezing time in contextual fear conditioning and a reduced social interaction duration and sociability index. Impairments in communication and social interaction are important symptoms of autism spectrum disorders (ASD), a group of common neuropsychiatric disorders17–20 in which environmental factors are thought to play an important aetiological role.21–23 Our findings of reduced social interaction duration and sociability index suggest that repeated neonatal exposure to sevoflurane and subsequent exposure to AE factors may predispose to ASD-like behaviour. These findings are in agreement with a previous report on the ability of neonatal sevoflurane to induce ASD-like behavior in mice.9 It will be important to test whether mouse strains genetically predisposed to ASD, are more vulnerable to the developmental effects of neonatal anaesthesia and whether such effects could be alleviated by placing the animals in an EE. Enriched housing provides enhanced possibilities of complex physical and social stimulations that increase learning experiences, visuals inputs, and social interactions. The exposure to EE induces plastic changes, particularly at the level of the hippocampus and cerebral cortex.16
Our findings of the important role of later-in-life environmental factors in determining the future developmental outcome of neonatal exposure to general anaesthetics, not only support the reports from other laboratories that delayed environmental enrichment may ameliorate sevoflurane-induced deficiencies in rodents,6–8 but further widen their conclusions by suggesting that subjects with a subsequent stressful lifestyle may be especially vulnerable to the detrimental effects of neonatal anaesthesia. The EE conditions in this study consisted of paired housing, voluntary wheel running, social interaction, environmental complexity, and environmental novelty. Obviously, environmental enrichment is not an issue for the majority of human patients because this is typical for most children. However, many children who had general anaesthesia-requiring procedures during the early postnatal period can be exposed to a variety of stressful factors during post-anaesthesia life (e.g. hunger, pain, maternal deprivation, baby abandonment, psychological isolation stress, fear and anxiety). Our findings suggest that there is a possibility that individuals exposed to neonatal anaesthesia may be differentially affected by neonatal anaesthetic depending on their future health status not necessarily directly related to anaesthesia exposure, and other adverse life events. It would be important to consider this possibility during selection of study subjects for human retrospective analyses of delayed neurocognitive effects of neonatal anaesthesia. It will also be important to test whether the alleviating effect of the EE is permanent, or, in other words, whether placement of the rats housed for one month in the EE back in the AE will unmask detrimental effects of neonatal anaesthesia.
Sevoflurane-exposed rats showed a decline in the concentrations of BDNF in the neonatal brain hippocampus and demonstrated an environment-dependent decline in the concentrations of BDNF after growing up. BDNF is one of the most well-studied neurotrophins and exerts a variety of neurotrophic and neuroprotective functions in the brain development.24,25 For instance, BDNF serves as a pivotal mediator of synaptic plasticity and hippocampal neurogenesis, and is implicated in learning and memory. EE treatment can restore the reduced hippocampal neurogenesis and protein concentrations of BDNF.26 BDNF is required for the enhancement of hippocampal neurogenesis after environmental enrichment.27 BDNF expression is vulnerable to many stress procedures, such as isolation rearing, which significantly decreases BDNF mRNA and protein expression concentration in the rat hippocampus.28–30 It is plausible that the reduction in concentrations of the hippocampal BDNF may play a mechanistic role in the sevoflurane-induced abnormalities observed in this study. However, further studies, such as intrahippocampal injection of BDNF, will be needed to prove this possibility.
Collectively, the results of this study provide evidence that the expression of the neurobehavioural abnormalities induced by repeated neonatal exposure to sevoflurane may critically depend on environmental factors presented later in life. Specifically, rodents living in an EE may exhibit neurobehavioural activity indistinguishable from non-exposed controls, while those living in stressful conditions may exhibit profound neurobehavioural deficiencies.
Authors' contributions
Study design/planning: M.Q.Z., J.J.Y., A.E.M.
Study conduct: M.H.J., Q.S.Z.
Data analysis: M.Q.Z., M.H.J., Q.S.Z., L.Q., J.J.Y., Y.G.P.
Writing paper: M.Q.Z.
Revising paper: all authors
Declaration of interest
None declared.
Funding
This work was supported by the National Natural Science Foundation of China (Grant nos. 81271216, 81300946 and 81471105) and by the National Institutes of Health (R01GM93036 and R01NS091542 to A.E.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Sanders RD, Hassell J, Davidson AJ, Robertson NJ, Ma D. Impact of anaesthetics and surgery on neurodevelopment: an update. Br J Anaesth 2013; 110: i53–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology 2012; 117: 494–503 [DOI] [PubMed] [Google Scholar]
- 3.Sprung J, Flick RP, Katusic SK, et al. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc 2012; 87: 120–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 2009; 110: 796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu C, Gao J, Karlsson N, et al. Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab 2010; 30: 1017–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen X, Dong Y, Xu Z, et al. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology 2013; 118: 502–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shih J, May LD, Gonzalez HE, et al. Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology 2012; 116: 586–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng H, Dong Y, Xu Z, et al. Sevoflurane anesthesia in pregnant mice induces neurotoxicity in fetal and offspring mice. Anesthesiology 2013; 118: 516–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satomoto M, Satoh Y, Terui K, et al. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology 2009; 110: 628–37 [DOI] [PubMed] [Google Scholar]
- 10.Moy S, Nadler J, Perez A, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 2004; 3: 287–302 [DOI] [PubMed] [Google Scholar]
- 11.Ji M, Dong L, Jia M, et al. Epigenetic Enhancement of Brain-Derived Neurotrophic factor signaling pathway improves cognitive impairments induced by isoflurane exposure in aged rats. Mol Neurobiol 2014; 50: 937–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Xu Z, Wang H, et al. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol 2012; 71: 687–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Z, Culley DJ, Dong Y, et al. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid β-protein level in vivo. Ann Neurol 2008; 64: 618–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christian KM, Song H, Ming GL. Functions and Dysfunctions of Adult Hippocampal Neurogenesis. Annu Rev Neurosci 2014; 37: 243–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus 2006; 16: 250–60 [DOI] [PubMed] [Google Scholar]
- 16.Hirase H, Shinohara Y. Transformation of cortical and hippocampal neural circuit by environmental enrichment. Neuroscience 2014; 280C: 282–98 [DOI] [PubMed] [Google Scholar]
- 17.Charman T. Autism spectrum disorders. Psychiatry 2008; 7: 331–4 [Google Scholar]
- 18.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol 2007; 17: 103–11 [DOI] [PubMed] [Google Scholar]
- 19.Johnson CP, Myers SM. Identification and evaluation of children with autism spectrum disorders. Pediatrics 2007; 120: 1183–215 [DOI] [PubMed] [Google Scholar]
- 20.Lord C, Cook EH. Autism spectrum disorders. Autism: The Science of Mental Health 2013; 28: 217 [Google Scholar]
- 21.Daniels JL. Guest Editorial: Autism and the Environment. Environ Health Persp 2006; 114: A396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurobiol 2010; 23: 103–10 [DOI] [PubMed] [Google Scholar]
- 23.James SJ, Melnyk S, Jernigan S, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet 2006; 141B: 947–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med 2004; 5: 11–25 [DOI] [PubMed] [Google Scholar]
- 25.Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience 2013; 239: 214–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jha S, Dong B, Sakata K. Enriched environment treatment reverses depression-like behavior and restores reduced hippocampal neurogenesis and protein levels of brain-derived neurotrophic factor in mice lacking its expression through promoter IV. Transl Psychiatry 2011; 1: e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi C, Angelucci A, Costantin L, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci 2006; 24: 1850–6 [DOI] [PubMed] [Google Scholar]
- 28.Biggio F, Pisu MG, Garau A, et al. Maternal separation attenuates the effect of adolescent social isolation on HPA axis responsiveness in adult rats. Eur Neuropsychopharmacol 2014; 24: 1152–61 [DOI] [PubMed] [Google Scholar]
- 29.Djouma E, Card K, Lodge DJ, Lawrence AJ. The CRF1 receptor antagonist, antalarmin, reverses isolation-induced up-regulation of dopamine D2 receptors in the amygdala and nucleus accumbens of fawn-hooded rats. Eur. J. Neurosci 2006; 23: 3319–27 [DOI] [PubMed] [Google Scholar]
- 30.Pisu MG, Dore R, Mostallino MC, et al. Down-regulation of hippocampal BDNF and Arc associated with improvement in aversive spatial memory performance in socially isolated rats. Behav Brain Res 2011; 222: 73–80 [DOI] [PubMed] [Google Scholar]