Abstract
Antibodies against cyclic citrullinated peptides (anti-CCP) are widely used for diagnosis of rheumatoid arthritis (RA). We performed a comparative analysis of antibodies targeting the citrullinating enzyme peptidylarginine deiminase type 4 (anti-PAD4) and mutated citrullinated vimentin (anti-MCV) with anti-CCP autoantibodies in RA patients and examined their relationships with clinical parameters, cytokine profiles and the PADI4 gene. Autoantibodies were examined by enzyme-linked immunosorbent assay (ELISA) in sera of 170 RA patients and 103 controls. Cytokine profiles were measured using a multiplex system. PADI4 polymorphisms (89G > A, 90T > C and 92G > C) were genotyped by polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP). Anti-PAD4, anti-MCV and anti-CCP autoantibodies were detected in 24, 61 and 74% of RA patients, respectively. Positive correlations were observed between anti-PAD4 and disease duration; anti-CCP and erythrocyte sedimentation rate (ESR); anti-MCV and ESR and C-reactive protein. Anti-MCV antibodies were associated with high disease activity score 28 (DAS-28) in early RA. Concentrations of T helper type 1 (Th1) [tumour necrosis factor (TNF)-α, interleukin (IL)-12, IL-2, IL-1β], Th2 (IL-4, IL-6, IL-10, IL-13) and Th17 (IL-17) cytokines were higher in RA than in controls. Th2 and, to a lesser extent, Th1-related cytokines, showed positive correlations with anti-MCV and anti-CCP. The GTG haplotype in PADI4 was associated with anti-CCP and anti-MCV, but not anti-PAD4 antibodies. In conclusion, anti-PAD4 antibodies are detected mainly in established RA, which is in contrast to the early detection of antibodies against citrullinated peptide/proteins (ACPAs). Among autoantibodies, anti-MCV appear to perform better as markers of disease activity. Furthermore, anti-CCP and anti-MCV are associated genetically with the citrullinating enzyme PAD4 and are related strongly to Th1 and Th2 cytokines, suggesting a feed-forward loop between cytokines and ACPA production.
Keywords: ACPA; citrullination, disease activity; PADI4; rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease that affects primarily the synovial tissue of the joints and can lead eventually to destruction of cartilage, bone erosions and substantial disability. RA is characterized by presence of autoantibodies and prominent production of inflammatory cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-17, which are all detected in the serum of patients 1,2. Although it is well known that cytokines are major drivers of the initiation and perpetuation of RA 3,4, their relationship with particular types of autoantibodies remains barely explored and evaluating these features might give insights into the pathogenic mechanisms of the disease and help to understand some of the clinical associations described for autoantibodies.
Antibodies against citrullinated peptide/proteins (ACPAs) represent the most specific markers for the diagnosis of RA 5. Current assays detect ACPA reactivity against epitopes on different citrullinated proteins. Among these, the anti-cyclic citrullinated peptide (anti-CCP) assay stands as the test used more frequently in the clinical practice due to its high specificity (>95%) and utility as an early diagnostic marker 6–8. Despite being a useful diagnostic tool, the peptides used in the anti-CCP assay do not correspond to in-vivo-generated citrullinated proteins and are therefore of limited adequacy for understanding the disease aetiology and pathogenesis of RA 9. Anti-mutated citrullinated vimentin (anti-MCV) antibodies have recently gained relevance in RA and several studies suggest better sensitivity and clinical performance in comparison to anti-CCP 10–12, whereas others have given conflicting results 13–16. Vimentin is an intermediate filament protein highly expressed by synoviocytes and macrophages of the joint 17. In the RA synovium, vimentin is released from macrophages undergoing apoptosis and experiences two modifications: citrullination and mutation 18. These changes generate neo-epitopes and may lead to the breaching of immunological tolerance in genetically predisposed individuals with the subsequent production of anti-MCV antibodies.
Protein citrullination, the post-translational modification of peptidyl-arginine residues to peptidyl-citrulline, is catalyzed by peptidylarginine deiminases (PADs) 19,20. Among five PAD enzymes, peptidylarginine deiminase type 4 (PAD4) is regarded as one of the major players in RA pathogenesis because it is highly expressed by the immune cells infiltrating the joint (monocytes, macrophages, neutrophils, T and B cells) as well as the RA synovial tissue, where it is located in the close vicinity of citrullinated proteins 21,22. Furthermore, PAD4 is itself a target of autoantibodies (anti-PAD4), reported in up to 50% of RA patients 23–25. Similar to what has been reported for anti-CCP and anti-MCV, the presence of anti-PAD4 autoantibodies has been associated with more severe disease and radiographic erosions 25–27, although the precise mechanisms that link autoantibodies with a more aggressive disease course are scarcely known.
Additionally, PAD4 has been associated genetically to RA in several populations 28–30. Three single nucleotide polymorphisms (SNPs) in PADI4 are in linkage disequilibrium and form a susceptibility haplotype (GTG) that leads to three amino acid substitutions in the enzyme 28. Studies suggest that this genetic variant have a functional effect that may impact on the generation of citrullinated autoantigens; the susceptibility haplotype carriers showed increased expression and stability of PADI4 mRNA 28,31, and in-vitro assays demonstrated increased interaction with some substrates and altered regulation of the enzyme at the protein level 32,33. We have reported previously that PADI4 is also a genetic marker for RA in a cohort of patients from western Mexico [odds ratio (OR) = 1·41, 95% confidence interval (CI) = 1·14–1·75; P < 0·001]. We detected that carriers of the susceptibility haplotype have higher frequency and titres of anti-CCP antibodies in comparison to carriers of the non-susceptibility haplotype (ACC) (OR = 3·27, 95% CI = 1·3–8·35; P = 0·004) 31; however, the relationship of this genetic marker with other ACPAs in RA, such as anti-MCV antibodies, has not been evaluated.
In this study, we analysed anti-PAD4, anti-MCV and anti-CCP autoantibodies in RA patients and compared its associations with the clinical parameters, cytokine profiles [T helper type 1 (Th1)/Th2/Th17] and the susceptibility genetic marker in PADI4 described previously in our population. An examination of autoantibodies and cytokines according to years of disease evolution was also performed. The results of the present report provide insights into the relationship of autoantibodies with clinical features and cytokines in RA, and provide additional links between genetics and serology of RA.
Subjects and methods
Patients and controls
We studied 170 patients who fulfilled the American College of Rheumatology 1987 criteria for the classification of RA 34. Patients were recruited from the rheumatology service of two hospitals in Jalisco, Mexico: Hospital Civil de Guadalajara ‘Fray Antonio Alcalde’ and Hospital General de Occidente, Secretaria de Salud.
The healthy control group (HC) comprised 103 individuals recruited from the general population. Control individuals had no history of autoimmune or inflammatory diseases and reported not being under medication. For the genetic analysis, only Mestizo unrelated subjects from western Mexico were included to avoid population heterogeneity. Mestizos are defined as those individuals born in Mexico with a Spanish-derived last name and Mexican ancestors at least three generations back. This population is a cross-breed of Amerindian, European, Asian and African genes 35.
The study was conducted according to the guidelines and recommendations stated in the Declaration of Helsinki and approved by the Ethics Committee of Universidad de Guadalajara. All the subjects signed a written informed consent prior to enrolment into the protocol.
Clinical assessment
A rheumatologist evaluated all the patients at the time of inclusion. Demographic, clinical and medication data were also obtained at the moment of blood sample collection. The clinical activity was estimated using the disease activity score 28 (DAS-28) 36, and is reported as a 0–10 scale. The functional disability was assessed through the application of the Health Assessment Questionnaire–Disability Index (HAQ-DI, Spanish version) 37, and is reported as a 0–3 scale. High-sensitivity C-reactive protein (hsCRP) was measured by a turbidimetric assay (COD31029; BioSystems, Barcelona, Spain) using automatized equipment (BS-120; Mindray, Shenzhen, China) (reported in mg/l). The erythrocyte sedimentation rate (ESR) was assessed using the Wintrobe method (reported in mm/h).
Quantification of autoantibodies
Rheumatoid factor [RF; isotypes immunoglobulin (Ig)M, IgG and IgA] was quantified using a turbidimetric assay (COD31030; BioSystems, Barcelona, Spain) on automatic equipment (BS-120; Mindray Shenzhen, China). The cut-off value for RF positivity was 30 IU/ml, which gave a specificity of 95%. Anti-MCV antibodies (isotype IgG) were quantified using an enzyme-linked immunosorbent assay (ELISA) (ORG 548; Orgentec Diagnostika, Mainz, Germany), according to the manufacturer's instructions. The cut-off value for anti-MCV positivity was 20 U/ml, which gave a specificity of 100%. Anti-CCP antibodies (isotype IgG) were measured with a second-generation ELISA kit (FCCP600; Axis-Shield Diagnostics, Dundee, UK), following the instructions provided by the manufacturer. The cut-off value for anti-CCP positivity was 5 U/ml, providing a specificity of 100%. Anti-PAD4 autoantibodies (isotypes IgM, IgA and IgG) were detected using an ELISA kit that recognizes autoantibodies against human recombinant PAD4 (Cayman Chemical, Ann Arbor, MI, USA). This assay uses an affinity-purified antibody isolated from the plasma of a RA patient as standard. Samples were processed according to the instructions provided in the kit, except for slight modifications in dilution of samples (1:100 or 1:500 for RA and 1:100 for controls). As there is no standard cut-off value for anti-PAD4 positivity, the cut-off was set as the value corresponding to the 95th centile of the control group (4749 U/ml), which gave a specificity of 95%.
Quantification of cytokines
From the total of 170 patients and 103 healthy controls included in the genetic and autoantibody analysis, we strictly selected 77 RA patients and 30 HC age- and sex-matched for the cytokine analysis. Exclusion criteria in HC were: pregnancy or endocrine alterations, inflammatory (such as obesity, diabetes, cancer) or infectious diseases. The RA patients were stratified into three subgroups: RA of < 2 years disease duration (n = 32); RA of > 2 years disease duration (n = 29) and RA untreated (RA-UTX, n = 16). This last group included patients who did not receive treatment with disease modifying anti-rheumatic drugs (DMARDs) or corticosteroids in the previous 6 months. The 69% (11 of 16) of the RA-UTX group were patients with < 2 years disease duration, whereas the remainder (31%) had long-standing disease and left the treatment.
Cytokines TNF-α, IL-12 (p40/p70), IFN-γ, IL-2, IL-1β, IL-4, IL-6, IL-10, IL-13 and IL-17, were measured using a custom-designed bead-based multiplex assay (Cytokine Human Magnetic Custom 10-Plex Panel; Invitrogen Life Technologies, Frederick, MD, USA) and the MAGPIX® System (Luminex, Austin, TX, USA). Serum samples were stored at −80°C until the day of the assay and then thawed and processed according to the manufacturer's instructions, except for the inclusion of an initial centrifugation step at 19700 g for 10 min at 4°C to remove debris. The fluorescence values of 100 events per region were considered as quantification criteria. Serial dilutions of the recombinant standards provided in the assay were performed in duplicate to generate standard curves of the cytokines and subsequently adjusted to a logistic regression model (five parameters). All the curves showed a correlation coefficient (R2) above 0·95. Quantitative levels of cytokines in the samples were interpolated from the standard curves and reported in pg/ml; those samples with cytokine concentrations below the lowest point on the standard curve were reported with the lowest value. To corroborate our results, the cytokines TNF-α (Invitrogen, Waltham, MA, USA), IL-10 and IFN-γ (both from R&D Systems, Minneapolis, MN, USA) were measured additionally in some samples with conventional ELISA kits; the cytokine values obtained with ELISA were correlated highly with this bead-based assay, which is in agreement with previous studies 38,39.
Genotyping of the PADI4 SNPs
The PADI4 89G>A, PADI4 90T>C and PADI4 92G>C exonic SNPs (reference sequences: rs11203366, rs11203367 and rs874881, respectively) were genotyped by the polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) method using the reagents and conditions described previously 31.
Statistical analysis
The distributions of all variables were examined using the Shapiro–Wilk normality test. Continuous variables distributed normally were expressed as mean ± standard deviation (s.d.) and those non-normally distributed were expressed as median and 5–95th centiles. Categorical variables were expressed as percentage and absolute frequency. Student's t-test or Mann–Whitney U-test (as applicable to data distribution) were used to evaluate differences between two groups. The Kruskal–Wallis test was used to analyse differences between three or more groups (for variables distributed non-normally) followed by Dunn's adjustment for multiple comparisons. Linear correlation coefficients were examined using the Pearson's or Spearman's correlation test (as appropriate). For the genetic analysis, comparison of allele and genotype distributions between groups was evaluated using the χ2 test. The OR and 95% CIs were estimated to analyse the risk of PADI4 genotypes associated with RA. All analyses were performed using Stata version 11·0 and GraphPad Prism version 5·0 software. Throughout, a probability (P) value of < 0.05 was considered significant.
Results
Patient characteristics and autoantibody positivity
The demographic and clinical data of the total 170 RA patients are presented in Table1. Patients had a mean age of 47 ± 12 years, 88% were female and 25% reported current or past smoking habits. Controls had a mean age of 38 ± 12 years and 70% were female. Inflammation markers in controls were found within normal values [median (5–95th centiles): ESR = 16 3–37 and hsCRP = 2 (0·1–8)] and both were significantly lower in comparison to RA (P < 0·001). On average, patients had moderate activity (mean DAS-28 = 4·3 ± 1.3) and mild disability (mean HAQ-DI = 0·7 ± 0·6). Most patients (91%) were receiving treatment with disease-modifying anti-rheumatic drugs (DMARDs), either as monotherapy or polytherapy. As the diagnostic performance of autoantibodies in RA may be influenced importantly by the disease phase (very early, early or long-standing RA) 16, we decided to analyse the positivity to autoantibodies by years of RA duration to achieve a clearer analysis [< 2 years (mean 1 ± 0·5; n = 80) and > 2 years (mean 8 ± 7; n = 90)]. In general, the demographic and clinical characteristics were similar in both subgroups (Table1).
Table 1.
Demographic and clinical features of rheumatoid arthritis (RA) patients.
| RA by years of disease duration | |||
|---|---|---|---|
| RA total | <2 years | >2 years | |
| Variables | n = 170 | n = 80 | n = 90 |
| Demographics | |||
| Age, mean ± s.d., years | 47 ± 12 | 45 ± 13 | 49 ± 12 |
| Female gender, % (n) | 88 (149) | 90 (72) | 86 (77) |
| Ever smoking, % (n) | 25 (43) | 26 (21) | 24 (22) |
| Inflammation markers | |||
| ESR, median (5–95th centiles), mm/h | 35 (11–55) | 32 (11–58) | 38 (12–54) |
| hsCRP, median (5th–95th centiles), mg/l | 27 (2–238) | 20 (2–287) | 30 (6–238) |
| Clinical activity and disability | |||
| RA duration, mean ± s.d., years | 5 ± 6 | 1 ± 0·5 | 8 ± 7 |
| DAS-28, mean ± s.d., 0–10 score | 4·3 ± 1·3 | 4·3 ± 1·4 | 4·3 ± 1·3 |
| HAQ-DI, mean ± s.d., 0–3 score | 0·7 ± 0·6 | 0·6 ± 0·5 | 0·7 ± 0·6 |
| Drug treatment,* % (n) | 91 (154) | 86 (69) | 94 (85) |
| NSAIDs, % (n) | 71 (121) | 76 (61) | 67 (60) |
| DMARDs | |||
| Methotrexate, % (n) | 76 (130) | 79 (63) | 74 (67) |
| Hydroxy/chloroquine, % (n) | 39 (67) | 40 (32) | 39 (35) |
| Leflunomide, % (n) | 1 (2) | 0 (0) | 2 (2) |
| Sulphasalazine, % (n) | 37 (63) | 36 (29) | 38 (34) |
| Prednisone,† % (n) | 36 (62) | 38 (30) | 36 (32) |
DAS-28 = disease activity score 28; DMARDs = disease-modifying anti-rheumatic drugs; ESR = erythrocyte sedimentation rate; HAQ-DI = health assessment questionnaire–disability index (Spanish version); hsCRP = high sensitivity C-reactive protein; s.d. = standard deviation; NSAIDs = non-steroidal anti-inflammatory drugs.
Any treatment with DMARDs involving monotherapy or polytherapy.
Doses of < 15 mg daily.
The median concentrations and positivity for RF, anti-CCP, anti-MCV and anti-PAD4 autoantibodies are shown in Table2. Consistent with previous studies 40, patients increased their positivity to all autoantibodies according to RA duration despite being under treatments (Table2). RF and anti-CCP positivity was similar to that reported extensively in previous studies 41,42, whereas anti-MCV antibodies were detected in a lower percentage of RA patients in comparison to anti-CCP (61 versus 74%, respectively), even when stratification by RA duration was performed. Thus, we did not confirm previous reports of a better sensitivity for anti-MCV antibodies in comparison to anti-CCP 10,18. Anti-PAD4 autoantibodies were detected in 24% of the RA patients, which is lower than the general prevalence reported in previous studies (30–50%) 24,25,43. However, analysis by RA duration showed positivity in 18% in RA < 2 years, which is similar to a report in preclinical RA samples (18%) 44, and 29% in RA > 2 years, consistent with most of the studies performed in established RA patients 25,26.
Table 2.
Positivity to autoantibodies in patients subgrouped by rheumatoid arthritis (RA) duration.
| Autoantibody | RA total | RA < 2 years | RA > 2 years | P value* |
|---|---|---|---|---|
| n = 170 | n = 80 | n = 90 | < 2 versus > 2 years | |
| RF, IU/ml | 85 (32–300) | 70 (29–300) | 94 (44–300) | 0·221 |
| Positives, % (n) | 76 (130) | 71 (57) | 81 (73) | |
| Anti-CCP, U/ml | 54 (5–130) | 28 (3–61) | 62 (22–170) | 0·0006 |
| Positives, % (n) | 74 (125) | 67 (54) | 79 (71) | |
| Anti-MCV, U/ml | 49 (7–385) | 23 (4–129) | 78 (13–626) | 0·001 |
| Positives, % (n) | 61 (103) | 51 (41) | 69 (62) | |
| Anti-PAD4, U/ml | 2090 (939–4445) | 1632 (942–3399) | 2550 (797–7110) | 0·046 |
| Positives, % (n) | 24 (40) | 18 (14) | 29 (26) |
Concentrations of autoantibodies are shown as median (25–75th centiles).
Mann–Whitney U-test. CCP = cyclic citrullinated peptide; MCV = mutated citrullinated vimentin; PAD4 = peptidylarginine deiminase type 4; RF = rheumatoid factor.
Regarding specificity, anti-CCP and anti-MCV antibodies displayed a specificity of 100%, whereas anti-PAD4 and RF showed a specificity of 95% (see the Methods section for cut-off values).
Combinations of autoantibody positivity in RA
To examine previously described associations among autoantibodies in our RA group and determine whether novel associations exist, we analysed the combinations of positivity to autoantibodies in the patients (Table3). The major groups were: triple-positives (RF, anti-CCP, anti-MCV; 38%); positives to all autoantibodies (18%), RF single-positive (11%), double-positives (RF, anti-CCP; 7%) and seronegatives (12%).
Table 3.
Combinations of autoantibody positivity in rheumatoid arthritis (RA).
| Seropositivity to | RA total, n = 170 | RA < 2 years, n = 80 | RA > 2 years, n = 90 |
|---|---|---|---|
| % (n) | % (n) | % (n) | |
| None | 12 (20) | 14 (11) | 10 (9) |
| RF | 11 (19) | 14 (11) | 9 (8) |
| Anti-CCP | 5 (9) | 8·5 (7) | 2 (2) |
| Anti-PAD4 | 2 (4) | 2·5 (2) | 2 (2) |
| RF, anti-CCP | 7 (11) | 10 (8) | 3 (3) |
| RF, anti-MCV | 1 (2) | 2·5 (2) | 0 (0) |
| Anti-CCP, anti-MCV | 3 (5) | 2·5 (2) | 3 (3) |
| Anti-PAD4, ACPA* | 1 (2) | 1 (1) | 1 (1) |
| RF, anti-CCP, anti-MCV | 38 (64) | 31 (25) | 44 (39) |
| RF, anti-CCP, anti-PAD4 | 2 (3) | 0 (0) | 3 (3) |
| All autoantibodies | 18 (31) | 14 (11) | 23 (20) |
Frequencies were obtained by direct counting.
Anti-cyclic citrullinated peptide (CCP) or anti-mutated citrullinated vimentin (MCV). PAD4 = peptidylarginine deiminase type 4; RF = rheumatoid factor; ACPA = antibodies against citrullinated peptide/proteins.
Anti-PAD4 positivity (n = 40) was observed mainly in patients who also were positive for the rest of the autoantibodies (n = 31). A strong association was found between anti-PAD4 and anti-CCP (OR = 4·15, 95% CI = 1·3–16·98; P = 0.007), which is consistent with previous reports 25,26. Because anti-MCV autoantibodies are closely related to anti-CCP, we sought to define the relationship between anti-MCV and anti-PAD4; a novel association was detected for these two autoantibodies (OR = 3·32, 95% CI = 1·35–8·94; P = 0·005). No association was found between RF and anti-PAD4.
Several studies suggest that testing of anti-MCV might add substantial diagnostic value for identifying RA patients seronegative to RF or anti-CCP 10,45; however, anti-MCV positivity was generally in combination with anti-CCP in our cohort (in 98% of the individuals), and single anti-MCV positivity was not detected. Of interest, four patients (2%) displayed single anti-PAD4 positivity (Table3).
Correlations between autoantibodies and clinical features of RA
Previous studies have suggested that anti-MCV antibodies could provide additional value over anti-CCP as markers of more persistent disease activity in patients with early and established RA 10–12. For anti-PAD4, a correlation with the DAS-28 was reported 24. We performed an analysis of correlation between serum autoantibody levels and the clinical features of RA to explore further the possible relationship between these parameters. As shown in Table4, anti-MCV antibodies displayed significant correlations with ESR, hsCRP and disease duration (rs = 0·194, P = 0·012; rs = 0·174, P = 0·025; rs = 0·201, P = 0·009, respectively), whereas anti-CCP with ESR (rs = 0·206, P = 0·008) and disease duration (rs = 0·238, P = 0·001). Conversely, anti-PAD4 antibodies only showed a correlation with the duration of RA (Table4; rs = 0·162, P = 0·034).
Table 4.
Correlations between autoantibodies and clinical features in rheumatoid arthritis (RA).
| Anti-PAD4 | Anti-MCV | Anti-CCP | ||||
|---|---|---|---|---|---|---|
| Variable | rs | P | rs | P | rs | P |
| Smoking, years | 0·004 | 0·965 | 0·032 | 0·733 | −0·007 | 0·937 |
| Inflammation markers | ||||||
| ESR, mm/h | 0·01 | 0·899 | 0·194 | 0·012 | 0·206 | 0·008 |
| hsCRP, mg/l | 0·008 | 0·917 | 0·174 | 0·025 | 0·064 | 0·412 |
| Clinical activity and disability | ||||||
| RA duration, years | 0·162 | 0·034 | 0·201 | 0·009 | 0·238 | 0·001 |
| DAS-28, 0–10 scale | −0·023 | 0·760 | 0·098 | 0·206 | 0·032 | 0·679 |
| HAQ-DI, 1–3 scale | −0·003 | 0·969 | 0.049 | 0.529 | −0·028 | 0·713 |
rs = Spearman's correlation coefficient. PAD4 = peptidylarginine deiminase type 4; MCV = mutated citrullinated vimentin; CCP = cyclic citrullinated peptide; ESR = erythrocyte sedimentation rate; DAS28 = disease activity score 28; HAQ-DI = health assessment questionnaire–disability index; hsCRP = high sensitivity C-reactive protein.
The relationship between the DAS-28 with positivity to each autoantibody is shown in Fig. 1. Irrespective of disease duration, analysis did not allow us to identify any significant difference, but when stratification by disease duration was performed we revealed that in RA with < 2 years disease duration, anti-MCV-positive patients had a significantly higher DAS-28 score than the anti-MCV negative group (Fig. 1b). The combinations of autoantibody positivity (e.g. single- versus double- or triple-positives) in relation to the DAS-28 were also evaluated, but no significant differences were detected, possibly due to small number of patients in some of the subgroups (see Supporting information, Fig. S1).
Figure 1.
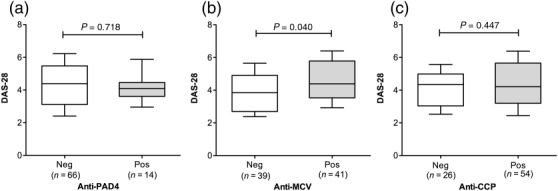
Relationship between autoantibody positivity and the disease activity score 28 (DAS-28). The DAS-28 is increased significantly in rheumatoid arthritis (RA) patients positive to anti-mutated citrullinated vimentin (MCV) with disease duration of < 2 years (b). Horizontal lines indicate median DAS-28 score for each group; whiskers depict the 10–90th centiles. Statistical analysis was performed using Student's t-test.
Autoantibody titres were correlated between each other; the highest coefficients of correlation were observed between anti-CCP and anti-MCV (rs = 0·75, P < 0·001) and between RF and both ACPAs (rs = 0·55, P < 0·001), whereas anti-PAD4 antibodies showed correlations of low magnitude with RF and anti-MCV (rs = 0·17, P = 0·02 and rs = 0·25, P = 0·001) and a marginal correlation with anti-CCP (rs = 0·15, P = 0·05).
Cytokine analysis in RA and their relationship with autoantibodies
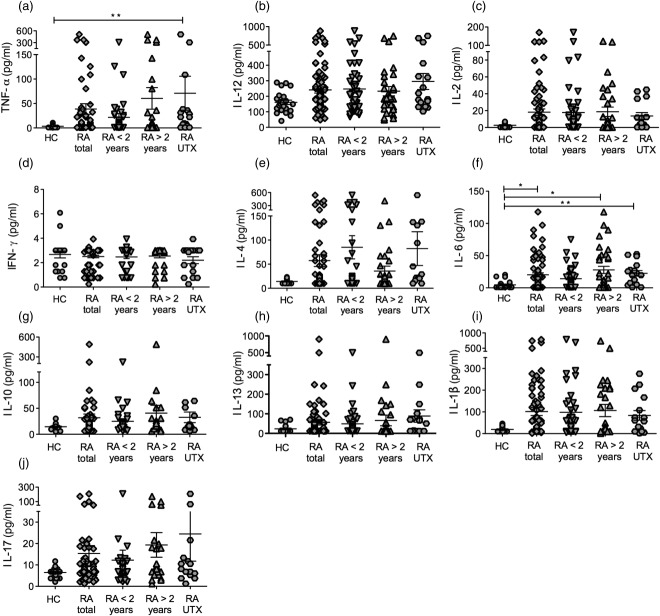
The concentrations of 10 cytokines related with the Th1, Th2 and Th17 profiles were measured using a multiplex bead-based system in serum samples of RA patients and matched healthy controls. Patients were stratified according to disease duration (<2 and >2 years) and a group of untreated patients was also included (see the Methods section for details). As shown in Fig. 2, increased concentrations of the Th1 (TNF-α, IL-12, IL-2 and IL-1β)-, Th2 (IL-4, IL-6, IL-10 and IL-13)- and Th17 (IL-17)-related cytokines were seen in RA patients in comparison to controls. A remarkable heterogeneity among the patients was also observed, and only TNF-α and IL-6 reached significant differences when analysing total concentrations (Fig. 1a,f). Nevertheless, all cytokines (except IFN-γ) were increased in a significantly higher proportion of patients in comparison to controls (‘increase’ was defined as concentrations above the 95th percentile in controls; data not shown). Unexpectedly, IFN-γ concentrations (a hallmark Th1-related cytokine) were very similar in controls and patients, even in the untreated RA group (Fig. 2d).
Figure 2.
Cytokine concentrations in rheumatoid arthritis (RA) patients and controls. Shown are serum concentrations (pg/ml) of the cytokines tumour necrosis factor (TNF)-α (a), interleukin (IL)-12 (b), IL-2 (c), interferon (IFN)-γ (d), IL-4 (e), IL-6 (f), IL-10 (g), IL-13 (h), IL-1β (i) and IL-17 (j). Cytokines were analysed by multiplex cytokine assay. Groups: healthy controls (HC); RA total (all patients); RA < 2 years (subgrouped by disease duration); RA > 2 years; RA UTX (untreated RA patients). Lines depict the mean and standard error of the mean (s.e.m.). Statistical analysis was performed using the Kruskal–Wallis test followed by Dunn's multiple comparison test. *P < 0·05; **P < 0·01.
Patients who displayed high concentrations of Th1-related cytokines (TNF-α, IL-1β and IL-12) also exhibited increased concentrations of the antagonistic Th2-related cytokines (IL-10, IL-4 and IL-13), which is in agreement with previous reports 39,46. This association was also supported by the fact that concentrations of all cytokines showed moderate to high correlations between one another (e.g. IL-1β and TNF-α, rs = 0·71, P < 0·001; IL-6 and IL-10, rs = 0·51, P < 0·001), even when evaluating cytokines with antagonistic effects [e.g. Th1/Th2 (IL-1β and IL-10, rs = 0·48, P < 0·001); Th17/Th2 (IL-17 and IL-10, rs = 0·27, P < 0·01)].
The relationship between cytokines and the clinical features of RA was also examined (see Supporting Information, Table S1). TNF-α and IL-12 were among the most significant markers of RA because both correlated positively with DAS-28 and HAQ-DI, as well as IL-6 (correlated with ESR, hsCRP, DAS-28 and HAQ-DI) and IL-1β (correlated with DAS-28 and ESR). In contrast, IL-17 and IL-10 were correlated only with hsCRP and HAQ-DI, respectively.
To explore further the relationship between autoantibodies and cytokine concentrations in RA patients, we performed a linear correlation analysis. Anti-MCV and anti-CCP antibodies showed significant correlations with almost the same Th1- and Th2-related cytokines (IL-10, IL-6, IL-4, IL-1β and TNF-α), but not with Th17 (Table5). Of note, the correlation coefficients were slightly higher for anti-MCV than for anti-CPP, and among all cytokines IL-10 and IL-6 displayed the best correlations with both ACPAs. In contrast, anti-PAD4 antibodies did not correlate with any cytokine measured in our study.
Table 5.
Relationship between autoantibodies and cytokine concentrations in rheumatoid arthritis (RA).
| Anti-PAD4 | Anti-MCV | Anti-CCP | ||||
|---|---|---|---|---|---|---|
| Cytokine | rs | P | rs | P | rs | P |
| IL-10 | 0·146 | 0·203 | 0·409 | <0·001 | 0·381 | <0·001 |
| IL-6 | −0·061 | 0·594 | 0·347 | 0·002 | 0·309 | 0·006 |
| IL-4 | 0·087 | 0·448 | 0·329 | 0·003 | 0·281 | 0·013 |
| IL-1β | −0·065 | 0·573 | 0·343 | 0·002 | 0·238 | 0·036 |
| TNF-α | 0·056 | 0·623 | 0·301 | 0·013 | 0·285 | 0·007 |
| IL-2 | −0·038 | 0·742 | 0·277 | 0·016 | 0·195 | 0·087 |
| IL-12 | 0·046 | 0·688 | 0·187 | 0·106 | 0·161 | 0·16 |
| IFN-γ | 0·029 | 0·798 | 0·023 | 0·842 | 0·135 | 0·241 |
| IL-13 | −0·125 | 0·278 | 0·059 | 0·614 | −0·056 | 0·627 |
| IL-17 | 0·064 | 0·578 | 0·075 | 0·519 | 0·194 | 0·089 |
rs = Spearman's correlation coefficient (values are shown in descending order). IL = interleukin; TNF = tumour necrosis factor; IFN = interferon; MCV = mutated citrullinated vimentin; CCP = cyclic citrullinated peptide; PAD4 = peptidylarginine deiminase type 4.
Relationship between autoantibodies and the PADI4 haplotypes
A genetic association between the citrullinating enzyme PAD4 with antibodies to citrullinated filaggrin as well as anti-CCP was reported previously in Asians 28,47 and in our Mexican population 31. Because anti-MCV antibodies are related closely to anti-CCP, we hypothesized that these antibodies are also associated with PADI4 gene. We genotyped three SNPs in PADI4 in order to identify the homozygote individuals carrying the haplotypes of susceptibility (GTG/GTG) and non-susceptibility (ACC/ACC) to RA, and evaluate its relationship with autoantibodies. By applying a dominant genetic model, the three SNPs were associated individually with RA susceptibility in our population, thus confirming the findings described previously by our group (see Supporting Information, Table S2). As depicted in Fig. 3, titres of anti-MCV and anti-CCP autoantibodies were increased significantly in the carriers of the PADI4 susceptibility haplotype (GTG/GTG) in comparison to the non-susceptibility haplotype carriers (ACC/ACC). Analysis by positivity also revealed a significant association of both ACPAs with the susceptibility haplotype (anti-CCP: OR = 5·6, 95% CI = 1·4–26·5, P = 0·004; anti-MCV: OR = 2·7, 95% CI = 0·9–8·1, P = 0·04). Conversely, anti-PAD4 antibodies did not show any relationship with the PADI4 haplotypes.
Figure 3.
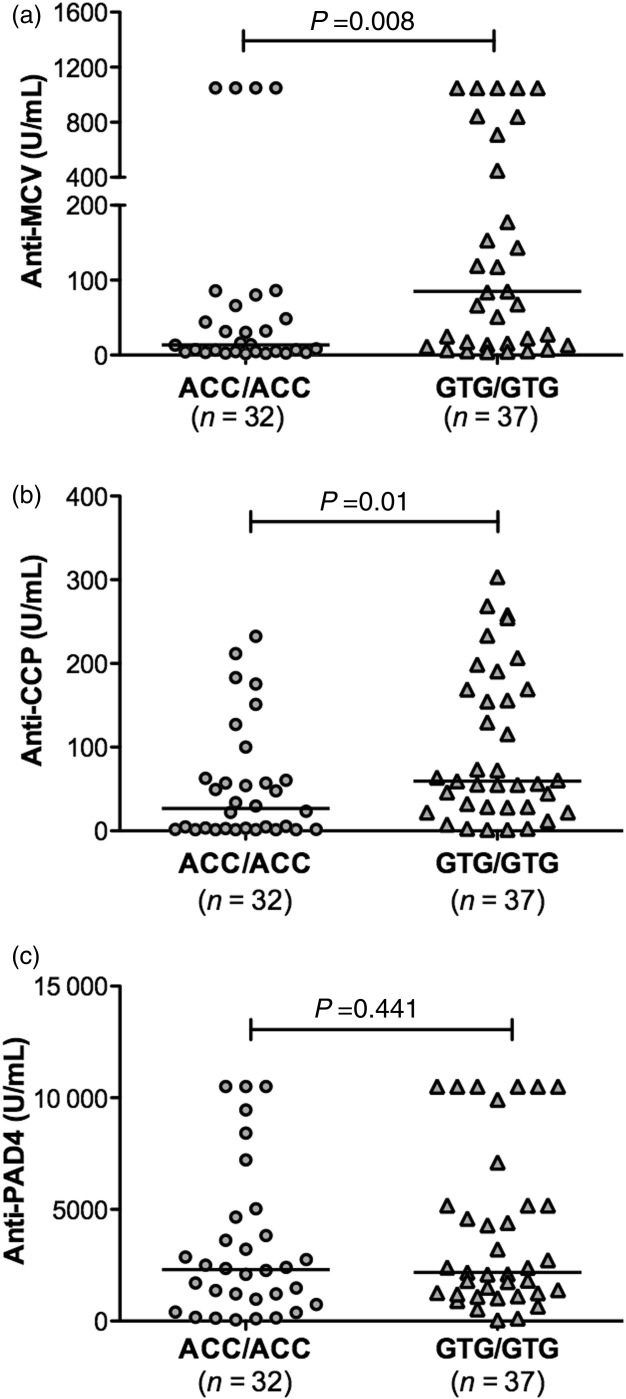
Autoantibody concentrations in rheumatoid arthritis (RA) patients according to the PADI4 haplotypes. Anti-mutated citrullinated vimentin (MCV) and anti-cyclic citrullinated peptide (CCP) (a,b), but not anti-peptidylarginine deiminase type 4 (PAD4) autoantibodies (c), were increased significantly in the homozygous carriers of the susceptibility PADI4 haplotype (GTG/GTG) in comparison to the carriers of the non-susceptibility PADI4 haplotype (ACC/ACC). Horizontal lines indicate median autoantibody concentrations. Statistical analysis was performed using the Mann–Whitney U-test.
Discussion
Autoantibodies are the hallmark of autoimmune diseases. For RA they represent promising tools for early diagnosis, prognosis and therapeutic management of the disease. In addition to the widely used RF and anti-CCP autoantibody assay, several tests have emerged recently: anti-MCV antibodies, which have been suggested to possess clinical relevance mainly for the diagnosis of RA 10,18, and anti-PAD4 autoantibodies, which have been proposed to allow the identification of a subgroup of RA patients with more severe disease 25,26. In this study, we performed a comparative analysis of anti-PAD4 and anti-MCV antibodies with respect to anti-CCP, and examined their relationship with the clinical parameters, cytokine profiles and genetic features to gain insights into the diagnostic and clinical performance of these autoantibody systems and provide additional links between serology and genetics of RA.
Positivity to RF and anti-CCP in our cohort of patients was similar to that reported extensively in prior studies 7. Analysis by RA duration (<2 versus >2 years) showed a marked increase in positivity for all autoantibodies. Recent studies have demonstrated that epitope spreading (the expansion of T and B cell immune responses against different autoantigens) can be detected several years prior to RA clinical onset 48. Additionally, reports suggest that once anti-CCP antibodies appear these remain positive in about 97% of patients independently of DMARD treatment 8,49. Taking into account these findings, our results suggest that despite the effect of treatments, there is a sustained immune response against citrullinated proteins in RA accompanied by expansion of B cell reactivity against citrullinated antigens (which is reflected by increase in anti-CCP positivity) throughout disease course.
For direct comparison between anti-MCV and anti-CCP sensitivities, we set the cut-off values of both autoantibodies to obtain equal specificity (100%) using a healthy subjects group as control. In contrast to previous studies 10–12, positivity to anti-MCV was found to be lower than anti-CCP, even when analysis by disease duration was performed. Therefore, these antibodies do not appear to provide additional diagnostic performance over anti-CCP in our RA cohort. Additionally, we neither confirmed a diagnostic value of anti-MCV for identifying patients seronegatives to RF nor anti-CCP, as described previously 10,45, because single anti-MCV positivity was not detected.
Instead, anti-MCV showed the best correlations with the parameters of clinical activity in comparison to anti-CCP and anti-PAD4, and a relationship with positivity and increased DAS-28 in RA of <2 years duration was also detected. In agreement with our findings, studies have reported a positive correlation between anti-MCV and DAS-28 (rs = 0·533, P = 0·0003) 18, as well as a correspondence between changes in anti-MCV levels with changes in DAS-28 and its individual components (ESR and CRP) 10. In contrast, the association between anti-CCP antibodies and RA disease activity has yielded conflicting results 11,50 and was not confirmed in the present study. It is unknown why anti-MCV antibodies represent better markers of disease activity than anti-CCP, but a possible explanation may be the fact that vimentin is a native protein of the RA synovium and, as a consequence of the oxidative stress and inflammatory process, undergoes mutation and citrullination by PADs 18 triggering the production of these antibodies, whereas the anti-CCP assay uses an artificial cyclic citrullinated peptide as antigen and may not reflect accurately the underlying inflammatory process involved in the pathogenesis of RA, although its diagnostic performance is undisputable.
The prevalence of anti-PAD4 autoantibodies (24%) in RA patients was slightly lower than that reported in former studies (35–50%) 23–26, but analysis by disease duration (<2 versus >2 years) exposed a substantial increase in positivity according to RA evolution (from 18 to 29%). These findings, together with the fact that most individuals with anti-PAD4 antibodies were also positive for anti-CCP, support the notion that anti-PAD4 immune response takes place mainly in established RA 25 after the production of ACPAs has started. However, this hypothesis should be confirmed in proper longitudinal studies addressing the development of these autoantibodies during the disease course.
Although it is not clear what drives the immune response against PAD4 in RA, it has been demonstrated that anti-PAD4 antibodies may have an activating or inhibitory effect on PAD4 enzymatic function, depending on the epitopes targeted by an individual 27,51. Based on our ELISA screening, we were unable to identify specifically which epitopes of PAD4 were targeted by anti-PAD4 antibodies, but the positive correlation as well as the strong association between anti-PAD4 with both ACPAs may suggest that anti-PAD4 antibodies, or at least a subset of them, exert an activating effect on PAD4, which promotes the generation of citrullinated epitopes and the production of ACPAs.
Previous studies have reported that antibodies to PAD4 are related to increased DAS-28 score, CRP and ESR values 24,26, and may identify a subgroup of patients with more severe joint damage as assessed through the Sharp–van der Heijde score 25. Nevertheless, we performed an extensive analysis and did not detect any significant association or correlation between anti-PAD4 and the parameters of clinical activity (DAS-28) and disability (HAQ-DI). A possibility for this lack of association may be related to the fact that antibodies to PAD4 were detected in a small group of individuals or to modifications in the clinical activity of the patients, because most of them were under treatment; however, is interesting to note that the relationship between anti-MCV with the clinical parameters was preserved despite treatment. Another important aspect to consider is that radiographic data for most of the patients were lacking and we did not address the relationship with this parameter. In this regard, Darrah et al. recently discovered a subset of anti-PAD4 antibodies that cross-react with PAD3, which are associated with more erosive disease and that markedly enhance PAD4 activity by increasing calcium sensitivity 27. Future studies addressing the contribution of these cross-reactive autoantibodies against PADs to disease activity or severity in other cohorts of RA patients will be of interest to validate the utility of these antibodies as a severity marker.
Analysis of cytokine concentrations in patients versus matched controls confirmed a prominent increase in Th1 (TNF-α, IL-12, IL-2 and IL-1β), Th2 (IL-4, IL-6, IL-10 and IL-13) and Th17 (IL-17)-related cytokines. In line with previous studies 39,46, we distinguished subsets of patients who had low levels of cytokines or high levels of both Th1- and Th2-related cytokines, evidencing the heterogeneity of RA and the parallel production of cytokines with antagonistic effects. The cytokines that most reflected the disease activity of RA (as measured by CRP, ESR, DAS-28 and HAQ-DI) were TNF-α, IL-6, IL-1β and IL-12, which supports the pathogenic role of these inflammatory cytokines in RA and the utility of biological therapy to block these key mediators of cartilage and bone destruction.
Correlation analysis between autoantibodies and cytokines showed that both anti-CCP and anti-MCV were correlated strongly with Th2 (IL-2, IL-4, IL-6, IL-10)- and Th1-related cytokines (TNF-α, IL-1β), but not with IL-17. Strikingly, anti-PAD4 antibodies did not exhibit a significant relationship with any of the cytokines analysed. It is possible that the low prevalence of anti-PAD4, together with the fact that quantification of cytokines was performed only in a subgroup of patients, may have provided limited statistical power to detect any association. The other possibility is that anti-PAD4 antibodies contribute to RA pathogenesis mainly through mechanisms other than inflammation (e.g. by modifying the catalytic function of PAD4, as described recently 27). Defining additional effector mechanisms of these autoantibodies, such as immune complex formation, will clarify further their role in RA pathogenesis.
The strongest correlations of both ACPAs were those with IL-10 and IL-6. Although IL-10 has potent immunoregulatory effects and can antagonize the differentiation of Th1 cells and the secretion of inflammatory cytokines such as TNF-α and IL-1β 52, its strong association with both ACPAs reflects that in the setting of RA, IL-10 may be promoting the differentiation and survival of B cells as well as the production of autoantibodies 53. Conversely, IL-6 is recognized as a key cytokine in RA with widespread inflammatory and systemic effects. Earlier studies identified IL-6 as a B cell differentiating factor and recent findings suggest a role in the development of T follicular helper cells (Tfh) 54,55, providing more evidence for IL-6 contribution on the induction of plasmatic cells and the production of autoantibodies.
Antibodies against citrullinated fibrinogen have the ability to activate complement and to form immune complexes that induce the production of TNF-α by activation of Toll-like receptor (TLR)-4 in macrophages 56,57. A recent study by Khandpur et al. demonstrated that RF and antibodies against citrullinated vimentin enhance the formation of neutrophil extracellular traps (NETs), and these in turn augment the production of IL-6 and IL-8 by synovial fibroblasts 58. In support of these findings in our study, anti-MCV and anti-CCP were correlated positively with the inflammatory cytokines TNF-α, IL-6 and IL-1β. These cytokines probably represent not only a downstream event of ACPAs; evidence suggests that inflammation is the common trigger for the generation of citrullinated proteins. Therefore TNF-α, IL-6 and IL-1β may also promote the generation of more citrullinated autoantigens creating a feed-forward loop of autoantibodies and inflammation.
The PADI4 gene has been associated with RA susceptibility in several Asian and Caucasian populations (OR = 1.1–1.8) 29,59. Results from this and our previous larger study confirm that PADI4 is also a genetic marker for RA in a population from western Mexico 31. We corroborated the relationship of the homozygous susceptibility haplotype (GTG/GTG) with the presence and increased levels of anti-CCP antibodies in comparison to the homozygous non-susceptibility haplotype (ACC/ACC) and, importantly, we detected a novel association with anti-MCV. Similarly, Suzuki et al. reported for the first time an association of PADI4 haplotype with antibodies against citrullinated filaggrin 28, although some other studies have failed in replicating this association 25,60. Conversely, Harris et al. described an association of the PADI4 susceptibility haplotype with anti-PAD4 antibodies in a Caucasian cohort; this association was particularly evident on heterozygote carriers 25. It is important to mention that we did not find any significant relationship of PADI4 gene with autoantibodies when analysing heterozygote carriers. We focused on the contribution of PADI4 homozygote haplotypes because in heterozygote individuals the haplotypes can only be inferred, and the true contribution of each allele on autoantibody production cannot be defined precisely. Furthermore, as there is no standardized assay to measure anti-PAD4 antibodies, discrepancies exist regarding the methods used for their detection (e.g. ELISAs and immunoprecipitation). It is unknown if these dissimilarities between studies are due to effects of the ethnic background of the populations, experimental variances in the quantification of autoantibodies, lack of genetic association and/or low frequency of the susceptibility haplotypes in the populations under study providing insufficient statistical power. Nevertheless, we detected a consistent relationship between PADI4 susceptibility haplotype and both ACPAs (anti-MCV and anti-CCP) in our population, which reinforces the idea of a functional effect of PADI4 polymorphisms that leads to increased ACPA production.
In this regard, the susceptibility haplotype in PADI4 is associated with increased expression and stability of mRNA 28,31, and at the protein level, the PAD4 polymorphic variant appears to have increased interaction with some substrates, including histones (H3, CitH3) and HDAC1 33. Considering that PAD4 also regulates gene expression and is essential in the formation of NETs 61, an interesting immunological mechanism that is being involved increasingly in autoimmunity, we cannot exclude an influence of the PAD4 polymorphic variant in these processes.
Finally, as several reports have demonstrated a strong link between ACPA levels with some of the human leucocyte antigen (HLA) shared epitope (SE) alleles 62,63, a limitation of our study was the lack of those genetic data. Thus, we cannot discard a genetic interaction of HLA–SE alleles with the PADI4 gene in our population. Additionally, the absence of radiographic data for most patients did not allow us to address the relationship of autoantibodies with erosive disease.
Conclusions
Our results suggest that anti-MCV antibodies do not provide superior diagnostic value over anti-CCP in RA; instead, they seem to perform better as markers of disease activity. In contrast to the early detection of anti-CCP and anti-MCV, anti-PAD4 autoantibodies were more frequent in established RA, suggesting that they are generated mainly in advanced stages of disease pathogenesis, although they do not appear to provide significant relevance as markers of disease activity in our cohort of patients.
A strong relationship was confirmed between both ACPAs (anti-MCV and anti-CCP) with Th1- and Th2-related cytokines, which may indicate that ACPAs are inducing the secretion inflammatory cytokines in RA, whereas Th2 cytokines may further promote the differentiation of B cells and autoantibody production.
Anti-CCP and anti-MCV, but not anti-PAD4 antibodies, are associated genetically with the citrullinating enzyme PAD4. The precise mechanisms by which the PAD4 polymorphic variant leads to increased ACPA production remain unclear and future studies will be necessary to solve these aspects.
Acknowledgments
Z.R.C. designed the study, performed the experiments, analysed the data and wrote the article. C.A.P.S. and G.E.M.B. provided patient samples and were involved in the interpretation of data. I.P.R., S.T.A. and M.G.R.D. were involved in the interpretation of data and revision of the manuscript. G.O.B. provided help with the immunoassays and genotyping of samples. J.F.M.V. had main responsibility for the design and coordination of the study and critical revision of the paper. All authors read and approved the final manuscript. This study was supported by funding from the National Council of Science and Technology (CONACYT) grant no. 161749 (Fondo Sectorial Secretaría de Salud-IMSS-ISSSTE-CONACYT-Mexico-Universidad de Guadalajara) assigned to J.F.M.V. The funding source had no involvement in any phase of the study.
Disclosure
The authors declare that they have no conflicts of interest.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Table S1. Correlation analysis between cytokines and clinical features of rheumatoid arthritis (RA).
Table S2. Distribution of PADI4 single nucleotide polymorphisms (SNPs) among rheumatoid arthritis (RA) patients and controls.
Fig. S1. Combinations of autoantibody positivity and its relationship with the disease activity score 28 (DAS-28). Analysis of positivity to autoantibody combinations showed no significant differences in relation to the DAS-28. Horizontal lines indicate median DAS-28 score for each group; whiskers depict the 10–90th centiles; the mean is represented with a cross (+). Statistical analysis was performed using Student's t-test.
References
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nat Rev Rheumatol. 2012;8:573–86. doi: 10.1038/nrrheum.2012.134. [DOI] [PubMed] [Google Scholar]
- Raza K, Falciani F, Curnow SJ, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7:R784–95. doi: 10.1186/ar1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester GR, Feist E, Dörner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol. 2013;10:77–88. doi: 10.1038/nrrheum.2013.168. [DOI] [PubMed] [Google Scholar]
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- Van Venrooij WJ, van Beers JJ, Pruijn GJ. Anti-CCP antibodies: the past, the present and the future. Nat Rev Rheumatol. 2011;7:391–8. doi: 10.1038/nrrheum.2011.76. [DOI] [PubMed] [Google Scholar]
- Avouac J, Gossec L, Dougados M. Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2005;65:845–51. doi: 10.1136/ard.2006.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnelid J, Wick MC, Lampa J, et al. Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis. 2005;64:1744–9. doi: 10.1136/ard.2004.033571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirke A-M, Fisher BAC, Kinloch AJ, Venables PJ. Citrullination of autoantigens: upstream of TNFα in the pathogenesis of rheumatoid arthritis. FEBS Lett. 2011;585:3681–8. doi: 10.1016/j.febslet.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Mathsson L, Mullazehi M, Wick MC, et al. Antibodies against citrullinated vimentin in rheumatoid arthritis: higher sensitivity and extended prognostic value concerning future radiographic progression as compared with antibodies against cyclic citrullinated peptides. Arthritis Rheum. 2008;58:36–45. doi: 10.1002/art.23188. [DOI] [PubMed] [Google Scholar]
- Innala L, Kokkonen H, Eriksson C, Jidell E, Berglin E, Dahlqvst SR. Antibodies against mutated citrullinated vimentin are a better predictor of disease activity at 24 months in early rheumatoid arthritis than antibodies against cyclic citrullinated peptides. J Rheumatol. 2008;35:1002–8. [PubMed] [Google Scholar]
- Wagner E, Skoumal M, Bayer PM, Klaushofer K. Antibody against mutated citrullinated vimentin: a new sensitive marker in the diagnosis of rheumatoid arthritis. Rheumatol Int. 2009;29:1315–21. doi: 10.1007/s00296-009-0854-2. [DOI] [PubMed] [Google Scholar]
- Ursum J, Nielen MM, van Schaardenburg D, et al. Antibodies to mutated citrullinated vimentin and disease activity score in early arthritis: a cohort study. Arthritis Res Ther. 2008;10:R12. doi: 10.1186/ar2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luime JJ, Colin EM, Hazes JMW, Lubberts E. Does anti-mutated citrullinated vimentin have additional value as a serological marker in the diagnostic and prognostic investigation of patients with rheumatoid arthritis? A systematic review. Ann Rheum Dis. 2010;69:337–44. doi: 10.1136/ard.2008.103283. [DOI] [PubMed] [Google Scholar]
- Raza K, Mathsson L, Buckley CD, Filer A, Ronnelid J. Anti-modified citrullinated vimentin (MCV) antibodies in patients with very early synovitis. Ann Rheum Dis. 2010;69:627–8. doi: 10.1136/ard.2009.118448. [DOI] [PubMed] [Google Scholar]
- Bartoloni E, Alunno A, Bistoni O, et al. Diagnostic value of anti-mutated citrullinated vimentin in comparison to anti-cyclic citrullinated peptide and anti-viral citrullinated peptide 2 antibodies in rheumatoid arthritis: an Italian multicentric study and review of the literature. Autoimmun Rev. 2012;11:815–20. doi: 10.1016/j.autrev.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Vossenaar ER, Després N, Lapointe E, et al. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther. 2004;6:R142–50. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang H, Egerer K, Gauliard A, et al. Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis. Arthritis Rheum. 2007;56:2503–11. doi: 10.1002/art.22817. [DOI] [PubMed] [Google Scholar]
- Vossenaar ER, Zendman AJ, van Venrooij WJ. Citrullination, a possible functional link between susceptibility genes and rheumatoid arthritis. Arthritis Res Ther. 2004;6:1–5. doi: 10.1186/ar1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicker KL, Thompson PR. The protein arginine deiminases: structure, function, inhibition, and disease. Biopolymers. 2013;99:155–63. doi: 10.1002/bip.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulquier C, Sebbag M, Clavel C, et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007;56:3541–53. doi: 10.1002/art.22983. [DOI] [PubMed] [Google Scholar]
- Kinloch A, Lundberg K, Wait R, et al. Synovial fluid is a site of citrullination of autoantigens in inflammatory arthritis. Arthritis Rheum. 2008;58:2287–95. doi: 10.1002/art.23618. [DOI] [PubMed] [Google Scholar]
- Takizawa Y, Sawada T, Suzuki A, Yamada R, Inoue T, Yamamoto K. Peptidylarginine deiminase 4 (PADI4) identified as a conformation-dependent autoantigen in rheumatoid arthritis. Scand J Rheumatol. 2005;34:212–5. doi: 10.1080/03009740510026346-1. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhao Y, He J, Jia R, Li Z. Prevalence and significance of anti-peptidylarginine deiminase 4 antibodies in rheumatoid arthritis. J Rheumatol. 2008;35:969–74. [PubMed] [Google Scholar]
- Harris ML, Darrah E, Lam GK, et al. Association of autoimmunity to peptidyl arginine deiminase type 4 with genotype and disease severity in rheumatoid arthritis. Arthritis Rheum. 2008;58:1958–67. doi: 10.1002/art.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen EH, Pollmann S, Gilboe I-M, et al. Serum IgG antibodies to peptidylarginine deiminase 4 in rheumatoid arthritis and associations with disease severity. Ann Rheum Dis. 2008;67:414–7. doi: 10.1136/ard.2007.080267. [DOI] [PubMed] [Google Scholar]
- Darrah E, Giles JT, Ols ML, Bull HG, Andrade F, Rosen A. Erosive rheumatoid arthritis is associated with antibodies that activate PAD4 by increasing calcium sensitivity. Sci Transl Med. 2013;5:186ra65–186ra65. doi: 10.1126/scitranslmed.3005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Yamada R, Chang X, et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet. 2003;34:395–402. doi: 10.1038/ng1206. [DOI] [PubMed] [Google Scholar]
- Iwamoto T. Association between PADI4 and rheumatoid arthritis: a meta-analysis. Rheumatology. 2006;45:804–7. doi: 10.1093/rheumatology/kel023. [DOI] [PubMed] [Google Scholar]
- Eyre S, Bowes J, Diogo D, et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet. 2012;44:1336–40. doi: 10.1038/ng.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán-Guzmán IP, Reyes-Castillo Z, Muñoz-Barrios S, et al. Polymorphisms and functional haplotype in PADI4: further evidence for contribution on rheumatoid arthritis susceptibility and anti-cyclic citrullinated peptide antibodies in a western Mexican population. Immunol Lett. 2015;163:214–20. doi: 10.1016/j.imlet.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Andrade F, Darrah E, Gucek M, Cole RN, Rosen A, Zhu X. Autocitrullination of human peptidyl arginine deiminase type 4 regulates protein citrullination during cell activation. Arthritis Rheum. 2010;62:1630–40. doi: 10.1002/art.27439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JL, Jones LE, Bhatia MM, Thompson PR. Autodeimination of protein arginine deiminase 4 alters protein–protein interactions but not activity. Biochemistry (Mosc) 2011;50:3997–4010. doi: 10.1021/bi200309e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F, Edworthy S, Bloch D, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Martínez-Cortés G, Salazar-Flores J, Fernández-Rodríguez L, et al. Admixture and population structure in Mexican-Mestizos based on paternal lineages. J Hum Genet. 2012;57:568–74. doi: 10.1038/jhg.2012.67. [DOI] [PubMed] [Google Scholar]
- Prevoo M, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte L, van Riel P. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- Cardiel M, Abello-Banfi M, Ruiz-Mercado R, Alarcon-Segovia D. How to measure health status in rheumatoid arthritis in non-English speaking patients: validation of a Spanish version of the Health Assessment Questionnaire Disability Index (Spanish HAQ-DI) Clin Exp Rheumatol. 1993;11:117–21. [PubMed] [Google Scholar]
- Kokkonen H, Söderström I, Rocklöv J, Hallmans G, Lejon K, Dahlqvist SR. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010;62:383–91. doi: 10.1002/art.27186. [DOI] [PubMed] [Google Scholar]
- Alex P, Szodoray P, Knowlton N, et al. Multiplex serum cytokine monitoring as a prognostic tool in rheumatoid arthritis. Clin Exp Rheumatol. 2007;25:584. [PubMed] [Google Scholar]
- Nielen MMJ, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–6. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- Schellekens GA, Visser H, De Jong BA, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–63. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Van Boekel MA, Vossenaar ER, Van Den Hoogen FH, van Venrooij WJ. Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value. Arthritis Res Ther. 2002;4:87–93. doi: 10.1186/ar395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger I, Charpin C, Balandraud N, Martin M, Roudier J. Autoantibodies to PAD4 and BRAF in rheumatoid arthritis. Autoimmun Rev. 2012;11:801–3. doi: 10.1016/j.autrev.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Kolfenbach JR, Deane KD, Derber LA, et al. Autoimmunity to peptidyl arginine deiminase type 4 precedes clinical onset of rheumatoid arthritis. Arthritis Rheum. 2010;62:2633–9. doi: 10.1002/art.27570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland PN, Mignot SG, Bruns A, et al. Antibodies to mutated citrullinated vimentin for diagnosing rheumatoid arthritis in anti-CCP-negative patients and for monitoring infliximab therapy. Arthritis Res Ther. 2008;10:R142. doi: 10.1186/ar2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueber W, Tomooka BH, Zhao X, et al. Proteomic analysis of secreted proteins in early rheumatoid arthritis: anti-citrulline autoreactivity is associated with up regulation of proinflammatory cytokines. Ann Rheum Dis. 2007;66:712–9. doi: 10.1136/ard.2006.054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S, Choi C-B, Han T-U, Kang CP, Kang C, Bae S-C. Association of anti–cyclic citrullinated peptide antibody levels with PADI4 haplotypes in early rheumatoid arthritis and with shared epitope alleles in very late rheumatoid arthritis. Arthritis Rheum. 2007;56:1454–63. doi: 10.1002/art.22570. [DOI] [PubMed] [Google Scholar]
- Sokolove J, Bromberg R, Deane KD, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLOS ONE. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastbom A. Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project) Ann Rheum Dis. 2004;63:1085–9. doi: 10.1136/ard.2003.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner A, Plischke H, Kellner H, Gruber R. Association of anti-cyclic citrullinated peptide antibodies, anti-citrullin antibodies, and IgM and IgA rheumatoid factors with serological parameters of disease activity in rheumatoid arthritis. Ann NY Acad Sci. 2005;1050:295–303. doi: 10.1196/annals.1313.031. [DOI] [PubMed] [Google Scholar]
- Auger I, Martin M, Balandraud N, Roudier J. Rheumatoid arthritis specific autoantibodies to peptidyl arginine deiminase type 4 inhibit citrullination of fibrinogen. Arthritis Rheum. 2010;62:126–31. doi: 10.1002/art.27230. [DOI] [PubMed] [Google Scholar]
- Asadullah K. Interleukin-10 therapy – review of a new approach. Pharmacol Rev. 2003;55:241–69. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–42. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- Hashizume M, Mihara M. The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthritis. 2011;2011:1–8. doi: 10.1155/2011/765624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly – TFH cells in human health and disease. Nat Rev Immunol. 2013;13:412–26. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcγ receptor. Arthritis Rheum. 2011;63:53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel C, Nogueira L, Laurent L, et al. Induction of macrophage secretion of tumor necrosis factor α through Fcγ receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008;58:678–88. doi: 10.1002/art.23284. [DOI] [PubMed] [Google Scholar]
- Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snir O, Gomez-Cabrero D, Montes A, et al. Non-HLA genes PTPN22, CDK6 and PADI4 are associated with specific autoantibodies in HLA-defined subgroups of rheumatoid arthritis. Arthritis Res Ther. 2014;16:414. doi: 10.1186/s13075-014-0414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantaert T. Functional haplotypes of PADI4: relevance for rheumatoid arthritis specific synovial intracellular citrullinated proteins and anticitrullinated protein antibodies. Ann Rheum Dis. 2005;64:1316–20. doi: 10.1136/ard.2004.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–62. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Helm-van Mil AHM, Verpoort KN, Breedveld FC, Huizinga TWJ, Toes REM, de Vries RRP. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 2006;54:1117–21. doi: 10.1002/art.21739. [DOI] [PubMed] [Google Scholar]
- Lundberg K, Bengtsson C, Kharlamova N, et al. Genetic and environmental determinants for disease risk in subsets of rheumatoid arthritis defined by the anticitrullinated protein/peptide antibody fine specificity profile. Ann Rheum Dis. 2012;72:652–8. doi: 10.1136/annrheumdis-2012-201484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Correlation analysis between cytokines and clinical features of rheumatoid arthritis (RA).
Table S2. Distribution of PADI4 single nucleotide polymorphisms (SNPs) among rheumatoid arthritis (RA) patients and controls.
Fig. S1. Combinations of autoantibody positivity and its relationship with the disease activity score 28 (DAS-28). Analysis of positivity to autoantibody combinations showed no significant differences in relation to the DAS-28. Horizontal lines indicate median DAS-28 score for each group; whiskers depict the 10–90th centiles; the mean is represented with a cross (+). Statistical analysis was performed using Student's t-test.