Abstract
There is a need for cellular biomarkers to differentiate patients with sepsis from those with the non-infectious systemic inflammatory response syndrome (SIRS). In this double-blind study we determined whether the expression of known (CD11a/b/c, CD62L) and putative adhesion molecules [CD64, CD97 and epidermal growth factor (EGF)-like molecule containing mucin-like hormone receptor (EMR2)] on blood neutrophils could serve as useful biomarkers of infection and of non-infectious SIRS in critically ill patients. We studied 103 patients with SIRS, 83 of whom had sepsis, and 50 healthy normal subjects, using flow cytometry to characterize neutrophils phenotypically in whole blood samples. Patients with SIRS had an increased prevalence of neutrophils expressing CD11c, CD64 and EMR2 in comparison with healthy subjects (P < 0·001), but normal expression of CD11a, CD11b, CD62L and CD97. An increase in the percentage of neutrophils bearing CD11c was associated with sepsis, EMR2 with SIRS and CD64 with sepsis and SIRS. Neutrophils expressing CD11c had the highest sensitivity (81%) and specificity (80%) for the detection of sepsis, and there was an association between the percentage of neutrophils expressing EMR2 and the extent of organ failure (P < 0·05). Contrary to other reports, we did not observe an abnormal expression of CD11b or CD62L on neutrophils from patients with SIRS, and suggest that this discrepancy is due to differences in cell processing protocols. We propose that blood neutrophils expressing CD11c and EMR2 be considered as potential biomarkers for sepsis and SIRS, respectively.
Keywords: biomarkers; diagnosis, neutrophils; sepsis; systemic inflammatory response syndrome
Introduction
Sepsis is the systemic inflammatory response syndrome (SIRS) induced by infections, 90% of which are bacterial in origin. The rapid administration of appropriate antibiotics is critical to patient survival 1,2, and a test that rapidly discriminates patients with sepsis from those with SIRS induced by non-infectious stimuli (e.g. trauma, pancreatitis and cardiopulmonary bypass surgery) would be advantageous to clinical management within intensive care units (ICUs) 3,4. Several studies have tried to differentiate sepsis from non-infectious SIRS by using soluble biomarkers such as C-reactive protein (CRP), procalcitonin and soluble triggering receptor expressed on myeloid cells-1 (sTREM-1), but overall results have not had the anticipated impact 5,6. The prevailing view is that no single biomarker is able to differentiate SIRS from sepsis 7.
As neutrophils eliminate pathogenic bacteria and contribute to tissue damage 8, we reasoned that surface molecules that participate in these events could serve as biomarkers of sepsis and systemic inflammation. Germane to bacterial clearance and to the elicitation of tissue insult is the attachment of neutrophils to the walls of the microvasculature, which is controlled by the sequential activities of two families of adhesion molecules: the selectins and the integrins 9. The initial adhesion is governed partly by CD62L (L-selectin), recognizing complementary carbohydrate receptors on the endothelial surface, whereas the subsequent firm attachment depends upon the interactions of the integrins CD11a and CD11b with the vascular ligand CD54. Blood neutrophils from patients with SIRS/sepsis are reported to have an up-regulation of CD11b and a down-regulation of CD62L 10–13, a phenotype that is synonymous with cell activation, although one study reported a decreased expression of CD11b 14. Another marker of neutrophil activation is CD64, the high-affinity receptor for IgG, whose expression is increased on blood neutrophils from patients with sepsis and which we have shown to be associated with neutrophil binding to endothelium 15,16.
We consider it likely that other adhesion-like molecules may act as neutrophil biomarkers in SIRS/sepsis. Potential candidates include the β2 integrin CD11c, which shares 63% amino acid homology with CD11b and also recognizes CD54 17, and members of the adhesion G-protein coupled receptor (GPCR) family. One GPCR subgroup, characterized by an epidermal growth factor (EGF) like-domain coupled to a seven-transmembrane spanning (TM7) region, is the EGF-TM7 family 18. It is expressed predominantly by leucocytes, and members include CD97 and the epidermal growth factor (EGF)-like molecule containing mucin-like hormone receptor (EMR2) 19,20. Our earlier work showed that the expression of EMR2 was increased on blood neutrophils from patients with SIRS and that ligation increased neutrophil adhesion, migration and anti-microbial mediator production 21.
The purpose of the current study was to determine if the neutrophil integrins (CD11a, CD11b and CD11c), the selectin CD62L and the putative adhesion-like molecules EMR2, CD97 and CD64 could serve as biomarkers in the clinical stratification of patients with sepsis and those with non-infectious SIRS. Accordingly, we undertook flow cytometric analysis of neutrophils in whole blood samples from patients with a clinical suspicion of sepsis. Associations were sought between these potential biomarkers and laboratory indices of infection and inflammation and also with measurements of disease activity and severity.
Materials and methods
Patients and controls
The study included 103 patients with SIRS 22 and 50 healthy control subjects. Blood samples were taken within 72 h of ICU admission and Acute Physiology and Chronic Health Evaluation (Apache II) score, neutrophil counts and CRP levels recorded. An additional six patients were included in a longitudinal analysis where changes in the distribution of subsets of neutrophils were related to the sequential organ failure assessment scores (SOFA). Patients were categorized retrospectively into those with sepsis and those with non-infectious SIRS by a microbiologist and an intensivist, neither of whom had knowledge of the neutrophil results. Clinical and laboratory details of the patients are shown in Table1a and characterization of patients with sepsis in Table1b. Of the 83 patients with sepsis, 57 had positive microbiological cultures from a sterile (e.g. blood) or non-sterile site (e.g. bronchoalveolar lavage) and 26 patients presented a strong clinical suspicion with a suspected bacterial focus 22. Twenty patients were defined as non-infectious SIRS (neither clinical suspicion nor microbiological evidence of infection). The study protocol received ethical approval from the local hospital ethics committee and next of kin provided informed consent for patient inclusion into the study.
Table 1.
Demographic data of patients studied
| (a) | Patients with SIRS | Healthy control subjects |
|---|---|---|
| n = 103 | n = 50 | |
| Age (years) | 57 ± 16 | 36 ± 10 |
| Male (%) | 60% | 40% |
| WBC × 109l | 18.3 ± 10.8 | 6.8 ± 2.4 |
| PMNs × 109l | 15.9 ± 11.4 | 4.1 ± 1.9 |
| Platelets × 109/l | 203 ± 129 | NT |
| CRP (μg/ml) | 152 ± 87 | < 1 |
| Survival after 28 days | 74% | n.a. |
| Apache II score | 18 ± 5 | n.a. |
| (b) Categorization of patients with sepsis (n = 83) | |||
|---|---|---|---|
| Microbiological confirmation | Clinical suspicion | ||
| Gram-negative (n = 23) | Gram-positive (n = 34) | n = 26 | |
| Bacterioides fragilis | Streptococcus pyogenes | ||
| Escherichia coli | Enterococcus sp. | ||
| Klebsiella sp. | Staphylococcus aureus (MRSA) | ||
| Morgonella sp. | Staphylococcus aureus | ||
| Serratia marcescens | Staphylococcus epidermidis | ||
| Pseudomonas aeruginosa | Streptococcus pneumonia | ||
Results are expressed as means ± standard deviation; n.a. = not applicable; NT = not tested; MRSA = methicillin-resistant Staphylococcus aureus; APACHE = Acute Physiology and Chronic Health Evaluation.
Flow cytometric analysis of neutrophil surface molecules
For each analysis, peripheral blood was collected first in a precooled K3-ethylenediamine tetraacetic acid (EDTA) VacutainerTM (Becton Dickinson, Oxford, UK) before transfer into citrate-coated Vacutainer tubes (CTADTM), according to a protocol that prevents modification of adhesion molecule expression 23. All samples were maintained at 4°C. Whole blood was incubated in the dark for 20 min with 2% mouse serum and 0·1 μg/ml saturated methanolic LDS-751 (Molecular Probes, Eugene, OR, USA) nuclear stain to prevent recording of anuclear cells. Sample aliquots (5 μl) were added to 100 μl Hanks's balanced salt solution (HBSS), Ca2+/Mg2+free (Bio-Whittaker) and incubated for 20 min with 0·1 μg/ml fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies against CD11a, CD11b, CD62L, CD66b (Beckman Coulter, High Wycombe, UK), CD11c (Dako, Glostrup, Denmark) and CD64 (Becton Dickinson). Conjugated mouse immunoglobulin (Ig)G1 and IgG2a immunoglobulins served as controls. Antibodies against CD97 and EMR2 were a generous gift from J. Hamann (AMC University of Amsterdam). Aliquots were washed twice with HBSS and diluted with 500 μl IsotonTM sheath fluid (Beckman Coulter). Samples were analysed in a Beckman Coulter EPICS-XL-MCLTM flow cytometer equipped with System IITM software. Neutrophils were identified by their characteristic forward- and side-scatter distributions (Fig. 1a) and by staining with anti-CD66b antibodies (Fig. 1b). The cursor was set so that fewer than 1% of the cells in the negative control sampled stained positively with the isotype control antibody. Compensations were applied to correct for LDS-751 emissions entering the FL1 channel. Results, generated within 3 h of blood collection, were presented as either the percentage of cells bearing surface molecules or as the mean fluorescence intensity (MFI) and displayed in single-parameter histograms of FL-1 (FITC) channel fluorescence (log10 scale). Examples of neutrophils expressing CD11c, EMR2 and CD64 are shown in Fig. 1c–e.
Figure 1.
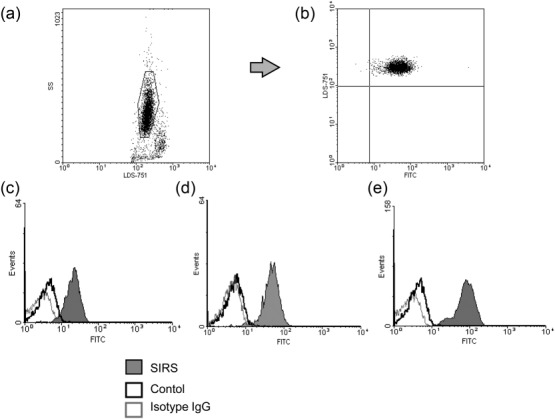
Identification of neutrophils stained by antibodies against CD11c, CD64 and epidermal growth factor (EGF)-like molecule containing mucin-like hormone receptor (EMR2). Flow cytometric analysis identified neutrophils in whole blood samples according to (a) their characteristic granularity (side-scatter), nuclear staining (positive for LDS-751) and (b) staining with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody against the neutrophil marker CD66b. Profiles of neutrophils from patients with systemic inflammatory response syndrome (SIRS) (shaded histograms) and healthy control subjects (open histograms) stained with antibodies against (c) CD11c, (d) CD64 and (e) EMR2 (dark lines) and isotype control antibodies (light lines).
The effect of cell processing on neutrophil adhesion molecule expression
To investigate whether a procedure for the fixation of neutrophils altered the expression of CD11b and CD62L, blood samples from an additional nine healthy subjects and nine patients with SIRS (six with sepsis) were subjected to erythrocyte lysis and fixation with paraformaldehyde (TQ-PREPTM; Beckman Coulter). Samples were processed before labelling with CD11b and CD62L monoclonal antibodies (prefixation) or after labelling (post-fixation). Flow cytometric analysis was undertaken 3 and 24 h later (samples held at 4°C and protected from light) and results compared with those of unfixed cells that were analysed immediately after antibody labelling.
Statistical analysis
Where data fitted a Gaussian distribution, Student's unpaired t-test was used and results presented as the mean ± standard deviation (s.d.). Non-parametric data were analysed with the Mann–Whitney U-test and presented as mean ± standard error of the mean (s.e.m.). For the assessment of associations between surface molecule expression and clinical and laboratory parameters, Pearson's product–moment correlation coefficient was calculated and tested for significance (two-tailed) using GraphPad Prism™ version 5·01 and Minitab™ version 10·51 statistical software and reference tables.
Results
Neutrophils expressing CD11c, EMR2 and CD64 as biomarkers of sepsis and non-infectious SIRS
Figure 2a shows that, in comparison with healthy control subjects, the patients with SIRS had an increased percentage of neutrophils bearing CD11c (mean 61 ± 25% versus mean 41 ± 28% controls; P < 0·001), EMR2 (mean 37 ± 23% versus mean 15 ± 9%; P < 0·001) and CD64 (mean 39 ± 30% versus mean 4 ± 7%; P < 0·001). In contrast, it is apparent from Fig. 2b that there was no difference between patients and healthy subjects in the prevalence of neutrophils bearing CD62L (mean 89 ± 16% in SIRS versus mean 92 ± 14% for controls) and CD97 (mean 58 ± 25% versus mean 46 ± 26%). Because CD11a and CD11b are expressed constitutively on neutrophils their results are presented as the MFI; both groups of subjects had similar expression of these molecules.
Figure 2.
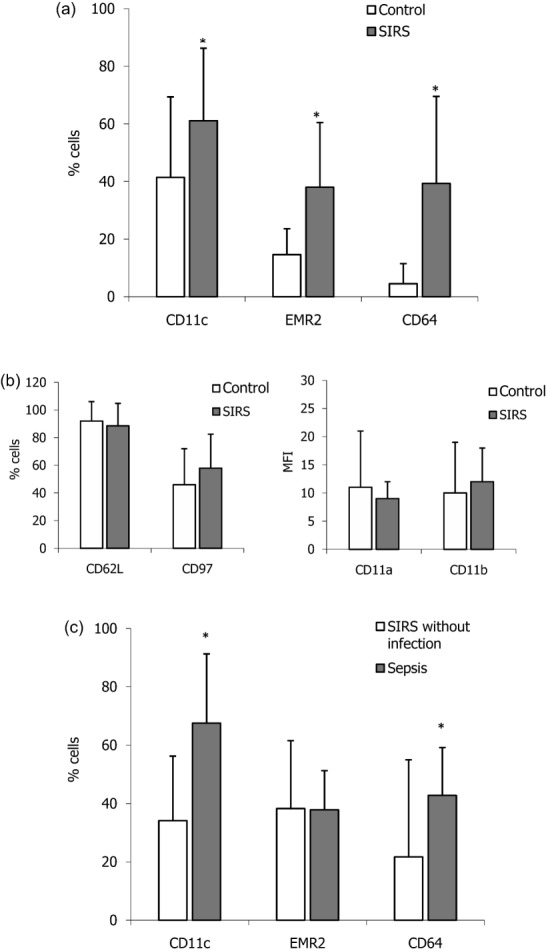
Increased prevalence of neutrophils expressing CD11c, epidermal growth factor (EGF)-like molecule containing mucin-like hormone receptor (EMR2) and CD64 in patients with the systemic inflammatory response syndrome (SIRS) and sepsis. Results are expressed as either the mean percentage of neutrophils ± standard deviation (s.d.) or as the mean fluorescence intensity (MFI) ± s.d. In (a) the percentage of neutrophils bearing CD11c, EMR2 and CD64 was higher in 103 patients with SIRS when compared with 50 healthy control subjects. *P < 0·001. (b) The percentage of neutrophils bearing CD62L and CD97 and the MFIs of CD11a and CD11b were similar for both groups of subjects. (c) When the patients with SIRS were categorized into those without infection (n = 20) and those with sepsis (n = 83), the prevalence of neutrophils bearing CD11c or CD64 was greater in the sepsis group when compared with patients without infection. *P < 0·001.
To ascertain if increases in the prevalence of neutrophils bearing CD11c, EMR2 and CD64 were a consequence of bacterial infection or of systemic inflammation the patients were differentiated retrospectively into those with sepsis and those with non-infectious SIRS. Figure 2c shows that in comparison with SIRS without infection, patients with sepsis had an increased percentage of neutrophils expressing CD11c (mean 68 ± 23% versus 34 ± 22%; P < 0·001) and CD64 (mean 43 ± 17% versus 28 ± 33%; P < 0·01). As the mean values for CD64+ neutrophils in the sepsis and non-sepsis patients were far higher than control levels, this strengthens the view that CD64 is a marker of bacterial infection and of SIRS. Neither CD11c nor CD64 discriminated between different organisms or groupings of bacteria. The observation that the percentage of neutrophils expressing EMR2 was similar in patients with sepsis (mean 38 ± 14%) and with non-infectious SIRS patients without infection (mean 37 ± 23%) suggests that elevated levels of EMR2-bearing neutrophils are a feature of systemic inflammation rather than of bacterial infection.
To determine the diagnostic accuracy of neutrophils expressing CD11c and CD64 in differentiating patients with sepsis from those with non-infective SIRS, we performed receiver operator characteristic (ROC) analysis. Figure 3 shows that the optimal cut-off point of 49% returned a sensitivity of 81% [95% confidence intervals (CI) = 69·2–87·6] and a specificity of 80% (CI = 68·3–98·8) for the diagnosis of sepsis. Applying ROC analysis to neutrophils expressing CD64 generated a sensitivity of 51% (CI = 39·7–62·8) and a specificity of 83% (CI = 61·2–95·1). We also included plasma CRP levels in this assessment, and found that they provided the lowest diagnostic accuracy with a sensitivity of 55% (CI = 42·8–65·9) and specificity of 67% (CI = 41·0–86·7) for the identification of sepsis.
Figure 3.
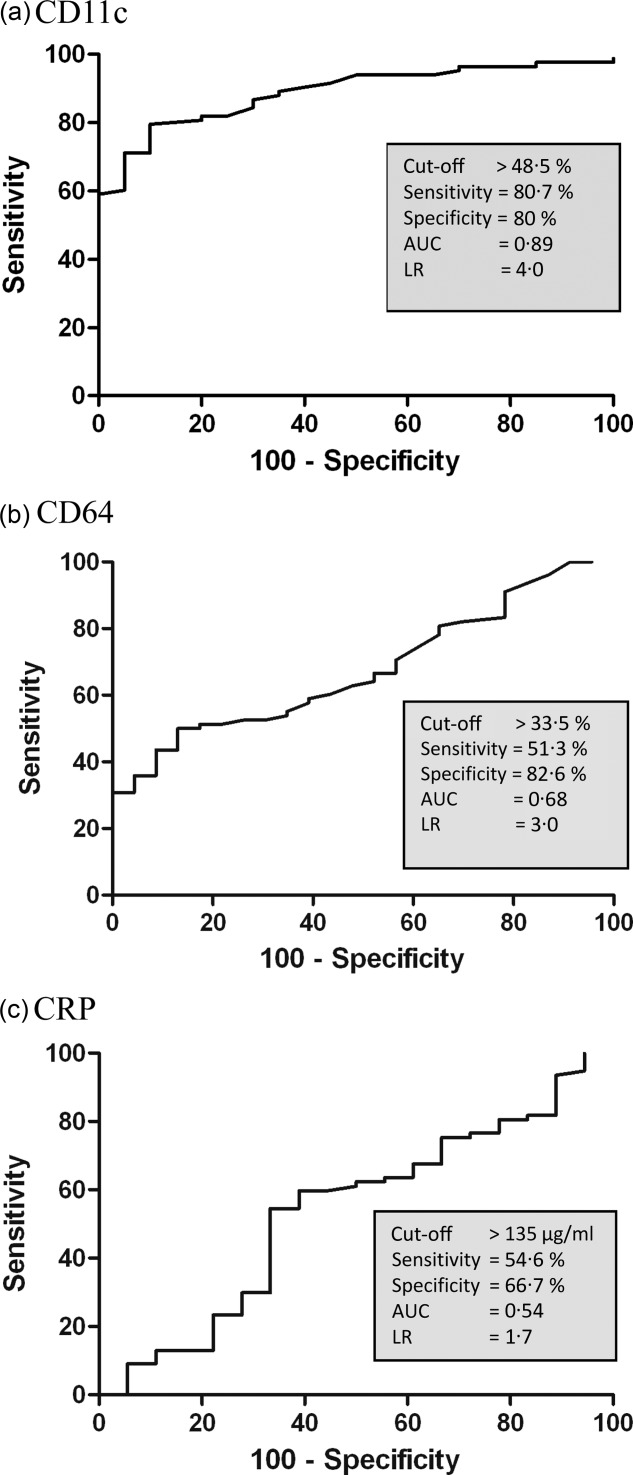
Diagnostic accuracy of neutrophils expressing CD11c, CD64 and of plasma C-reactive protein (CRP) levels for sepsis. Receiver operator characteristic (ROC) curves are presented for (a) CD11c+ neutrophils (b) CD64+ neutrophils and (c) plasma CRP levels. In differentiating patients with sepsis from those with N-I systemic inflammatory response syndrome (SIRS), sepsis was defined as either positive microbiology or a strong clinical suspicion. Sensitivity and specificity values are shown together with optimal cut-off points, the areas under curve (AUC) and likelihood ratios (LR).
Associations between CD11c, CD64 and EMR2 and laboratory indices of inflammation and infection, disease activity and severity
Increased numbers of blood neutrophils and high concentrations of circulating CRP (> 5 µg) are considered to be indices of inflammation and infection and both were raised frequently in the patients investigated. Figure 4 shows a weak association between the percentage of CD64+ neutrophils and plasma levels of CRP (r = 0·37; P < 0·01), but no further relationships were noted between CD11c, CD64 and EMR2 and the concentrations of CRP and neutrophil counts. Levels of CRP were unrelated to the numbers of neutrophils and the percentages of neutrophils bearing CD11c, CD64 and EMR2 were not associated with the Apache II score (data not shown).
Figure 4.
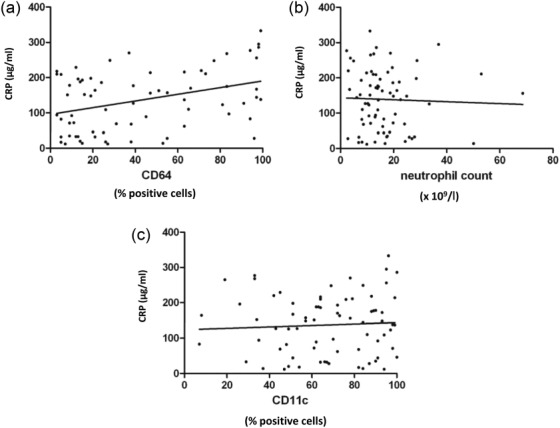
Relationship between the distribution of neutrophils expressing CD11c, CD64 with C-reactive protein (CRP) levels and blood neutrophil numbers. There was a positive association between (a) the % CD64+ neutrophils and plasma CRP levels (r = 0·37; P < 0·01), but there were no relationships between (b) the % CD11c+ neutrophils and concentrations of CRP or between (c) the number of blood neutrophils and CRP levels.
A sequential study was undertaken to determine if changes in the levels of neutrophils expressing EMR2, CD64 and CD11c were associated with organ failure (SOFA score). Serial blood samples were obtained from six patients with sepsis for up to 2 weeks after ICU entry. Towards the end of the study two patients had a high SOFA with more than 60% of the neutrophils expressing EMR2 (Fig. 5a,b), and both died within 2 days of provision of the last blood samples. For another patient (Fig. 5c), a relatively stable SOFA score corresponded with low percentages of EMR2+ neutrophils. The other three patients had increased levels of EMR2+ neutrophils and high SOFA scores at ICU entry (Fig. 5d–f), but thereafter these values declined and the patients were discharged onto general hospital wards within a few days of the final blood analysis. The percentage of neutrophils expressing EMR2 was related to the SOFA score (Pearson's correlation coefficient = 0·396; P < 0·05), but similar associations did not occur with neutrophils bearing CD11c and CD64.
Figure 5.
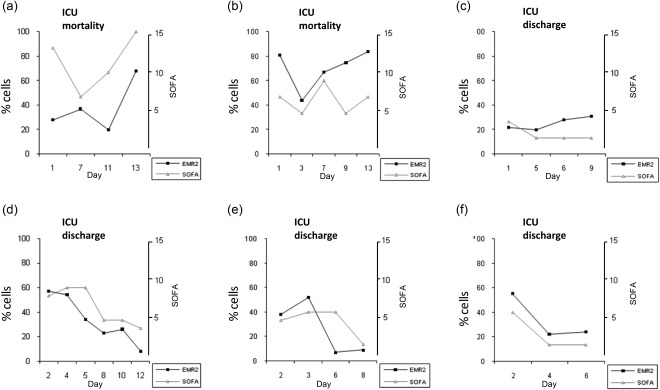
Changes in the distribution of neutrophils expressing epidermal growth factor (EGF)-like molecule containing mucin-like hormone receptor (EMR2) correspond to the extent of organ dysfunction [sequential organ failure assessment (SOFA) score]. The distribution of neutrophils expressing EMR2 in six patients with sepsis (a–f) was examined sequentially for up to 2 weeks following entry into the intensive care unit (ICU) and related to the SOFA score, assessed on the day of blood sample collection. There was a direct correlation between the percentage of neutrophils bearing EMR2 and SOFA (r = 0·396; P < 0·05).
Expression of CD11b and CD62L on neutrophils from patients with SIRS is modified by neutrophil fixation procedures
The observation that CD11b and CD62L were not expressed abnormally on neutrophils from patients with SIRS differs from other reports stating that they are up- and down-regulated, respectively 10–13. In past investigations, neutrophils in whole blood samples were often fixed in paraformaldehyde by a procedure that included erythrocyte lysis, either prior to or after antibody labelling, and stored for up to 48 h before flow cytometric analysis 11,13. Our results were generated within 3 h of collection of whole blood samples using a staining protocol that did not include fixation and erythrocyte lysis. To determine if fixation altered the expression of CD11b and CD62L, we applied a commercially available fixation procedure to whole blood samples from nine patients with SIRS (six of whom had sepsis) and nine healthy control subjects. Figure 6a shows that pre- or post-fixation of the patients’ neutrophils increased CD11b expression in samples stored for 3 or 24 h. Neither procedure modified the expression of CD11b on neutrophils from healthy subjects. Pre- but not post-fixation labelling also led to a reduction in the percentage of patient neutrophils bearing CD62L that were stored for 3 or 24 h and in control samples kept for 24 h (Fig. 6b). These findings show that CD11b and CD62L expression on neutrophils from patients with SIRS are susceptible to modification following erythrocyte lysis and paraformaldehyde fixation.
Figure 6.
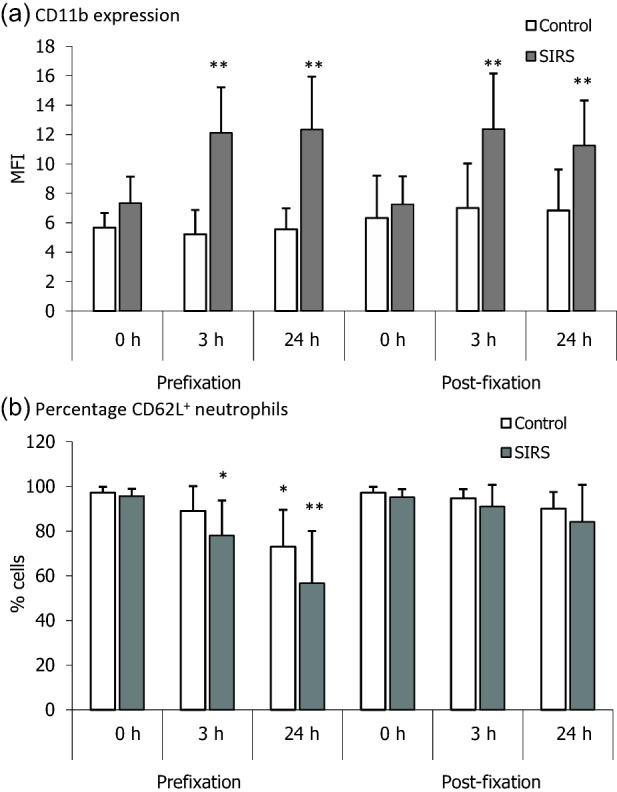
Fixation of neutrophils from patients with systemic inflammatory response syndrome (SIRS) increases CD11b expression and down-regulates CD62L. Neutrophils in whole blood samples from nine healthy control subjects and nine patients with SIRS (six with sepsis) were fixed with paraformaldehyde either before (pre-fixation) or after (post-fixation) labelling with anti-CD11b and anti-CD62L antibodies, respectively. Samples were examined by flow cytometric analysis after storage at 4ºC for the times shown. Results are expressed as either the mean fluorescence intensity (MFI) of CD11b or the percentage of neutrophils bearing CD62L. Vertical bars denote standard deviations (s.d.). (a) The expression of CD11b on neutrophils from patients with SIRS, but not those from healthy subjects, was increased by the pre- and post-fixation procedure. (b) A decrease in the percentage of CD62L+ neutrophils was associated mainly with SIRS patients. *P < 0·01 and **P < 0·001 compared with unfixed cells, which were analysed immediately after labelling.
Discussion
A major challenge in critical care medicine is to differentiate patients with sepsis from those with non-infectious SIRS in order to allow a more discriminative approach to clinical management. In this study we found that an increase in the prevalence of neutrophils bearing the biomarkers CD11c and CD64 were associated predominantly with sepsis, whereas elevated levels of neutrophils expressing EMR2 were a feature of systemic inflammation and poor patient outcome. The markers CD11a, CD11b, CD62L and CD97 did not aid patient classification. For the identification of sepsis, neutrophils expressing CD11c had a sensitivity and specificity of 80%. The diagnostic accuracy for neutrophil CD64 was lower than that reported by others 6,24–27, but broadly agrees with a recent report 28. Possible reasons for these differences include the methodology for flow cytometric expression and patient cohort selection. With regard to the latter consideration, the current investigation compared sepsis patients (i.e. SIRS and infection) with those who had non-infective SIRS, whereas one study compared patients with sepsis with all non-sepsis patients admitted to the ICU 6. In the current investigation, measurements of CRP provided the poorest discrimination of systemic inflammation from sepsis, which is in agreement with earlier published reports of the limited performance of CRP as a diagnostic test for sepsis 29,30.
The finding that an increased incidence of neutrophils bearing CD11c in sepsis did not correspond with an up-regulation of CD11b was unexpected, as both molecules are translocated from cytoplasmic vesicles to the cell surface within minutes of neutrophil activation 9. However, a similar independent up-regulation of CD11c was seen in neutrophils from diabetic patients with vascular complications 31, and low concentrations of bacterial products are reported to increase CD11c expression 32,33. Elevated levels of neutrophils expressing CD11c could enhance microvascular sequestration and infiltration into inflammatory sites and increase the potential for cell-mediated damage 34. Ligands for CD11c include fibrinogen and the complement breakdown product C3bi, both of which induce cell activation 9, together with CD54 17, whose expression on endothelial cells is up-regulated by inflammatory factors. Binding of neutrophils to fibrinogen via CD11c is enhanced following stimulation by cytokines [e.g. tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6] or chemoattractants such as C5a and IL-8, all of which are elevated in the circulation of patients with sepsis 35,36. Cross-linking of CD11c produces a chloride ion efflux 37 and engagement with platelet-expressed fibrinogen generates an oxidative burst 38, which is in accord with the increased oxidative activity of blood neutrophils in SIRS 39.
Both EMR2 and CD97 are members of the EGF-TM7 family, which are expressed on myeloid cells 40. We noted that neutrophils bearing EMR2 were more prevalent in patients with SIRS than in healthy individuals, whereas CD97 was distributed similarly in both groups of subjects. That neutrophils bearing EMR2 were not increased further in patients with sepsis suggests that this molecule is a biomarker of systemic inflammation. In our sequential study of a small group of patients with sepsis, the percentage of neutrophils expressing EMR2 was associated with the SOFA score, the highest levels being recorded in two patients shortly before their demise from multiple organ failure. Thus, an expansion in the population of neutrophils bearing EMR2 may indicate disease progression and poor patient outcome, but further experiments are needed to substantiate this proposal. The ligand for EMR2 is chondroitin sulphate, an extracellular matrix component of blood vessel walls, which co-localizes with EMR2 at sites of leucocyte infiltration 41. We have demonstrated recently that ligation of EMR2 enhances neutrophil adhesion to endothelium, chemotaxis and degranulation of proteolytic enzymes 21. As EMR2 signalling also enhances the release of proinflammatory cytokines from lipopolysaccharide (LPS)-primed neutrophils 42,43, activation of EMR2+ blood neutrophils in patients with sepsis could exacerbate tissue insult. We are currently investigating the prognostic implications of neutrophils expressing EMR2 by undertaking a longitudinal study of a large cohort of ICU patients.
The observation that levels of CD64+ neutrophils were increased in SIRS and elevated further in sepsis supports and extends our earlier observations and those of others 15. Neutrophils expressing CD64 are highly adherent to endothelium 14, and signalling through CD64 results in the extracellular release of several inflammatory factors 44. Reports of elevated levels of CD64+ neutrophils in the joints of patients with rheumatoid arthritis 45, in the circulation of sickle cell patients during crisis 16 and in the blood of patients with bacterial infections 46,47 furthers the view that CD64 is a marker of both inflammation and infection. The percentage of neutrophils bearing CD64 was associated with the levels of circulating CRP, which are also increased in SIRS induced by infectious and non-infectious stimuli. There were no additional relationships between neutrophil biomarkers and circulating neutrophil numbers, CRP concentrations or disease activity as measured by Apache II score (data not shown). Levels of CRP and numbers of blood neutrophils were not indicative of bacterial infection.
The current demonstration, that blood neutrophils from SIRS patients had a normal expression of CD11b and CD62L, contrasts with earlier studies which reported an up-regulation of CD11b and a down-regulation of CD62L, both of which are synonymous with cell activation 10–13. Differences in experimental protocols may account for these discrepancies, as illustrated by CD11b and CD62L being increased and decreased, respectively, by neutrophil isolation and erythrocyte lysis 48 and by changes in temperature and the choice of anti-coagulant 23. Earlier investigations of neutrophils in patients with SIRS/sepsis often used paraformaldehyde fixation of the cells and lysis of erythrocytes prior to or following antibody labelling. Thereafter, the fixed neutrophils were stored for up to 48 h before analysis by flow cytometry. Our analysis of whole blood samples did not involve fixation of the neutrophils or erythrocyte lysis. We noted that prefixation of neutrophils (i.e. prior to antibody staining) from SIRS patients, but not those from normal subjects, increased the expression of CD11b and decreased that of CD62L. This alteration of CD11b and CD62L expression may be due to neutrophil priming by inflammatory factors in the circulation of SIRS patients 35, so that when these neutrophils are subjected to the physical and chemical procedures of cell fixation the expression of CD11b and CD62L changes due to the lower threshold stimulus required for cell activation. Supporting this proposal is the observation that antibody labelling procedures which incorporate erythrocyte lysis augment the expression of CD11b on neutrophils in paediatric patients with infections 49. We therefore propose that an accurate assessment of the expression of integrins and selectins on neutrophils in SIRS requires a protocol that is independent of cell fixation.
In the search for novel biomarkers of sepsis and of SIRS the present study provides CD11c and EMR2 as two potential new candidates that could be used in conjunction with other markers to improve patient stratification 6. Neutrophils expressing high levels of CD11c were a feature of sepsis and had a greater diagnostic accuracy for sepsis than neutrophils bearing CD64. An increased prevalence of neutrophils expressing EMR2 was associated with systemic inflammation and with organ failure. The latter observation may have prognostic implications, and supports the emerging view that there are distinct populations of neutrophils with discrete functional characteristics. For example, it is proposed that there are two forms of tumour-associated neutrophils, one which has immunostimulatory activity and another which possesses immunosuppressive properties 50. Moreover, in systemic lupus erythematosus there is a low-density subset of neutrophils that are regarded as proinflammatory due to their high expression of type 1 interferons (IFNs), TNF-α and IFN-γ, whereas patients with systemic inflammation have a subpopulation of neutrophils that suppress T cell responses 51,52.
Acknowledgments
This work was supported by the British Heart Foundation, The Henry Smith Charity and the Guy's and St Thomas’ Charity.
Author contributions
S. M. L. undertook the experimental analysis of neutrophils, contributed to the experimental design and was involved with the writing and assembly of the manuscript. G. M. performed the flow cytometric analysis of fixed samples. D. F. T. and J. E. were responsible for the enrolment of patients into the study and for the retrospective characterization of patients with sepsis and with non-infective SIRS. C. S. B. participated in the acquisition and analysis of data and its presentation. T. A. M. performed an extensive review of the literature and was involved actively in the drafting and revision of the manuscript. M. S. contributed to the planning of the work, data interpretation and formulating the manuscript. R. B. contributed to the writing and revision of the manuscript. K. A. B. was involved in formulating the hypothesis, devising the experimental design and in the writing of the manuscript.
Disclosure
R.B's Department has received government grants to study the behaviour of neutrophils in sepsis to support this research work. S.M.L. and K.A.B. are co-inventors of patent no.US#8,476,028 and EP 1984733B1 which are owned by King's College London.
References
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J-L, Opal SO, Marshall JC, Tracey KJ. Sepsis definitions, time for a change. Lancet. 2013;381:774–5. doi: 10.1016/S0140-6736(12)61815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson RL, Webster NR. Sepsis and the systemic inflammatory response syndrome. J R Coll Surg Edinb. 2000;45:178–82. [PubMed] [Google Scholar]
- Su L, Han B, Liu C, et al. Value of soluble TREM-1, procalcitonin, and C-reactive protein serum levels as biomarkers for detecting bacteremia among sepsis patients with new fever in intensive care units: a prospective cohort study. BMC Infect Dis. 2012;12:157. doi: 10.1186/1471-2334-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibot S, Béné MC, Noel R, et al. Combination biomarkers to diagnose sepsis in the critically ill patient. Am J Respir Crit Care Med. 2012;186:65–71. doi: 10.1164/rccm.201201-0037OC. [DOI] [PubMed] [Google Scholar]
- Bloos F. Clinical diagnosis of sepsis and the combined use of biomarkers and culture- and non-culture-based assays. Methods Mol Biol. 2015;1237:247–60. doi: 10.1007/978-1-4939-1776-1_19. [DOI] [PubMed] [Google Scholar]
- Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368:157–69. doi: 10.1016/S0140-6736(06)69005-3. [DOI] [PubMed] [Google Scholar]
- Langer HF, Chavakis T. Leukocyte-Endothelial Interactions in Inflammation. J Cell Mol Med. 2009;13:1211–20. doi: 10.1111/j.1582-4934.2009.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RY, Astiz ME, Saxon JC, Rackow EC. Altered leukocyte immunophenotypes in septic shock. Studies of HLA-DR, CD11b, CD14, and IL-2R expression. Chest. 1993;104:847–53. doi: 10.1378/chest.104.3.847. [DOI] [PubMed] [Google Scholar]
- Rosenbloom AJ, Pinsky MR, Napolitano C, et al. Suppression of cytokine-mediated beta2 -integrin activation on circulating neutrophils in critically ill patients. J Leukoc Biol. 1999;66:83–9. doi: 10.1002/jlb.66.1.83. [DOI] [PubMed] [Google Scholar]
- Muller Kobold AC, Tulleken JE, Zijlstra JG, et al. Leukocyte activation in sepsis; correlations with disease state and mortality. Intensive Care Med. 2000;26:883–92. doi: 10.1007/s001340051277. [DOI] [PubMed] [Google Scholar]
- Chishti AD, Shenton BK, Kirby JA, Baudouin SV. Neutrophil chemotaxis and receptor expression in clinical septic shock. Intensive Care Med. 2004;30:605–11. doi: 10.1007/s00134-004-2175-y. [DOI] [PubMed] [Google Scholar]
- Tansho-Nagakawa S, Ubagai T, Kikuchi-Ueda T, Koshio O, Koshibu Y. Kikuchi H. Analysis of membrane antigens on neutrophils from patients with sepsis. J Infect Chemother. 2012;18:646–56. doi: 10.1007/s10156-012-0386-7. [DOI] [PubMed] [Google Scholar]
- Qureshi SS, Lewis SM, Gant VA, Treacher D, Davis BH, Brown KA. Increased distribution and expression of CD64 on blood polymorphonuclear cells from patients with the systemic inflammatory response syndrome (SIRS) Clin Exp Immunol. 2001;125:258–65. doi: 10.1046/j.1365-2249.2001.01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadlon E, Vordermeier S, Pearson TC, et al. Blood polymorphonuclear leukocytes from the majority of sickle cell patients in the crisis phase of the disease show enhanced adhesion to vascular endothelium and increased expression of CD64. Blood. 1998;91:266–74. [PubMed] [Google Scholar]
- Frick C, Odermatt A, Zen K, et al. Interaction of ICAM-1 with beta 2-integrin CD11c/CD18: characterization of a peptide ligand that mimics a putative binding site on domain D4 of ICAM-1. Eur J Immunol. 2005;35:3610–21. doi: 10.1002/eji.200425914. [DOI] [PubMed] [Google Scholar]
- Bjarnadóttir TK, Fredriksson R, Höglund PJ, Gloriam DE, Lagerström MC, Schiöth HB. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics. 2004;84:23–33. doi: 10.1016/j.ygeno.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kwakkenbos MJ, Kop EN, Stacey M, et al. The EGF-TM7 family: a postgenomic view. Immunogenetics. 2004;55:655–66. doi: 10.1007/s00251-003-0625-2. [DOI] [PubMed] [Google Scholar]
- Stacey M, Chang G-W, Davies JQ, et al. The epidermal growth factor-like domains of the human EMR2 receptor mediate cell attachment through chondroitin sulfate glycosaminoglycans. Blood. 2003;102:2916–24. doi: 10.1182/blood-2002-11-3540. [DOI] [PubMed] [Google Scholar]
- Yona S, Lin H-H, Dri P, et al. Ligation of the adhesion-GPCR EMR2 regulates human neutrophil function. Faseb J. 2008;22:741–51. doi: 10.1096/fj.07-9435com. [DOI] [PubMed] [Google Scholar]
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- Macey M, McCarthy D, Azam U, Milne T, Golledge P, Newland A. Ethylenediamine tetraacetic acid plus citrate-theophylline-adenosine-dipyridamole (EDTA-CTAD): a novel anticoagulant for the flow cytometric assessment of platelet and neutrophil activation ex vivo in whole blood. Cytometry. 2003;51:30–40. doi: 10.1002/cyto.b.10001. [DOI] [PubMed] [Google Scholar]
- Icardi M, Erickson Y, Kilborn S, Stewart B, Grief B, Scharnweber G. CD64 index provides simple and predictive testing for detection and monitoring of sepsis and bacterial infection in hospital patients. J Clin Microbiol. 2009;47:3914–9. doi: 10.1128/JCM.00628-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KH, Chan MC, Wang JM, Lin LY, Wu CL. Comparison of Fcγ receptor expression on neutrophils with procalcitonin for the diagnosis of sepsis in critically ill patients. Respirology. 2011;16:152–60. doi: 10.1111/j.1440-1843.2010.01876.x. [DOI] [PubMed] [Google Scholar]
- Rogina P, Stubljar D, Lejko-Zupanc T, Osredkar J, Skvarc M. Expression of CD64 on neutrophils (CD64 index): diagnostic accuracy of CD64 index to predict sepsis in critically ill patients. Clin Chem Lab Med. 2015;53:e89–91. doi: 10.1515/cclm-2014-0814. [DOI] [PubMed] [Google Scholar]
- Stubljar D, Skvarc M. Expression of CD64 on neutrophils can be used to predict the severity of bloodstream infection before broad range 16S rRNA PCR. Folia Microbiol. 2014;60:111–8. doi: 10.1007/s12223-014-0346-y. [DOI] [PubMed] [Google Scholar]
- Gros A, Roussel M, Sauvadet E, et al. The sensitivity of neutrophil CD64 expression as a biomarker of bacterial infection is low in critically ill patients. Intensive Care Med. 2012;38:445–52. doi: 10.1007/s00134-012-2483-6. [DOI] [PubMed] [Google Scholar]
- Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206–17. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- Ten OJ, Tromp M, Bleeker-Rovers CP, et al. Combination of biomarkers for the discrimination between bacterial and viral lower respiratory tract infections. J Infect. 2012;13:6–11. doi: 10.1016/j.jinf.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Caimi G, Montana M, Citarrella R, Porretto F, Catania A, Lo Presti R. Polymorphonuclear leukocyte integrin profile in diabetes mellitus. Clin Hemorheol Microcirc. 2002;27:83–9. [PubMed] [Google Scholar]
- Hansen PS, Madsen PH, Petersen SB, Nielsen H. Inflammatory activation of neutrophils by Helicobacter pylori; a mechanism insensitive to pertussis toxin. Clin Exp Immunol. 2001;123:73–80. doi: 10.1046/j.1365-2249.2001.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Ambriz J, Rodríguez-Orozco AR, Béjar-Lozano C, Cortés-Rojo C. The increased expression of CD11c and CD103 molecules in the neutrophils of peripheral blood treated with a formula of bacterial ribosomes and proteoglycans of Klebsiella pneumoniae. Arch Bronconeumo. 2012;48:316–9. doi: 10.1016/j.arbres.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Pan W, Shi C, Tian M, Yu J. Anti-CD11c antibody, Efalizumab attenuates ventilator-induced lung injury. Eur Rev Med Pharmacol Sci. 2014;18:2182–90. [PubMed] [Google Scholar]
- Netea M, van der Meer JWM, van Deuren M, Kullberg BJ. Proinflammatory cytokines and sepsis syndrome: not enough, or too much of a good thing? Trends Immunol. 2003;24:254–8. doi: 10.1016/s1471-4906(03)00079-6. [DOI] [PubMed] [Google Scholar]
- Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4:133–42. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- Menegazzi R, Busetto S, Decleva E, Cramer R, Dri P, Patriarca P. Triggering of chloride ion efflux from human neutrophils as a novel function of leukocyte beta2 integrins: relationship with spreading and activation of the respiratory burst. J Immunol. 1999;162:423–34. [PubMed] [Google Scholar]
- Ruf A, Patscheke H. Platelet-induced neutrophil activation: platelet-expressed fibrinogen induces the oxidative burst in neutrophils by an interaction with CD11C/CD18. Br J Haematol. 1995;90:791–6. doi: 10.1111/j.1365-2141.1995.tb05197.x. [DOI] [PubMed] [Google Scholar]
- Nakamori Y, Koh T, Ogura H, et al. Enhanced expression of intranuclear NF-kappa B in primed polymorphonuclear leukocytes in systemic inflammatory response syndrome patients. J Trauma. 2003;54:253–60. doi: 10.1097/01.TA.0000037096.73714.E6. [DOI] [PubMed] [Google Scholar]
- Lin HH, Stacey M, Hamann J, Gordon S, McKnight AJ. Human EMR2, a novel EGF-TM7 molecule on chromosome 19p13.1, is closely related to CD97. Genomics. 2000;67:188–200. doi: 10.1006/geno.2000.6238. 15. [DOI] [PubMed] [Google Scholar]
- Kop EN, Kwakkenbos MJ, Teske GJD, et al. Identification of the epidermal growth factor-TM7 receptor EMR2 and its ligand dermatan sulfate in rheumatoid synovial tissue. Arthritis Rheum. 2005;52:442–50. doi: 10.1002/art.20788. [DOI] [PubMed] [Google Scholar]
- Chen T-Y, Hwang T-L, Lin C-Y, et al. EMR2 receptor ligation modulates cytokine secretion profiles and cell survival of lipopolysaccharide-treated neutrophils. Chang Gung Med J. 2011;34:468–77. [PubMed] [Google Scholar]
- Huang Y-S, Chiang N-Y, Hu C-H, et al. Activation of myeloid cell-specific adhesion class G protein-coupled receptor EMR2 via ligation-induced translocation and interaction of receptor subunits in lipid raft microdomains. Mol Cell Biol. 2012;32:1408–20. doi: 10.1128/MCB.06557-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Guyre PM, Fanger MW. Polymorphonuclear leukocyte function triggered through the high affinity Fc receptor for monomeric IgG. J Immunol. 1987;139:534–8. [PubMed] [Google Scholar]
- Quayle JA, Watson F, Bucknall RC, Edwards SW. Neutrophils from the synovial fluid of patients with rheumatoid arthritis express the high affinity immunoglobulin G receptor, Fc gamma RI (CD64): role of immune complexes and cytokines in induction of receptor expression. Immunol. 1997;91:266–73. doi: 10.1046/j.1365-2567.1997.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herra CM, Keane CT, Whelan A. Increased expression of Fc gamma receptors on neutrophils and monocytes may reflect ongoing bacterial infection. J Med Microbiol. 1996;44:135–40. doi: 10.1099/00222615-44-2-135. [DOI] [PubMed] [Google Scholar]
- Leino L, Sorvajärvi K, Katajisto J, et al. Febrile infection changes the expression of IgG Fc receptors and complement receptors in human neutrophils in vivo. Clin Exp Immunol. 1997;107:37–43. doi: 10.1046/j.1365-2249.1997.d01-899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey MG, McCarthy DA, Vordermeier S, Newland AC, Brown KA. Effects of cell purification methods on CD11b and L-selectin expression as well as the adherence and activation of leucocytes. J Immunol Methods. 1995;181:211–9. doi: 10.1016/0022-1759(95)00003-s. [DOI] [PubMed] [Google Scholar]
- Alvarez-Larrán A, Toll T, Rives S, Estella J. Assessment of neutrophil activation in whole blood by flow cytometry. Clin Lab Haematol. 2005;27:41–6. doi: 10.1111/j.1365-2257.2004.00661.x. [DOI] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil (TAN) phenotype by TGF-β: ‘N1’ versus ‘N2’ TAN. Cancer Cell. 2010;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny MF, Yalavartha S, Zhao W, Thakar SG, Anderson M. Sandy AR. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I interferons. J Immunol. 2010;184:3284–97. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay J, Kamp VM, van Hoffen E, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–35. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]


