Abstract
Noroviruses (NoV) are the most common cause of epidemic gastroenteritis worldwide. The acute immune response to NoV in humans is poorly understood, hindering research on prevention and treatment. To elucidate the acute immune response and test for cytokine predictors of susceptibility to infection, serum samples from two human NoV challenge studies were tested for 16 cytokines. Subjects who became infected (n = 26) were age-matched with subjects who remained uninfected following NoV challenge (n = 26). Samples were tested from prechallenge and days 1-4 post-challenge. Cytokine responses were compared between infected and uninfected groups. Overall, infected individuals exhibited an elevation in T helper type 1 (Th1) and Th2 cytokines, as well as chemokines interleukin (IL)-8 and monocyte chemoattractant protein (MCP-1), compared to uninfected individuals (all P < 0·05). Most cytokines peaked on day 2 post-challenge in infected subjects, and tumour necrosis factor (TNF)-α, IL-8, and IL-10 remained elevated to day 3. The only cytokine elevated significantly among infected subjects to day 4 post-challenge was IL-10 (P = 0·021). Prechallenge cytokine concentrations were not predictive of infection status post-challenge. There were no significant changes in serum cytokines among NoV-challenged subjects who remained uninfected. These results suggest that NoV infection elicits a Th1-type response, with some Th2 activation. Persistent elevation of IL-10 among infected subjects is consistent with activation of adaptive immune responses, such as B cell expansion, as well as down-regulation of Th1 cytokines. This study presents the first comprehensive description of the acute cytokine response to GI.1 NoV in humans.
Keywords: adaptive immunity, caliciviruses, innate immunity
Introduction
Noroviruses (NoV) comprise seven genogroups in the positive-sense RNA virus family Caliciviridae 1,2. NoV are responsible for 18% of gastroenteritis worldwide 3. Despite this broad impact, human NoV immunology is poorly understood 4,5, in part because until recently there was no small animal model or reliable cell culture system for human NoV 6,7. Furthermore, there are few prospective experimental studies on the acute human innate and cellular immune response to NoV.
The genetic determinants of NoV infection (e.g. secretor blood group antigens) are relatively well characterized, but many aspects of protective innate and cellular immunity have not been described 8,9. Histological studies 4,10–12 and in-vitro tests of peripheral blood mononuclear cells 13 suggest cytotoxic and CD8+ T cell activation with some cells sensitized to NoV challenge antigens, but little work has been conducted in vivo to describe the cytokine response to NoV infection. In particular, there is a gap in understanding the acute immune response to NoV. Prior studies have shown that adaptive immunity is critical for clearing the infection 14, but there is a gap in the knowledge about early pathogenesis of NoV infection. Key cytokines for consideration include acute response-related proinflammatory cytokines [e.g. interleukin (IL)-1, IL-6 and IL-12], chemokines involved in neutrophil and monocyte recruitment [e.g. IL-8 and major chemoattractant protein 1 (MCP)-1] and T helper type 1 (Th1)- and Th2-related cytokines [e.g. interferon (IFN)-γ, IL-2 and tumour necrosis factor (TNF)-α and IL-4, IL-5 and IL-10, respectively] involved in the cellular immune response and initiation of adaptive immune mechanisms.
The goal of this study was twofold. The first goal was to describe serum cytokine responses to NoV infection, including the temporal trends in serum cytokines during the acute phase of human NoV infection. The second goal was to use cytokine concentrations prechallenge to predict infection post-challenge. Human serum samples from two prior NoV challenge studies that used the same Norwalk virus (GI.1) inoculum were analysed for a broad panel of relevant cytokines 15,16. The results have important implications for determining the role of specific cytokines in NoV infection.
Materials and methods
Population and samples
The samples tested for this study were collected from subjects involved in two separate NoV challenge studies, described previously 15,16. However, these studies could use the same inoculum preparation, were conducted at the same institution and used very similar protocols. Key differences between these two challenge studies (i.e. inoculum dose) were accounted for in the modelling strategy of this study. Briefly, the first study enrolled healthy secretor-positive adult volunteers, who were challenged with Norwalk virus 8FIIb inoculum at Emory University Hospital's Clinical Interaction Site, part of the Atlanta Clinical and Translational Science Institute Clinical Interaction Network, between May 2006 and December 2006. Volunteers ingested filtered groundwater seeded artificially with 6·5 × 107 genomic equivalent copies (GEC) of NoV inoculum, which had been incubated at room temperature in the dark for different set lengths of time. Prior to challenge, serum and stool samples were collected. During the first 4 days post-challenge, serum samples were collected daily, as were all stool samples.
The second study enrolled healthy secretor-positive adult volunteers, who were also challenged with Norwalk virus 8FIIb inoculum at Emory University Hospital's Clinical Interaction Site between February 2008 and September 2009. They were randomized into control and intervention groups and administered oysters seeded with 1 × 104 GEC of virus, which had been treated with high hydrostatic pressure processing for 5 min (intervention) or left untreated (control). Prechallenge stool and serum samples were collected before challenge. During the first 4 days post-challenge serum samples were collected daily, as were all stool samples.
The Emory University Institutional Review Board approved both studies, and both were registered on Clinical Trials.gov (identifiers NCT00313404 and NCT00674336). All subjects consented to the future use of all biological specimens from the studies. All specimens were stored at −80°C.
Of the initial participants in both NoV challenge studies, a total of 26 became infected as defined by a NoV-positive stool or emesis sample tested by reverse transcription–polymerase chain reaction (RT–PCR) (limit of detection: 3570 GEC/g stool) 15,17. These 26 were pair-matched by age to 26 of the challenge study participants who remained uninfected following NoV challenge. Some infected subjects from one challenge group were matched with uninfected subjects from a different challenge group, because within the same group there were no uninfected individuals within 3 years of age of the infected individual. The total sample size for this study was 52 participants (13 from the 2006 trial, 39 from the 2008–09 trial). Because the focus of this study is the acute immune response, only serum samples from prechallenge and days 1–4 post-challenge were included, for a total of five longitudinal samples per subject.
Inoculum
The two NoV challenge studies included participants who ingested water or shellfish seeded with 8FIIb NoV inoculum. The water samples were seeded with identical amounts of NoV and stored at room temperature in the dark for varying lengths of time. They showed no evidence of titre attenuation over time, as measured by RT–PCR 15. Therefore, for these subjects, the regression models did not control for study arm. The shellfish samples were seeded with identical amounts of NoV and treated under varying processing conditions. The inocula showed some evidence of attenuation for some treatments. For subjects from this study, all adjusted models controlled for study arm as a surrogate for dose.
Detection of viral shedding
NoV presence was measured by RT–PCR for all stool and emesis samples at all time-points during the original challenge studies. A subject with a positive sample at any time-point, even after day 4 post-exposure, as determined in the original or subsequent studies, was defined as infected.
Detection of cytokines
Serum samples from prechallenge and days 1–4 post-challenge (five total samples) were analysed from all study subjects. Serum was collected during the studies, processed and stored at −80°C until testing. Samples were tested by a commercial laboratory (EMD Millipore Corporation Discovery and Development Solutions; Millipore Corporation, Billerica, MA, USA) using a Milliplex human cytokine 16-plex assay for IFN-α2, IFN-γ, IL-1a, IL-1b, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p40, IL-12p70, MCP-1, TNF-α, and TNF-β. Samples were run in duplicate with controls. Standard curves were generated using recombinant cytokines and calculated using a five-parameter logistic model for each cytokine. Lower limits of detection (LLOD) were 3·2 pg/ml for IFN-γ, IL-10, IL-12p70, IL-1b, IL-2, IL-5, IL-6, IL-8, MCP-1 and TNF-α; 16·0 pg/ml for TNF-β; and 80·0 pg/ml for IFN-α2, IL-12p40, IL-1ra, IL-1a and IL-4. Values below LLOD were assigned the value of the LLOD.
Statistical analysis
The data were analysed using sas version 9·4 (SAS Institute, Cary, NC, USA). When possible, age and inoculum were controlled for in adjusted analyses. Because the exact amount of infectious NoV in the inocula for study arms with attenuation was unknown, inoculum was controlled for using a categorical variable for each study and study arm. The Wilcoxon signed-rank test was to test for the significance of the unadjusted difference between pre- and post-challenge serum cytokine levels. Conditional logistic regression was used to evaluate the association between pre- and post-challenge fold changes in serum cytokine levels (i.e. ratio between pre- and post-challenge cytokine concentrations), controlling for inoculum and age. To summarize the differences between infected and uninfected individuals’ responses over time while accounting for correlation between time-points, a mixed linear model was used to test the association between log10 cytokine concentration and day post-challenge, stratified by infection status with a random effect by subject, autoregressive correlation between different time-points taken for the same individual and fixed effect for inoculum. The parameter estimates for the effect of each day on log10 cytokine concentration for each stratum were averaged and Student's t-test was used to compare infected and uninfected subjects’ cytokine responses over time. Conditional logistic regression adjusted for inoculum and age was also used to assess whether three measures of early cytokine levels or responses could be used as predictors of post-challenge infection. The measures assessed were prechallenge log10 cytokine concentration, prechallenge to day 1 post-challenge log10 cytokine change and prechallenge to day 1 post-challenge fold change. All cytokines were modelled individually. Differences between groups were considered statistically significant at P < 0·05.
Results
To determine the acute serum cytokine response to GI.1 NoV infection, serum cytokine responses in individuals who became infected following experimental challenge were compared to those who remained uninfected after challenge, as determined by RT–PCR detection of GI.1 NoV RNA in stool or emesis samples. Immune responses may differ with age, therefore the infected and uninfected subjects were matched for age (Table1). The study population was relatively young, with a mean age of 26·7 years and a median age of 25 years. There was also a similar distribution of sex and race between infected and uninfected subjects selected for the study, although neither was a matching factor. Some uninfected subjects exhibited symptoms, including fever and diarrhoea, which is consistent with past NoV challenge studies 16. All infected individuals had higher cumulative shedding than the inoculum dose (data not shown), reducing the likelihood of misclassification of infection status because of detection of inoculum post-challenge in the absence of infection. Serum samples were available for prechallenge to day 4 post-challenge (five time-points) and all samples were tested for 16 serum cytokines. Two subjects did not have samples for day 3, and values for these samples were imputed using the average of neighbouring observations for the same subject. A total of 10 subjects had invalid test results for one or more cytokine tested (29 total observations). Using imputation, all but one of these invalid results was estimated to be below the LLOD.
Table 1.
Characteristics of norovirus-challenged individuals included in analytical sample
| Total (n = 52) | Infected (n = 26) | Uninfected (n = 26) | |
|---|---|---|---|
| Age (mean, s.d.) | 26·7 (7·6) | 26·7 (7·5) | 26·7 (7·9) |
| Female | 32 (61·5%) | 17 (65·4%) | 15 (57·7%) |
| Race | |||
| White | 25 (48·1%) | 15 (57·7%) | 10 (38·5%) |
| Black | 21 (40·4%) | 8 (30·8%) | 13 (50·0%) |
| Other | 6 (11·5%) | 3 (11·5%) | 3 (11·5%) |
| AGE symptoms* | 19 (33·9%) | 17 (65·4%) | 2 (7·7%) |
| Modified Vesikari score (mean, s.d.) | 2·6 (2·4) | 4·1 (2·4) | 1·1 (0·9) |
AGE = acute gastroenteritis; s.d. = standard deviation.
AGE symptoms defined as diarrhoea (three or more or ≥ 400 g loose stools in 24 h) or emesis during days 1–4 post-challenge.
Studies of serum cytokine levels in humans often have high rates of observations below the LLOD 18. Overall of the 16 cytokines tested, nine had greater than 50% of observations above the LLOD, and four had greater than 75% of observations above the LLOD (Table2). In general, infected subjects had more samples above the LLOD.
Table 2.
Serum cytokine concentrations (pg/ml) of norovirus-challenged individuals pooled across days, stratified by infection status*
| All | Infected | Uninfected | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Median | IQR | % above LLOD | Median | IQR | % above LLOD | Median | IQR | % above LLOD |
| IFN-α2 | 80·0 | 80·0–80·0 | 24·8% | 80·0 | 80·0–133·0 | 27·9% | 80·0 | 80·0–80·0 | 21·7% |
| IFN-γ | 186·5 | 40·4–656·1 | 90·3% | 182·0 | 67·9–629·3 | 98·4% | 188·3 | 18·2–669·5 | 82·2% |
| IL-1ra | 80·0 | 80·0–999·3 | 54·3% | 80·0 | 80·0–389·1 | 73·6% | 80·0 | 80·0–1,993·9 | 34·9% |
| IL-1a | 163·4 | 80·0–433·2 | 24·7% | 179·8 | 80·0–433·2 | 28·5% | 80·2 | 80·0–433·55 | 21·1% |
| IL-1b | 3·2 | 3·2–10·3 | 58·9% | 3·3 | 3·2–18·45 | 70·2% | 3·2 | 3·2–7·2 | 48·1% |
| IL-2 | 4·6 | 3·2–30·1 | 47·8% | 7·5 | 3·2–38·2 | 48·0% | 3·6 | 3·2–17·6 | 47·6% |
| IL-4 | 80·0 | 80·0–99·9 | 54·7% | 80·0 | 80·0–170·9 | 59·4% | 80·0 | 80·0–80·0 | 50·0% |
| IL-5 | 3·2 | 3·2–3·9 | 38·4% | 3·2 | 3·2–6·0 | 48·1% | 3·2 | 3·2–3·2 | 28·7% |
| IL-6 | 23·9 | 3·2–120·2 | 52·5% | 27·8 | 3·2–93·0 | 55·5% | 21·4 | 3·2–161·1 | 49·6% |
| IL-8 | 66·5 | 20·7–198·1 | 25·4% | 81·7 | 34·2–198·0 | 28·9% | 45·0 | 11·5–202·4 | 21·9% |
| IL-10 | 4·4 | 3·2–29·5 | 26·8% | 17·0 | 3·2–50·3 | 33·9% | 3·2 | 3·2–6·2 | 19·7% |
| IL-12p40 | 80·0 | 80·0–80·0 | 70·7% | 80·0 | 80·0–85·0 | 69·5% | 80·0 | 80·0–80·0 | 71·9% |
| IL-12p70 | 6·1 | 3·2–89·8 | 92·2% | 19·5 | 3·2–86·6 | 96·9% | 3·2 | 3·2–96·0 | 87·6% |
| MCP-1 | 612·8 | 397·1–832·5 | 100·0% | 693·5 | 510·8–944·3 | 100·0% | 495·1 | 329·4–673·1 | 100·0% |
| TNF-α | 14·3 | 6·0–27·2 | 85·3% | 15·1 | 8·3–25·2 | 87·6% | 14·0 | 4·9–36·2 | 82·9% |
| TNF-β | 16·0 | 16·0–58·5 | 37·2% | 16·0 | 16·0–100·5 | 35·7% | 16·0 | 16·0–33·4 | 38·8% |
IQR = interquartile range; LLOD = lower limit of detection; IFN = interferon; IL = interleukin; MCP = monocyte chemoattractant protein; TNF = tumour necrosis factor.
Values below LLOD included in estimates as LLOD value.
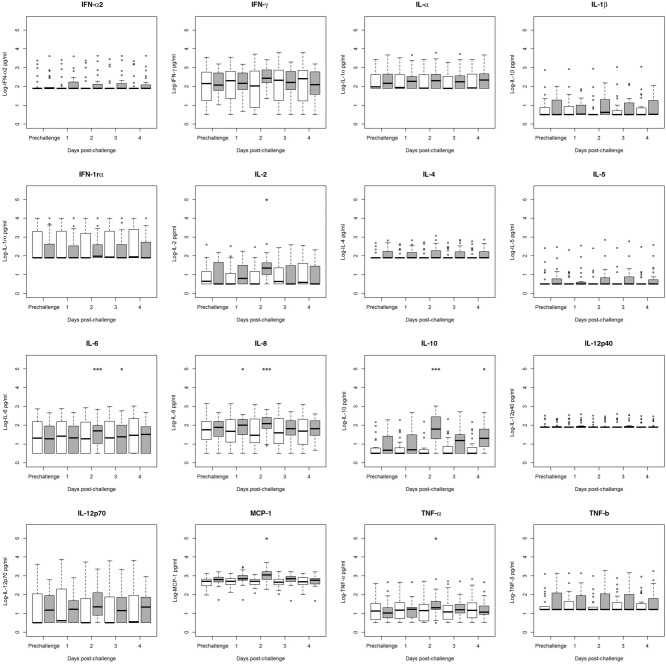
To assess whether serum cytokines exhibited a change in concentration following NoV challenge, the Wilcoxon signed-rank test was used, stratifying by infection status. Among infected individuals, there were significant increases in IL-2, IL-10, MCP-1 and TNF-α in the days post-challenge (Fig. 1, all P < 0·05). Among uninfected individuals, there were no significant changes in serum cytokine concentrations post-challenge compared to prechallenge. Based on conditional logistic regression models controlling for age and inoculum, there were no significant differences between infected and uninfected individuals’ changes in serum cytokine concentrations between prechallenge and day 1 post-challenge (data not shown). However, infected individuals were significantly more likely to have increased IFN-γ, IL-6, IL-8, IL-12p70, MCP-1 and TNF-α between prechallenge and day 2 post-challenge compared to uninfected individuals (Table3). Infected individuals were also significantly more likely to have increased levels of TNF-α, IL-8 and IL-10 from prechallenge to day 3 post-challenge (all P < 0·05; data not shown). At day 4 post-challenge, the only cytokine elevated significantly over prechallenge levels was IL-10, which was elevated significantly in infected individuals [odds ratio (OR) of infection based on a twofold increase in IL-10 from prechallenge to day 4 post-challenge = 3·92, P = 0·049; data not shown].
Figure 1.
Comparison of pre- to post-challenge serum cytokine concentrations in norovirus-challenged individuals, stratified by infection status (infected n = 26, uninfected n = 26). Values below the lower limit of detection (LLOD) were assigned to the value of the LLOD. Significance of change from prechallenge value was tested using Wilcoxon's test and is denoted by an asterisk above the relevant category. Interquartile ranges (IQRs) for infected individuals (grey boxes) and for uninfected individuals (white boxes) are shown. Dark lines indicate median values. Whiskers indicate most extreme value that is no more than 1·5 times the IQR away from the bound of the IQR. Circles indicate outliers. *P < 0·05, **P < 0·01, ***P < 0·001.
Table 3.
Table comparing results from this study to prior studies of cytokine responses to human norovirus (NoV) infection. An ‘x’ indicates significant elevation compared to uninfected controls; grey cells indicate that no testing was conducted for that cytokine
| Study | This study* | Lindesmith 2005 12 | Long 2011 22 | Chen 2012 37 | Souza 2008 18 | Souza 2007 19 |
|---|---|---|---|---|---|---|
| NoV genogroup | GI.1 | GII.2 | Mixed | Mixed | GII.4 | GII.4 |
| Subjects | Human | Human | Human | Human | Gn Calf | Gn Pig |
| Study design | Challenge | Challenge | Observational cohort | Cross-sectional | Challenge | Challenge |
| Cytokine | ||||||
| IFN-α | x | |||||
| IFN-α2 | n.s. | |||||
| IFN-γ | x | x | n.s. | x† | x | |
| IL-1ra | n.s. | |||||
| IL-1a | n.s. | |||||
| IL-1b | n.s. | |||||
| IL-2 | x | x | ||||
| IL-4 | n.s. | n.s. | n.s. | x† | x | |
| IL-5 | n.s. | x | x | |||
| IL-6 | x | n.s. | x | x | ||
| IL-8 | x | x | x | |||
| IL-10 | x | n.s. | n.s. | x† | x | |
| IL-12 | x† | x | ||||
| IL-12p40 | n.s. | |||||
| IL-12p70 | x | |||||
| MCP-1 | x | x | ||||
| TNF-α | x | n.s. | n.s. | x† | ||
| TNF-β | n.s. | |||||
NoV = norovirus; Gn = gnotobiotic; ns, not significant; IL = interleukin; IFN = interferon; TNF = tumour necrosis factor; MCP = monocyte chemoattractant protein.
Modelled using conditional logistic regression conditional on inoculum dose and adjusted for age.
Cytokine concentrations were elevated compared to controls but no statistical testing was conducted.
Differences between infected and uninfected individuals over time
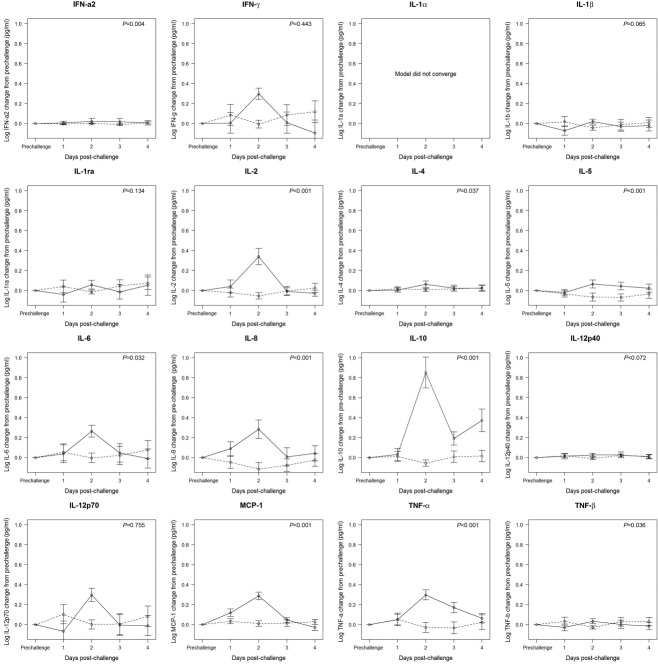
Overall trends in serum cytokine response were analysed using mixed models to account for correlation within subject over time (Fig. 2). For infected individuals compared to uninfected individuals, there were statistically significant fold changes across the 4 days post-challenge from prechallenge levels in most serum cytokines measured (i.e. IFN-α2, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, MCP-1, TNF-α and TNF-β, Fig. 2), with all but TNF-β significantly higher in infected individuals compared to uninfected individuals. All cytokines peaked at day 2 post-challenge among infected subjects, with the exception of IL-1ra, but many were elevated by day 1 post-challenge (i.e. IL-2, IL-6, IL-8, MCP-1 and TNF-α) or remained elevated to day 4 post-challenge (i.e. IL-10) (Fig. 2).
Figure 2.
Mixed linear model results for the association between log10 cytokine change from pre- to post-challenge by day, with fixed effects for inoculum dose and day and a random effect by individual subject. Solid line = infected individuals; dashed line = uninfected individuals. Points represent estimates of the effect of day on change in cytokine concentration. Error bars indicate one standard error. P-value indicates significance of overall elevation in cytokine concentration across days.
Prechallenge predictors of infection
To determine if prechallenge serum cytokine concentrations or early cytokine responses could predict infection status following challenge, the associations were estimated between infection status and three measures of serum cytokines: log10 serum cytokine concentration, log10 difference between prechallenge and day 1 post-challenge serum cytokine concentrations and prechallenge to day 1 post-challenge fold change (i.e. ratio between prechallenge and day 1 post-challenge concentrations). Across these measures, the only significant predictor of infection status was prechallenge to day 1 post-challenge log10 change in MCP-1 concentration (data not shown). An increase of 1 log10 over this time-period was associated with an OR of infection of 5·74 (P = 0·018). No other measures of early cytokine response were statistically significant predictors of infection.
Discussion
This study found overall significant elevation in IFN-α2, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, MCP-1, TNF-α and TNF-β in infected subjects compared to uninfected subjects. Among infected individuals, these cytokines peaked on day 2 post-challenge. The results suggest that NoV infection elicits a Th1- and Th2-type response. There also was persistent elevation of IL-10 among infected subjects, suggestive of an evolving early adaptive immune response and down-regulation of Th1 cytokines. This study represents the first comprehensive description of the dynamics of serum cytokines in the acute time-period following NoV challenge, including the largest number of cytokines described simultaneously for NoV infection (Table3).
We found that before challenge, individuals who became infected following NoV challenge were similar to individuals who did not become infected with regard to all 16 cytokines tested. From these similar prechallenge features, differences began to emerge as early as 24 h post-challenge and persisted to day 4 post-challenge. As documented previously with human Snow Mountain strain (SMV; GII·2) infection, there was a significant Th1 response (i.e. IFN-γ, IL-2 and TNF-α) with significant elevation of one Th2-related cytokine (Table3) 13. In this study, some Th2 and other cytokines (e.g. IL-4, IL-5, IL-6, IL-8 and IL-10) were also elevated significantly following NoV challenge in infected individuals.
The cytokine responses also resembled results from animal studies (Table3), but differed in the timing and magnitude of cytokine response 19,20. These results suggest that the immune response by gnotobiotic animals to human NoV may be substantially different from the response in humans, due perhaps to the gnotobiotic animals’ immature immune systems 21 or because of the use of a different NoV inoculum.
Initial response: IL-8, MCP-1
We found that IL-8 levels increased in infected subjects by day 1 post-challenge and remained elevated to day 3 post-challenge. IL-8 elevation has been identified in prior field studies of NoV infection (Table3) 22,23. IL-8 is a powerful chemoattractant for neutrophils 24,25 and intraepithelial lymphocytes 26,27, and a key mediator of the immune response to other gastrointestinal pathogens 28. Histological findings from previous human NoV challenge studies show increases in granulocyte and monocyte cells in the lamina propria of the small intestine 12–48 h after challenge in infected subjects 29. Based on the rise in IL-8 alongside an increase in MCP-1 and a later rise in IL-6, two monocyte chemoattractants, these observations are consistent with neutrophil recruitment acting in concert with monocyte response to NoV infection. Earlier work found that GI and GII NoV infection is associated with elevated fecal MCP-1, suggesting local monocyte activation 23, but to our knowledge, this study is the first to show elevated serum MCP-1, suggesting a systemic response as well.
TNF-α and symptoms
In this study, the peak serum TNF-α concentration at 48 h post-challenge corresponded with the time that most NoV-infected individuals were symptomatic. TNF-α increases cellular permeability, leading to oedema mucosal damage 30. Elevated TNF-α has also been associated with NoV infection in past human and animal studies (Table3) 19,23. In some gastrointestinal infections, TNF-α is associated with symptoms 31–33. The association between TNF-α and NoV symptoms is an important area for future research.
IL-6
IL-6 is a multi-functional cytokine with strong proinflammatory effects. It is associated with damage to the intestinal mucosa 29 and increased frequency of diarrhoea 22, suggesting a role in pathogenesis and clinical severity of illness. In this study, IL-6 was elevated significantly on day 2 post-challenge. This confirms some clinical studies’ identification of the association between IL-6 and NoV infection, but contrasts with the results from a SMV challenge study (Table3) 13,22,23 and suggests possible strain-related differences in immune response. This study reported a median maximum serum IL-6 level that was higher than prior studies have identified 22,34, but this discrepancy may be the result of differences in sample collection.
Persistently elevated IL-10
Although IL-10 peaked on day 2 post-challenge, it was elevated significantly to day 4 post-challenge. This suggests an ongoing role in NoV infection beyond the acute time-period. IL-10 is involved in the Th2 response and B cell development 35, so its elevation may be associated with the development of NoV-specific antibodies, which begin to be detectable around or before day 7 post-challenge 36,37. Elevated IL-10 in the context of NoV infection has been described in prior studies of human NoV (Table3). IL-10 may also play an anti-inflammatory role in down-regulating Th1 cytokine production. The significant elevation of IL-10 in infected subjects following the elevation of Th1 cytokines supports the conclusion that NoV elicits a Th1-type response. Furthermore, IL-10-deficient mice exhibit mucosal inflammation and epithelial barrier dysfunction following mouse norovirus (MNV) challenge, whereas wild-type mice do not 38. It is possible that IL-10 plays a similar protective role in NoV-challenged humans.
Strengths and limitations
This study used rigorously collected human NoV challenge study data, which provided a detailed, longitudinal data set. It is the largest collection of human NoV challenge subjects yet studied for serum cytokine response. It represents the broadest panel of cytokines yet examined at one time for human NoV infection. Some limitations of this study are that it may have been underpowered to detect some changes in serum cytokines, the exposure history of subjects prior to NoV challenge was unknown and there is the possibility that the duration of sample storage may have caused cytokine degradation 39. An additional limitation of the study is that although RT–PCR is the gold standard for NoV testing, there may have been individuals who were misclassified as uninfected because they shed virus at levels below the limit of detection.
Conclusion
Although NoV remains a major cause of morbidity worldwide, some aspects of the immune response continue to be poorly understood. To develop effective vaccines and prophylaxis, it is important to understand the human acute immune response to NoV. This study demonstrated a Th1- and Th2-type response to GI.1 NoV infection, early elevation of chemokines IL-8 and MCP-1 and ongoing elevation of IL-10. This work confirms earlier findings of the increase in MCP-1 associated with NoV infection, suggesting macrophage and dendritic cell involvement in NoV infection. These findings buttress the existing findings from MNV models and enhance the depth of knowledge regarding the human cytokine response to NoV. Differences between the results of this study and those of other human and animal work may be attributable to strain-related variability in immune response. Future work should consider the interplay between the immune response and both clinical outcomes and viral shedding to understand NoV pathogenesis and help to develop clinical and public health strategies to reduce NoV transmission.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K.L.N., grant 1F30DK100097; C.A.P. grants R01DK072564, R01DK061379, andR01DK079392), the ARCS Foundation (K.L.N), the National Institute of Allergy and Infectious Diseases (J.S.L., grant 1K01AI087724), the Emory University Global Health Institute (J.S.L.), and NoroCORE (K.L.N., J.S.L., A.E.K., C.L.M, FDA grant 2011-68003-30395). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or United States Food and Drug Administration.
Disclosure
None to report.
References
- Vinje J. Advances in laboratory methods for detection and typing of norovirus. J Clin Microbiol. 2015;53:373–81. doi: 10.1128/JCM.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KY. Caliciviridae: the Noroviruses. In: Knipe DM, Howley P, editors. Fields virology. Philadelphia, PA: Lippincott Williams and Wilkins; 2013. pp. 582–608. [Google Scholar]
- Ahmed SM, Hall AJ, Robinson AE, et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:725–30. doi: 10.1016/S1473-3099(14)70767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon JSSM, Qiuhong W, Smith ER, Saif LJ, Moe CL. Immunology of norovirus infection. In: Vajdy M, editor. Immunity against mucosal pathogens. New York: Springer; 2008. pp. 219–62. [Google Scholar]
- Lindesmith LC, Donaldson E, Leon J, et al. Heterotypic humoral and cellular immune responses following Norwalk virus infection. J Virol. 2010;84:1800–15. doi: 10.1128/JVI.02179-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube S, Kolawole AO, Hohne M, et al. A mouse model for human norovirus. mBio. 2013;4:e00450–13. doi: 10.1128/mBio.00450-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MK, Watanabe M, Zhu S, et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science. 2014;346:755–9. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Jiang X. Norovirus gastroenteritis, carbohydrate receptors, and animal models. PLOS Pathog. 2010;6:e1000983. doi: 10.1371/journal.ppat.1000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson AM, Airaud F, LePendu J, Estes MK, Atmar RL. Norwalk virus infection associates with secretor status genotyped from sera. J Med Virol. 2005;77:116–20. doi: 10.1002/jmv.20423. [DOI] [PubMed] [Google Scholar]
- Parrino TA, Schreiber DS, Trier JS, Kapikian AZ, Blacklow NR. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N Engl J Med. 1977;297:86–9. doi: 10.1056/NEJM197707142970204. [DOI] [PubMed] [Google Scholar]
- Schreiber DS, Blacklow NR, Trier JS. The small intestinal lesion induced by Hawaii agent acute infectious nonbacterial gastroenteritis. J Infect Dis. 1974;129:705–8. doi: 10.1093/infdis/129.6.705. [DOI] [PubMed] [Google Scholar]
- Troeger H, Loddenkemper C, Schneider T, et al. Structural and functional changes of the duodenum in human norovirus infection. Gut. 2009;58:1070–7. doi: 10.1136/gut.2008.160150. [DOI] [PubMed] [Google Scholar]
- Lindesmith L, Moe C, Lependu J, Frelinger JA, Treanor J, Baric RS. Cellular and humoral immunity following Snow Mountain virus challenge. J Virol. 2005;79:2900–9. doi: 10.1128/JVI.79.5.2900-2909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachu KA, Strong DW, LoBue AD, Wobus CE. Baric RS, Virgin HW. Antibody is critical for the clearance of murine norovirus infection. J Virol. 2008;82:6610–7. doi: 10.1128/JVI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz SR, Leon JS, Schwab KJ, et al. Norovirus infectivity in humans and persistence in water. Appl Environ Microbiol. 2011;77:6884–8. doi: 10.1128/AEM.05806-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon JS, Kingsley DH, Montes JS, et al. Randomized, double-blinded clinical trial for human norovirus inactivation in oysters by high hydrostatic pressure processing. Appl Environ Microbiol. 2011;77:5476–82. doi: 10.1128/AEM.02801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby AE, Shi J, Montes J, Lichtenstein M, Moe CL. Disease course and viral shedding in experimental Norwalk virus and Snow Mountain virus infection. J Med Virol. 2014;86:2055–64. doi: 10.1002/jmv.23905. [DOI] [PubMed] [Google Scholar]
- Wong HL, Pfeiffer RM, Fears TR, Vermeulen R, Ji S, Rabkin CS. Reproducibility and correlations of multiplex cytokine levels in asymptomatic persons. Cancer Epidemiol Biomarkers Prev. 2008;17:3450–6. doi: 10.1158/1055-9965.EPI-08-0311. [DOI] [PubMed] [Google Scholar]
- Souza M, Azevedo MS, Jung K, Cheetham S, Saif LJ. Pathogenesis and immune responses in gnotobiotic calves after infection with the genogroup II.4-HS66 strain of human norovirus. J Virol. 2008;82:1777–86. doi: 10.1128/JVI.01347-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza M, Cheetham SM, Azevedo MS, Costantini V, Saif LJ. Cytokine and antibody responses in gnotobiotic pigs after infection with human norovirus genogroup II.4 (HS66 strain) J Virol. 2007;81:9183–92. doi: 10.1128/JVI.00558-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RD. Effects of microbiota on GI health: gnotobiotic research. Adv Exp Med Biol. 2008;635:41–56. doi: 10.1007/978-0-387-09550-9_4. [DOI] [PubMed] [Google Scholar]
- Chen SM, Lin CP, Tsai JD, Chao YH, Sheu JN. The significance of serum and fecal levels of interleukin-6 and interleukin-8 in hospitalized children with acute rotavirus and norovirus gastroenteritis. Pediatr Neonatol. 2014;55:120–6. doi: 10.1016/j.pedneo.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Long KZ, Garcia C, Ko G, et al. Vitamin A modifies the intestinal chemokine and cytokine responses to norovirus infection in Mexican children. J Nutr. 2011;141:957–63. doi: 10.3945/jn.110.132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzik T, Hudson JT, III, Lügering A, et al. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut. 2005;54:1565–72. doi: 10.1136/gut.2004.061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307:97–101. doi: 10.1016/0014-5793(92)80909-z. [DOI] [PubMed] [Google Scholar]
- Roberts AI, Bilenker M, Ebert EC. Intestinal intraepithelial lymphocytes have a promiscuous interleukin-8 receptor. Gut. 1997;40:333–8. doi: 10.1136/gut.40.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert EC. Human intestinal intraepithelial lymphocytes have potent chemotactic activity. Gastroenterology. 1995;109:1154–9. doi: 10.1016/0016-5085(95)90573-1. [DOI] [PubMed] [Google Scholar]
- Casola A, Estes MK, Crawford SE, et al. Rotavirus infection of cultured intestinal epithelial cells induces secretion of CXC and CC chemokines. Gastroenterology. 1998;114:947–55. doi: 10.1016/s0016-5085(98)70314-2. [DOI] [PubMed] [Google Scholar]
- Schreiber DS, Blacklow NR, Trier JS. The mucosal lesion of the proximal small intestine in acute infectious nonbacterial gastroenteritis. N Engl J Med. 1973;288:1318–23. doi: 10.1056/NEJM197306212882503. [DOI] [PubMed] [Google Scholar]
- Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6–19. doi: 10.1038/mi.2013.73. [DOI] [PubMed] [Google Scholar]
- Peterson KM, Shu J, Duggal P, Haque R, Mondal D, Petri WA., Jr Association between TNF-alpha and Entamoeba histolytica diarrhea. Am J Trop Med Hyg. 2010;82:620–5. doi: 10.4269/ajtmh.2010.09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls S, Stephens S, Braegger CP, Walker-Smith JA, MacDonald TT. Cytokines in stools of children with inflammatory bowel disease or infective diarrhoea. J Clin Pathol. 1993;46:757–60. doi: 10.1136/jcp.46.8.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DE, Jiang ZD, Steffen R, Verenker MP, DuPont HL. Markers of inflammation in bacterial diarrhea among travelers, with a focus on enteroaggregative Escherichia coli pathogenicity. J Infect Dis. 2002;185:944–9. doi: 10.1086/339617. [DOI] [PubMed] [Google Scholar]
- Chen SM, Ku MS, Lee MY, Tsai JD, Sheu JN. Diagnostic performance of serum interleukin-6 and interleukin-10 levels and clinical predictors in children with rotavirus and norovirus gastroenteritis. Cytokine. 2012;59:299–304. doi: 10.1016/j.cyto.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Sabat R, Grutz G, Warszawska K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–44. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Ramani S, Neill FH, Opekun AR, et al. Mucosal and cellular immune responses to Norwalk virus. J Infect Dis. 2015;212 doi: 10.1093/infdis/jiv053. :397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol. 2003;108:241–7. doi: 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- Basic M, Keubler LM, Buettner M, et al. Norovirus triggered microbiota-driven mucosal inflammation in interleukin 10-deficient mice. Inflamm Bowel Dis. 2014;20:431–43. doi: 10.1097/01.MIB.0000441346.86827.ed. [DOI] [PubMed] [Google Scholar]
- de Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009;10:52. doi: 10.1186/1471-2172-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]