Abstract
Matrix metalloproteases (MMPs) are increased in different infections due to their role in controlling immune responses and are regulated by tissue inhibitors (TIMPs). Different MMP promoter single nucleotide polymorphisms (SNPs) induce changes in MMP genes, mRNA and protein expression. Gender might also modify MMP plasma levels. In order to determine the weight of these variables on MMP secretion we studied MMP-1, -2, -3, -8, -9, -10, -13 and TIMP-1, -2, -4 plasma levels in 90 patients with severe bacterial sepsis, 102 with anti-retroviral (ARV)-treated HIV monoinfection, 111 with ARV-treated HIV–hepatitis C virus (HCV) co-infection and 86 non-infected controls (45 stroke and 41 trauma patients). MMP-1(-1607 1G/2G), MMP-3(-1612 5A/6A), MMP-8(-799C/T), MMP-9(-1562 C/T) and MMP-13(-77A/G) SNPs were genotyped. MMP-3 plasma levels were significantly higher in men than in women in each diagnostic group, and MMP-3 SNP allele 6A carriers also had higher levels than allele 5A carriers, an effect that was magnified by sepsis. Independent predictors of higher MMP-3 levels were male gender (P = 0·0001), MMP-3(-1612 5A/6A) SNP (P = 0·001), higher levels of TIMP-4 (P = 0·004) and MMP-8 (P = 0·006) and lower levels of MMP-1 (P = 0·03) by multivariate analysis. No strong associations with gender or SNPs were observed for other MMPs or TIMPs. In conclusion, male gender and MMP-3(-1612 5A/6A) 6A allele carriage increased MMP-3 plasma levels significantly, especially in patients with severe bacterial sepsis. This confounding gender effect needs to be addressed when evaluating MMP-3 plasma levels in any infectious or non-infectious condition.
Keywords: bacterial, gender, hepatitis C, HIV, human, MMPs, polymorphisms, sepsis, TIMPs
Introduction
Matrix metalloproteases (MMPs) are a family of zinc- and calcium-dependent endopeptidases that degrade extracellular matrix (ECM) and are regulated by tissue inhibitors (TIMPs). MMPs also play an important role in the normal immune response to infection. MMPs facilitate leucocyte recruitment, cytokine and chemokine processing and defensin activation, in addition to ECM remodelling 1,2. Among MMPs, MMP-3 (stromelysin-1) plays a key role. MMP-3 is involved in the turnover of ECM in different human tissues, but also in the activation of other MMPs (pro-MMP-1, -9, -8, -9 and -13) as well as in the autoactivation of pro-MMP3. MMPs promoter single nucleotide polymorphisms (SNPs) induce changes in MMPs genes, mRNA and protein expression. The gene coding for MMP-3 is located on the long arm of chromosome 11 in regions 11q22.2-22.3. A common polymorphism has been identified in the promoter region of the MMP-3 gene located 1612 base pairs (bp) upstream of the transcription start site, with one allele containing a run of five adenosines (5A) and the other allele containing a run of six adenosines (6A). Transient transfection experiments have indicated that the 5A allele expresses a twofold higher activity of the reporter gene than does the 6A allele, a finding suggesting that carriers of the 5A allele exhibit a higher MMP-3 promoter activity 3. However, some in vivo studies have found, surprisingly, that carriers of the 6A allele of this MMP-3 SNP had higher plasma MMP-3 levels than those with the 5A allele in patients with coronary disease and also in healthy populations 4,5. No MMP-3 plasma levels differences among MMP-3 (-1612 5A/6A) SNP genotype carriers were observed by others, however 6,7. MMP-3 (-1612 5A/6A) SNP has been associated with coronary disease, vascular aneurysms, brain stroke, cancer and arthritis, but not so far with infections 8–13.
Some isolated studies, focused on arthritis, tuberculosis and myocardial infarction, have reported a gender effect on plasma levels of MMP-3, MMP-8 and MMP-9 4,14–16. However, to our knowledge no study has been devoted specifically to analyse the possible impact of gender in MMPs plasma levels.
The aim of this study was to analyse the effect of gender and different MMPs SNPs on MMPs and TIMPs secretion in different infectious and non-infectious conditions. MMP-1, -2, -3, -8, -9, -10, -13 and TIMP-1, -2, -4 plasma levels were measured, and MMP-1(-1607 1G/2G), MMP-3(-1612 5A/6A) MMP-8(-799C/T), MMP-9(-1562 C/T) and MMP-13(-77A/G) SNPs genotyped in a large cohort of patients with severe bacterial sepsis, anti-retroviral (ARV)-treated HIV with/without hepatitis C virus (HCV) co-infection, and brain stroke and severe trauma patients as non-infectious controls.
Patients and methods
Patients
Adult Caucasian patients with severe microbiologically demonstrated bacterial sepsis, admitted to the intensive care unit (ICU) of the Hospital Universitario Central de Asturias (HUCA) in Oviedo, Spain, were included into the study. They were considered as septic if they fulfilled the diagnosis of severe sepsis according to the 1992 International Sepsis Definitions Conference Criteria modified in 2003 17,18. HIV-infected patients, with/without HCV co-infection, on ARV for at least 34 months and with a therapy adherence > 75%, were also enrolled from the HIV out-patients clinic of the HUCA. Patients admitted to the ICU because of severe trauma and brain strokes were used as non-infectious controls. Patients and controls were members of a homogeneous Caucasian population, and were residents of the same region (Asturias, northern Spain) that has a small foreign immigrant population (less than 5%). Each participant or their legal representatives gave informed consent for the study, which was approved by the Ethics Committee of the HUCA. Detailed demographic and clinical characteristics of the patients and controls have been published elsewhere 19,20.
Methods
Plasma MMPs and TIMPs
All diagnostic groups underwent measurements of MMP-1, -2, -3, -8, -9, -10, -13 and TIMP-1, -2, -4 plasma levels. Plasma levels of MMPs-1, -2, -3, -8, - 9, -10 and -13 and TIMP -1, -2 and -4 were measured using the Quantibody™ human MMP Array 1 from Raybiotech (Raybiotech, Norcross, GA, USA), according to the manufacturer's instructions. Plasma MMPs and TIMPs levels were measured in two different batches, the first involving all HIV-infected patients, and the second involving all the non-HIV-infected individuals. Plasma samples of septic, stroke and trauma patients were obtained at ICU admission and those of HIV mono/co-infected patients at a random clinical visit. Plasma samples were diluted two to 10-fold for MMP array assay as needed. QuantibodyTM human MMP Array 1 standards ranged from 0 to 100 ng/ml for MMP-1, 0 to 50 ng/ml for MMP-2, 0 to 30 ng/ml for MMP-3, 0 to 80 ng/ml for MMP-8, 0 to 1200 ng/ml for MMP-9, 0 to 100 ng/ml for MMP-10, 0 to 20 ng/ml for MMP-13, 0 to 400 ng/ml for TIMP-1, to 200 ng/ml for TIMP-2 and 0 to 50 ng/ml for TIMP-4.
MMPs SNPs genotyping
The following SNPs related to MMPs were also determined in all groups: MMP-1 (-1607 1G/2G, rs 11292517), MMP-8 (-799C/T, rs 11225395), MMP-9 (-1562 C/T, rs 34016235) and MMP (-13-77A/G, rs 2252070) by polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP). The MMP-3 (-1612 5A/6A, rs35068180) SNP was also determined, but only in septic, stroke and trauma patients. Oligonucleotide primer sequences, PCR conditions and restriction enzymes used for genotyping and sequencing the different MMPs SNPs studied are described in detail elsewhere 21–24.
Statistical analysis
Values of continuous variables are expressed as median and interquartile range (IQR), and categorical variables as percentages. Comparison of continuous variables was carried out by the Mann–Whitney test, if two groups, and by the Kruskal–Wallis test to evaluate the MMPs and TIMPs plasma levels of the different SNP genotypes. Categorical variables were compared with the χ2 test and Fisher's exact test, as appropriate. A stepwise multiple regression analysis was used to evaluate the independent predictors of MMP-3 plasma levels, and a multivariate stepwise logistic regression to assess the factors associated independently with gender. Statistical calculations were performed with spss version 22 software (IBM, Armonk, NY, USA). All P-values reported are two-sided. The limit of statistical significance was established at the P < 0·05 level.
Results
A total of 389 patients were included into the study; 253 men (65.0%) and 136 women. The median age was 47·5 years (IQR = 41·0–59·0). All patients were classified into the following five diagnostic categories: ARV-treated HIV/HCV co-infection (111 patients), ARV-treated HIV monoinfection (102 patients), severe bacterial sepsis (90 patients), brain stroke (45 patients) and severe trauma (41 patients).
Table1 shows the age, MMPs and TIMPs values of the patients classified according to gender and diagnostic groups. It can be appreciated that MMP-3 plasma levels were significantly higher in men than in women in each diagnostic group, with absolute values of approximately twofold in men compared to women. No other MMP or TIMP showed such consistent differences, although MMP-9 was significantly higher in men than in women only in ARV-treated HIV+/HCV+, MMP-8 in HIV+/HCV– and MMP-1 in septic patients. Women had significantly higher levels than men only for MMP-10 in the group of septic patients.
Table 1.
Age, matrix metalloprotease (MMP) and tissue inhibitors (TIMP) plasma values of the patients according to diagnostic categories and gender*
| All patients (n = 389) | HIV+/HCV+ (n = 111) | HIV+/HCV– (n = 102) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Male (n = 253) | Female (n = 136) | P | Male (n = 75) | Female (n = 36) | P | Male (n = 69) | Female (n = 33) | P | |
| Age (years) | 47·0 (40·5–58·0) | 49·0 (42·0–65·0) | 0·3 | 44·0 (40·0–48·0) | 44·0 (41·3–47·0) | 0·9 | 45·0 (40·0-52·0) | 44·0 (38·0-52·0) | 0·6 |
| MMP-1 | 29·9 (11·6–57·8) | 23·5 (12·7–41·3) | 0·3 | 38·4 (18·8–64·7) | 4·6 (2·7–75·3) | 0·06 | 3·9 (2·4-11·6) | 10·9 (3·2300-73·5) | 0·5 |
| MMP-2 | 0·76 (0·43–1·33) | 0·82 (0·44–1·48) | 0·6 | 0·88 (0·57–1·28) | 0·96 (0·48–1·38) | 0·7 | 0·48 (0·22-0·83) | 0·85 (0·24-1·51) | 0·3 |
| MMP-3 | 18·3 (10·8–33·1) | 11·31 (5·9–19·0) | <0·0001 | 17·6 (13·4–31·7) | 13·7 (7·3–22·3) | 0·049 | 16·5 (10·0-27·2) | 8·8 (5·4-16·3) | 0·0007 |
| MMP-8 | 0·87 (0·03–26·18) | 0·97 (0·02–34·4) | 0·9 | 0·04 (0·02–0·14) | 0·03 (0·01–0·05) | 0·08 | 0·03 (0·02-0·05) | 0·015 (0·01-0·03) | 0·003 |
| MMP-9 | 87·3 (22·3–565·5) | 99·0 (18·3–546·0) | 0·5 | 29·2 (12·0–54·0) | 13·6 (6·5–30·4) | 0·01 | 21·9 (11·3-52·2) | 24·5 (9·5-36·7) | 0·6 |
| MMP-10 | 11·22 (2·19–47·69) | 24·60 (3·33–75·18) | 0·08 | 2·05 (0·84–5·89) | 2·21 (0·83–3·16) | 0·9 | 1·50 (0·76-2·68) | 2·24 (0·98-4·42) | 0·2 |
| MMP-13 | 0·11 (0·06–0·31) | 0·12 (0·06–0·25) | 0·8 | 0·59 (0·07–1·64) | 0·12 (0·06–0·13) | 0·3 | 0·07 (0·02-0·19) | 0·13 (0·05-0·22) | 0·5 |
| TIMP-1 | 129·9 (53·3–262·2) | 146·1 (49·3–251·7) | 0·6 | 59·2 (38·3–75·9) | 47·8 (29·3–71·4) | 0·1 | 51·5 (36·4-75·4) | 49·4 (29·4-65·1) | 0·3 |
| TIMP-2 | 22·5 (7·9–89·6) | 31·4 (7·9–87·9) | 0·97 | 8·5 (5·9–13·4) | 8·8 (5·9–12·3) | 0·7 | 8·7 (5·2-11·3) | 7·6 (3·0-12·0) | 0·2 |
| TIMP-4 | 0·55 (0·05–2·95) | 1·36 (0·11–4·25) | 0·047 | 0·06 (0·03–0·17) | 0·09 (0·03–0·25) | 0·4 | 0·04 (0·02-0·09) | 0·09 (0·04-0·24) | 0·005 |
| Septics (n = 90) | Stroke (n = 45) | Trauma (n = 41) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Male (n = 50) | Female (n = 40) | P | Male (n = 26) | Female (n = 19) | P | Male (n = 33) | Female (n = 8) | P | |
| Age (years) | 58·0 (45·8–72·0) | 65·0 (41·3–75·0) | 0·5 | 68·0 (56·0–72·3) | 65·0 (59·0–74·0) | 0·8 | 50·0 (35·0-65·0) | 60·5 (39·5-68·8) | 0·4 |
| MMP-1 | 34·3 (19·9–72·1) | 22·8 (11·8–36·2) | 0·03 | 33·7 (19·0–54·7) | 37·7 (22·5–55·5) | 0·6 | 19·6 (5·35-52·5) | 20·2 (13·5-56·7) | 0·4 |
| MMP-2 | 1·08 (0·54–2·59) | 0·69 (0·50–2·22) | 0·3 | 0·73 (0·44–1·37) | 0·76 (0·47–1·54) | 0·9 | 0·64 (0·32-1·98) | 0·59 (0·28-1·39) | 0·7 |
| MMP-3 | 36·0 (16·06–74·0) | 14·1 (9·5–23·5) | 0·0001 | 12·6 (7·0–22·3) | 5·9 (4·3–12·2) | 0·01 | 13·4 (7·9-26·7) | 5·8 (3·9-9·1) | 0·003 |
| MMP-8 | 48·7 (34·0–75·2) | 43·5 (32·4–70·2) | 0·5 | 12·9 (9·0–25·1) | 13·8 (9·5–25·3) | 0·8 | 14·7 (8·3-26·4) | 15·7 (4·0-29·0) | 0·7 |
| MMP-9 | 467·7 (226·4–641·3) | 362·8 (165·7–587·4) | 0·17 | 660·0 (530·3–1012·2) | 719·9 (643·0–920·5) | 0·3 | 630·4 (387·1-852·3) | 538·3 (246·2-690·2) | 0·4 |
| MMP-10 | 44·9 (11·6–147·5) | 82·7 (36·7–186·8) | 0·03 | 34·9 (11·9–96·4) | 23·6 (8·0–27·9) | 0·12 | 16·7 (10·4-45·4) | 10·2 (8·6-33·2) | 0·3 |
| MMP-13 | 0·16 (0·08–0·26) | 0·16 (0·08–0·28) | 0·8 | 0·12 (0·05–0·38) | 0·07 (0·04–0·39) | 0·5 | 0·06 (0·04-0·26) | 0·04 (0·03-0·35) | 0·5 |
| TIMP-1 | 265·5 (233·2–314·6) | 246·9 (222·6–285·9) | 0·15 | 264·7 (224·2–309·5) | 255·9 (225·8–308·4) | 0·8 | 263·3 (201·5-304·0) | 237·6 (169·8-315·2) | 0·7 |
| TIMP-2 | 104·8 (74·4–128·5) | 92·9 (68·3–124·4) | 0·4 | 88·3 (50·1–140·5) | 68·7 (52·6–119·2) | 0·6 | 77·9 (61·0-89·4) | 67·0 (45·6-115·5) | 0·7 |
| TIMP-4 | 3·43 (2·05–6·38) | 4·06 (2·10–7·78) | 0·6 | 3·89 (2·15–8·32) | 4·25 (2·17–5·80) | 0·8 | 2·43 (1·24-3·63) | 2·05 (1·35-3·71) | 0·7 |
MMPs and TIMPs are expressed as ng/ml. HIV+ = human immunodeficiency virus infection; HCV+ = hepatitis C virus infection. HIV+/HCV− and HIV+/HCV+ were anti-retroviral (ART)-treated.
Figure 1 depicts the MMP-3 plasma levels according to each diagnostic group and gender. Male patients had substantially higher levels than women in each diagnostic group, and septic patients, especially septic men, had the highest MMP-3 values among the different diagnostic groups.
Figure 1.

Matrix metalloprotease 3 (MMP)-3 plasma levels according to diagnosis and gender. M = male; F = female.
Table2 shows the genotypic and allelic frequencies of different MMPs SNPs. None of the SNPs studied showed significant differences among men and women.
Table 2.
Genotypical and allelic frequencies of different matrix metalloprotease (MMP) single nucleotide polymorphisms (SNPs) according to gender
| Genotypical frequencies | Allelic frequencies | |||||||
|---|---|---|---|---|---|---|---|---|
| SNP | Genotype | Male n (%) | Female n (%) | P | Allele | Male n (%) | Female n (%) | P |
| MMP-1 (-1607 1G/2G) | 1G1G | 44 (26·5%) | 28 (28·9%) | 0·078 | 1G | 149 (44·9%) | 79 (40·7%) | 0·4 |
| 1G2G | 61 (36·7%) | 23 (23·7%) | 2G | 183 (55·1%) | 115 (59·3%) | |||
| 2G2G | 61 (36·7%) | 46 (47·4%) | ||||||
| MMP-3 (-1612 5A/6A) | 5A5A | 31 (28·4%) | 23 (34·3%) | 0·7 | 5A | 113 (51·8%) | 75 (56·0%) | 0·5 |
| 5A6A | 51 (46·8%) | 29 (43·3%) | 6A | 105 (48·2%) | 59 (44·0%) | |||
| 6A6A | 27 (24·8%) | 15 (22·4%) | ||||||
| MMP-8 (-799C/T) | CC | 52 (26·8%) | 24 (21·8%) | 0·6 | C | 195 (50·3%) | 102 (46·4%) | 0·4 |
| CT | 91 (46·9%) | 54 (49·1%) | T | 193 (49·7%) | 118 (53·6%) | |||
| TT | 51 (26·3%) | 32 (29·1%) | ||||||
| MMP-9 (-1562 C/T) | CC | 152 (79·2%) | 88 (80·7%) | 0·7 | C | 343 (89·3%) | 197 (90·4%) | 0·7 |
| CT | 39 (20·3%) | 21 (19·3%) | T | 41 (10·7%) | 21 (9·6%) | |||
| TT | 1 (0·5%) | 0 (0·0%) | ||||||
| MMP-13 (-77A/G) | AA | 123 (59·7%) | 77 (64·7%) | 0·5 | A | 328 (79·6%) | 196 (82·4%) | 0·4 |
| AG | 82 (39·8%) | 42 (35·3%) | G | 84 (20·4%) | 42 (17·7%) | |||
| GG | 1 (0·5%) | 0 (0·0%) | ||||||
MMP-3(-1612 5A/6A) SNP determination was only available for septic, stroke and trauma patients. Figure 2 depicts the MMP-3 levels according to the MMP-3(-1612 5A/6A) genotypes. It can be appreciated that the homozygous 6A/6A genotype had consistently higher MMP-3 levels than the heterozygous 5A/6A, and both genotypes had higher levels than the homozygous 5A/5A genotype in all diagnostic groups, although the differences were statistically significant only in the patients as a whole and in the septic patients, probably because of the relatively small sample sizes of the stroke and trauma groups.
Figure 2.
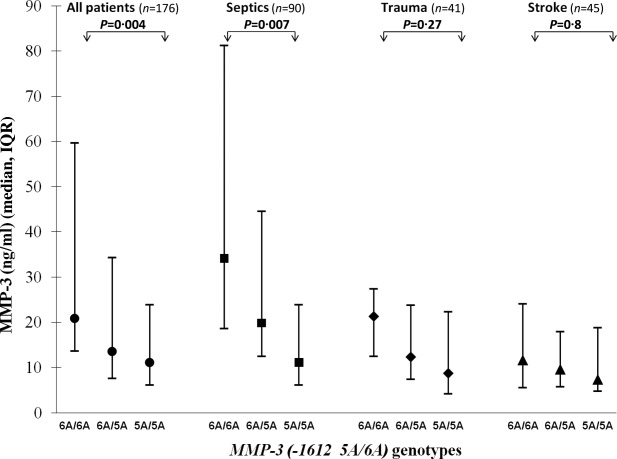
Matrix metalloprotease 3 (MMP)-3 plasma levels according to diagnosis and the MMP-3(-1612 5A/6A) genotypes.
Figure 3 shows the MMP-3 plasma levels classified according to the MMP-3(-1612 5A/6A) genotypes and gender. Males had significantly higher levels than females in each of the three genotypes.
Figure 3.

Matrix metalloprotease 3 (MMP)-3 plasma levels according to the MMP-3(-1612 5A/6A) genotypes and gender. M = male; F = female.
Regarding other SNPs genotyped, and referred to the same diagnostic groups (septic, stroke and trauma patients), only the MMP-9(-1562 C/T) SNP had a marginally significant association (P = 0·042) with MMP-9 plasma levels [CC 547·5 ng/ml (310·9–719·8), CT 641·4 ng/ml (473·6–863·8]; no patient had the TT genotype.
Neither the MMP-1(-1607 1G/2G) SNP [1G/1G 30·8 ng/ml (15·6–66·4), 1G/2G 32·3 ng/ml (13·7–56·3), 2G/2G 24·4 ng/ml (11·2–44·8), P = 0·5], the MMP-8(-799C/T) SNP [CC 30·3 ng/ml (11·5–60·3), CT 27·7 ng/ml (12·3–48·2), TT 32·1 ng/ml (14·9–61·0), P = 0·7] nor the MMP-13(-77A/G) SNP [AA 0·108 ng/ml (0·058–0·207), AG 0·131 ng/ml (0·058–0·366) were associated with their respective MMPs plasma levels; there was only one patient with the GG genotype, P = 0·3]. When all five diagnostic groups were considered altogether, no significant associations among SNPs and their respective MMPs were found (P = 0·14–0·28).
Multivariate analyses were carried out to identify the parameters independently associated with gender and with MMP-3 plasma levels, after adjustment for the two batches of determination. The variables associated with male gender were higher levels of MMP-3 [odds ratio (OR) = 1·04, 95% confidence interval (CI) = 1·021–1·059, P < 0·0001] and lower levels of TIMP-4 (OR = 0·926, 95% CI = 0·870–0·985, P = 0·015). Similarly, the independent predictors of higher MMP-3 levels were male gender (P = 0·0001), MMP-3(-1612 5A/6A) SNP (P = 0·001), higher levels of TIMP-4 (P = 0·004) and MMP-8 (P = 0·006) and lower levels of MMP-1 (P = 0·03).
Discussion
In our study we found that male gender was associated significantly with higher MMP-3 plasma levels than women in all clinical conditions evaluated, both infectious and non-infectious. Similarly, lower TIMP-4 levels were associated independently with male gender. Conversely, a similar increasing effect of the carriage of the MMP-3(-1612 5A/6A) SNP 6A allele on MMP-3 plasma levels was also evident in all diagnostic groups, although the differences were statistically significant only in the septic group, which displayed the largest sample size and the highest levels of MMP-3.
Although some authors reported higher MMPs plasma levels in men compared with women 4,14–16, these studies were not focused to analyse and clarify this point or underwent adjustment for covariates, and were limited to particular pathologies. Therefore, this is the first study devoted specifically to evaluate the gender differences in plasma MMPs or TIMPs in a substantial cohort of patients with a variety of infectious (bacterial and viral) and non-infectious conditions.
A Swedish study found higher MMP-3 levels in men than in women with myocardial infarction and healthy controls 4. Another study from the United States found similar findings in patients with diverse rheumatic diseases and in healthy controls 14. Other authors also found higher levels of MMP-3, MMP-8 and MMP-9 in British men with rheumatoid arthritis compared with women 15. Finally, another study reported higher levels of MMP-8 in Peruvian male patients with tuberculosis 16. However, as these studies were not focused on the gender effect on MMPs plasma levels, their findings were neither discussed nor analysed in depth, nor adjusted for confounding factors, an important aspect as MMPs and TIMPs correlated among them and are modified by diverse circumstances. Therefore, the results of these studies should be interpreted cautiously.
Our study, centred specifically on evaluating the influence of gender in infectious and non-infectious conditions, found significantly higher levels of MMP-3 in each of the diverse diagnostic groups analysed. We also found that men, compared with women, had significantly higher plasma levels of MMP-8 and lower of TIMP-4 in ARV-treated HIV-monoinfected patients, higher levels of MMP-9 in ARV-treated HIV/HCV-co-infected patients and higher levels of MMP-1 and lower of MMP-10 in septic patients, although only MMP-3 and TIMP-4 were associated independently with gender in the multivariate analysis.
Our study also suggests that the gender effect on MMP-3 is independent of the underlying pathologies, a suggestion reinforced by the finding of higher MMP-3 levels in healthy men than in healthy women in other studies 4,14.
The reasons for this imbalance between men and women are unclear, although they may be related to hormonal issues. Thus, males seem to mount more aggressive and damaging inflammatory immune responses to microbial stimuli than women of reproductive age 25. Also, decreased MMP-9 and TNF-α expression by neutrophils were observed in women during the periovulatory period, when the oestrogen levels are higher 26.
Experimental studies reported somewhat conflicting results. Thus, the secretion by osteoarthritic chondrocytes of MMP-3, MMP-13 or TIMP-1 was not significantly different after exposure to 17 beta-oestradiol, either in the presence or absence of cytokine stimulation. On the contrary, the secretion of MMP-1 was reduced significantly after oestrogen exposure in chondrocytes, either stimulated or not with cytokines 24.
Conversely, oestradiol administration reduced hepatic mRNA for types I and III procollagens and TIMP-1 in rat male livers, an effect that was reverted by the concomitant administration of neutralizing antibodies against oestradiol. Similarly, ovariectomy in the female rat model induced the hepatic expression of both types of procollagen and TIMP-1 27.
Regardless of the particular MMPs or TIMPs implicated, and of the complexity of the stimulatory and inhibitory pathways and mediators involved in the inflammatory and immune responses to different stimuli, these experimental studies in human and rat cells suggest at least a certain role of sexual hormones in the secretion of these substances: a role that could, hypothetically, be minimized by the strongest effect of concomitant infections or autoimmune conditions, but that persist despite them in some MMPs. In fact, we found lower MMP-3 levels in women than in men in each pathological group analysed, including bacterial and viral, acute and chronic infections, as well as in non-infectious conditions. In this regard, our findings are remarkable in severely septic patients, who had the highest MMP-3 levels and who also displayed the greatest differences between genders, suggesting either a decreased response in women or, more probably, an exacerbated response in males. Different gender profiles, more favourable to women, have also been observed by our group in diverse viroimmunological aspects of HIV infection 28 and liver fibrosis caused by HCV infection 29. However, our clinical study was not designed to investigate the basic mechanisms underlying the different MMPs responses in males and females and, consequently, the causes of this imbalance should be clarified by further studies.
Some authors reported higher plasma levels of MMP-3 associated with the 6A allele of the MMP-3(-1612 5A/6A) SNP in patients with coronary artery disease and healthy controls of Swedish and Indian extraction 4,5. Other authors did not find differences among the diverse genotypes in the MMP-3 expression of vascular smooth muscle cells of English patients undergoing coronary artery bypass surgery 7, or even an apparently enhancing effect of the 5A allele in MMP-3 expression of fibroblasts and vascular smooth muscle cells 3. Finally, an Iranian study found higher MMP-3 plasma levels associated with the 6A allele, compared with the 5A allele, of the MMP-3(-1171 5A/6A) SNP in healthy individuals, but the opposite pattern was observed in patients with acute myocardial infarction 6. However, once again these findings were observed in pathologies related exclusively to coronary artery disease and were not adjusted for covariates.
In our study we found an enhancing effect of the MMP-3(-1612 5A/6A) SNP 6A allele on MMP-3 plasma levels in all the conditions evaluated, although it was only significant in patients with severe bacterial sepsis, the group who had the largest sample size and experienced the greatest MMP-3 differences among genotypes – an effect, independent of other covariates, that was even gradually progressive and clearly related to the number of 6A alleles. Perhaps the increased cytokine levels found in sepsis, interleukin (IL)-1, IL-6 and especially tumour necrosis factor (TNF)-α might magnify the effect of the MMP-3 SNP on MMP-3 plasma levels. In this regard, it has been reported an increase in MMP-3 expression in macrophages of uninfected individuals after stimulation with cytokines 30.
Finally, we also found that the differences between men and women in MMP-3 plasma levels were statistically significant in each of the three genotypes of the MMP-3(-1612 5A/6A) SNP, supporting, once again, the decisive influence of gender, beyond the genetic conditionings of the MMP-3 production.
We conclude that male gender and MMP-3(-1612 5A/6A) allele 6A carriage were associated with higher MMP-3 plasma levels, especially in patients with severe bacterial sepsis. These confounding and additive gender and genetic effects need to be addressed when evaluating MMP-3 plasma levels in patients with any infectious or non-infectious condition. Consequently, comparison groups in further studies need to be matched for gender and MMP-3 genotype to prevent biased results.
Acknowledgments
This work was supported by the Oviedo University research grants (UNIOV-12-MA-03 and SV-PA-13-ECOEMP-57) given to V. A. and by grants from Janssen Pharmaceuticals and Red Temática de Investigación de SIDA (RIS), Instituto de Salud Carlos III (ISCIII), Plan Estatal I+D+i and European Regional Development Fund (ERDF) ‘Una manera de hacer Europa’, grant (RD06/0006/0010). These results will presented in part at the 55th ICAAC Meeting, ASM, San Diego, California, USA, 17–21 September 2015, abstract control number 991.
Disclosure
None.
References
- Parks WC, Wilson CL, López-Boado YS. Matrix metaloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–29. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Elkington PT, O'Kane CM, Friedland JS. The paradox of matrix metalloproteinases in infectious disease. Clin Exp Immunol. 2005;142:12–20. doi: 10.1111/j.1365-2249.2005.02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Eriksson P, Hamsten A, Kurkinen M, Humphries SE, Henney AM. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression. J Biol Chem. 1996;271:13055–60. doi: 10.1074/jbc.271.22.13055. [DOI] [PubMed] [Google Scholar]
- Samnegård A, Silveira A, Lundman P, et al. Serum matrix metalloproteinase-3 concentration is influenced by MMP-3-1612 5A/6A promoter genotype and associated with myocardial infarction. J Intern Med. 2005;258:411–9. doi: 10.1111/j.1365-2796.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- Shalia KK, Shah VK, Mashru MR, et al. Matrix metalloproteinase-3 (MMP-31612 5A/6A promoter polymorphism in coronary artery disease in Indian population. Indian J Clin Biochem. 2010;25:133–40. doi: 10.1007/s12291-010-0025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderian SM, Akbarzadeh Najar R, Tabatabaei Panah AS. Genetic polymorphisms and plasma levels of matrix metalloproteinases and their relationships with developing acute myocardial infarction. Coron Artery Dis. 2010;21:330–5. doi: 10.1097/MCA.0b013e32833ce065. [DOI] [PubMed] [Google Scholar]
- Maqbool A, Keswani A, Galloway S, et al. MMP-3 (5A/6A) polymorphism does not influence human smooth muscle cell invasion. J Surg Res. 2012;175:343–9. doi: 10.1016/j.jss.2011.03.043. [DOI] [PubMed] [Google Scholar]
- Terashima M, Akita H, Kanazawa K, et al. Stromelysin promoter 5A/6A polymorphism is associated with acute myocardial infarction. Circulation. 1999;99:2717–9. doi: 10.1161/01.cir.99.21.2717. [DOI] [PubMed] [Google Scholar]
- Deguara J, Burnand KG, Berg J, et al. An increased frequency of the 5A allele in the promoter region of the MMP3 gene is associated with abdominal aortic aneurysms. Hum Mol Genet. 2007;16:3002–7. doi: 10.1093/hmg/ddm258. [DOI] [PubMed] [Google Scholar]
- Sherva R, Ford CE, Eckfeldt JH, Davis BR, Boerwinkle E, Arnett DK. Pharmacogenetic effect of the stromelysin (MMP3) polymorphism on stroke risk in relation to antihypertensive treatment: the genetics of hypertension associated treatment study. Stroke. 2011;42:330–5. doi: 10.1161/STROKEAHA.110.593798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jin X, Fang S, et al. The functional SNP in the matrix metalloproteinase-3 promoter modifies susceptibility and lymphatic metastasis in esophageal squamous cell carcinoma but not in gastric cardiac adenocarcinoma. Carcinogenesis. 2004;25:2519–24. doi: 10.1093/carcin/bgh269. [DOI] [PubMed] [Google Scholar]
- Holliday DL, Hughes S, Shaw JA, Walker RA, Jones JL. Intrinsic genetic characteristics determine tumor-modifying capacity of fibroblasts: matrix metalloproteinase-3 5A/5A genotype enhances breast cancer cell invasion. Breast Cancer Res. 2007;9:R67. doi: 10.1186/bcr1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin A, Lauwers-Cancès V, Navaux F, et al. Stromelysin 1 (matrix metalloproteinase 3) and HLA-DRB1 gene polymorphisms: association with severity and progression of rheumatoid arthritis in a prospective study. Arthritis Rheum. 2002;46:1754–62. doi: 10.1002/art.10336. [DOI] [PubMed] [Google Scholar]
- Zucker S, Lysik RM, Zarrabi MH, et al. Elevated plasma stromelysin levels in arthritis. J Rheumatol. 1994;21:2329–33. [PubMed] [Google Scholar]
- Mattey DL, Nixon NB, Dawes PT. Association of circulating levels of MMP-8 with mortality from respiratory disease in patients with rheumatoid arthritis. Arthritis Res Ther. 2012;14:R204. doi: 10.1186/ar4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyamoorthy T, Sandhu G, Tezera LB, et al. Gender-dependent differences in plasma matrix metalloproteinase-8 elevated in pulmonary tuberculosis. PLOS ONE. 2015;10:e0117605. doi: 10.1371/journal.pone.0117605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RC, Sibald WJ, Spring CL. The ACCP-SCCM Consensus Conference on sepsis and organ failure. Chest. 1992;101:1481–3. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- Martin G, Asensi V, Montes AH, et al. Role of plasma matrix-metalloproteases (MMPs) and their polymorphisms (SNPs) in sepsis development and outcome in ICU patients. Sci Rep. 2014;4:5002. doi: 10.1038/srep05002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Zarracina T, Valle-Garay E, Collazos J, et al. Didanosine (ddI) associates with increased liver fibrosis in adult HIV-HCV coinfected patients. J Viral Hepatol. 2012;19:685–93. doi: 10.1111/j.1365-2893.2012.01596.x. [DOI] [PubMed] [Google Scholar]
- Ye S. Influence of matrix metalloproteinase genotype on cardiovascular disease susceptibility and outcome. Cardiovasc Res. 2006;69:636–45. doi: 10.1016/j.cardiores.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Kader AK, Shao L, Dinney CP, et al. Matrix metaloproteinase polymorphisms and bladder cancer risk. Cancer Res. 2006;66:11644–8. doi: 10.1158/0008-5472.CAN-06-1212. [DOI] [PubMed] [Google Scholar]
- Borghese B, Chiche JD, Vernerey D, et al. Genetic polymorphisms of matrix metalloproteinase 12 and 13 genes are implicated in endometriosis progression. Hum Reprod. 2008;23:1207–13. doi: 10.1093/humrep/den007. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Lee EB, Kwon YE, et al. Effect of estrogen on the expression of matrix metalloproteinase (MMP)-1, MMP-3, and MMP-13 and tissue inhibitor of metalloproternase-1 in osteoarthritis chondrocytes. Rheumatol Int. 2003;23:282–8. doi: 10.1007/s00296-003-0312-5. [DOI] [PubMed] [Google Scholar]
- Marriott I, Huet-Hudson YM. Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res. 2006;34:177–92. doi: 10.1385/IR:34:3:177. [DOI] [PubMed] [Google Scholar]
- Smith JM, Shen Z, Wira CR, Fanger MW, Shen L. Effects of menstrual cycle status and gender on human neutrophil phenotype. Am J Reprod Immunol. 2007;58:111–9. doi: 10.1111/j.1600-0897.2007.00494.x. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29:719–27. doi: 10.1002/hep.510290307. [DOI] [PubMed] [Google Scholar]
- Collazos J, Asensi V, Carton JA. Sex differences in the clinical, immunological and virological parameters of HIV-infected patients treated with HAART. Aids. 2007;21:835–43. doi: 10.1097/QAD.0b013e3280b0774a. [DOI] [PubMed] [Google Scholar]
- Collazos J, Carton JA, Asensi V. Gender differences in liver fibrosis and hepatitis C virus-related parameters in patients coinfected with human immunodeficiency virus. Curr HIV Res. 2011;9:339–45. doi: 10.2174/157016211797635982. [DOI] [PubMed] [Google Scholar]
- Uzui H, Harpf A, Liu M, et al. Increased expression of membrane type 3-matrix metalloproteinase in human atherosclerotic plaque: role of activated macrophages and inflammatory cytokines. Circulation. 2002;106:3024–30. doi: 10.1161/01.cir.0000041433.94868.12. [DOI] [PubMed] [Google Scholar]


