Abstract
Purpose
To investigate the efficacy and toxicity of postoperative intensity-modulated radiation therapy (IMRT) for patients with soft tissue sarcomas (STSs) in the extremities and trunk wall.
Patients and methods
Eighty patients with localized STSs in the extremities and trunk wall treated with function-conserving surgery and postoperative IMRT were analyzed. The primary locations were in the extremities in 51 patients and in the trunk wall in 29 patients. The margins were positive in nine patients and negative in 71 patients. The median dose of IMRT was 64 Gy.
Results
At a median follow-up time of 38 months, eight patients developed local recurrences. The 5-year local control (LC) rate was 88.1%. The patients with negative margins exhibited much better LC than did those with positive margins (90% vs 64.8%, P=0.023). Multivariate analysis revealed that positive margin was an independent risk factor for LC. The 5-year distant metastasis-free survival, disease-free survival, and overall survival rates were 75.2%, 72.6%, and 83.6%, respectively. Large tumor size (>5 cm) was significantly associated with poor overall survival. Edema and joint stiffness were observed in 17.6% and 9.8% of patients with extremity STSs, respectively.
Conclusion
IMRT provides excellent LC and low toxicity for patients with STSs in the extremities and trunk wall.
Keywords: soft tissue sarcoma, extremities and trunk wall, intensity-modulated radiation therapy, local control, toxicities
Introduction
In the past 30 years, the management of soft tissue sarcoma (STS) had evolved from amputation and similar radical resection approaches to more conservative, function-preserving surgeries combined with radiotherapy (RT). Evidence from two randomized trials demonstrated that RT in combination with limb-sparing surgery achieves local control (LC) and overall survival (OS) rates that are comparable to those of amputation.1,2 In both of these trials, conventional two-dimensional (2D) external beam RT or brachytherapy technique was used.
Standard of practice of STS has changed to intensity-modulated radiation therapy (IMRT) within the past decade, as two dosimetric studies revealed that IMRT has the advantage of improving the dose distribution to large tumor targets while sparing the normal tissue in STSs in the thigh.3,4 Although various studies have demonstrated the improved survival and decreased toxicity that are associated with the use of IMRT in the treatment of other malignant tumors,5,6 some previous studies have reported on the favorable outcomes achieved with IMRT for pediatric and adult patients with STS.7–10 Thus, whether IMRT can be as effective in the treatment of STS patients like 2D-RT without posing risks to LC remains an important issue. In this large series of patients with STSs in the extremities and trunk wall, we evaluated the outcomes and toxicities of adjuvant IMRT following function-preserving surgery.
Patients and methods
Patients
This study was approved by the Institutional Ethic Committee of Cancer Hospital & Institute, Chinese Academy of Medical Sciences (CAMS) in October 2012. As the study was a retrospective study, there was no intervention regarding the patients and their treatment. Only the clinical data were reviewed, and patient confidentiality was upheld. Therefore, written patient consent was deemed unnecessary. The patients with STS treated with function-preserving surgery and RT in our institution between July 2005 and November 2011 were identified. Eighty patients who met the following criteria were included in this study: tumor located in the extremities or trunk wall, treated with postoperative IMRT, no distant metastasis, and no previous RT. The patients were staged according to the Seventh American Joint Committee on Cancer staging system.11
Surgery and IMRT
All patients received wide local excisions. The majority of patients (n=71, 88.8%) underwent R0 resections (>1 mm margin), and nine patients received R1 (≤1 mm margin or microscopic residual disease) or R2 (gross residual disease) resection.
RT was administered 4–6 weeks after surgery. The patients were immobilized with a board-fixed shoe in T-shape shoe and vacuum cushion for patients with tumors in extremities and trunks, respectively. Computed tomography simulation was performed. The clinical target volume (CTV) was defined by tumor bed plus 3–4 cm margins in the superior and inferior directions and 1.0–1.5 cm margins in the medial and lateral directions without expanding beyond any anatomical barrier. The surgical scar and drain sites were included in the CTV. The first planning target volume 1 (PTV1) was produced by expanding the CTV by 0.5–1.0 cm. The PTV2 was defined as the PTV1 after reductions of 3–4 cm in the superior and inferior directions. A total dose of 50 Gy in 25 fractions was delivered to PTV1 and then an additional 10–16 Gy was applied to the PTV2. The PTV coverage criteria was D95 (dose covering 95% volume) and V95 (volume receiving at least 95% of dose), both ≥95%. Patients with positive margins received a boost dose of 16–20 Gy. Based on our previous experience, five to seven fields, 6 MV, coplanar, IMRT plans were generated on the Pinnacle system, Version 3.0 (Pinnacle Systems, Inc., Mountain View, CA, USA). For patients with sarcomas in the extremities, all beams were arranged on one side of the extremity to spare total circumference irradiation of bone, joints, and soft tissues.
In patients with target volume on trunk wall, dose constraints for organ at risk (OAR) were as follows: V20 (volume receiving ≥40 Gy) <30% for the liver; V20<30% for both kidneys or a mean dose of <20 Gy; V30<30% for the heart; V20<15% for lungs; the maximal dose for the spinal cord ≤40 Gy; and V50<10% for the small bowel and colon. In patients with target volume in extremities, dose constraints of OAR were as follows: V40<60% for bones; if 0%–50% bone circumference within PTV, 100% bone cortex <50 Gy; if >50% bone circumference within PTV, try to spare at least one third bone cortex outside of PTV1; spare approximately 50% of the joint and the skin over the length of PTV1 within the field; no beams entering or exiting through contralateral leg if possible; no hot spots (≥107% of the prescribed dose) are allowed to be located on the bone.
Follow-up, end points, and statistics
The follow-up schedule consisted of clinical evaluations that included toxicity assessments every 3 months for the first 2 years and imaging of the primary lesion site and chest every 6 months. These examinations were subsequently repeated every 6 months for 3 years and yearly thereafter.
The OS, disease-free survival (DFS), LC, and distant metastasis-free survival (DMFS) were calculated from the date of surgery. Local recurrence was defined as any recurrence in the primary site irrespective of distant metastasis. Morbidity was assessed using the Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0.12
Survival time was calculated using the Kaplan–Meier method. The survival differences were compared with log-rank tests. Independent prognostic factors were identified using Cox stepwise regression analysis.
Results
Clinical characteristics
Table 1 summarizes the patient characteristics. The most common histologies were malignant fibrous histiocytomas (23.8%, n=19) and liposarcomas (23.8%, n=19), followed by fibrosarcomas (15.0%, n=12) and synovial sarcomas (15.0%, n=12). The median age was 50 years (range: 5–74).
Table 1.
Characteristics of the patients with soft tissue sarcomas in the extremities and trunk wall
| Patients, N (%) | |
|---|---|
| Sex | |
| Male | 49 (61.3) |
| Female | 31 (38.7) |
| Age | |
| ≥50 years | 41 (51.2) |
| <50 years | 39 (48.8) |
| Location | |
| Extremities | 51 (63.8) |
| Trunk wall | 29 (36.2) |
| Size | |
| ≤5 cm | 39 (48.7) |
| >5 cm | 41 (51.3) |
| Depth | |
| Superficial | 30 (37.5) |
| Deep | 50 (62.5) |
| Stage | |
| I | 19 (23.8) |
| II | 60 (75.0) |
| III | 1 (1.2) |
| Tumor grade | |
| Grade 1 | 18 (22.5) |
| Grade 2 | 50 (62.5) |
| Grade 3 | 12 (15.0) |
| Surgery | |
| R0 resection | 71 (88.8) |
| R1 resection | 5 (6.3) |
| R2 resection | 4 (5.0) |
Local control
Among the 80 patients, eight patients developed local recurrence with (n=2) or without (n=6) distant metastasis. The actual 3-year LC rate was 92.9%, and the estimated 5-year LC rate was 88.1% for all patients (Figure 1). Incomplete resection and male sex were associated with local recurrence (Table 2). Patient with positive margins exhibited significantly more local recurrence than did those with negative margins. The 5-year LC rates were 90% for the patients with negative margins (R0 resection) and 64.8% for those with positive margins (R1 + R2 resection, P=0.023). The 5-year LC rates were 100% for women and 80.0% for men (P=0.032). Multivariate analysis revealed that only positive margin (hazard ratio 5.33, 95% confidence interval 1.19–23.86, P=0.029) exerted an independent adverse influence on LC.
Figure 1.
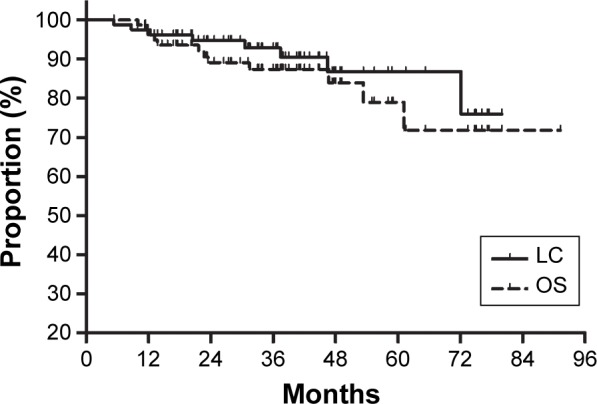
Local control (LC) and overall survival (OS) for all patients.
Table 2.
Treatment outcomes of the patients with soft tissue sarcomas in the extremities and trunk wall following IMRT
| Estimated 5-year LC
|
Estimated 5-year OS
|
|||
|---|---|---|---|---|
| % | P-value | % | P-value | |
| Sex | ||||
| Male | 80.0 | 0.032 | 76.6 | 0.77 |
| Female | 100 | 86.2 | ||
| Age | ||||
| ≥50 years | 80.7 | 0.12 | 68.8 | 0.46 |
| <50 years | 91.8 | 87.9 | ||
| Location | ||||
| Extremities | 91.0 | 0.24 | 79.2 | 0.46 |
| Trunk wall | 81.6 | 78.4 | ||
| Size | ||||
| ≤5 cm | 97.2 | 0.18 | 94.7 | 0.041 |
| >5 cm | 78.0 | 68.9 | ||
| Depth | ||||
| Superficial | 91.3 | 0.87 | 79.3 | 0.92 |
| Deep | 78.3 | 79.4 | ||
| Stage | ||||
| I | 94.7 | 0.50 | 88.2 | 0.72 |
| II + III | 84.8 | 76.9 | ||
| Tumor grade | ||||
| Grade 1 | 94.4 | 0.80 | 88.2 | 0.88 |
| Grade 2 | 84.4 | 75.8 | ||
| Grade 3 | 90.0 | 91.7 | ||
| Margin | ||||
| Negative | 90.0 | 0.023 | 76.1 | 0.80 |
| Positive | 64.8 | 31.5 | ||
Abbreviations: IMRT, intensity-modulated radiation therapy; LC, local control; OS, overall survival.
Survival
Twelve patients died due to their diseases. At a median follow-up time of 38 months for the surviving patients, the actual 3-year OS, DFS, and DMFS rates were 87.4%, 77.3%, and 81.2%, respectively. The estimated 5-year OS, DFS, and DMFS rates were 83.6%, 72.6%, and 75.2%, respectively (Figure 1). The patient characteristics were evaluated for prognostic significance for OS (Table 2). Large tumor size was associated with poor OS. The 5-year OS rates were 68.9% for the patients with tumors larger than 5 cm and 94.7% for those with tumors of 5 cm or less (P=0.041). No factors were found to be related to OS in the multivariate analysis.
Morbidity
The late complications are shown in Table 3. Among the 51 patients with extremity STSs, nine (17.6%) had grade 1–4 edema, and five (9.8%) had grades 1–3 joint stiffness. Only one patient developed grade 3 joint stiffness. This patient presented with recurrent bulky disease localized in his hip joint was covered with CTV at a total dose of 50 Gy. Another patient developed grade 3 acute dermatitis and a wound complication, and he lived with a chronic nonhealing ulcer. Other severe late toxicities, such as bone fracture and secondary neoplasm, did not occur in any patients.
Table 3.
Late postoperative morbidities of IMRT in 51 patients with soft tissue sarcomas in the extremities
| Late radiation morbidity | N | % |
|---|---|---|
| Edema | ||
| Grade 1 | 2 | 3.9 |
| Grade 2 | 3 | 5.9 |
| Grade 3 | 3 | 5.9 |
| Grade 4 | 1 | 2.0 |
| Joint stiffness (≥ CTCAE grade 2) | ||
| Grade 1 | 2 | 3.9 |
| Grade 2 | 2 | 3.9 |
| Grade 3 | 1 | 2.0 |
Abbreviations: IMRT, intensity-modulated radiation therapy; CTCAE, Common Terminology Criteria for Adverse Events.
Discussion
To our knowledge, our study represents one of the largest studies of IMRT for the postoperative treatment of STS in the modern era. IMRT produced favorable LC and OS among patients with STSs in the extremities and trunk wall. Positive margin independently and adversely influenced LC in the multivariate analysis. Furthermore, the late morbidity of IMRT was low compared with those reported in previous studies that have utilized 2D radiation techniques.13–15
The clinical importance of adjuvant IMRT for STS is not well defined.8–10 One large study from the Memorial Sloan-Kettering Cancer Center reported favorable prognoses following preoperative and postoperative IMRT in 41 adult patients with extremity STSs. The 5-year LC and OS rates for these patients were 94% and 64%, respectively (median follow-up time, 35 months).8 Similarly, the treatment outcomes following postoperative IMRT in patients with STSs in the extremities and trunk wall were excellent in this series. The 5-year LC rates in this series were 88.1% for all patients and 91.0% for the patients with extremity STSs, and the 5-year OS rate was 83.6%. The high OS rate observed in the study can be partially explained by the low proportions of large-sized and high-grade tumors.8 Furthermore, the LC and OS in this series were similar to those reported in other studies that utilized conventional radiation techniques.2,16,17 Consistent with previous studies of conventional RT,18–23 our study clearly demonstrated that patients with positive margins exhibited significantly more local recurrence that did those with negative margins in both the univariate and the multivariate analyses. This finding indicates that IMRT might not compensate for poor surgeries with positive margins. In contrast, two other studies of the use of IMRT for extremity STSs achieved excellent LCs for both patients with negative and positive margins.8,24
The possible advantage of IMRT in the treatment of STS is the reduction in late toxicities.25 Compared with conventional RT and 3D conformal RT, IMRT provides homogeneous CTV coverage and greater sparing of the surrounding normal tissues.3,4,26 Compared with the early studies of conventional RT,8,13–15,27 the postoperative IMRT in the current study resulted in acceptably lower rates of late complications, including a 17.7% rate of edema and a 9.8% rate of joint stiffness. Severe late complications were rare, and no patient experienced bone fracture. In contrast, Davis et al reported that 23.3% of patients experienced edema and 23.3% had joint stiffness following treatment with 2D-RT.15 Cannon et al showed that the 20-year chronic radiation-related rate of limb complications was 13% in patients with primary lower extremity STSs.27 The reported overall fracture rates range from 1.2% to 6.3%.8,13,14,27
This study is limited by its retrospective nature. Prospective assessment of the functional outcomes was not possible, which might have introduced bias into the results. Additionally, because the median follow-up time was relatively short, the long-term complications of IMRT for STS need further evaluation.
Conclusion
Postoperative IMRT provides excellent LC and low late morbidity rates in patients with STSs in the extremities and trunk wall. These findings clearly indicate the feasibility and efficiency of IMRT in clinical practice. As observed in previous studies of the use of IMRT for other malignancies,5,6 the precise dose distributions achieved with IMRT in the treatment of STS produced favorable outcomes by sparing the surrounding normal tissues.
Acknowledgments
The article was accepted as an oral presentation at the 55th Annual Meeting of ASTRO on September 24, 2013, in Atlanta, USA.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14(3):859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 2.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16(1):197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 3.Hong L, Alektiar KM, Hunt M, Venkatraman E, Leibel SA. Intensity-modulated radiotherapy for soft tissue sarcoma of the thigh. Int J Radiat Oncol Biol Phys. 2004;59(3):752–759. doi: 10.1016/j.ijrobp.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Stewart AJ, Lee YK, Saran FH. Comparison of conventional radiotherapy and intensity-modulated radiotherapy for post-operative radiotherapy for primary extremity soft tissue sarcoma. Radiother Oncol. 2009;93(1):125–130. doi: 10.1016/j.radonc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Bi XW, Li YX, Fang H, et al. High-dose and extended-field intensity modulated radiation therapy for early-stage NK/T-cell lymphoma of Waldeyer’s ring: dosimetric analysis and clinical outcome. Int J Radiat Oncol Biol Phys. 2013;87(5):1086–1093. doi: 10.1016/j.ijrobp.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 6.Nutting CM, Morden JP, Harrington KJ, et al. PARSPORT trial management group Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alektiar KM, Hong L, Brennan MF, Della-Biancia C, Singer S. Intensity modulated radiation therapy for primary soft tissue sarcoma of the extremity: preliminary results. Int J Radiat Oncol Biol Phys. 2007;68(2):458–464. doi: 10.1016/j.ijrobp.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 8.Alektiar KM, Brennan MF, Healey JH, Singer S. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol. 2008;26(20):3440–3444. doi: 10.1200/JCO.2008.16.6249. [DOI] [PubMed] [Google Scholar]
- 9.Lin C, Donaldson SS, Meza JL, et al. Effect of radiotherapy techniques (IMRT vs 3D-CRT) on outcome in patients with intermediate-risk rhabdomyosarcoma enrolled in COG D9803 – a report from the children’s oncology group. Int J Radiat Oncol Biol Phys. 2012;82(5):1764–1770. doi: 10.1016/j.ijrobp.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang JC, Dharmarajan KV, Wexler LH, La Quaglia MP, Happersett L, Wolden SL. Intensity modulated radiation therapy with dose painting to treat rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2012;84(3):e371–e377. doi: 10.1016/j.ijrobp.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Soft Tissue Sarcomas . NCCN Guidelines Version 3. 2012 Staging. National Comprehensive Cancer Network; 2012. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#sarcoma. [Google Scholar]
- 12.National Institute of Health . Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0. Bethesda: National Institute of Health; 2003. [Accessed September 24, 2015]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. [Google Scholar]
- 13.Holt GE, Griffin AM, Pintilie M, et al. Fractures following radiotherapy and limb-salvage surgery for lower extremity soft-tissue sarcomas. A comparison of high-dose and low-dose radiotherapy. J Bone Joint Surg Am. 2005;87(2):315–319. doi: 10.2106/JBJS.C.01714. [DOI] [PubMed] [Google Scholar]
- 14.Alektiar KM, Leung D, Zelefsky MJ, Healey JH, Brennan MF. Adjuvant brachytherapy for primary high-grade soft tissue sarcoma of the extremity. Ann Surg Oncol. 2002;9(1):48–56. doi: 10.1245/aso.2002.9.1.48. [DOI] [PubMed] [Google Scholar]
- 15.Davis AM, O’Sullivan B, Turcotte R, et al. Canadian Sarcoma Group, NCI Canada Clinical Trial Group Randomized Trial Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75(1):48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Sampath S, Schultheiss TE, Hitchcock YJ, Randall RL, Shrieve DC, Wong JY. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma: multi-institutional analysis of 821 patients. Int J Radiat Oncol Biol Phys. 2011;81(2):498–505. doi: 10.1016/j.ijrobp.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 17.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 18.Biau DJ, Ferguson PC, Chung P, et al. Local recurrence of localized soft tissue sarcoma: a new look at old predictors. Cancer. 2012;118(23):5867–5877. doi: 10.1002/cncr.27639. [DOI] [PubMed] [Google Scholar]
- 19.Jebsen NL, Engellau J, Engström K, et al. Patterns of local recurrence and dose fractionation of adjuvant radiation therapy in 462 patients with soft tissue sarcoma of extremity and trunk wall. Int J Radiat Oncol Biol Phys. 2013;86(5):949–955. doi: 10.1016/j.ijrobp.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Stojadinovic A, Leung DH, Hoos A, Jaques DP, Lewis JJ, Brennan MF. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235(3):424–434. doi: 10.1097/00000658-200203000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gronchi A, Casali PG, Mariani L, et al. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: a series of patients treated at a single institution. J Clin Oncol. 2005;23(1):96–104. doi: 10.1200/JCO.2005.04.160. [DOI] [PubMed] [Google Scholar]
- 22.Delaney TF, Kepka L, Goldberg SI, et al. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007;67(5):1460–1469. doi: 10.1016/j.ijrobp.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 23.Dagan R, Indelicato DJ, McGee L, et al. The significance of a marginal excision after preoperative radiation therapy for soft tissue sarcoma of the extremity. Cancer. 2012;118(12):3199–3207. doi: 10.1002/cncr.26489. [DOI] [PubMed] [Google Scholar]
- 24.Alektiar KM, Brennan MF, Singer S. Local control comparison of adjuvant brachytherapy to intensity-modulated radiotherapy in primary high-grade sarcoma of the extremity. Cancer. 2011;117(14):3229–3234. doi: 10.1002/cncr.25882. [DOI] [PubMed] [Google Scholar]
- 25.O’Sullivan B, Griffin AM, Dickie CI, et al. Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma. Cancer. 2013;119(10):1878–1884. doi: 10.1002/cncr.27951. [DOI] [PubMed] [Google Scholar]
- 26.O’Sullivan B, Ward I, Haycocks T, Sharpe M. Techniques to modulate radiotherapy toxicity and outcome in soft tissue sarcoma. Curr Treat Options Oncol. 2003;4(6):453–464. doi: 10.1007/s11864-003-0046-3. [DOI] [PubMed] [Google Scholar]
- 27.Cannon CP, Ballo MT, Zagars GK, et al. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer. 2006;107(10):2455–2461. doi: 10.1002/cncr.22298. [DOI] [PubMed] [Google Scholar]


