Abstract
Background
Antiretroviral therapy (ART) initiation for HIV-1 infection is associated with 2-6% loss in bone mineral density (BMD).
Objective
To evaluate vitamin D3 (4000 IU daily) plus calcium (1000 mg calcium carbonate daily) supplementation on bone loss associated with ART initiation.
Design
48-week prospective, randomized, double-blind, placebo-controlled study.
Setting
Thirty nine AIDS Clinical Trials Network research units.
Participants
ART-naïve HIV-infected adults.
Measurements
BMD by dual-energy X-ray absorptiometry (DXA); 25-hydroxy vitamin D (25(OH)D) levels, parathyroid hormone (PTH), phosphate metabolism, markers of bone turnover and systemic inflammation.
Results
165 eligible subjects were randomized (79 Vitamin D/calcium (VitD/Cal); 86 placebo); 142 subjects with evaluable DXA data were included in the primary analysis. The study arms were well-balanced at baseline: median age 33 years; 90% male; 33% non-Hispanic black; median CD4 count 341 cells/mm3; and median 25(OH)D 23 ng/mL (57 nmol/L). At 48 weeks, subjects receiving placebo had greater decline in total hip BMD than VitD/Cal: −3.19% median change (1st-3rd quartile (Q1, Q3) −5.12%, −1.02%) vs. (−1.46% −3.16%,−0.40%). respectively (p=0.001). Lumbar spine BMD loss for the two groups was similar: −2.91% (−4.84%, −1.06%) vs. −1.41% (−3.78%, 0.00%), (p=0.085). At week 48, 90% of participants achieved HIV-1 RNA <50 copies/mL. Levels of 25(OH)D3 increased in the VitD/Cal but not the placebo group: median change of 24.5 (14.6, 37.8) vs. 0.7 (−5.3, 4.3) ng/mL, respectively (p<0.001). Additionally, increases in markers of bone turnover were blunted in the VitD/Cal group.
Limitations
No international sites were included; only 48 weeks of follow up
Conclusion
Vitamin D/calcium supplementation mitigates the loss of BMD seen with initiation of efavirenz/emtricitabine/tenofovir, particularly at the total hip, which is the site of greatest concern for fragility fracture.
Primary Funding Source
National Institute of Allergy and Infectious Diseases, Bristol-Meyers Squibb, Gilead Sciences.
Introduction
Antiretroviral therapy (ART) has transformed HIV infection from a terminal disease to a manageable chronic illness. While incidence of AIDS-defining conditions has declined, other comorbidities have increased (1), including osteoporosis and fragility fractures (2-7). Both viral and host factors likely contribute to bone loss and fracture risk: HIV infection mediated by certain viral proteins, HIV-associated inflammation, lifestyle and behavioral factors, underlying genetic predisposition, comorbidities, and ART (8-14).
ART initiation studies have confirmed that 2-6% loss of hip and spine BMD occurs over the first 24-48 weeks after ART initiation with subsequent stabilization (15-18). The magnitude of bone loss is similar to that observed with glucocorticoids or during the first year of menopausal transition (19,20). This initial bone loss is marked by an increase in serum bone resorption markers followed by a delayed compensatory increase in bone formation markers [21]; therefore, this catabolic window, a high bone turnover state with excess bone resorption, may be a central mechanism of bone loss with ART initiation.
Tenofovir (TDF), a nucleotide analogue reverse transcriptase inhibitor (NRTI), has been associated with greater bone loss than other NRTIs (15,16). TDF use is associated with increased PTH, elevated vitamin D binding protein and reduced free 1,25-dihydroxy vitamin D (1,25(OH)2D3) levels (22,23), suggesting that functional vitamin D deficiency with TDF use potentially contributes to excess bone loss. Initiation of efavirenz (EFV), a non-nucleoside reverse transcriptase inhibitor, is associated with a 2.5-5 ng/ml (6.2-12.5nmol/L) decrease in 25(OH)D levels (24,25). Efavirenz induces cytochrome P450 enzymes involved in vitamin D metabolism and may accelerate the catabolism of 25(OH)D and 1,25(OH)2D3, the latter being the active vitamin D metabolite (26). These two ART agents are combined with emtricitabine (FTC), another NRTI, into a fixed dose combination (FDC) once-a-day pill (EFV/FTC/TDF) that is highly effective for treating HIV infection (27).
Beyond bone metabolism effects, vitamin D has immunomodulatory effects mediated through the vitamin D receptor present on cells in both the innate and the adaptive immune system (28,29). Vitamin D increases monocyte expression of CD14 and cathelicidin, molecules involved in innate immune responses (30,31), and down-regulates cytokine expression in activated T cells and suppresses T cell proliferation and the production of IFN-gamma and IL-2, thus reducing net state of inflammation(32,33). These pathways are particularly relevant in HIV infection where excess monocyte activation and T cell activation are important drivers of morbidity and mortality (34-37).
We hypothesized that bone loss associated with ART initiation with EFV/FTC/TDF would be attenuated with high dose vitamin D and calcium supplementation. Additionally, we evaluated immunomodulatory effects of vitamin D in the setting of treatment of HIV infection. Herein, we report the results of ACTG A5280: a multi-center, randomized, double-blind, placebo-controlled study assessing the effect of daily oral 4000 IU vitamin D3 and 1000 mg calcium carbonate in HIV-infected adults initiating their first ART regimen with EFV/FTC/TDF.
Methods
HIV-infected subjects, naïve to ART without evidence of resistance to the antiretrovirals in the regimen and with HIV-1 RNA>1000 copies/ml, were eligible if they met the following criteria: screening 25(OH)D level ≥ 10 and <75 ng/mL (≥25 and <188 nmol/L), CrCl ≥60 ml/min by Cockcroft-Gault, and serum calcium <10.5 mg/dl. We excluded subjects with daily calcium supplementation >500mg, daily vitamin D supplementation >800 IU, any bisphosphonate use, recent steroid or chemotherapy treatments, clinically active thyroid disease, active substance or alcohol abuse, history of fragility fracture, documented osteoporosis, nephrolithiasis, or weight>300 lbs (limit of DXA scanner). Pregnant and breastfeeding women were excluded. We performed three-day dietary recalls at entry to estimate vitamin D and calcium intake. Participants were randomized to 4000 IU cholecalciferol (vitamin D3) daily plus 500mg calcium carbonate twice daily or identically matching placebos (Tishcon Corporation, Westbury, NY), and counseled to take with food to facilitate absorption. Some experts consider the current upper U.S. Dietary Reference Intake of 2,000 IU below actual physiologic requirements; therefore, we tested the highest supplementation without risk of toxicity (39-41). Vitamin D3 (4000 IU/day) is the highest daily dose considered safe for adults by the Institute of Medicine (38) and has previously been evaluated in HIV-infected persons with excellent tolerability and safety data (42). We randomized subjects to treatment arms in a 1:1 ratio using permuted blocks stratified by screening serum 25(OH)D (≤ 20 and >20 ng/mL (50nmol/L)). The Institutional Review Boards of all participating sites approved the study; all subjects provided written informed consent. (clinicalTrials.gov Identifier NCT01403051)
Primary endpoint was percentage change in total hip BMD from baseline to 48 weeks. Secondary endpoints included percentage change in lumbar spine BMD at 48 weeks, change in plasma 25(OH)D, PTH, markers of bone turnover, soluble inflammatory biomarkers, and CD4 cell counts at 24 and 48 weeks. Incidence of hypercalcemia and nephrolithiasis were monitored. All DXA scans were read in a blinded fashion at the Body Composition Analysis Center at Tufts Medical Center (Boston, MA) using a standardized protocol.
Biomarker Assays
Screening laboratories were performed at local CLIA certified laboratories. Serum samples were stored at −70°C until batched analysis at the Irving Institute Biomarkers Core at Columbia University Medical Center (New York, NY). We measured 25(OH) D2 and D3 by liquid chromatography tandem mass spectrometry; intact PTH (radioimmunoassay; Scantibodies, Santee, CA); N-terminal propeptide of procollagen type 1 (P1NP; RIA; IDS, Scottsdale, AZ); C-telopeptide (CTX; ELISA; IDS Scottsdale, AZ); IL-6 (ELISA; R&D Systems, Minneapolis, MN); soluble receptors of TNFα (sTNFr-I and –II; ELISA; R&D Systems, Minneapolis, MN), and soluble CD14 (sCD14, ELISA; R&D Systems, Minneapolis, MN). Inflammatory biomarkers were chosen based upon association with relevant endpoints (43-46). Except for 25(OH)D, biomarkers were measured in duplicate and values averaged for analysis.
Statistical Analysis
To provide an intent-to-treat interpretation, analyses were performed regardless of status regardless of status on randomized treatment. Two subjects who did not have the correct Vitamin D test performed at baseline were excluded from the efficacy analyses. The primary analysis utilized a multiple imputation approach to impute missing data with a Markov chain Monte Carlo (MCMC) method. These analyses used 5 imputations based upon screening 25(OH)D stratum, age, sex, and race for the primary outcomes of change in hip and spine BMD from baseline to 48 weeks. A pre-specified complete case approach was utilized for analyses by stratum and to assess interactions. Stratified Wilcoxon rank sum tests were used to test for distribution shifts between the treatment groups, stratified by screening vitamin D levels. Fisher's exact tests and Wilcoxon rank sum tests were used to evaluate for differences between groups for categorical and continuous secondary outcomes, respectively. Wilcoxon signed rank test was used to evaluate within treatment group change. The 95% confidence intervals for median changes within treatment group were estimated using distribution-free method via percentiles. For the BMD outcomes modification of the treatment effect by screening vitamin D stratum was evaluated via linear regression. All statistical tests were two-sided and interpreted at the 5% nominal level of significance without adjustment for multiple comparisons. Analyses were performed using the following procedures in SAS version 9.2 and Cytel Proc StatXact package version 9: FREQ, STRATIFY, PAIRED, UNIVARIATE, REG, MI, and MIANALYZE).
Role of the Funding Source
The National Institute of Allergy and Infectious Diseases funded the study. Industry sponsors provided antiretrovirals, vitamin D, calcium and matching placebos and additional funding was provided for completion of DXA scans and laboratory assays. Representatives from Bristol Meyers Squibb and Gilead Sciences served as members of the study team. NIAID had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The manuscript was reviewed by industry sponsors prior to submission.
Results
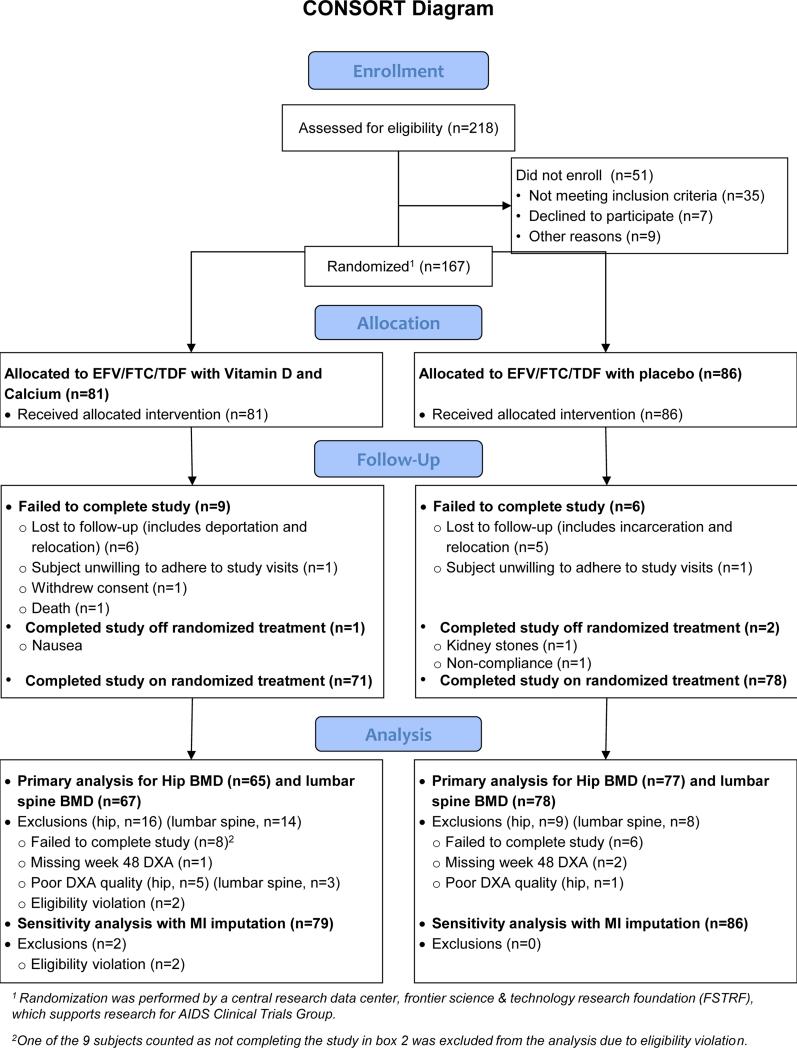
We enrolled 167 subjects between September 2011 and February 2012, (81 in VitD/Cal group and 86 in placebo group) from 39 sites in the US and Puerto Rico. Two subjects, both from VitD/Cal group, were found to have eligibility violations due to incorrect screening test being performed (1,25(OH)2D rather than 25(OH)D). At the time of discovery and blinded to treatment group assignment and all on-study data, the protocol team decided that these subjects could continue to receive study treatment, but their data would not be included in efficacy analyses. Baseline demographic, immunologic, virologic, and other parameters of the 165 eligible study subjects are summarized in table 1. Overall, the cohort was 90% male, 33% non-Hispanic black, 37% non-Hispanic white, and 25% Hispanic; median CD4 cell count of 341 cells/mm3 (230, 490 cells/mm3); and no subjects reported HBV or HCV co-infection. Overall, 148 subjects (90%) completed study follow-up with 9 discontinuations in VitD/Cal and 8 in placebo group. Loss to follow-up (including relocation and incarceration) and noncompliance were the most commonly cited reasons; additionally 3 subject (1 in VitD/Cal, 2 in placebo group) completed follow-up off study treatment. Twenty-five subjects discontinued EFV/FTC/TDF prematurely (13 in VitD/Cal and 12 in placebo group). While week 48 outcomes were unavailable for a greater number of subjects in the VitD/Cal group compared to the placebo, reasons for missing outcomes did not appear related to any of the outcomes of interest (figure 1).
Table 1.
Baseline Demographics
| Parameter | Vitamin D / Calcium (n=79) | Placebo (n=86) |
|---|---|---|
| Age in years* | 36 (28, 47) | 31 (25, 44) |
| Race/ethnicity | ||
| White non-Hispanic | 28 (35%) | 33 (38%) |
| Black non-Hispanic | 24 (30%) | 30 (35%) |
| Hispanic | 23 (29%) | 18 (21%) |
| Other | 4 (6%) | 5 (6%) |
| Male sex | 72 (91%) | 77 (90%) |
| BMI (kg/m2)* | 25.0 (22.5, 28.2) | 24.0 (21.7, 27.2) |
| Plasma HIV RNA (log10cp/mL)* | 4.5 (4.1, 5.1) | 4.5 (4.0, 5.1) |
| > 100,000 cp/mL | 21 (27%) | 22 (26%) |
| CD4 cell count (cells/μl)* | 339 (230, 500) | 342 (232, 454) |
| < 200 cells/ μl | 17 (22%) | 15 (17%) |
| BMD at hip (g/cm2)* | 1.08 (0.96, 1.19) | 1.02 (0.94, 1.11) |
| Z score for BMD at hip* | 0.10 (−0.70, 0.70) | −0.20 (−0.80, 0.40) |
| < −2.0 | 4 (5%) | 5 (6%) |
| BMD at lumbar spine (g/cm2)* | 1.15 (1.03, 1.26) | 1.07 (0.99, 1.21) |
| Z score for BMD at lumbar spine* | 0.00 (−0.90, 0.70) | −0.50 (−1.30, 0.40) |
| < −2.0 | 7 (9%) | 9 (10%) |
| Estimated daily Ca intake (mg)*# | 813 (492, 1303) | 811 (365, 1227) |
| Estimated daily VitD intake (IU)*# | 120 (62, 215) | 137 (59, 279) |
Denotes median value with 25th and 75th percentile value in parentheses.
We performed three-day dietary recalls at entry to estimate daily vitamin D and calcium intake.
Note: Two ineligible subjects are not included in the table. These 2 subjects are both non-Hispanic males, ages 30 and 41,and were randomized to the Vitamin D/Calcium group.
Abbreviations: BMI: body mass index
Figure 1.
Details and Disposition of Study Participants.
No footnotes.
Vitamin D Changes
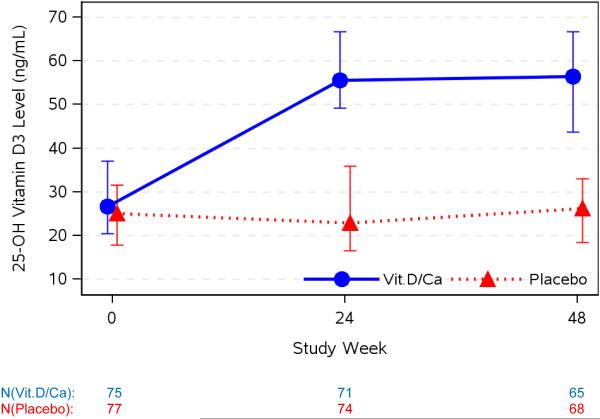
Median screening 25(OH)D level was 23 ng/mL (Q1,Q3: 18, 31 ng/mL) (57nmol/L; 45, 77 nmol/L). The median (Q1,Q3) estimated daily dietary calcium and vitamin D intake at entry were 813 mg (435, 1239 mg) and 131 IU (59, 260 IU), respectively. Of the entire cohort, 18% and 22% reported calcium and vitamin D supplementation, respectively, within 30 days of baseline. As expected, from entry to weeks 24 and 48, median 25(OH)D3 levels did not change in the placebo group: −0.6 (−5.9, 5.0) and 0.7 (−5.3, 4.3) ng/mL, respectively. Levels of 25(OH)D3 increased in the VitD/Cal group from entry to weeks 24 and 48: 28.6 (15.0, 38.0) and 24.5 (14.6, 37.8) ng/mL, respectively (p<0.001 at both time points). Group 25(OH)D3 distributions differed at both weeks 24 and 48 ( p<0.001 for both, figure 2).
Figure 2. 25(OH) Vitamin D3 Levels at Baseline, 24 and 48 Weeks.
25(OH) vitamin D3 levels at baseline, weeks 24 and 48 are presented in by study group. Data is presented as median value in ng/mL with error bars representing first and third quartiles (25th and 75th percentiles). From entry to weeks 24 and 48, 25(OH)D3 levels did not significantly change in the placebo group (median change (Q1,Q3): −0.6 (−5.9, 5.0) and 0.7 (−5.3, 4.3) ng/mL, respectively) but levels increased significantly in the VitD/Cal group (median change: 28.6 (15.0, 38.0) and 24.5 (14.6, 37.8) ng/mL, respectively; p<0.001 at both time points). The consequence of these changes between group differences in the 25(OH)D3 distributions was significant at both weeks 24 and 48 ( p<0.001 for both).
BMD Changes
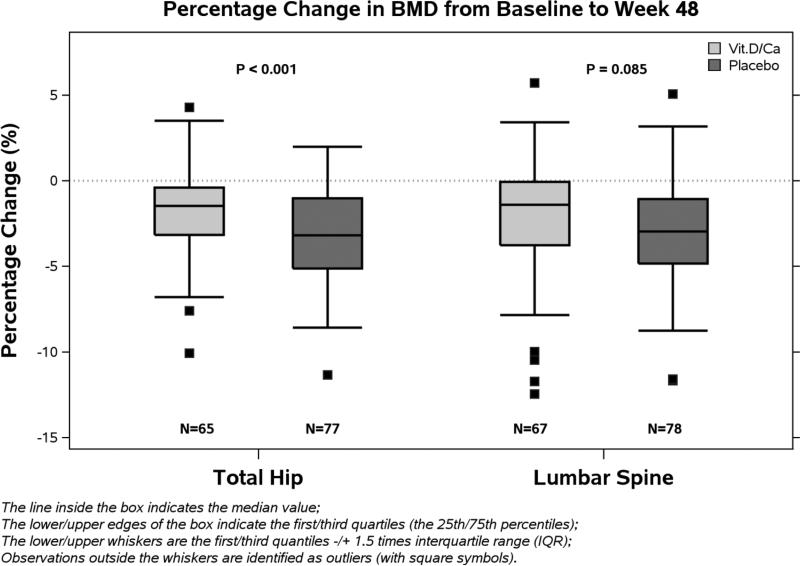
At baseline, median BMD (Q1,Q3) for the entire cohort at the total hip and lumbar spine were 1.05 g/cm2 (0.96, 1.17 g/cm2) and 1.11 g/cm2 (1.02, 1.21 g/cm2), respectively, with similar distributions between groups (table 1). Median Z-scores (Q1,Q3) for the entire cohort at hip and lumbar spine were 0.00 (−0.80, 0.50) and −0.30 (−1.20, 0.50), respectively, with similar distributions between groups. At 48 weeks, both groups demonstrated significant decreases in BMD at total hip from baseline (p< 0.001, for both). Using the multiple imputations approach, percentage decline in BMD at total hip was smaller in the VitD/Cal group compared to the placebo group: median (Q1, Q3) −1.36% (−3.43, 0.50%) and −3.22% (−5.56, −0.88%), respectively (P=0.004). Between-group differences at the lumbar spine were similar in magnitude to differences at the total hip (−1.23%, (−3.73, 0.20%) and −2.94% (−4.87, −0.94%), respectively) (p=0.033).
Our pre-specified complete case analysis approach yielded similar results; median (Q1, Q3) at the hip −1.46% (−3.16, −0.40%) versus −3.19% (−5.12, −1.02%) (p= 0.001) and (−1.41%; −3.78, 0.00% versus −2.91% (−4.84, −1.06%) (p=0.085) (figure 3). To evaluate whether changes in BMD were related to 25(OH)D levels, we assessed the changes in total hip BMD by screening 25(OH)D strata: 25(OH)D 10-20 ng/ml (25-50 nmol/L), or 20-75ng/mL (50-188 nmol/L). For the VitD/Cal group, the median decline (Q1,Q3) at 48 weeks was −1.33% (−3.24, −0.00%) in the low stratum (n=20) and −1.48% (−3.13, −0.66%) in the high stratum (n=45). For the placebo group, the median decline was −2.87% (−5.20, −1.34%) in the low stratum (n=24) and −3.19 (−5.12, −1.02%) in the high stratum (n=53). Modification of the treatment effect by screening 25(OH)D stratum was not apparent (interaction p=0.15).
Figure 3. Percent Change in Bone Mineral Density from Baseline to week 48.
Percent changes in bone mineral density from baseline to week 48 are presented in this figure: total hip on the left and lumbar spine on the right. For both groups there was a significant decline in median percent BMD change (p<0.001 for both sites and both groups). The percentage decline in BMD at total hip was significantly smaller in the VitD/Cal group (−1.46%; −3.16, −0.40%) compared to the placebo group (−3.19%; −5.12, −1.02%) (p= 0.001). The difference in BMD at lumbar spine between arms was of similar magnitude as the total hip, it did not achieve statistical significance: VitD/Cal group (−1.41%; −3.78, 0.00%) compared to the placebo group (−2.91%; −4.84, −1.06%) (p=0.085).
Bone Turnover Marker and PTH Changes
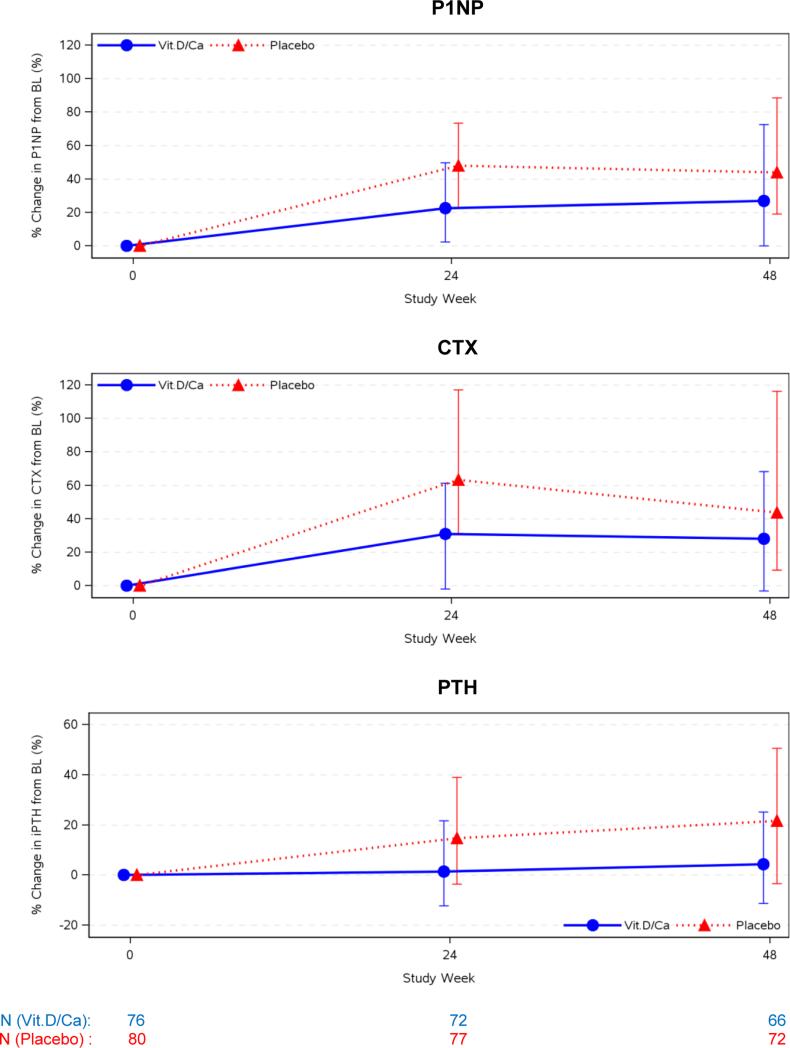
At baseline, bone turnover marker distributions were similar in the VitD/Cal and placebo groups for both P1NP, a bone formation marker (52 ng/mL (41, 67ng/mL) versus 45 ng/mL (36, 65 ng/mL), respectively) and CTX, a bone resorption marker (0.41 ng/mL (0.30, 0.53 ng/mL) versus 0.34 ng/mL (0.25, 0.51 ng/mL), respectively). Both groups experienced increases in these markers at 24 and 48 weeks after ART initiation but increases were attenuated in the VitD/Cal group compared to the placebo group at week 24 (p=0.002 and 0.005 for P1NP and CTX, respectively) (figure 4, table 2). Also, PTH increased 15% and 22% from baseline to week 24 and week 48 (p≤0.001 for both) in the placebo group, while a change from baseline was not apparent in the VitD/Ca group (1.4%, p=0.18 and 4.3%, p=0.17), (between group comparison: P<0.011, figure 4). Changes in urinary fractional excretion of phosphate from baseline to week 24 or 48 were not apparent in either group (Table 2).
Figure 4. Percent Change in Bone Turnover Markers and PTH at 24 and 48 Weeks.
Percent changes in biomarkers related to bone turnover from baseline to week 24 and 48 are presented in this figure: N-terminal propeptides of procollagen type 1 (P1NP), C-telopeptide (CTX), and parathyroid hormone (PTH) are presented in the top, middle, and bottom panels, respectively. Data is presented as median percent increase from baseline with error bars representing first and third quartiles (25th and 75th percentiles. In the upper and middle panels, P1NP and CTX increased significantly from baseline to week 24 and 48 in both groups (p< 0.001, for all). At week 24, the between group differences were also statistically significant (p= 0.002 for P1NP, p=0.005 for CTX) but the differences were not statistically significant at week 48 (p> 0.088 for both). The lower panel displays the percent change in PTH. In the VitD/Cal group, there was no significant change in PTH at week 24 or 48 (p>0.31 for both), while the placebo group experienced a significant increase at both 24 and 48 weeks (p< 0.001, for both). The between group differences were statistically significant for both time points (p=0.011 at 24 weeks and p=0.004 at 48 weeks)
Table 2.
Summary of BMD, Vitamin D, Bone Turnover Markers, and Soluble Inflammatory Biomarkers at Baseline, 24 weeks, and 48 weeks
| Parameter | Group | Baseline | Week 24 | P value* | Week 48 | P value* |
|---|---|---|---|---|---|---|
| BMD at hip (g/cm2) | VitD/Ca | 1.08 (0.96, 1.19) | Not Assessed | N/A | 1.05 (0.98, 1.17)‡ | <0.001 |
| Placebo | 1.02 (0.94, 1.11) | 0.98 (0.90, 1.07)‡ | ||||
| BMD at lumbar spine (g/cm2) | VitD/Ca | 1.15 (1.04, 1.26) | Not Assessed | N/A | 1.12 (1.03, 1.24)‡ | 0.085 |
| Placebo | 1.07 (0.99, 1.21) | 1.05 (0.94, 1.18)‡ | ||||
| Total 25(OH) D3 (ng/mL) | VitD/Ca | 26.7 (20.4, 37.1) | 55.6 (49.1, 66.7)‡ | <0.001 | 56.4 (43.7, 66.6)‡ | <0.001 |
| Placebo | 25.1 (17.8, 31.5) | 22.9 (16.6, 35.9) | 26.2 (18.4, 32.9) | |||
| P1NP (ng/mL) | VitD/Ca | 52 (41, 67) | 72 (81, 86)‡ | 0.002 | 68 (48, 80)‡ | 0.088 |
| Placebo | 45 (36, 65) | 75 (55, 88)‡ | 73 (51, 91)‡ | |||
| CTX (ng/mL) | VitD/Ca | 0.41 (0.30, 0.53) | 0.53 (0.37, 0.74)‡ | 0.005 | 0.51 (0.37, 0.75)‡ | 0.12 |
| Placebo | 0.34 (0.25, 0.51) | 0.62 (0.37, 0.85)‡ | 0.56 (0.36, 0.74)‡ | |||
| PTH (pg/mL) | VitD/Ca | 28.3 (24.5, 34.5) | 29.2 (24.7, 35.5) | 0.011 | 30.0 (25.2, 37.2) | 0.004 |
| Placebo | 27.6 (22.1, 33.9) | 32.9 (25.7, 42.5)‡ | 33.1 (26.8, 42.1)‡ | |||
| Percent Fractional | VitD/Ca | 8.4 (5.9, 11.1) | 8.4 (6.1, 12.2) | 0.77 | 8.4 (6.2, 12.5) | 0.66 |
| Excretion of PO4 (%) | Placebo | 7.9 (4.9, 10.6) | 8.4 (5.7, 10.7) | 9.2 (5.9, 12.4) | ||
| CD4 Count (cells/μl) | VitD/Ca | 346 (238, 495) | 487 (346, 650)‡ | 0.73 | 551 (414, 733)‡ | 0.90 |
| Placebo | 337 (266, 468) | 507 (361, 646)‡ | 526 (410, 732)‡ | |||
| IL-6 (log10 pg/mL) | VitD/Ca | 0.08 (−0.10, 0.31) | 0.02 (−0.19, 0.26) | 0.98 | 0.00 (−0.12, 0.25) | 0.70 |
| Placebo | 0.12 (−0.09, 0.29) | 0.08 (−0.12, 0.23) | −0.01 (−0.20, 0.32) | |||
| sTNFr-I (log10 pg/mL) | VitD/Ca | 3.04 (2.97, 3.13) | 3.01 (2.95, 3.09) | 0.65 | 3.02 (2.97, 3.07)‡ | 0.81 |
| Placebo | 3.04 (2.98, 3.10) | 3.02 (2.98, 3.08)‡ | 3.03 (2.95, 3.08)‡ | |||
| sTNFr-II (log10 pg/mL) | VitD/Ca | 3.57 (3.44, 3.68) | 3.43 (3.33, 3.51)‡ | 0.76 | 3.39 (3.30, 3.49)‡ | 0.73 |
| Placebo | 3.59 (3.47, 3.68) | 3.43 (3.35, 3.54)‡ | 3.43 (3.31, 3.49)‡ | |||
| sCD14 (log10 ng/mL) | VitD/Ca | 3.14 (3.06, 3.26) | 3.19 (3.09, 3.31) | 0.76 | 3.22 (3.14, 3.33)‡ | 0.16 |
| Placebo | 3.20 (3.10, 3.29) | 3.22 (3.13, 3.34) | 3.24 (3.12, 3.30) |
All data presented as median values with 25th and 75th percentile value in parentheses.
denotes within-arm difference from baseline is statistically significant (p< 0.05).
P values -evaluate the difference in changes from baseline to week 24/48 between the two treatment groups, stratified by screening 25-OH vitamin D levels; statistically significant differences are bolded.
Abbreviations: P1NP: N-terminal propeptide of procollagen type 1; CTX: C-telopeptide; IL-6: interleukin 6; sTNFr-I and II: soluble receptors of tumor necrosis factor alpha; sCD14: soluble CD14
Inflammatory biomarker changes
At baseline, the distributions of IL-6, sTNFr-I, sTNFr-II, and sCD14, were similar between the two groups (Table 2). There were no significant changes in IL-6 at week 24 or 48 in either treatment group (p> 0.07 for all) or between groups at either time point (p≥0.70 for both). A within-group decline in sTNFr-I at week 24 and week 48 was seen in the placebo group (p =0.016, and 0.041, respectively) although changes were modest (median decrease: 64 and 62 pg/mL at week 24 and 48, respectively). For the VitD/Cal group, there was a similar modest decline at week 48 (median decrease: 52 and 94 pg/mL at week 24 and 48 (p=0.071 and 0.005, respectively). However, differences in magnitude of changes between groups were not apparent (p> 0.65 for both time points). Decreases in sTNFr-II were seen at weeks 24 and 48 for both treatment groups (p<0.001 for all), but there were no differences between arms (p> 0.73 for both). While a 17% increase in sCD14 from baseline to week 48 was observed in the VitD/Cal group (p=0.008), a change was not apparent in the placebo group (5% median increase, p=0.18). Differences in the change in sCD14 between groups at week 24 and 48 were not apparent (p>0.16 for both).
HIV disease parameters
Total CD4 T-cell count increased at weeks 24 and 48 in both groups (p<0.001 for all) with no differences between groups (p≥0.28 for both). At 48 weeks, the median gain (Q1,Q3) was 192 (113, 305) and 201 (108, 292) cells/μL in the VitD/Cal and placebo groups, respectively (Table 2). Overall, 90% of subjects achieved virologic suppression (plasma HIV RNA < 50cp/mL) at 48 weeks; four subjects in the VitD/Cal group and 10 subjects in the placebo group experienced virologic failure (defined as >200cp/mL confirmed with a second evaluation on or after week 24).
Safety
Overall, 103 subjects (62%) reported at least one adverse event during the study: 50 subjects in VitD/Cal group (33 Grade 1-2, 15 Grade 3, 2 Grade 4) and 53 subjects in placebo group (33 Grade 1-2, 15 Grade 3, 5 Grade 4). No clear pattern emerged with regards to AEs (Table 3). There were no cases of hypercalcemia, and 1 case of nephrolithiasis in the placebo group. One death occurred in the VitD/Cal group due to renal failure in the context of rapid HIV disease progression.
Table 3.
Summary of Grade 3 and 4 Adverse Events
| Primary event | Grade | VitD/Ca | Placebo |
|---|---|---|---|
| Headache | 3 | 0 | 1 |
| Seizure | 3 | 1 | 0 |
| Aseptic Meningitis | 3 | 1 | 0 |
| Depression | 3 | 1 | 1 |
| Mallory Weiss Tear | 3 | 1 | 0 |
| Gastroenteritis | 3 | 0 | 2 |
| Abdominal pain | 3 | 1 | 0 |
| Cholecystitis | 4 | 1 | 0 |
| Alcoholic Hepatitis | 3 | 0 | 1 |
| Anal AIN 3 | 3 | 0 | 1 |
| Asthma exacerbation | 1 | 0 | 1 |
| Bacterial pneumonia | 3 | 0 | 1 |
| Back pain | 3 | 0 | 1 |
| Shoulder dislocation | 3 | 0 | 1 |
| Left hand laceration | 3 | 1 | 0 |
| Right knee effusion | 3 | 0 | 1 |
| R foot abscess | 3 | 0 | 1 |
| Weight loss | 3 | 1 | 0 |
| Coagulopathy | 3 | 0 | 1 |
| Lyme Disease | 3 | 0 | 1 |
| Immune Reconstitution | 3 | 1 | 0 |
| Inflammatory syndrome | |||
| DRESS Syndrome | 3 | 0 | 1 |
| LAB ABNORMALITIES | |||
| Absolute Neutrophil Count | 3 | 1 | 3 |
| Serum Phosphate | 3 | 4 | 1 |
| ALT | 3 | 1 | 3 |
| AST | 3 | 1 | 2 |
| 4 | 0 | 1 | |
| Alkaline Phosphatase | 3 | 0 | 1 |
| Total Bilirubin | 3 | 1 | 0 |
| Triglycerides | 3 | 0 | 1 |
| Total Cholesterol | 3 | 1 | 0 |
| Glucose | 3 | 2 | 3 |
| 4 | 0 | 2 |
Discussion
Supplementation with high dose vitamin D3 (4000IU) and calcium carbonate (1000mg) with ART initiation increased 25(OH)D levels, attenuated bone turnover marker increases and bone loss at the hip and lumbar spine by approximately 50% at 48 weeks. These results mark the first successful intervention to attenuate bone loss with ART initiation and demonstrate the benefit of vitamin D and calcium supplementation to promote bone health in HIV-infected persons. The bone loss associated with ART initiation leaves HIV-infected persons with lower bone mass, potentially contributing to increased fracture risk (2-7). With the lifespan of HIV-infected persons now approaching that of seronegative individuals(47), the incidence of fragility fractures will undoubtedly increase (3,6). Few guidelines have addressed preventive measures in HIV-infected individuals (48).
Tenofovir (TDF) is associated with BMD decline (16) not only among HIV-infected persons starting ART but also in seronegative individuals using TDF as a component of Pre-Exposure Prophylaxis (PrEP) and in seronegative newborns exposed to TDF in utero (49,50). Recent studies have explored the association between TDF bone toxicity, proximal tubule toxicity and increased PTH levels (50). In our study, ART initiation with a TDF-based regimen increased PTH and vitamin D and calcium supplementation prevented increases in PTH levels, without altering urinary phosphate excretion, suggesting that TDF does not cause increase PTH through proximal renal tubule toxicity. Similar results were reported by Havens and colleagues (22,23) in a study of vitamin D supplementation, reporting that PTH levels decreased with vitamin D supplementation without change in urinary phosphate excretion, and the greatest effect on PTH was seen in persons receiving TDF-containing ART. Given that PTH secretion is regulated by serum calcium, it is plausible that increased intestinal calcium absorption mediated by vitamin D is the key mechanism blocking the negative bone effects of TDF.
Efavirenz is a potent inducer of cytochrome P450 enzymes. Several of these enzymes are involved in vitamin D metabolism and efavirenz may have the detrimental off-target effect of reducing available vitamin D substrate and active metabolites (25,51). In contrast to prior observations, we saw only a modest non-significant decline in the 25(OH)D levels at 24 weeks in the placebo group (24,25,52). In the group that received VitD/Cal, efavirenz did not prevent increases in 25(OH)D. We did not measure 1,25(OH)2D3, the active metabolite, and thus cannot comment how supplementation affected ongoing vitamin D metabolism.
Given recent data on the immunomodulatory effects of vitamin D (29,53), we explored whether the diminished BMD loss in the VitD/Cal group was immune-mediated. Immune reconstitution with ART-initiation may result in a surge in pro-resorptive cytokines, and potentially explain the relatively consistent magnitude of BMD decline within the first 24-48 weeks after initiation of ART across different regimens, and the more modest effect when ART is used as PrEP. In our study, sCD14 increased in the VitD/Cal group, likely related to vitamin D effects on monocyte CD14 expression (30), while TNFr levels decreased similarly from baseline to 48 weeks in both arms, likely related to virologic suppression, as previously reported (54). It is possible that the anti-inflammatory effects of ART initiation obscured any potential immunomodulatory effects of vitamin D. Additional research is needed to define the mechanism behind the salutary effect of vitamin D and calcium supplementation.
Our study has some limitations. We had an imbalance in the amount of missing data between groups, but a review of reasons for missing data (relocation, loss to follow up, death and missing or uninterpretable DXA data) did not reveal a systemic bias, but rather a chance imbalance in discontinuations. Furthermore, results for our primary outcome were similar when analyzed using a multiple imputation approach for missing data or a complete case approach that assumes that missing data was random. We followed subjects for only 48 weeks and did not evaluate longer-term effects of vitamin D supplementation on fracture prevention. Data on smoking status, alcohol intake, falls, and physical activity were not collected. We cannot determine if the beneficial effect was due to vitamin D, calcium or their combination. Our study was performed in the US and Puerto Rico and comprised of 90% male subjects, thus generalizability to other areas and females may be limited. We evaluated only one ART regimen (EFV/FTC/TDF); therefore, our conclusions may not be applicable to other antiretroviral regimens.
In summary, daily high dose vitamin D and calcium supplementation over 48 weeks attenuates BMD decline associated with ART initiation by approximately 50%. This beneficial effect corresponded with reductions in bone turnover markers and limited excursion of PTH levels. We did not observe a specific anti-inflammatory effect of vitamin D supplementation. Vitamin D and calcium is a low cost, well-tolerated intervention to prevent ART-related bone loss. Future studies will examine alternative vitamin D doses, effects when utilized with other ART regimen and in international settings, and longer-term efficacy.
Acknowledgements
We wish to gratefully acknowledge all of the study sites and study participants who have devoted their time and effort to this research endeavor. We also would like to acknowledge the additional members of the A5280 study team who contributed to the study: Jennifer Rothenberg, Kathy Watson, Lara Hosey, Tia Frazier, Bernadette Jarocki, Lynette Purdue, John Adams, Allan Tenorio, Laura Blair, and Amy Gonzalez. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Funding: The project described was supported by Award Number U01AI068636 from the National Institute of Allergy and Infectious Diseases. Bristol-Myers Squibb and Gilead funded the DXA scans and ancillary laboratory testing. Study medications were provided by Bristol-Myers Squibb, Gilead Sciences, and Tischcon Corporation.
Footnotes
clinicalTrials.gov Identifier NCT01403051
References
- 1.Weber R, Ruppik M, Rickenbach M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013;14:195–207. doi: 10.1111/j.1468-1293.2012.01051.x. [DOI] [PubMed] [Google Scholar]
- 2.Yin MT, Kendall MA, Wu X, et al. Fractures after Antiretroviral Initiation. AIDS. 2012 Nov 13;26(17):2175–84. doi: 10.1097/QAD.0b013e328359a8ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young B, Dao CN, Buchacz K, et al. HIV Outpatient Study (HOPS) Investigators Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000-2006. Clin Infect Dis. 2011 Apr 15;52(8):1061–8. doi: 10.1093/cid/ciq242. [DOI] [PubMed] [Google Scholar]
- 4.Womack JA, Goulet JL, Gibert C, et al. Increased risk of fragility fractures among HIV-infected compared to uninfected male veterans. PLoS One. 2011;6:e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedimo R, Maalouf NM, Zhang S, et al. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012 Apr 24;26(7):825–31. doi: 10.1097/QAD.0b013e32835192ae. [DOI] [PubMed] [Google Scholar]
- 6.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008 Sep;93(9):3499–504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen AB, Gerstoft J, Kronborg G, et al. Incidence of low and high-energy fractures in persons with and without HIV infection: a Danish population-based cohort study. AIDS. 2012 Jan 28;26(3):285–93. doi: 10.1097/QAD.0b013e32834ed8a7. [DOI] [PubMed] [Google Scholar]
- 8.Tebas P, Powderly WG, Claxton S, Marin D, Tantisiriwat W, Teitelbaum SL, Yarasheski KE. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS. 2000 Mar 10;14(4):F63–7. doi: 10.1097/00002030-200003100-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez-Vallejo SJ, Beaupere C, Larghero J, et al. HIV protease inhibitors induce senescence and alter osteoblastic potential of human bone marrow mesenchymal stem cells: beneficial effect of pravastatin. Aging Cell. 2013 Dec;12(6):955–65. doi: 10.1111/acel.12119. [DOI] [PubMed] [Google Scholar]
- 10.Yin MT, Lund E, Shah J, et al. Lower peak bone mass and abnormal trabecular and cortical microarchitecture in young men infected with HIV early in life. AIDS. 2014 Jan 28;28(3):345–53. doi: 10.1097/QAD.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown TT, Ross AC, Storer N, et al. Bone turnover, osteoprotegerin/RANKL and inflammation with antiretroviral initiation: tenofovir versus non-tenofovir regimens. Antivir Ther. 2011;16(7):1063–72. doi: 10.3851/IMP1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant PM, Kitch D, McComsey GA, et al. Low Baseline CD4+ Count Is Associated With Greater Bone Mineral Density Loss After Antiretroviral Therapy Initiation. Clin Infect Dis. 2013 Nov;57(10):1483–8. doi: 10.1093/cid/cit538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolland MJ, Grey AB, Gamble GD, Reid IR. Low body weight mediates the relationship between HIV infection and low bone mineral density: a meta-analysis. J Clin Endocrinol Metab. 2007;92:4522–8. doi: 10.1210/jc.2007-1660. [DOI] [PubMed] [Google Scholar]
- 14.Vikulina T, Fan X, Yamaguchi M, Roser-Page S, Zayzafoon M, Guidot DM, Ofotokun I, Weitzmann MN. Alterations in the immuno-skeletal interface drive bone destruction in HIV-1 transgenic rats. Proc Natl Acad Sci U S A. 2010 Aug 3;107(31):13848–53. doi: 10.1073/pnas.1003020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011 Jun 15;203(12):1791–801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, Coakley DF, Lu B, Toole JJ, Cheng AK, 903 Study Group Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004 Jul 14;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 17.Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51:963–72. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 18.Duvivier C, Kolta S, Assoumou L, Ghosn J, Rozenberg S, Murphy RL, Katlama C, Costagliola D. ANRS 121 Hippocampe study group. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS. 2009 Apr 27;23(7):817–24. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- 19.Recker R, Lappe J, Davies K, Heaney R. Characterization of perimenopausal bone loss: a prospective study. J Bone Mineral Res. 2000;15(10):1965–73. doi: 10.1359/jbmr.2000.15.10.1965. [DOI] [PubMed] [Google Scholar]
- 20.Aloia JF, Dhaliwal R, Shieh A, Mikhail M, Islam S, Yeh JK. Calcium and vitamin d supplementation in postmenopausal women. J Clin Endocrinol Metab. 2013 Nov;98(11):E1702–9. doi: 10.1210/jc.2013-2121. [DOI] [PubMed] [Google Scholar]
- 21.Cotter AG, Mallon PW. The effects of untreated and treated HIV infection on bone disease. Curr Opin HIV AIDS. 2014 Jan;9(1):17–26. doi: 10.1097/COH.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 22.Havens PL, Stephensen CB, Hazra R, Flynn PM, Wilson CM, Rutledge B, Bethel J, Pan CG, Woodhouse LR, Van Loan MD, Liu N, Lujan-Zilbermann J, Baker A, Kapogiannis BG, Mulligan K. Adolescent Medicine Trials Network (ATN) for HIV/AIDS Interventions 063 study team. Vitamin D3 decreases parathyroid hormone in HIV-infected youth being treated with tenofovir: a randomized, placebo-controlled trial. Clin Infect Dis. 2012 Apr;54(7):1013–25. doi: 10.1093/cid/cir968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havens PL, Kiser JJ, Stephensen CB, Hazra R, Flynn PM, Wilson CM, Rutledge B, Bethel J, Pan CG, Woodhouse LR, Van Loan MD, Liu N, Lujan-Zilbermann J, Baker A, Kapogiannis BG, Gordon CM, Mulligan K. Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) 063 Study Team. Association of higher plasma vitamin d binding protein and lower free calcitriol levels with tenofovir disoproxil fumarate use and plasma and intracellular tenofovir pharmacokinetics: cause of a functional vitamin D deficiency? Antimicrob Agents Chemother. 2013 Nov;57(11):5619–28. doi: 10.1128/AAC.01096-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther. 2010;15(3):425–9. doi: 10.3851/IMP1502. [DOI] [PubMed] [Google Scholar]
- 25.Wohl DA, Orkin C, Doroana M, Pilotto JH, Sungkanuparph S, Yeni P, Vanveggel S, Deckx H, Boven K. Change in vitamin D levels and risk of severe vitamin D deficiency over 48 weeks among HIV-1-infected, treatment-naive adults receiving rilpivirine or efavirenz in a Phase III trial (ECHO). Antivir Ther. 2014;19(2):191–200. doi: 10.3851/IMP2721. [DOI] [PubMed] [Google Scholar]
- 26.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. 2014 Jan;55(1):13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, Lu B, McColl D, Chuck S, Enejosa J, Toole JJ, Cheng AK, Study 934 Group Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006 Jan 19;354(3):251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 28.Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011 Mar;50(3):194–200. doi: 10.1016/j.jcv.2010.12.006. PMID:21242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang CL, Wang MH, Chiang CK, Lu KC. Vitamin D and the Immune System from the Nephrologist's Viewpoint. ISRN Endocrinol. 2014 Jan 22;2014:105456. doi: 10.1155/2014/105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, Tangpricha V. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009 Apr 23;7:28. doi: 10.1186/1479-5876-7-28. doi: 10.1186/1479-5876-7-28. PMID:19389235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009 Apr 1;182(7):4289–95. doi: 10.4049/jimmunol.0803736. PMID:19299728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005 Oct;97(1-2):93–101. doi: 10.1016/j.jsbmb.2005.06.002. PMID:16046118. [DOI] [PubMed] [Google Scholar]
- 33.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001 Nov 1;167(9):4974–80. doi: 10.4049/jimmunol.167.9.4974. PMID: 11673504. [DOI] [PubMed] [Google Scholar]
- 34.Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, Lederman MM, Storer N, Labbato DE, McComsey GA. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014 Apr 24;28(7):969–77. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206:1558–1567. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217:207–213. doi: 10.1016/j.atherosclerosis.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Adv Immunol. 2013;119:51–83. doi: 10.1016/B978-0-12-407707-2.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, FaNBIoM Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. National Academy Press; Washington, D.C.: 1997. [Google Scholar]
- 39.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007 Jan;85(1):6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 40.Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007 Mar;85(3):649–50. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 41.Mitka M. More evidence on low vitamin D levels fuels push to revise recommended intake. JAMA. 2009 Dec 16;302(23):2527–8. doi: 10.1001/jama.2009.1788. [DOI] [PubMed] [Google Scholar]
- 42.Longenecker CT, Hileman CO, Carman TL, Ross AC, Seydafkan S, Brown TT, Labbato DE, Storer N, Tangpricha V, McComsey GA. Vitamin D supplementation and endothelial function in vitamin D deficient HIV-infected patients: a randomized placebo-controlled trial. Antivir Ther. 2012;17(4):613–21. doi: 10.3851/IMP1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD. INSIGHT SMART Study Group. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008 Oct 21;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, Kuller LH, Pett SL, Ristola M, Ross MJ, Shlipak MG, Tracy R, Neaton JD. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010 Jun 15;201(12):1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar A, Abbas W, Herbein G. TNF and TNF receptor superfamily members in HIV infection: new cellular targets for therapy? Mediators Inflamm. 2013;2013:484378. doi: 10.1155/2013/484378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, Lederman MM, Storer N, Labbato DE, McComsey GA. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014 Apr 24;28(7):969–77. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sørensen HT, Vaeth M, Obel N. Survival of persons with and without HIV infection in Denmark, 1995-2005. Ann Intern Med. 2007 Jan 16;146(2):87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 48.McComsey GA, Tebas P, Shane E, et al. Bone Disease in HIV: A Practical Review and Recommendations for HIV Providers. CID. 2010;51:937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu AY, Vittinghoff E, Sellmeyer DE, Irvin R, Mulligan K, Mayer K, Thompson M, Grant R, Pathak S, O'Hara B, Gvetadze R, Chillag K, Grohskopf L, Buchbinder SP. Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco. PLoS One. 2011;6(8):e23688. doi: 10.1371/journal.pone.0023688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siberry GK, Jacobson DL, Kalkwarf H, Wu JW, DiMeglio LA, Yogev R, Knapp KM, Wheeler J, Hazra R, Rich KC. Lower Newborn Bone Mineral Content Associated with Maternal Use of Tenofovir Disoproxil Fumarate.. Program and Abstracts from Conference on Retroviruses and Opportunistic Infections 2014; Boston MA, USA. March 3-6, 2014; 2014. p. 35. Abstract 71. Published in Topics in Antiviral Medicine. [Google Scholar]
- 51.Rosenvinge MM, Gedela K, Copas AJ, Wilkinson A, Sheehy CA, Bano G, Hay PE, Pakianathan MR, Sadiq ST. Tenofovir-linked hyperparathyroidism is independently associated with the presence of vitamin D deficiency. J Acquir Immune Defic Syndr. 2010 Aug;54(5):496–9. doi: 10.1097/qai.0b013e3181caebaa. [DOI] [PubMed] [Google Scholar]
- 52.Welz T, Childs K, Ibrahim F, Poulton M, Taylor CB, Moniz CF, Post FA. Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. AIDS. 2010;24:1923–1928. doi: 10.1097/QAD.0b013e32833c3281. [DOI] [PubMed] [Google Scholar]
- 53.Khoo AL, Chai L, Koenen H, Joosten I, Netea M, van der Ven A. Translating the role of vitamin D3 in infectious diseases. Crit Rev Microbiol. 2012 May;38(2):122–35. doi: 10.3109/1040841X.2011.622716. [DOI] [PubMed] [Google Scholar]
- 54.McComsey GA, Kitch D, Daar ES, Tierney C, Jahed NC, Melbourne K, Ha B, Brown TT, Bloom A, Fedarko N, Sax PE. Inflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavir. AIDS. 2012 Jul 17;26(11):1371–85. doi: 10.1097/QAD.0b013e328354f4fb. [DOI] [PMC free article] [PubMed] [Google Scholar]