Abstract
Aim
Epidemiologic studies have demonstrated high rates of smoking among alcoholics, and neuroimaging studies have detected white matter atrophy and degeneration in both smokers and individuals with alcohol-related brain disease (ARBD). These findings suggest that tobacco smoke exposure may be a co-factor in ARBD. The present study examines the differential and additive effects of tobacco-specific nitrosamine (NNK) and ethanol exposures on the structural and functional integrity of white matter in an experimental model.
Methods
Adolescent Long Evans rats were fed liquid diets containing 0 or 26% ethanol for 8 weeks. In weeks 3–8, rats were treated with nicotine-derived nitrosamine ketone (NNK) (2 mg/kg, 3×/week) or saline by i.p. injection. In weeks 7–8, the ethanol group was binge-administered ethanol (2 g/kg; 3×/week).
Results
Ethanol, NNK and ethanol + NNK caused striking degenerative abnormalities in white matter myelin and axons, with accompanying reductions in myelin-associated glycoprotein expression. Quantitative RT-PCR targeted array and heatmap analyses demonstrated that ethanol modestly increased, whereas ethanol + NNK sharply increased expression of immature and mature oligodendroglial genes, and that NNK increased immature but inhibited mature oligodendroglial genes. In addition, NNK modulated expression of neuroglial genes in favor of growth cone collapse and synaptic disconnection. Ethanol- and NNK-associated increases in FOXO1, FOXO4 and NKX2-2 transcription factor gene expression could reflect compensatory responses to brain insulin resistance in this model.
Conclusion
Alcohol and tobacco exposures promote ARBD by impairing myelin synthesis, maturation and integrity via distinct but overlapping mechanisms. Public health measures to reduce ARBD should target both alcohol and tobacco abuses.
INTRODUCTION
Alcohol targets central nervous system (CNS) white matter (WM) oligodendrocytes and myelin
Alcohol abuse and addiction cause neurobehavioral abnormalities, impairments in cognitive and executive functions (Schmidt et al., 2005; Chanraud et al., 2007; Elofson et al., 2013; Jacobus et al., 2013; de la Monte and Kril, 2014), dementia and disability (Sutherland et al., 2013) which are associated with brain atrophy (Harper, 1982), selective loss of WM volume (de la Monte, 1988) and structural abnormalities in myelin and axons (de la Monte, 1988; Harper et al., 2003; Baker et al., 2013; de la Monte and Kril, 2014). High resolution magnetic resonance imaging (MRI) coupled with generalized fractional anisotropy revealed that alcohol-related brain degeneration (ARBD)-associated white matter atrophy is associated with reduced micro-structural integrity of the fibers (Bava et al., 2013). Furthermore, an etiopathic role for alcohol in ARBD-associated white matter atrophy was shown by the partial reversal of macro- and micro-structural lesions, metabolic dysfunction and neurobehavioral abnormalities that occur with abstinence (de la Monte and Kril, 2014).
Neuroimaging and postmortem studies showed that the severities of WM atrophy and degeneration correlate with maximum daily and lifetime alcohol exposures (Harper and Kril, 1985; Harper et al., 2003; Sutherland et al., 2013; de la Monte and Kril, 2014). The corpus callosum, one of the main targets, is significantly atrophied in alcoholics (Estruch et al., 1997; Pfefferbaum et al., 2007). Atrophy of the corpus callosum impairs inter-hemispheric communication, compromising exchange of sensory, motor and cognitive information. Besides the corpus callosum, prefrontal, temporal and cerebellar WM are also damaged by alcohol (Phillips et al., 1987; de la Monte and Kril, 2014). In both immature and mature brains, WM oligodendrocytes and myelin are targeted by alcohol (Harper et al., 1985; de la Monte, 1988; Kril et al., 1989; Kril and Halliday, 1999; de la Monte and Kril, 2014) due to its direct neurotoxic effects, and impairment of insulin and insulin-like growth factor (IGF) signaling (Cohen et al., 2007; de la Monte et al., 2009; de la Monte, 2012; Tong et al., 2015) needed for oligodendrocyte function, and myelin synthesis, maturation and maintenance (Chesik et al., 2008; Freude et al., 2008; Gong et al., 2008).
Oligodendrocyte development and function-effects of alcohol
Myelin is a specialized membrane that has a very high dry mass of lipids (70–85%) compared with proteins (15–30%). Oligodendrocytes produce and maintain CNS myelin which insulates and supports axons. Oligodendrocytes develop from oligodendrocyte precursor cells (OPC), which differentiate into immature followed by mature myelin producing oligodendrocytes. Mature oligodendrocytes express integral membrane proteins including myelin basic protein (MBP), myelin-associated glycoprotein (MAG), myelin oligodendrocyte glycoprotein (MOG) and proteolipid protein (PLP) (Bordner et al., 2011). Myelin PLP (30 kDa) is the most abundant protein in CNS myelin (Groseclose et al., 2007; Nicklay et al., 2013). Ethanol exposures adversely affect oligodendrocytes by delaying cellular maturation, developmental expression of MBP and MAG and de novo synthesis of myelin (Gnaedinger and Druse, 1984; Gnaedinger et al., 1984; Chiappelli et al., 1991). In addition, ethanol impairs insulin signaling along survival and metabolic pathways, in part by reducing insulin/IGF receptor binding (Soscia et al., 2006) and altering oligodendrocyte membrane phospholipid content and membrane fluidity (Wing et al., 1982; Harris et al., 1984; Qu et al., 1999). Moreover, ethanol inhibits de novo sphingolipid biosynthesis, reducing WM myelination and promoting cognitive impairment (Kwon et al., 1997).
Potential role of tobacco nitrosamine exposures on ARBD
Variability in the nature and severity of clinical and pathological features of ARBD suggests that co-factors contribute to its pathogenesis. Since a very high percentage of heavy drinkers/alcoholics (∼80%) also abuse tobacco products, typically by smoking cigarettes (Romberger and Grant, 2004; Kalman et al., 2010), and both heavy drinking and cigarette smoking adversely affect neurocognitive function (Durazzo et al., 2004, 2007a,b) and WM structure (neuroimaging) (Wang et al., 2009), consideration should be given to the concept that tobacco and its toxic metabolites may be co-factors in ARBD. Several neuroimaging studies have shown smoking-related brain abnormalities in humans (Brody et al., 2004; Gazdzinski et al., 2005; Gallinat et al., 2006; Almeida et al., 2008; Paul et al., 2008; Das et al., 2012; Durazzo et al., 2012, 2014; Liao et al., 2012; Fritz et al., 2014), and meta-analysis revealed smoking-related gray matter loss in structures typically targeted by alcohol (Pan et al., 2013). However, additional research is needed to evaluate the contributions of alcohol and/or tobacco smoke to the pathogenesis of ARBD. Furthermore, determining the agents in tobacco smoke that mediate ARBD and cognitive impairment could help with designing assays that monitor exposures and risk for neurodegeneration.
Adverse effects of nitrosamine ± ethanol exposures on the CNS
Alcohol-tobacco dual effects on carcinogenesis have been well described (Johnson et al., 1996; de Boer et al., 1997; Tramacere et al., 2010; Duell, 2012), particularly with respect to the tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its metabolites (Johnson et al., 1996; de Boer et al., 1997; Go et al., 2005; Tramacere et al., 2010; Duell, 2012). However, in low doses, nicotine-derived nitrosamine ketone (NNK) (Zabala et al., 2015) like other nitrosamines (Tong et al., 2009, 2010), has toxic-degenerative rather than carcinogenic effects. Previous reports showed that low-dose nitrosamine exposures, e.g. streptozotocin (Bolzan and Bianchi, 2002; Koulmanda et al., 2003; Wang et al., 2011) and N-nitrosodiethylamine (NDEA) (de la Monte and Tong, 2009; Tong et al., 2009) cause degenerative CNS effects mediated by insulin resistance, DNA damage, lipid peroxidation, mitochondrial dysfunction, ER stress and impairments in PI3K-Akt signaling (de la Monte and Tong, 2009; Tong et al., 2009). Further studies revealed: (a) NNK-induced neurotoxic effects in the brain (Ghosh et al., 2009); (b) additive and interactive effects of ethanol and NDEA on brain development in experimental fetal alcohol spectrum disorder (Andreani et al., 2014) and (c) additive or independent adverse effects of ethanol and NNK (sub-mutagenic) on cognitive function and insulin/IGF signaling in adolescent brains (Tong et al., 2015).
Study objectives
The present study tests the hypothesis that sub-mutagenic doses of NNK are sufficient to cause WM degeneration and possibly exacerbate adverse effects of alcohol with respect to the expression of oligodendrocyte myelin-associated genes and proteins needed for myelin synthesis and maintenance. NNK rather than tobacco smoke was used because smoking could confound the results by causing pulmonary disease and hypoxia (Gan et al., 2011; McCaskill et al., 2011). This work is an extension of a larger effort in which we have already characterized the effects of ethanol and NNK on liver structure and function (Zabala et al., 2015).
METHODS
Experimental model
The model and detailed methods including data analysis are described under Supplementary (S) Methods. In brief, Long Evans 4-week old male rats were chronically fed with liquid diets containing 0 or 26% ethanol by caloric content, and sub-groups (n = 8–10) were further treated with NNK (2 mg/kg), and/or ethanol binges (2 g/kg), or vehicle as control. Temporal lobes with hippocampi were used for molecular and histological studies.
Enzyme-linked immunosorbent assays (ELISAs)
Direct binding duplex ELISAs were used to measure immunoreactivity to MAG-1 and glial fibrillary acidic protein (GFAP), in which results were normalized to large acidic ribosomal protein (RPLPO) (Longato et al., 2012).
Targeted glial gene arrays
Targeted quantitative RT-PCR arrays were used to measure expression of genes linked to oligodendrocyte and astrocyte function (Supplementary Tables S1 and S2). The objective was to evaluate ethanol and NNK exposure effects on genes that are relevant to CNS myelin and white matter, rather than the entire database of rat genes.
Statistics
Inter-group comparisons were made using two-way analysis of variance (ANOVA) with Tukey or linear trend post hoc tests (GraphPad Prism 6, San Diego, CA, USA). Heatmaps were constructed using Version 3.1 of R software.
RESULTS
Ethanol and NNK-associated white matter pathology
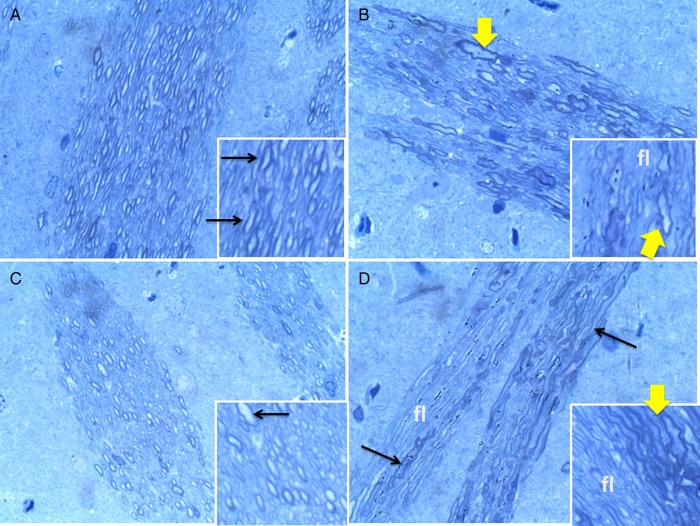
Brain tissue was fixed in glutaraldehyde and embedded in Epon resin (Ramirez et al., 2012). Toluidine Blue-stained 1-micrometer thick sections showed control white matter replete with well-myelinated fibers (Figure 1A). NNK reduced white matter fiber densities, and increased variation in fiber size, dystrophic enlargement of fibers and abundance of small, thinly myelinated fibers (Figure 1B). Ethanol reduced myelin thickness and decreased myelin staining intensity (Figure 1C). Ethanol + NNK produced striking abnormalities characterized by conspicuous loss of fibers, fiber atrophy, dystrophy and regenerative sprouting (Figure 1D). Altogether, ethanol caused demyelination or hypo-myelination and decreased large myelinated axons; NNK caused fiber loss and dystrophy, and dual exposures caused demyelination, axonal degeneration and fiber loss, i.e. the responses were additive or synergisic.
Fig. 1.
Ethanol and NNK cause degeneration of CNS myelinated fibers. Glutaraledhyde fixed, epon-embedded, 1 µM thick, Toluidine blue-stained sections of cerebral white matter demonstrating: (A) abundant large myelinated fibers in controls; (B) irregular dystrophic, dilated large myelinated fibers, small hypomyelinated fibers and fiber loss in NNK-exposed brains; (C) uniform populations of abundant small hypomyelinated axons in ethanol-fed rats and (D) fiber loss (fl), dystrophy and degeneration and fiber hypo-myelination in ethanol + NNK-treated rats (Solid arrows, swollen dystrophic axons; broad arrows, dystrophic axon). Original magnification, 650× (Insets, 1200×).
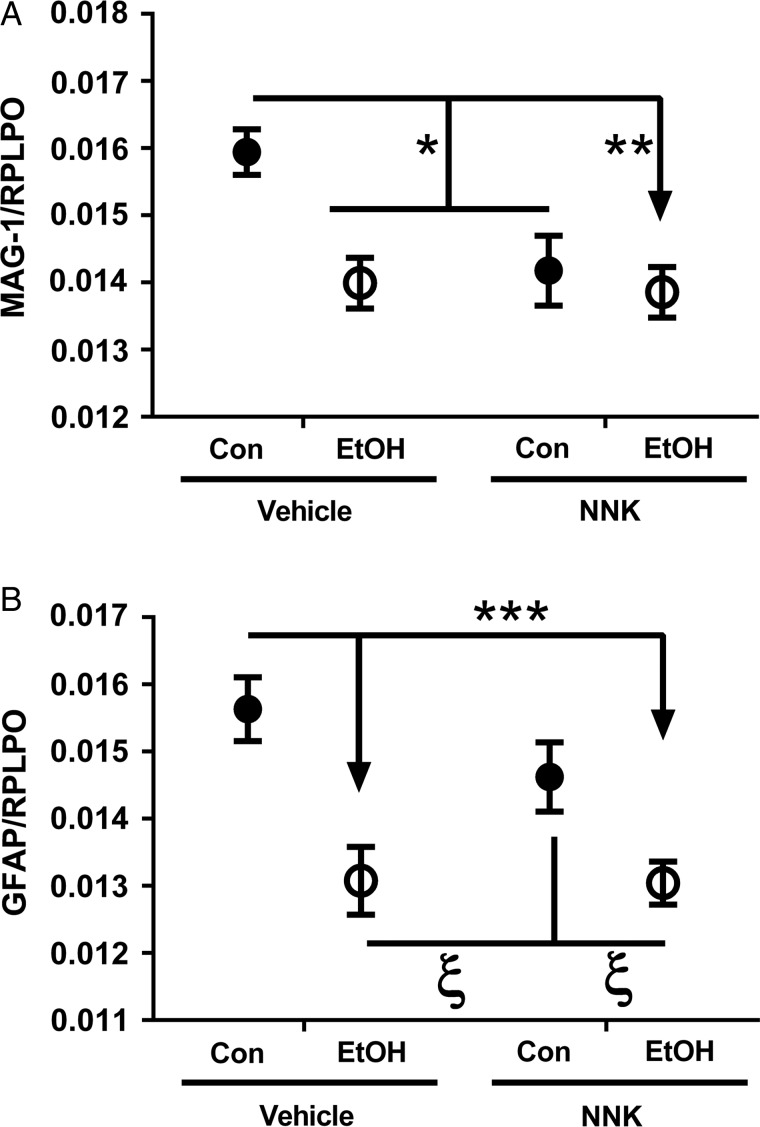
Ethanol and NNK inhibit MAG-1
Ethanol and NNK each had significant effects on MAG-1 protein expression, whereas only ethanol had a significant effect on GFAP (Supplementary Table S3). Post hoc tests demonstrated that MAG-1 expression was significantly reduced by ethanol, NNK and ethanol + NNK (Figure 2A). In addition, ethanol, with or without NNK, significantly reduced GFAP protein relative to control and reached a statistical trend relative to NNK (Figure 2B). These results suggest that ethanol and NNK have toxic/degenerative effects on oligodendroglia, and that ethanol but not NNK also impairs astrocyte function.
Fig. 2.
Ethanol and NNK effects on glial protein expression. Duplex ELISAs were used to measure immunoreactivity to (A) MAG-1 and (B) GFAP with results was normalized to large acidic ribonuclear protein (RPLPO). Inter-group comparisons were made by two-way ANOVA (Supplementary Table S2) with the Tukey post-test (*P < 0.05; **P < 0.01; ***P < 0.001; ξ 0.05 < P < 0.10).
Focused qRT-PCR arrays delineate differential effects of ethanol and NNK on oligodendroglial genes
PCR arrays were designed to measure mRNA transcripts of genes involved in glial and neuronal growth, maturation and function (Supplementary Table S1) using custom primers (Supplementary Table S2). Gene expression was calculated using the ΔΔ−Ct method with results normalized to HPRT1. Data were analyzed by two-way ANOVA and the Tukey post-test. Clustered or patterned results were visualized with heatmaps. As markers of immature oligodendroglia, we measured nestin, vimentin, 2′,3′-Cyclic Nucleotide 3′ Phosphodiesterase (CNP), platelet-derived growth factor receptor-alpha (PDGFR-α) and Group-specific component (GC). For mature myelinating oligodendroglia, we measured proteolipid protein 1 (PLP), MOG, MAG, MBP and Reticulon 4 (RTN4) (Table 1 and Supplementary Figures S1 and S2). NNK had significant effects on immature oligodendroglial genes including nestin, PDGFR-α and GC, but not mature oligodendroglial genes. Ethanol had significant effects on nestin and PLP, and trend effects on MBP. Ethanol × NNK interactive trend effects occurred for PLP and MBP.
Table 1.
Ethanol, NNK and ethanol × NNK effects on temporal lobe expression of glial and neuronal genes
| Protein | NNK effect |
Ethanol effect |
Ethanol × NNK effect |
|||
|---|---|---|---|---|---|---|
| F-ratio | P-value | F-ratio | P-value | F-ratio | P-value | |
| Immature oligodendroglia | ||||||
| Nestin | 15.50 | 0.002 | 5.52 | 0.037 | 0.05 | NS |
| Vimentin | 0.30 | NS | 0.14 | NS | 0.01 | NS |
| CNP | 0.76 | NS | 1.59 | NS | 0.25 | NS |
| PDGFR-α | 7.83 | 0.016 | 0.68 | NS | 1.82 | NS |
| GC | 12.29 | 0.004 | 0.47 | NS | 2.85 | 0.117 |
| Mature oligodendroglia | ||||||
| PLP | 0.08 | NS | 17.41 | 0.0013 | 4.28 | 0.061 |
| MOG | 0.05 | NS | 1.06 | NS | 0.18 | NS |
| MAG-1 | 1.51 | NS | 1.97 | NS | 0.06 | NS |
| MBP | 0.28 | NS | 3.34 | 0.096 | 3.89 | 0.072 |
| RTN4 | 1.61 | NS | 0.38 | NS | 0.33 | NS |
| Neuroglial markers | ||||||
| CSPG4 | 10.43 | 0.007 | 0.13 | NS | 0.08 | NS |
| GFAP | 0.38 | NS | 1.05 | NS | 1.44 | NS |
| NCAM | 27.90 | 0.0002 | 0.78 | NS | 1.13 | NS |
| NTRK2 | 4.17 | 0.067 | 0.21 | NS | 0.24 | NS |
| GSTP2 | 1.16 | NS | 0.716 | NS | 0.01 | NS |
| GPD1 | 0.01 | NS | 0.17 | NS | 0.87 | NS |
| Transcription factors | ||||||
| FOXO1 | 13.65 | 0.003 | 1.95 | NS | 2.38 | NS |
| FOXO4 | 7.99 | 0.015 | 0.13 | NS | 1.14 | NS |
| Olig1 | 0.64 | NS | 0.79 | NS | 2.10 | NS |
| Olig2 | 0.06 | NS | 0.44 | NS | 1.81 | NS |
| NKX2-2 | 13.48 | 0.003 | 1.27 | NS | 1.32 | NS |
| SOX9 | 0.60 | NS | 0.30 | NS | 0.14 | NS |
Temporal lobe RNA was analyzed using a targeted PCR array to examine ethanol, NNK and ethanol × NNK interactive effects on oligodendroglial gene expression. Data were analyzed by Two-way ANOVA. Tukey post hoc test results are depicted in Supplementary Figures S1–S4.
Higher temporal lobe levels of nestin were measured in the NNK and ethanol + NNK groups relative to control or ethanol exposures (Supplementary Figure S1A). PDGFR-α (Supplementary Figure S1D) and GC (Supplementary Figure S1E) expression increased progressively from control to ethanol and then NNK (P < 0.0001 by linear trend analysis), but did not further increase following dual exposures. These responses resulted in higher levels of PDGFR-α and GC expression in the NNK and ethanol + NNK groups relative to control or ethanol treatment. In contrast, vimentin (Supplementary Figure S1B) and CNP (Supplementary Figure S1C) mRNA levels were not altered by ethanol and/or NNK exposures. With regard to the mature oligodendroglial genes, the main effects observed were that NNK inhibited PLP (Supplementary Figure S2A) and MBP (Supplementary Figure S2D). Although ethanol alone had no effect, its co-administration with NNK blocked NNK's inhibitory effects on PLP and MBP. MOG (Supplementary Figure S2B), MAG-1 (Supplementary Figure S2C) and RTN4 (Supplementary Figure S2E) were expressed at similar levels across the four groups.
Effects of ethanol and NNK on neural-glial gene expression
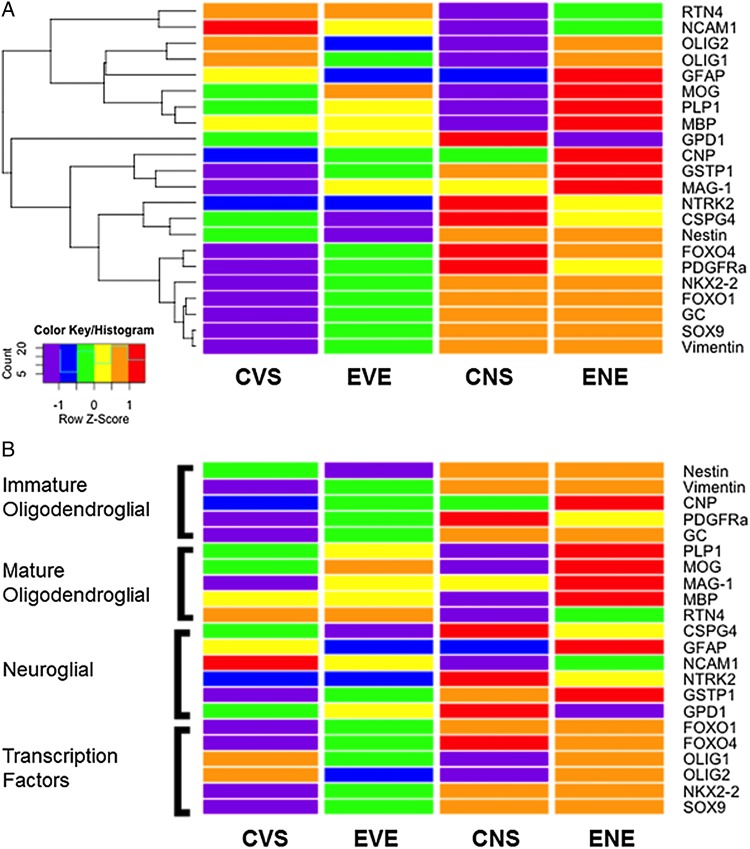
We extended our analysis to examine selected neuronal and astrocytic genes to assess how ethanol and NNK might alter function of other CNS cell types. For this component of the study, we measured: Chondroitin Sulfate Proteoglycan 4 (CSPG4), GFAP, neural cell adhesion molecule (NCAM), neurotrophic tyrosine kinase receptor, Type 2 (NTRK2), Glutathione S-Transferase Pi-1 (GSTP1) and Glycerol-3-phosphate dehydrogenase 1-soluble (GPD1) (Table 1, Supplementary Figure S3). NNK had significant effects on CSPG4 and NCAM, and a trend effect on NTRK2. No other significant or trend effects of NNK, ethanol or ethanol × NNK interactions were observed (Table 1). NNK and ethanol + NNK significantly reduced NCAM expression relative to control and ethanol exposures (Supplementary Figure S3C). In addition, generally higher mean levels of CSPG4 (Supplementary Figure S3A) and NTRK2 (Supplementary Figure S3D) were observed in the NNK and ethanol + NNK. In addition, ethanol + NNK modestly increased GFAP (Supplementary Figure S3B) and GSTP1 (Supplementary Figure S3E), and reduced GPD1 (Figure S3F) expression relative to the other groups. Although those individual differences were not statistically significant, the aggregate effects of NNK, ethanol and ethanol + NNK on neuroglial gene expression were better revealed with heatmaps (Figure 3).
Fig. 3.
Heatmap illustrating (A) hierarchical clustering and (B) grouping according to gene function. The heatmap was generated using Version 3.1 of R software. Results shown with the 6 tone palette correspond to z-scores, which were scaled to have a mean of 0 and S.D. of 1. (A) A hierarchical clustering algorithm was applied using the Euclidean distance function on the overall table to display a dendrogram of mRNAs. (B) Results shown in Panel A were sorted to juxtapose data corresponding to immature oligodendroglial genes, mature oligodendroglial genes, neuroglial genes and transcription factors. CVS, control diet with vehicle treatment and saline binge treatment; EVE, ethanol diet with vehicle treatment and ethanol binge; CNS, control diet with NNK treatment and saline binge treatment; ENE, ethanol diet with NNK treatment and ethanol binge.
Glial transcription factor gene expression is more prominently altered by NNK than ethanol
To examine effects of ethanol and NNK on the regulators of glial gene expression and function, we measured mRNA levels of glial-associated transcription factors including, Forkhead Box O1 (FOXO1), FOXO4, oligodendrocyte transcription factor 1 (Olig1), Olig2, NK2 Homeobox 2 (NKX2-2) and Sex determining region Y-Box 9 (SOX9). NNK had significant effects on FOXO1, FOXO4 and NKX2-2, but not Olig1, Olig2 or SOX9 (Table 1). In contrast, no significant ethanol or ethanol × NNK interactive effects or trends were observed for any of the transcription factors studied. Progressively higher levels of FOXO1 (Supplementary Figure S4A), FOXO4 (Supplementary Figure S4B) and NKX2-2 (Supplementary Figure S4E) from control, to ethanol and then NNK exposures were observed (P < 0.0001 by linear trend analysis), but similarly high mRNA levels were present in the NNK and ethanol + NNK brains. Post hoc tests demonstrated that the differences between control and NNK or ethanol + NNK were significant for FOXO1 and NKX2-2, and had a trend effect for control versus NNK with respect to FOXO4. In contrast, Olig1 expression was modestly reduced in all experimental groups relative to control (Supplementary Figure S4C), Olig2 expression was lowest in the NNK group (Supplementary Figure S4D) and SOX9 (Supplementary Figure S4F) was similarly expressed in all groups.
Heatmaps depict differential ethanol, NNK and ethanol × NNK interactive effects on gene expression
Heatmaps with hierarchical clustering helped illustrate overall and trend effects of ethanol, NNK or both exposures on temporal lobe expression of immature and mature oligodendroglial, neuroglial and transcription factor genes (Figure 3). Two dominant hierarchical clusters were detected. The upper one included: RTN4 NCAM1, Olig2, Olig1, GFAP, MOG, PLP1 and MBP. The lower one included GPD1, CNP, GSTP1, MAG-1, NTRK2, CSPG4, Nestin, FOXO4, PDGFR-α, NKX2-2, FOXO1, GC, SOX9 and Vimentin. For the lower cluster, controls generally had the lowest levels of gene expression, followed by ethanol treatment, and then NNK. Gene expression in the ethanol + NNK group was either similar to or reduced relative to NNK (not control or ethanol). CNP, GSTP1 and MAG1 were exceptions in that they were expressed at higher levels in the ethanol + NNK than in the NNK-only group. In addition, GPD1 expression was lowest overall in the ethanol + NNK group.
The upper hierarchical cluster had two subgroupings: the top subgroup, included RTN4, NCAM1, Olig2 and Olig1, and the bottom subgroup included GFAP, MOG, PLP1 and MBP (Figure 3A). For the top sub-group, the heatmap revealed ethanol- and NNK-associated inhibition of gene expression relative to control, and either inhibition or unchanged levels of gene expression in ethanol + NNK relative to control. With regard to the lower subgroup, gene expression was inhibited by NNK and increased by ethanol + NNK, while ethanol effects varied. Ethanol increased MOG and PLP1 expression relative to control and NNK, reduced GFAP to levels observed with NNK, and had no effect on MBP relative to control. Since the degrees to which ethanol + NNK inhibited RTN4 and NCAM1 were intermediate between the responses to ethanol and NNK, and in contrast to the ethanol and NNK, ethanol + NNK either had no effect (Olig2 and Olig1) or sharply increased (GFAP, MOG, PLP1 and MBP) gene expression relative to control, it is likely that ethanol + NNK exposures had interactive effects on these target genes.
The heatmap was reconfigured to visualize treatment effects according to target gene function (Figure 3B). Immature oligodendroglial genes (Nestin, vimentin, CNP, PDGFR-α, GC) were consistently expressed at low levels in control samples, and their expression levels increased with ethanol, NNK or ethanol + NNK exposures. In contrast, for mature oligodendroglial genes (PLP1, MOG, MAG, MBP, RTN4), ethanol caused the most consistent increases in gene expression (except for RTN4), while NNK had mainly inhibitory effects (except for MAG) relative to control. Combined ethanol + NNK exposures further increased gene expression relative to ethanol alone. RTN4 was an exception in that its expression was modestly inhibited by NNK and ethanol + NNK.
With regard to neuroglial markers (CSPG4, GFAP, NCAM1, NTRK2, GSTP1, GPD1): (a) ethanol inhibited while NNK and ethanol + NNK increased expression of CSPG4; (b) ethanol and NNK inhibited while ethanol + NNK increased GFAP, (c) ethanol, NNK and ethanol + NNK inhibited NCAM1 relative to control, although NCAM1 levels in the ethanol + NNK group were intermediate between ethanol's and NNK's; (d) ethanol had no effect on NTRK2 while NNK and ethanol + NNK increased NTRK2 expression; (e) ethanol, NNK and ethanol + NNK increased GSTP1 and (f) ethanol and NNK increased GPD1, while ethanol + NNK had opposite effects and profoundly inhibited GPD1 expression relative to all other groups. In essence, effects of ethanol, NNK or both exposures failed to follow any consistent trends. In contrast, ethanol and/or NNK effects on transcription factors were more uniform since ethanol, NNK and ethanol + NNK increased FOXO1, FOXO4, NKX2-2 and SOX9 expression, while ethanol and NNK (but not ethanol + NNK) inhibited Olig1 and Olig2.
DISCUSSION
Clinical and epidemiologic relevance of the model
This study examined the independent and interactive effects of ethanol and NNK exposures on the expression of proteins and genes that regulate white matter structure and function. The rat model mimics effects of chronic plus binge drinking from human adolescence to young adulthood, and NNK tobacco-specific nitrosamine exposures from late adolescence to early adulthood. The chronic low-dose NNK exposures were used to test the hypothesis that tobacco-specific nitrosamines contribute to the pathogenesis of ARBD. This concept was drawn into focus based on epidemiological evidence that a very high proportion of heavy drinkers also smoke cigarettes (Romberger and Grant, 2004), and prior studies showing that exposures to sub-mutagenic doses of other nitrosamines cause developmental and degenerative effects that overlap with ARBD (de la Monte and Tong, 2009; Tong et al., 2009). Our working hypothesis was that, like alcohol, chronic low-dose NNK exposures can impair white matter structure and function during adolescent brain development. In addition, dual exposures could have additive neurotoxic effects on white matter. Although the ultimate concern is tobacco smoke, which contains thousands of chemicals, we hypothesized that the tobacco-specific nitrosamines mediate adverse effects on the brain. Moreover, we studied NNK rather than tobacco smoke to avoid unrelated effects due to pulmonary disease or hypoxia (Gan et al., 2011; McCaskill et al., 2011).
White matter is targeted by ethanol and NNK
Histological studies demonstrated differential effects of ethanol and NNK on white matter in that ethanol mainly caused demyelination while NNK caused axonal degeneration and fiber loss. Combined exposures had additive effects. These findings illustrate the likelihood that heavy alcohol consumption and smoking can damage the brain by damaging structural connections needed for functions such as learning and memory. Follow-up studies will determine the degree to which these abnormalities can be reversed by cessation of alcohol/tobacco consumption.
Initial studies showed that both ethanol and NNK inhibit MAG-1, while ethanol and not NNK depresses GFAP protein. MAG-1 is an oligodendrocyte glycoprotein that facilitates cell–cell interactions between neurons and myelinating cells. GFAP is the main intermediate filament protein of mature astrocytes. The inhibitory effects of ethanol on MAG-1 protein correspond with the white matter hypotrophy or atrophy and reduced myelin integrity associated with chronic and/or repeated binge alcohol exposures (de la Monte and Kril, 2014). Ethanol's inhibition of GFAP suggests its toxic, metabolic and degenerative effects extend to astrocytes. Therefore, in addition to disrupting myelin integrity, ethanol can compromise various astrocytic functions, including those needed to maintain blood–brain barrier integrity, and regulate electrical impulses, transmitter homeostasis and metabolic support. Targeted arrays helped delineate abnormalities in glial gene expression caused by ethanol, NNK or both exposures.
Oligodendroglial genes: lineage, maturation and function
Oligodendroglial cells generate and maintain CNS myelin, whose main function is to ensure nerve cell conduction. Oligodendrocytes develop from OPC's that differentially express CNP, NG2 proteoglycan CSPG4, PDGFR-α, Olig2, Dlx2 Homeobox and NK2 Homeobox (Nkx) (Supplementary Table S1). OPCs differentiate into immature oligodendroglia that express CNP, Olig1 and low levels of Olig2, followed by CNP, Olig1, low Olig2 and RTN4 (Campagnoni and Macklin, 1988; Gordon et al., 1990; Boulanger and Messier, 2014). Mature myelinating oligodendroglia express distinct integral membrane proteins including MBP, MAG, MOG and PLP (Le Bras et al., 2005; Bordner et al., 2011). Chronic ethanol exposure delays the time course of oligodendroglial maturation, expression of MBP (Chiappelli et al., 1991) and MAG-1 (Gnaedinger et al., 1984), and de novo synthesis of myelin (Gnaedinger and Druse, 1984).
Ethanol and/or NNK impair myelin maintenance, maturation and function
Targeted arrays were mainly focused on oligodendroglial-associated genes. The results were interpreted by clustering data according to: (a) genes expressed in immature versus mature oligodendrocytes; (b) other neuroglial genes and (c) transcription factors. The combined utilization of ANOVA tests and heatmaps facilitated identification of major trends. Although ethanol and NNK both increased expression of genes that mark immature oligodendroglia, the responses were more pronounced in NNK-treated rats, independent of ethanol co-exposure, particularly with respect to Nestin, GC and PDGFRα. Nestin regulates vimentin intermediate filament disassembly during growth and is needed for survival, renewal and proliferation of neural progenitor cells. Inhibition of Nestin corresponds with prior reports of impaired neurogenesis following chronic ethanol exposure (Crews and Nixon, 2009; Singh et al., 2009). GC is a Vitamin D-binding nuclear hormone receptor that interacts with RXR (Adams et al., 2003; Christakos et al., 2003) and regulates maturation of immature oligodendroglial precursors into myelin-generating oligodendrocytes (Baumann and Pham-Dinh, 2001). PDGFRα is a cell surface receptor tyrosine kinase expressed in oligo-progenitor cells. PDGF stimulation of PDGFRα activates proliferation pathways. Altogether, the findings suggest that NNK has growth promoting effects on immature oligodendroglia.
Analysis of the mature oligodendroglial gene cluster revealed mainly stimulatory effects of ethanol, with or without NNK and principally inhibitory effect of NNK. Ethanol modestly increased expression of immature oligodendroglial genes, but robustly increased expression of mature oligodendroglial genes. In contrast, NNK's effects on these clusters were reciprocal in that it stimulated immature and inhibited mature oligodendroglial genes. A ceiling effect of NNK most likely accounts for the similar levels of immature oligodendroglial gene expression in NNK-only and ethanol + NNK-exposed brains. In contrast, additive or synergistic responses to dual exposures were manifested by higher levels of PLP, and to some extent MBP relative to ethanol only or vehicle. The elevated levels of oligodendroglial mRNA transcripts vis-à-vis reduced levels of MAG-1 protein in ethanol-exposed brains suggest that the inhibitory effects on MAG-1 were mediated by post-translational processes such as enhanced myelin degradation. In contrast, the reduced levels of both mature myelin mRNA transcripts and MAG-1 protein expression in NNK-exposed brains suggest that the inhibitory effects of NNK were mediated at the level of transcription. The generally higher levels of both immature and mature myelin gene expression in ethanol + NNK-exposed temporal lobes are not readily explained, but could reflect compensatory responses to progressive myelin degeneration.
Complex effects of ethanol and/or NNK exposures on neuroglial genes
The collective responses of neuroglial genes to ethanol and/or NNK were largely lacking in pattern. Only CSPG4 and NCAM were significantly modulated by NNK, with or without ethanol co-exposures, and those responses were opposite to one another. NNK up-regulated CSPG4, had an upward trend effect on NTRK2, and down-regulated NCAM. CSPG4 inhibits neurite outgrowth and promotes growth cone collapse during axon regeneration (Ghosh and David, 1997; Nash et al., 2009), while NCAM1 mediates neuronal adhesion and neurite outgrowth (Skaper, 2005, 2012), and NTRK2 is a positive regulator of synapse formation and plasticity (Skaper, 2012). Therefore, we suggest that chronic NNK exposure via tobacco smoke, with or without heavy alcohol consumption, causes cognitive deficits and neurodegeneration by impairing processes needed for neuronal plasticity and repair.
Ethanol and NNK effects on transcription factor genes—potential relevance to insulin signaling abnormalities
The main findings with respect to transcription factors were that FOXO1, FOXO4 and NKKX2-2 expression increased progressively from control to ethanol, and then NNK, although additive or interactive effects of ethanol and NNK did not occur. Both FOXO1 and FOXO4 target insulin signaling (Webb and Brunet, 2014). NKX2-2 supports insulin-producing cells (Habener et al., 2005), myelin maturation from OPCs (Syed et al., 2008) and genes that have important roles in axonal guidance (Croizier et al., 2011). Therefore, our findings suggest that modulations in transcription factor gene expression may represent compensatory efforts to attenuate ethanol- and NNK-mediated impairments in myelin maturation and maintenance.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at Alcohol and Alcoholism online.
FUNDING
Supported by AA-11431, AA-12908 and Diversity Supplement to AA-11431 from the National Institutes of Health.
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENT
The authors are very grateful to Emre Gundogan (emre@gundogan.us) for his contribution in applying R software to generate the heatmap of gene expression data.
REFERENCES
- Adams JS, Chen H, Chun RF, et al. (2003) Novel regulators of vitamin D action and metabolism: lessons learned at the Los Angeles zoo. J Cell Biochem 88:308–14. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Garrido GJ, Lautenschlager NT, et al. (2008) Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. Am J Geriatr Psychiatry 16:92–8. [DOI] [PubMed] [Google Scholar]
- Andreani T, Tong M, de la Monte SM. (2014) Hotdogs and beer: dietary nitrosamine exposure exacerbates neurodevelopmental effects of ethanol in fetal alcohol spectrum disorder. J Drug Alchol Dis 3:1–9. [Google Scholar]
- Baker ST, Yucel M, Fornito A, et al. (2013) A systematic review of diffusion weighted MRI studies of white matter microstructure in adolescent substance users. Neurosci Biobehav Rev 37:1713–23. [DOI] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871–927. [DOI] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer RE, et al. (2013) Longitudinal changes in white matter integrity among adolescent substance users. Alcohol Clin Exp Res 37 Suppl 1:E181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzan AD, Bianchi MS. (2002) Genotoxicity of streptozotocin. Mutat Res 512:121–34. [DOI] [PubMed] [Google Scholar]
- Bordner KA, George ED, Carlyle BC, et al. (2011) Functional genomic and proteomic analysis reveals disruption of myelin-related genes and translation in a mouse model of early life neglect. Front Psychiatry 2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger JJ, Messier C. (2014) From precursors to myelinating oligodendrocytes: contribution of intrinsic and extrinsic factors to white matter plasticity in the adult brain. Neuroscience 269C:343–66. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, et al. (2004) Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry 55:77–84. [DOI] [PubMed] [Google Scholar]
- Campagnoni AT, Macklin WB. (1988) Cellular and molecular aspects of myelin protein gene expression. Mol Neurobiol 2:41–89. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, et al. (2007) Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology 32:429–38. [DOI] [PubMed] [Google Scholar]
- Chesik D, De Keyser J, Wilczak N. (2008) Insulin-like growth factor system regulates oligodendroglial cell behavior: therapeutic potential in CNS. J Mol Neurosci 35:81–90. [DOI] [PubMed] [Google Scholar]
- Chiappelli F, Taylor AN, Espinosa de los Monteros A, et al. (1991) Fetal alcohol delays the developmental expression of myelin basic protein and transferrin in rat primary oligodendrocyte cultures. Int J Dev Neurosci 9:67–75. [DOI] [PubMed] [Google Scholar]
- Christakos S, Barletta F, Huening M, et al. (2003) Vitamin D target proteins: function and regulation. J Cell Biochem 88:238–44. [DOI] [PubMed] [Google Scholar]
- Cohen AC, Tong M, Wands JR, et al. (2007) Insulin and insulin-like growth factor resistance with neurodegeneration in an adult chronic ethanol exposure model. Alcohol Clin Exp Res 31:1558–73. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. (2009) Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol 44:115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croizier S, Amiot C, Chen X, et al. (2011) Development of posterior hypothalamic neurons enlightens a switch in the prosencephalic basic plan. PLoS One 6:e28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D, Cherbuin N, Anstey KJ, et al. (2012) Lifetime cigarette smoking is associated with striatal volume measures. Addict Biol 17:817–25. [DOI] [PubMed] [Google Scholar]
- de Boer MF, Sanderson RJ, Damhuis RA, et al. (1997) The effects of alcohol and smoking upon the age, anatomic sites and stage in the development of cancer of the oral cavity and oropharynx in females in the south west Netherlands. Eur Arch Otorhinolaryngol 254:177–9. [DOI] [PubMed] [Google Scholar]
- de la Monte SM. (1988) Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch Neurol 45:990–2. [DOI] [PubMed] [Google Scholar]
- de la Monte SM. (2012) Alcohol-induced liver and brain degeneration: roles of insulin resistance, toxic ceramides, and endoplasmic reticulum stress. In Sova MS. (ed), Alcohol, Nutrition, and Health Consequences. New York: Humana Press. [Google Scholar]
- de la Monte SM, Tong M. (2009) Mechanisms of nitrosamine-mediated neurodegeneration: potential relevance to sporadic Alzheimer's disease. J Alzheimers Dis 17:817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Kril JJ. (2014) Human alcohol-related neuropathology. Acta Neuropathol 127:71–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Longato L, Tong M, et al. (2009) Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs 10:1049–60. [PMC free article] [PubMed] [Google Scholar]
- Duell EJ. (2012) Epidemiology and potential mechanisms of tobacco smoking and heavy alcohol consumption in pancreatic cancer. Mol Carcinog 51:40–52. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, et al. (2004) Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin Exp Res 28:1849–60. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Meyerhoff DJ. (2007a) The neurobiological and neurocognitive consequences of chronic cigarette smoking in alcohol use disorders. Alcohol Alcohol 42:174–85. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Cardenas VA, et al. (2007b) Chronic cigarette smoking and heavy drinking in human immunodeficiency virus: consequences for neurocognition and brain morphology. Alcohol 41:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. (2012) A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depend 122:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW, et al. (2014) Smoking and increased Alzheimer's disease risk: a review of potential mechanisms. Alzheimers Dement 10:S122–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elofson J, Gongvatana W, Carey KB. (2013) Alcohol use and cerebral white matter compromise in adolescence. Addict Behav 38:2295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch R, Nicolas JM, Salamero M, et al. (1997) Atrophy of the corpus callosum in chronic alcoholism. J Neurol Sci 146:145–51. [DOI] [PubMed] [Google Scholar]
- Freude S, Leeser U, Muller M, et al. (2008) IRS-2 branch of IGF-1 receptor signaling is essential for appropriate timing of myelination. J Neurochem 107:907–17. [DOI] [PubMed] [Google Scholar]
- Fritz HC, Wittfeld K, Schmidt CO, et al. (2014) Current smoking and reduced gray matter volume-a voxel-based morphometry study. Neuropsychopharmacology 39:2594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, et al. (2006) Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci 24:1744–50. [DOI] [PubMed] [Google Scholar]
- Gan G, Hu R, Dai A, et al. (2011) The role of endoplasmic reticulum stress in emphysema results from cigarette smoke exposure. Cell Physiol Biochem 28:725–32. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, et al. (2005) Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin Exp Res 29:1484–95. [DOI] [PubMed] [Google Scholar]
- Ghosh A, David S. (1997) Neurite growth-inhibitory activity in the adult rat cerebral cortical gray matter. J Neurobiol 32:671–83. [PubMed] [Google Scholar]
- Ghosh D, Mishra MK, Das S, et al. (2009) Tobacco carcinogen induces microglial activation and subsequent neuronal damage. J Neurochem 110:1070–81. [DOI] [PubMed] [Google Scholar]
- Gnaedinger JM, Druse MJ. (1984) Glycoproteins and proteins in an axolemma-enriched fraction and myelin from developing rats: effect of maternal ethanol consumption. J Neurosci Res 12:633–45. [DOI] [PubMed] [Google Scholar]
- Gnaedinger JM, Noronha AB, Druse MJ. (1984) Myelin gangliosides in developing rats: the influence of maternal ethanol consumption. J Neurochem 42:1281–5. [DOI] [PubMed] [Google Scholar]
- Go VL, Gukovskaya A, Pandol SJ. (2005) Alcohol and pancreatic cancer. Alcohol 35:205–11. [DOI] [PubMed] [Google Scholar]
- Gong X, Xie Z, Zuo H. (2008) Invivo insulin deficiency as a potential etiology for demyelinating disease. Med Hypotheses 71:399–403. [DOI] [PubMed] [Google Scholar]
- Gordon MN, Kumar S, Espinosa de los Monteros A, et al. (1990) Developmental regulation of myelin-associated genes in the normal and the myelin deficient mutant rat. Adv Exp Med Biol 265:11–22. [DOI] [PubMed] [Google Scholar]
- Groseclose MR, Andersson M, Hardesty WM, et al. (2007) Identification of proteins directly from tissue: in situ tryptic digestions coupled with imaging mass spectrometry. J Mass Spectrom 42:254–62. [DOI] [PubMed] [Google Scholar]
- Habener JF, Kemp DM, Thomas MK. (2005) Minireview: transcriptional regulation in pancreatic development. Endocrinology 146:1025–34. [DOI] [PubMed] [Google Scholar]
- Harper C. (1982) Neuropathology of brain damage caused by alcohol. Med J Aust 2:276–82. [DOI] [PubMed] [Google Scholar]
- Harper C, Kril J. (1985) Brain atrophy in chronic alcoholic patients: a quantitative pathological study. J Neurol Neurosurg Psychiatry 48:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Holloway RL. (1985) Brain shrinkage in chronic alcoholics: a pathological study. Br Med J 290:501–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Dixon G, Sheedy D, et al. (2003) Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog Neuropsychopharmacol Biol Psychiatry 27:951–61. [DOI] [PubMed] [Google Scholar]
- Harris RA, Baxter DM, Mitchell MA, et al. (1984) Physical properties and lipid composition of brain membranes from ethanol tolerant-dependent mice. Mol Pharmacol 25:401–9. [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Bava S, et al. (2013) White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Res 214:374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NW, Warnakulasuriy S, Tavassoli M. (1996) Hereditary and environmental risk factors; clinical and laboratory risk matters for head and neck, especially oral, cancer and precancer. Eur J Cancer Prev 5:5–17. [PubMed] [Google Scholar]
- Kalman D, Kim S, DiGirolamo G, et al. (2010) Addressing tobacco use disorder in smokers in early remission from alcohol dependence: the case for integrating smoking cessation services in substance use disorder treatment programs. Clin Psychol Rev 30:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulmanda M, Qipo A, Chebrolu S, et al. (2003) The effect of low versus high dose of streptozotocin in cynomolgus monkeys (Macaca fascilularis). Am J Transplant 3:267–72. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM. (1999) Brain shrinkage in alcoholics: a decade on and what have we learned? Prog Neurobiol 58:381–7. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Gundlach AL, Dodd PR, et al. (1989) Cortical dihydropyridine binding sites are unaltered in human alcoholic brain. Ann Neurol 26:395–7. [DOI] [PubMed] [Google Scholar]
- Kwon OS, Schmued LC, Slikker W., Jr (1997) Fumonisin B1 in developing rats alters brain sphinganine levels and myelination. Neurotoxicology 18:571–9. [PubMed] [Google Scholar]
- Le Bras B, Chatzopoulou E, Heydon K, et al. (2005) Oligodendrocyte development in the embryonic brain: the contribution of the plp lineage. Int J Dev Biol 49:209–20. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tang J, Liu T, et al. (2012) Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol 17:977–80. [DOI] [PubMed] [Google Scholar]
- Longato L, Ripp K, Setshedi M, et al. (2012) Insulin resistance, ceramide accumulation, and endoplasmic reticulum stress in human chronic alcohol-related liver disease. Oxi Med Cell Longev 2012:479348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaskill ML, Kharbanda KK, Tuma DJ, et al. (2011) Hybrid malondialdehyde and acetaldehyde protein adducts form in the lungs of mice exposed to alcohol and cigarette smoke. Alcohol Clin Exp Res 35:1106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash M, Pribiag H, Fournier AE, et al. (2009) Central nervous system regeneration inhibitors and their intracellular substrates. Mol Neurobiol 40:224–35. [DOI] [PubMed] [Google Scholar]
- Nicklay JJ, Harris GA, Schey KL, et al. (2013) MALDI imaging and in situ identification of integral membrane proteins from rat brain tissue sections. Anal Chem 85:7191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Shi H, Zhong J, et al. (2013) Chronic smoking and brain gray matter changes: evidence from meta-analysis of voxel-based morphometry studies. Neurol Sci 34:813–7. [DOI] [PubMed] [Google Scholar]
- Paul RH, Grieve SM, Niaura R, et al. (2008) Chronic cigarette smoking and the microstructural integrity of white matter in healthy adults: a diffusion tensor imaging study. Nicotine Tob Res 10:137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, et al. (2007) Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain 130:48–64. [DOI] [PubMed] [Google Scholar]
- Phillips SC, Harper CG, Kril J. (1987) A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain 110 (Pt 2):301–14. [DOI] [PubMed] [Google Scholar]
- Qu W, Zhang B, Wu D, et al. (1999) [Effects of alcohol on membrane lipid fluidity of astrocytes and oligodendrocytes]. Wei Sheng Yan Jiu 28:153–4. [PubMed] [Google Scholar]
- Ramirez T, Tong M, Ayala C, et al. (2012) Structural correlates of PPAR agonist rescue of experimental chronic alcohol-induced steatohepatitis. J Clin Exp Pathol 2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberger DJ, Grant K. (2004) Alcohol consumption and smoking status: the role of smoking cessation. Biomed Pharmacother 58:77–83. [DOI] [PubMed] [Google Scholar]
- Schmidt KS, Gallo JL, Ferri C, et al. (2005) The neuropsychological profile of alcohol-related dementia suggests cortical and subcortical pathology. Dement Geriatr Cogn Disord 20:286–91. [DOI] [PubMed] [Google Scholar]
- Singh AK, Gupta S, Jiang Y, et al. (2009) In vitro neurogenesis from neural progenitor cells isolated from the hippocampus region of the brain of adult rats exposed to ethanol during early development through their alcohol-drinking mothers. Alcohol Alcohol 44:185–98. [DOI] [PubMed] [Google Scholar]
- Skaper SD. (2005) Neuronal growth-promoting and inhibitory cues in neuroprotection and neuroregeneration. Ann N Y Acad Sci 1053:376–85. [DOI] [PubMed] [Google Scholar]
- Skaper SD. (2012) Neuronal growth-promoting and inhibitory cues in neuroprotection and neuroregeneration. Methods Mol Biol 846:13–22. [DOI] [PubMed] [Google Scholar]
- Soscia SJ, Tong M, Xu XJ, et al. (2006) Chronic gestational exposure to ethanol causes insulin and IGF resistance and impairs acetylcholine homeostasis in the brain. Cell Mol Life Sci 63:2039–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland GT, Sheahan PJ, Matthews J, et al. (2013) The effects of chronic alcoholism on cell proliferation in the human brain. Exp Neurol 247:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed YA, Baer AS, Lubec G, et al. (2008) Inhibition of oligodendrocyte precursor cell differentiation by myelin-associated proteins. Neurosurg Focus 24:E5. [DOI] [PubMed] [Google Scholar]
- Tong M, Neusner A, Longato L, et al. (2009) Nitrosamine exposure causes insulin resistance diseases: relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and alzheimer's disease. J Alzheimers Dis 17:827–44. [PMC free article] [PubMed] [Google Scholar]
- Tong M, Longato L, de la Monte SM. (2010) Early limited nitrosamine exposures exacerbate high fat diet-mediated type2 diabetes and neurodegeneration. BMC Endocr Disord 10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Yu R, Deochand C, et al. (2015) Differential contributions of alcohol and the nicotine-derived nitrosamine ketone (NNK) to insulin and insulin-like growth factor resistance in the adolescent brain. Alcohol Alcohol 50:670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramacere I, Negri E, Bagnardi V, et al. (2010) A meta-analysis of alcohol drinking and oral and pharyngeal cancers. Part 1: overall results and dose-risk relation. Oral Oncol 46:497–503. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Durazzo TC, Gazdzinski S, et al. (2009) MRSI and DTI: a multimodal approach for improved detection of white matter abnormalities in alcohol and nicotine dependence. NMR Biomed 22:516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Kamat A, Pergola P, et al. (2011) Metabolic factors in the development of hepatic steatosis and altered mitochondrial gene expression in vivo. Metabolism 60:1090–9. [DOI] [PubMed] [Google Scholar]
- Webb AE, Brunet A. (2014) FOXO transcription factors: key regulators of cellular quality control. Trends Biochem Sci 39:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing DR, Harvey DJ, Hughes J, et al. (1982) Effects of chronic ethanol administration on the composition of membrane lipids in the mouse. Biochem Pharmacol 31:3431–9. [DOI] [PubMed] [Google Scholar]
- Zabala V, Tong M, Yu R, et al. (2015) Potential contributions of the tobacco nicotine-derived nitrosamine ketone (NNK) in the pathogenesis of steatohepatitis in a chronic plus binge rat model of alcoholic liver disease. Alcohol Alcohol 50:118–31. [DOI] [PMC free article] [PubMed] [Google Scholar]