Abstract
Infants admitted to health-care centers with acute bronchiolitis are frequently monitored with a pulse oximeter, a noninvasive method commonly used for measuring oxygen saturation. The decision to hospitalize children with bronchiolitis has been largely influenced by pulse oximetry, despite its questionable diagnostic value in delineating the severity of the illness. Many health-care providers lack the appropriate clinical fundamentals and limitations of pulse oximetry. This deficiency in knowledge might have been linked to changes in the management of bronchiolitis. The aim of this paper is to provide the current evidence on the role of pulse oximetry in bronchiolitis. We discuss the history, fundamentals of operation, and limitations of the apparatus. A search of the Google Scholar, Embase, Medline, and PubMed databases was carried out for published articles covering the use of pulse oximetry in bronchiolitis.
Keywords: bronchiolitis, children, monitor, oxygen
Introduction
Bronchiolitis is a respiratory condition usually caused by viral lower respiratory tract infection in infants and young children. The pathophysiology of bronchiolitis commences with acute inflammation, followed by edema, and augmentation of mucus production.1 Bronchiolitis is the most common cause of infant hospitalization in the USA, costing the health-care system more than $1 billion annually.2 The American Academy of Pediatrics (AAP) strongly advocates that the history and physical examination form the focus of diagnostic procedures for bronchiolitis so as to produce the correct diagnosis and to evaluate the severity of the illness.1
The decision to hospitalize children with bronchiolitis has been largely influenced by pulse oximetry, despite its questionable diagnostic value in delineating the severity of the illness.3 Recently, infants admitted to health-care centers with acute bronchiolitis have been frequently monitored with a pulse oximeter,4 a noninvasive method commonly used for measuring oxygen saturation.5
Many health-care providers lack the appropriate clinical fundamentals and limitations of pulse oximetry.5,6 This deficiency in knowledge has been linked to changes in the management of bronchiolitis,5 perhaps because many emergency department physicians depend on oximetry when deciding to admit the infant to the hospital. Moreover, their decisions to admit might be based on only a 2% difference in oxygen saturation.3
Despite being considered as a weak prognosticator of respiratory distress, oxygen saturation is very closely linked to the increased rate of hospitalization of infants with acute bronchiolitis7 and has been considered a major factor in the length of stay in hospital.8,9 There is no consensus regarding the oxygen saturation cutoff value for the initiation of oxygen supplements in infants with bronchiolitis.10,11 In a study conducted by on clinical characteristics associated with hospitalization and length of stay, Corneli et al7 showed that among infants admitted with acute bronchiolitis, the unique saturation measurement of 97% or less at presentation. It has been postulated that if non-ill-appearing infants with mild-to-moderate bronchiolitis can be discharged home from the emergency department, fewer infants will be admitted to the hospital and lower health-care expenses will occur.4
Hemoglobin is usually considered functional if it has the capacity to bind and transport oxygen, producing oxyhemoglobin and deoxyhemoglobin.12–14 Failure of hemoglobin to bind or transport oxygen results in carboxyhemoglobin (COHb) and methemoglobin.13,14
A dangerous pitfall of pulse oximetry is that the presence of COHb can overestimate arterial oxygenation. This outcome is induced by the specific features of COHb, which displays red-light absorption analogous to that of oxyhemoglobin. The same concept applies to the presence of methemoglobin.6
PaO2, by definition, is the partial pressure of oxygen diffused in blood,13 while the calculation and measurement of the percentage of oxygen bound to hemoglobin in arterial blood is termed oxygen saturation (SpO2).12
Formerly, SpO2 measurement was obtained solely by drawing blood samples and measuring O2 levels directly. This approach was considered invasive and was inadequate to support real-time measurements.15 Pulse oximetry is a noninvasive technique for overseeing a human’s SpO2. The most common available device is the transmissive application mode, which uses a sensor device placed on a thin part of the patient’s body, such as the fingertip, earlobe, or across the foot.16
In this paper, we describe the physiologic principles, common applications, limitations, and clinical studies of pulse oximetry in bronchiolitis.
Methods
A search of the Google Scholar, Embase, Medline, and PubMed databases was carried out using different combinations of the following terms: pulse, oximetry, infants, children, operation, mode, and limitations. In addition, we searched the references of the identified articles for additional articles. The articles included in this review were restricted to randomized controlled trials, cross-sectional studies, well-designed prospective pilots, and guidelines from well-known international organizations such as the AAP, the Scottish Intercollegiate Guidelines Network (SIGN), and the National Institute for Health and Care Excellence (NICE). We also referred to websites for terminology and definitions. We did not count on case reports, studies funded by device manufacturers, and small studies. Finally, the search was limited to studies of disease that were published in English, Portuguese, and Spanish from 1895 until July 2015 (Figure 1).
Figure 1.
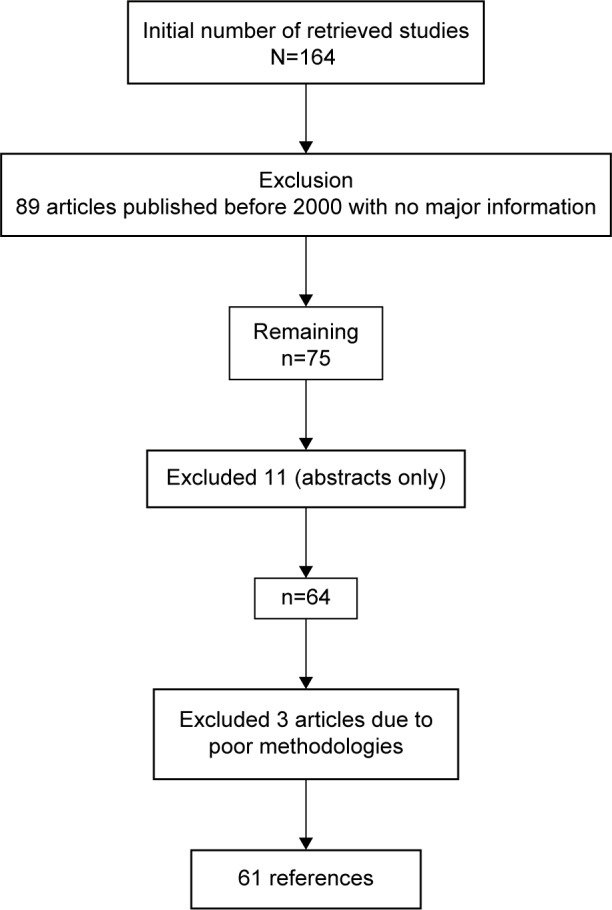
Flow diagram of selection of references.
Results
History
In 1935, Karl Matthes refined the first 2-wavelength ear O2 saturation meter with red and green filters, leading to the production of the first apparatus to measure O2 saturation. The evolution of oximetry was boosted during World War II, when an approach was needed to monitor oxygenation in aviators flying in high-pressure cockpits at high altitudes.17
In 1964, Robert Shaw put together the first complete reading ear oximeter by using eight wavelengths of light. It was made commercially available by Hewlett-Packard (Palo Alto, CA, USA) in 1970, but its use was limited to cardiac catheterization labs, perhaps due to cost and size.18
In 1970, investigations carried out by Takuo Aoyagi and Michio Kishi on dye-dilution techniques for measuring cardiac output resulted in the development of a photoplethysmogram, which produces time-dependent volumetric variations in living tissues, resulting in the development of a pulse oximeter.12,18
Thirteen years later, in 1983, Bill New introduced the Nellcor Pulse Oximeter (Covidien Corporation, Dublin, Ireland) for inpatient as well as outpatient settings.19 Pulse oximetry was of special interest in the neonatal intensive care unit where premature neonates have had a high rate of morbidity and mortality with inadequate oxygenation.20 One of the limitations of pulse oximetry was motion artifact, leading to false results. The logic behind the inaccurate results is that during movements, several pulse oximeter devices cannot differentiate between moving venous blood and pulsating arterial blood, resulting in underestimation of SpO2.21,22 These results prompted Masimo and his team to introduce Signal Extraction Technology that can accurately measure SpO2 during low perfusion or when the patient moves, by separating the arterial signal from the venous and other signals.23 Pulse oximeters that separate the arterial signal from the venous are now available and have greater sensitivity/specificity than older models.24
Principles of operation of pulse oximetry
A regular pulse oximeter uses an electronic processor and a pair of small light-emitting diodes facing a photodiode through a translucent part of the patient’s body, such as the fingertip or an earlobe. Typically, one light-emitting diode is red, with a wavelength of 660 nm, while the other is infrared with a wavelength of 940 nm. The absorption of light at these wavelengths is not similar between blood loaded with oxygen and blood lacking oxygen. Oxygenated hemoglobin permits more red light to cross and absorbs more infrared light. On the other hand, deoxygenated hemoglobin permits more infrared light to pass through and imbibes more red light.18,25 With every cardiac beat, there is an increase in arterial blood, which temporarily augments arterial blood volume across the measuring site. This will lead to increased light absorption during the surge, creating “waveform” by the photodetector. The term “pulse oximetry” was derived after the observation of peak blood flow with each heartbeat or pulse. The quantity of transmitted light is then measured. The ratio of the red light to the infrared light (ratio of oxygenated hemoglobin to deoxygenated hemoglobin) is then computed by the processor, producing a SpO2 measurement.25
Applications
Pulse oximetry has become ubiquitous in pediatrics. It is generally found in pediatric wards, emergency departments, and pediatric intensive care units.
In addition, pulse oximetry has been widely used in association with respiratory illnesses, cyanosis, during resuscitations, and as a screening tool in the emergency department. It has also been used in the detection of pulsus paradoxus, estimating perfusion, and for screening infants for congenital heart disease.26–39 In adults, the limited literature has shown that pulse oximetry can be used in patients with pulmonary embolism and chronic diseases.40–43
Limitations
Pulse oximeters have several limitations (Table 1). One of them is caused by inadequate signals, especially in cases of anemia, dark skin, bright external light, intravenous dye, nail polish, and low perfusion.21,25,44,45 Additionally, owing to the sigmoidal shape of the oxyhemoglobin dissociation curve, pulse oximetry might not detect hypoxemia in individuals with elevated arterial oxygen tension (PaO2) levels.25 Another limitation of the pulse oximeter is low reading in cases of venous pulsations such as tricuspid regurgitation, severe right heart failure, and tourniquet or blood pressure cuff above the site of the pulse oximeter.44 Furthermore, a pulse oximeter might provide unreliable readings (80%–85% saturation regardless of actual saturation) in cases such as methemoglobinemia.44
Table 1.
Limitations of a pulse oximeter
| Limitations | Condition(s) |
|---|---|
| Inadequate signal | • Anemia |
| • Dark skin | |
| • Bright external light | |
| • Intravenous dye | |
| • Nail polish | |
| • Low perfusion | |
| False hypoxemia | • Elevated arterial oxygen tension (PaO2) levels |
| Low reading | Venous pulsations such as |
| • Tricuspid regurgitation | |
| • Severe right heart failure | |
| • Tourniquet or blood pressure cuff above the site of pulse oximeter | |
| Unreliable readings | Dyshemoglobin |
| • Methemoglobin | |
| • Carboxyhemoglobin |
Bronchiolitis and pulse oximetry
The association between oxygen saturation and respiratory distress in young infants with respiratory infections is not well established.44 However, it has been established that the effect of oxygen saturation on respiratory drive is inferior to carbon dioxide concentrations in the blood.46 Moreover, while sleeping, children can have physiologic low oxygen saturation (SpO2), especially those living at higher altitudes.47
Although a pulse oximeter is a handy device to measure the percentage of oxygen-bound hemoglobin in children, it lacks precision particularly in the 76%–90% range.48 Currently, there is no international consensus on the SpO2 levels that are required to admit or discharge the pediatric patient with acute bronchiolitis.10,11,49–51 For instance, the NICE and the SIGN recommend giving oxygen supplementation to children with bronchiolitis if their oxygen saturation is persistently <92%.49,51 The AAP, on the other hand, suggests providing oxygen supplements to children with acute bronchiolitis for SpO2 of <90%.10,11
Oxygen saturation measurement has become part of the vital signs in the majority of health-care centers, and it is usually adopted by health-care providers to lead their clinical evaluation of respiratory status and their decisions about safe discharge.52 In the recent bronchiolitis guidelines, the AAP emphasized the importance of history and physical examination, and otherwise recommend little testing, treatment, or intervention including the use of continuous pulse oximetry.1,53
Transient desaturation is a normal variant in healthy infants. A study of 103 preterm infants born at <1,750 g and #34 weeks postmenstrual age, and 99 healthy term infants showed that episodes of intermittent hypoxemia occurred in three-quarters of preterm and almost two-thirds of term infants. The acute intermittent hypoxemia was defined as a decrease in absolute oxygen saturation that was sustained below 90% for ≥5 seconds.54
In a prospective study to assess the accuracy of pulse oximetry in the saturation range of 65%–97%, Ross et al48 included 225 mechanically ventilated children with an arterial catheter. For every arterial blood gas sample, SpO2 from pulse oximetry and arterial oxygen saturations from CO-oximetry (SaO2) were concurrently gathered if the SpO2 was #97%. The authors concluded that the accuracy of pulse oximetry varies considerably as a function of the SpO2 range. Saturations obtained by pulse oximetry embellish SaO2 from CO-oximetry in the SpO2 range of 76%–90%.
A retrospective study conducted by Schroeder et al4 on the impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations showed that 26% of children had delayed discharges from the hospital due to the perception of oxygen requirement based on pulse oximetry readings and not clinical findings.
A randomized, double-blind, parallel-group trial was conducted in Canada from 2008 to 2013 on the effect of pulse oximetry on bronchiolitis hospitalization. The study included 213 healthy infants aged 4 weeks to 1 year with mild-to-moderate bronchiolitis and genuine oxygen saturations of 88% or higher. The investigators deliberately increased the saturation values by 3 percentage points above the true values in the intervention group. The study concluded that among infants who are brought to the emergency department with mild-to-moderate bronchiolitis, infants with a deliberately augmented pulse oximetry reading were less likely to be admitted within 72 hours or to acquire health center care for more than 6 hours than those with genuine oximetry readings.2
Lowering the threshold of acceptable SpO2 at discharge could significantly decrease the length of stay for hospitalized infants with mild or moderate bronchiolitis. Cunningham and McMurray conducted a prospective observational study of two oxygen saturation targets (≥90% and ≥94%) for discharge in bronchiolitis. The study included 68 infants with a median age of 14 weeks. The time required for infants to achieve a stable SpO2≥90% was a median of 22 hours (interquartile range: 7–39 hours) sooner than the equivalent for stable SpO2 ≥94%.8
Perhaps simple interventions might ameliorate the issue. In a recently published paper on using quality improvement to reduce continuous pulse oximetry use in children with wheezing, Schondelmeyer et al55 were able to decrease the median duration on continuous pulse oximetry by 70%. The investigators used simple steps such as education about guidelines and acceptable goals for well-timed discontinuation of continuous pulse oximetry.
In addition to its questionable use in bronchiolitis, repeated alarms from pulse oximetry due to false measurement in an active infant or child might lead to alarm fatigue,56 which has been associated with dangerous safety occurrences.57–59 The Joint Commission stated in its report published in 2013 that 85%–99% of alarm signals are attributed to technical flaws and do not require clinical intervention. As a result, health-care providers become insensitive or develop tolerance to the sounds, leading to “alarm fatigue”. As a consequence, clinicians decrease the volume of the alarm or even turn it off, which can have eventually drastic and dreadful repercussions.60
In a 6-month pilot study, Martin et al assessed evidence-based protocols to guide pulse oximetry and oxygen weaning in inpatient children with asthma and bronchiolitis. An interprofessional team from Cook Children’s Medical Center in the State of Texas initially identified lack of guidelines and deficiencies in oxygen weaning protocols for respiratory therapists or nurses concerning the use of pulse oximetry in young children hospitalized for asthma or bronchiolitis. The team eventually developed evidence-based pulse oximetry and oxygen weaning protocols, and compared the outcomes of small samples (n=15, n=16) of the pilot group with those of children admitted with asthma or bronchiolitis prior to the protocol use.
The study concluded that these protocols decreased length of stay by 50% and shortened both the duration of oxygen supplementation and pulse oximetry monitoring.61
Conclusion
The introduction of pulse oximetry in clinical practice has permitted a plain and noninvasive mode of measuring arterial oxygen saturation. However, it has certain limitations. Considering the current technological progress, randomized controlled clinical trials on how to integrate pulse oximetry into evidence-based diagnostic and management algorithms in bronchiolitis are required.
Summary and recommendations
Intermittent hypoxemia physiologically occurs in three-quarters of preterm and almost two-thirds of term infants with bronchiolitis.
Although a pulse oximeter is a handy device to measure the percentage of oxygen-bound hemoglobin in children, it lacks precision, particularly in the 76%–90% range.
The diagnostic value of pulse oximetry in assessing the severity of bronchiolitis is questionable; hence history and physical examination is important to delineate the correct diagnosis and to evaluate the severity of the illness.
Overreliance on pulse oximetry measurements may lead to the overdiagnosis of illness severity and increased bronchiolitis admissions.
Repeated alarms from pulse oximetry by false measurement in an active infant or child might lead to alarm fatigue, leading to possible dreadful outcomes.
Pulse oximetry should be used only in patients with unstable respiratory status, and development of specific weaning protocols may limit unnecessary admissions, length of stay, cost, and prevent negative outcomes associated with alarm fatigue.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ralston SL, Lieberthal AS, Meissner HC, et al. American Academy of Pediatrics Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 2.Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312(7):712–718. doi: 10.1001/jama.2014.8637. [DOI] [PubMed] [Google Scholar]
- 3.Mallory MD, Shay DK, Garrett J, Bordley WC. Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics. 2003;111(1):e45–e51. doi: 10.1542/peds.111.1.e45. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder AR, Marmor AK, Pantell RH, Newman TB. Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158(6):527–530. doi: 10.1001/archpedi.158.6.527. [DOI] [PubMed] [Google Scholar]
- 5.Fouzas S, Priftis KN, Anthracopoulos MB. Pulse oximetry in pediatric practice. Pediatrics. 2011;128(4):740–752. doi: 10.1542/peds.2011-0271. [DOI] [PubMed] [Google Scholar]
- 6.Elliott M, Tate R, Page K. Do clinicians know how to use pulse oximetry? A literature review and clinical implications. Aust Crit Care. 2006;19(4):139–144. doi: 10.1016/s1036-7314(06)80027-5. [DOI] [PubMed] [Google Scholar]
- 7.Corneli HM, Zorc JJ, Holubkov R, et al. Bronchiolitis Study Group for the Pediatric Emergency Care Applied Research Network Bronchiolitis: clinical characteristics associated with hospitalization and length of stay. Pediatr Emerg Care. 2012;28(2):99–103. doi: 10.1097/PEC.0b013e3182440b9b. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham S, McMurray A. Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97(4):361–363. doi: 10.1136/adc.2010.205211. [DOI] [PubMed] [Google Scholar]
- 9.Unger S, Cunningham S. Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008;121(3):470–475. doi: 10.1542/peds.2007-1135. [DOI] [PubMed] [Google Scholar]
- 10.Zorc JJ, Hall CB. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125(2):342–349. doi: 10.1542/peds.2009-2092. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 12.Dresher RP, Mendelson Y. Reflectance forehead pulse oximetry: effects of contact pressure during walking. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3529–3532. doi: 10.1109/IEMBS.2006.260136. [DOI] [PubMed] [Google Scholar]
- 13.Pittman RN. Oxygen transport in the microcirculation and its regulation. Microcirculation. 2013;20(2):117–137. doi: 10.1111/micc.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thom CS, Dickson CF, Gell DA, Weiss MJ. Hemoglobin variants: biochemical properties and clinical correlates. Cold Spring Harb Perspect Med. 2013;3(3):a011858. doi: 10.1101/cshperspect.a011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel S, Park H, Bonato P, Chan L, Rodgers M. A review of wearable sensors and systems with application in rehabilitation. J Neuroeng Rehabil. 2012;9:21. doi: 10.1186/1743-0003-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brand TM, Brand ME, Jay GD. Enamel nail polish does not interfere with pulse oximetry among normoxic volunteers. J Clin Monit Comput. 2002;17(2):93–96. doi: 10.1023/a:1016385222568. [DOI] [PubMed] [Google Scholar]
- 17.Mechem CC. Pulse oximetry. [Accessed July 11, 2015]. Available from: http://www.uptodate.com/contents/pulse-oximetry.
- 18.Kamat V. Pulse oximetry. Indian J Anaesth. 2002;46(4):261–268. [Google Scholar]
- 19.History of pulse oximetry. [Accessed July 11, 2015]. Available from: http://www.oximetry.org/pulseox/history.htm.
- 20.Lin JC, Strauss RG, Kulhavy JC, et al. Phlebotomy overdraw in the neonatal intensive care nursery. Pediatrics. 2000;106(2):E19. doi: 10.1542/peds.106.2.e19. [DOI] [PubMed] [Google Scholar]
- 21.Barker SJ. “Motion-resistant” pulse oximetry: a comparison of new and old models. Anesth Analg. 2002;95(4):967–972. doi: 10.1097/00000539-200210000-00033. [DOI] [PubMed] [Google Scholar]
- 22.Gehring H, Hornberger C, Matz H, Konecny E, Schmucker P. The effects of motion artifact and low perfusion on the performance of a new generation of pulse oximeters in volunteers undergoing hypoxemia. Respir Care. 2002;47(1):48–60. [PubMed] [Google Scholar]
- 23.Jopling MW, Mannheimer PD, Bebout DE. Issues in the laboratory evaluation of pulse oximeter performance. Anesth Analg. 2002;94(1 Suppl):S62–S68. [PubMed] [Google Scholar]
- 24.Shah N, Ragaswamy HB, Govindugari K, Estanol L. Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers. J Clin Anesth. 2012;24(5):385–391. doi: 10.1016/j.jclinane.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Jubran A. Pulse oximetry. Crit Care. 2015;19:272. doi: 10.1186/s13054-015-0984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown L, Dannenberg B. Pulse oximetry in discharge decision-making: a survey of emergency physicians. CJEM. 2002;4(6):388–393. doi: 10.1017/s1481803500007880. [DOI] [PubMed] [Google Scholar]
- 27.Callahan JM. Pulse oximetry in emergency medicine. Emerg Med Clin North Am. 2008;26(4):869–879. vii. doi: 10.1016/j.emc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Choi J, Claudius I. Decrease in emergency department length of stay as a result of triage pulse oximetry. Pediatr Emerg Care. 2006;22(6):412–414. doi: 10.1097/01.pec.0000221340.26873.2f. [DOI] [PubMed] [Google Scholar]
- 29.Duke T, Subhi R, Peel D, Frey B. Pulse oximetry: technology to reduce child mortality in developing countries. Ann Trop Paediatr. 2009;29(3):165–175. doi: 10.1179/027249309X12467994190011. [DOI] [PubMed] [Google Scholar]
- 30.Kattwinkel J, Perlman JM, Aziz K, et al. Neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S909–S919. doi: 10.1161/CIRCULATIONAHA.110.971119. [DOI] [PubMed] [Google Scholar]
- 31.Altuncu E, Ozek E, Bilgen H, Topuzoglu A, Kavuncuoglu S. Percentiles of oxygen saturations in healthy term newborns in the first minutes of life. Eur J Pediatr. 2008;167(6):687–688. doi: 10.1007/s00431-007-0540-x. [DOI] [PubMed] [Google Scholar]
- 32.Kamlin CO, O’Donnell CP, Davis PG, Morley CJ. Oxygen saturation in healthy infants immediately after birth. J Pediatr. 2006;148(5):585–589. doi: 10.1016/j.jpeds.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 33.Mariani G, Dik PB, Ezquer A, et al. Pre-ductal and post-ductal O2 saturation in healthy term neonates after birth. J Pediatr. 2007;150(4):418–421. doi: 10.1016/j.jpeds.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Rabi Y, Yee W, Chen SY, Singhal N. Oxygen saturation trends immediately after birth. J Pediatr. 2006;148(5):590–594. doi: 10.1016/j.jpeds.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 35.Amoozgar H, Ghodsi H, Borzoee M, Amirghofran AA, Ajami G, Serati Z. Detection of pulsus paradoxus by pulse oximetry in pediatric patients after cardiac surgery. Pediatr Cardiol. 2009;30(1):41–45. doi: 10.1007/s00246-008-9274-4. [DOI] [PubMed] [Google Scholar]
- 36.Bendjelid K. The pulse oximetry plethysmographic curve revisited. Curr Opin Crit Care. 2008;14(3):348–353. doi: 10.1097/MCC.0b013e3282fb2dc9. [DOI] [PubMed] [Google Scholar]
- 37.Lima A, Bakker J. Noninvasive monitoring of peripheral perfusion. Intensive Care Med. 2005;31(10):1316–1326. doi: 10.1007/s00134-005-2790-2. [DOI] [PubMed] [Google Scholar]
- 38.Arlettaz R, Bauschatz AS, Monkhoff M, Essers B, Bauersfeld U. The contribution of pulse oximetry to the early detection of congenital heart disease in newborns. Eur J Pediatr. 2006;165:94–98. doi: 10.1007/s00431-005-0006-y. [DOI] [PubMed] [Google Scholar]
- 39.Hoffman JI. It is time for routine neonatal screening by pulse oximetry. Neonatology. 2011;99(1):1–9. doi: 10.1159/000311216. [DOI] [PubMed] [Google Scholar]
- 40.Cunningham S, McMurray A. The availability and use of oxygen saturation monitoring in primary care in order to assess asthma severity. Prim Care Respir J. 2006;15:98–101. doi: 10.1016/j.pcrj.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kline JA, Hernandez-Nino J, Newgard CD, Cowles DN, Jackson RE, Courtney DM. Use of pulse oximetry to predict in-hospital complications in normotensive patients with pulmonary embolism. Am J Med. 2003;115:203–208. doi: 10.1016/s0002-9343(03)00328-0. [DOI] [PubMed] [Google Scholar]
- 42.Kelly AM, McAlpine R, Kyle E. How accurate are pulse oximeters in patients with acute exacerbations of chronic obstructive airways disease? Respir Med. 2001;95:336–340. doi: 10.1053/rmed.2001.1046. [DOI] [PubMed] [Google Scholar]
- 43.Parameswaran GI, Brand K, Dolan J. Pulse oximetry as a potential screening tool for lower extremity arterial disease in asymptomatic patients with diabetes mellitus. Arch Intern Med. 2005;165:442–446. doi: 10.1001/archinte.165.4.442. [DOI] [PubMed] [Google Scholar]
- 44.Pope J, McBride J. Consultation with the specialist: respiratory failure in children. Pediatr Rev. 2004;25(5):160–167. doi: 10.1542/pir.25-5-160. [DOI] [PubMed] [Google Scholar]
- 45.Petterson MT, Begnoche VL, Graybeal JM. The effect of motion on pulse oximetry and its clinical significance. Anesth Analg. 2007;105(6 Suppl):S78–S84. doi: 10.1213/01.ane.0000278134.47777.a5. [DOI] [PubMed] [Google Scholar]
- 46.Kim V, Benditt JO, Wise RA, Sharafkhaneh A. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):513–518. doi: 10.1513/pats.200708-124ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gavlak JC, Stocks J, Laverty A, et al. The Young Everest Study: preliminary report of changes in sleep and cerebral blood flow velocity during slow ascent to altitude in unacclimatised children. Arch Dis Child. 2013;98(5):356–362. doi: 10.1136/archdischild-2012-302512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross PA, Newth CJL, Khemani RG. Accuracy of pulse oximetry in children. Pediatrics. 2014;133(1):22–29. doi: 10.1542/peds.2013-1760. [DOI] [PubMed] [Google Scholar]
- 49.National Institute for Health and Care Excellence (NICE) guidance Bronchiolitis in children. 2015. [Accessed July 19, 2015]. Available from: http://www.nice.org.uk/guidance/ng9.
- 50.Everard ML. Respiratory syncytial virus associated lower respiratory tract disease. In: Taussig LM, Landau LI, LeSouëf PN, Martinez FD, Morgan WJ, Sly PD, editors. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby-Elsevier; 2008. pp. 491–499. [Google Scholar]
- 51.Scottish Intercollegiate Guidelines Network Bronchiolitis in children: a national clinical guideline. [Accessed June 7, 2015]. Available from: http://www.sign.ac.uk.
- 52.Vinci R, Bauchner H. Bronchiolitis, deception in research, and clinical decision making. JAMA. 2014;312(7):699–700. doi: 10.1001/jama.2014.8638. [DOI] [PubMed] [Google Scholar]
- 53.Quinonez RA, Schroeder AR. Safely doing less and the new AAP bronchiolitis guideline. Pediatrics. 2015;135(5):793–795. doi: 10.1542/peds.2014-3703. [DOI] [PubMed] [Google Scholar]
- 54.Hunt CE, Corwin MJ, Weese-Mayer DE, et al. Collaborative Home Infant Monitoring Evaluation (CHIME) Study Group Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. J Pediatr. 2011;159(3):377–383.e1. doi: 10.1016/j.jpeds.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044–e1051. doi: 10.1542/peds.2014-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lutter NO, Urankar S, Kroeber S. False alarm rates of three third-generation pulse oximeters in PACU, ICU and IABP patients. Anesth Analg. 2002;94(1 Suppl):s69–s75. [PubMed] [Google Scholar]
- 57.Jones K. Alarm fatigue a top patient safety hazard. CMAJ. 2014;186(3):178. doi: 10.1503/cmaj.109-4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keesler J. The dangers of alarm fatigue. Nurs Manage. 2014;45(4):8, 10. [PubMed] [Google Scholar]
- 59.Ulrich B. Alarm fatigue: a growing problem. Nephrol Nurs J. 2013;40(4):293, 346. [PubMed] [Google Scholar]
- 60.Joint Commission National patient safety goal on alarm management. [Accessed July 20, 2015];Joint Commission Perspectives. 2013 33(7):1–4. Available from: http://www.jointcommission.org/assets/1/18/JCP0713_Announce_New_NSPG.pdf. [Google Scholar]
- 61.Martin S, Martin J, Seigler T. Evidence-based protocols to guide pulse oximetry and oxygen weaning in inpatient children with asthma and bronchiolitis: a pilot project. J Pediatr Nurs. 2015 doi: 10.1016/j.pedn.2015.02.003. [DOI] [PubMed] [Google Scholar]


