Summary
Background
Within the development and approval of several new direct-acting antivirals (DAA) against hepatitis C virus (HCV), a new era of hepatitis C therapy has begun. Even more treatment options are likely to become available during the next 1-2 years.
Methods
A summary of the current phase II and III trials investigating DAA and a review of the recent HCV guidelines was conducted.
Results
With the development of new potent DAA and the approval of different DAA combinations, cure rates of HCV infection of >90% are achievable for almost all HCV genotypes and stages of liver disease. Currently available DAA target different steps in the HCV replication cycle, in particular the NS3/4A protease, the NS5B polymerase, and the NS5A replication complex. Treatment duration varies between 8 and 24 weeks depending on the stage of fibrosis, prior treatment, HCV viral load, and HCV genotype. Ribavirin is required only for some treatment regimens and may be particularly beneficial in patients with cirrhosis. DAA resistance influences treatment outcome only marginally; thus, drug resistance testing is not routinely recommended before treatment. In the case of treatment failure, however, resistance testing should be performed before re-treatment with other DAA is initiated.
Conclusion
With the new, almost side effect-free DAA treatment options chronic HCV infection became a curable disease. The clinical benefit of DAA combination therapies in patients with advanced cirrhosis and the effects on incidence rates of hepatocellular carcinoma remain to be determined.
Keywords: Hepatitis C, Direct-acting antivirals, DAA, Cirrhosis, Drug resistance
Introduction
An estimated 300,000-500,000 individuals in Germany are affected by persistent hepatitis C virus (HCV) infection. The main clinical problems of chronic hepatitis C include the development of liver cirrhosis, hepatocellular carcinoma (HCC), or the need for liver transplantation. HCV infections are associated not only with increased liver-related mortality but also with higher extrahepatic mortality [1,2]. The HCV-associated disease burden in Germany will progress without more intense therapeutic intervention during the next 5-10 years [3]. An association between sustained virological response (SVR) and a prolonged overall survival in hepatitis C patients with advanced liver fibrosis has recently been established [4]. Until 2011, the existing standard therapy consisting of the administration of pegylated interferon α (peg-IFN) in combination with ribavirin (RBV) reached SVR rates from 30 to 90% depending on HCV genotype (GT) and stage of liver disease [5]. Despite SVR rates of up to 90%, patients had to face sometimes severe side effects (flu-like symptoms, leukopenia, thrombocytopenia, depression, etc.), and for many patients peg-IFN and/or RBV were contraindicated. Thus, there was an urgent need to develop new therapeutic strategies.
In the recent years, the development and clinical testing of highly effective direct-acting antivirals (DAA) for HCV launched a new era in the treatment of hepatitis C. In 2014/2015, seven new DAA obtained the approval of the European Medicines Agency (EMA), and IFN-free treatments became available for the first time. Further approvals of additional DAA in various combinations are expected in the coming months and years, likely enabling curative, side effect-free therapies for every patient. Mathematical modeling to forecast HCV disease burden already shows an increased efficacy of DAA with higher treatment rates to decrease the number of HCC, decompensated and compensated cirrhosis, and consecutive liver-related deaths by 75% in the next 15 years [6].
There may be economic considerations that peg-IFN-based therapies are still reasonable in some situations. For example, dual peg-IFN/RBV may still be the preferred treatment option for GT3 patients with low baseline viral load and mild fibrosis where SVR rates can be >90% within just 16 weeks of therapy if patients achieve a rapid virological response (RVR) (HCV RNA-negative after 4 weeks of therapy) [7]. In contrast, peg-IFN/RBV and sofosbuvir (SOF, SOVALDI®) for 12 weeks in GT1 is not recommended as a first choice of treatment in Germany anymore because the fixed-dose combination of SOF and ledipasvir (LDV, co-formulated with SOF as HARVONI®) is available at a similar price. Therefore, we will focus only on IFN-free regimes in this article.
Direct-Acting Antivirals against Hepatitis C Virus
Requirements for the clinical development of DAA included an understanding of each step of the HCV replication cycle. Through the breakdown of the structure of HCV replicons detailed molecular studies allowed an in vitro screening of small molecules with activity against HCV [8]. Understanding the individual steps of the HCV replication cycle, main targets for DAA such as the NS3/4A protease, NS5B polymerase, and the NS5A replication complex could be identified and ultimately led to the identification of many new substances, which have been tested in clinical trials and have already been approved (table 1).
Table 1.
Approved ‘direct-acting antivirals’ for the treatment of chronic hepatitis C (5/2015) (modified according to [44])
| Medication | HCV genotype | Dosing |
|---|---|---|
| HCV NS3/4A protease inhibitors | oral | |
| Boceprevir (VICTRELIS®) | 1 | not recommended anymore |
| Telaprevir (INCIVEK®, INCIVO®) | 1 | not recommended anymore |
| Simeprevir (OLYSIO® (US), SOVRIAD® (Japan), GALEXOS® (Canada)) | 1 & 4 | 150 mg (1 × 150 mg capsules) once daily; 100 mg in Japan |
| Paritaprevir (co-formulated with ritonavir and ombitasvir as VIEKIRAX®) | 1 & 4 | 150 mg once daily (2 × 75 mg, 2 tablets once daily; only in combination with dasabuvir or ribavirin) |
| Asunaprevir (SUNVEPRA®) | 1 & 4 | 100 mg (1 × 100 mg capsules) twice daily (only available in Japan in combination with daclatasvir) |
| HCV NS5B polymerase inhibitors | oral | |
| Sofosbuvir (SOVALDI®) (nucleotide analogue) | 1–6 | 400 mg (1 × 400 mg tablets) once daily |
| Dasabuvir (EXVIERA®) (non-nucleoside analogue) | 1 | 250 mg (1 × 250 mg tablets) twice daily (only in combination with VIEKIRAX®) |
| HCV NS5A replication complex inhibitor | oral | |
| Daclatasvir (DAKLINZA®) | 1–6 | 60 mg (1 × 60 mg tablets) once daily |
| Ledipasvir (co-formulated with sofosbuvir as HARVONI®) | 1, 3 & 4 | 90 mg (1 × 90 mg tablets) once daily |
| Ombitasvir (co-formulated with paritaprevir/ ritonavir as VIEKIRAX®) | 1 & 4 | 25 mg once daily (2 × 12.5 mg, 2 tablets once daily) |
Protease Inhibitors (-previrs)
For the cleavage of HCV polyproteins the multifunctional protein NS3 with a serine protease activity is essential. In 2002, the first NS3 protease inhibitor (BILN 2061, CILUPREVIR®), developed by Boehringer Ingelheim, was tested in clinical trials. Within 48 h HCV viral load decreased vastly by several logs but side effects stopped further development of this compound [9,10]. As a result, it took nine more years of intensive research before the first two HCV protease inhibitors could finally be approved for the treatment of chronic HCV infection in 2011. The combination of boceprevir (BOC, VICTRELIS®) or telaprevir (TLV, INCIVEK®, INCIVO®) with peg-IFN and RBV as a ‘triple therapy’ were used for 24 to 48 weeks in patients with HCV GT1 infection and raised the cure rate up to 75% in treatment-naïve GT1-infected HCV patients. However, additional side effects like rash, pruritus, or anemia were recorded [11,12]. A further problem was dosing and pill burden, requiring an intake of 750 mg TLV (one tablet 375 mg) or 800 mg boceprevir (one tablet 200 mg) 3 times a day (2 or 4 tablets 7-9 h apart) plus 1,200 mg of RBV (weight-dependent; one tablet 200 mg) [13]. Thus, the patients had to take 9-18 tablets a day in total for at least 12 weeks. Moreover, treatment was response-guided based on early viral response, which means that the duration of the therapy was based on the HCV RNA level response at weeks 4 and 12 (i.e. an extended rapid virologic response (eRVR)) of therapy [14,15].
However, with the approval of new DAA, ‘old’ triple therapies with peg-IFN, RBV and the protease inhibitors BOC or TLV are no longer recommended for the treatment of chronic hepatitis C in Germany according to the current guidelines of the German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS) (www.dgvs.de).
Simeprevir
In May 2014, the EMA approved the first once-daily, second-generation NS3/4A protease inhibitor simeprevir (SMV, OLYSIO®, SOVRIAD®). SMV has a significant advantage over BOC or TLV regarding dose (one tablet 150 mg, once daily) and side effects (no anemia, rarely rush). The approval includes HCV infections with GT1 and GT4, but small pilot studies also showed an efficacy against GT2 and GT6 but not GT3. A phase III study for approval was performed in combination with peg-IFN-α-2a or −2b and RBV and showed an increased SVR up to 80-81% versus 50% for the traditional treatment with peg-IFN and RBV alone [16,17].
However, this triple therapy treatment option is not recommended as first-line therapy option because of the necessity of at least 24 weeks of peg-IFN/RBV treatment. Fortunately, the EMA approved an IFN-free combination therapy of SMV and SOF for urgent treatment for patients with a contraindication for IFN, and this treatment regimen has been widely used in the United States since December 2013 [18].
Further -previrs
Other protease inhibitors were tested in IFN-free phase III studies. In January 2015, a combination regimen of three DAA including a ritonavir-boosted protease inhibitor was approved by the EMA for GT1 patients. This therapy consists of VIEKIRAX®, the fixed-dose combination of the NS3/4A protease inhibitor paritaprevir boosted with ritonavir (PTV/r), co-formulated with the NS5A inhibitor ombitasvir (OBV), and EXVIERA®, the non-nucleoside NS5B polymerase inhibitor dasabuvir (DSV) with or without RBV. An approval for VIEKIRAX with RBV was also given for GT4. Asunaprevir (ASV, SUNVEPRA®) is developed in combination with the NS5A inhibitor daclatasvir (DCV, DAKLINZA®) for GT1b [19]. This combination is already approved in Japan.
Further development includes the combination of grazoprevir (formerly MK-5172) with elbasvir (NS5A inhibitor; formerly MK-8742). Potential benefits of grazoprevir and elbasvir include sensitivity against HCV variants, which are resistant to other DAA and the hepatic metabolisation and elimination, probably without dose adaption in patients with chronic kidney insufficiency or hemodialysis (C-SURFER).
NS5A Inhibitors (-asvirs)
The non-structural protein NS5A plays an important role in the building of replication complexes, viral packaging, and mounting of the HCV. In contrast to the protease inhibitors, inhibition of NS5A is possible in picomolar concentrations, and NS5A inhibitors have therefore shown the strongest antiviral efficacy until now [20]. Thus, NS5A inhibitors are components of almost all approved IFN-free DAA regimes or evaluated in phase III studies.
Daclatasvir
In August 2014, based on a phase II trial, the first NS5A inhibitor DCV (DAKLINZA) has been approved by the EMA in combination with SOF for the therapy of patients without cirrhosis in GT1 and GT4 (only based on extrapolated GT1 data) as well as for GT3 in combination with RBV [21]. The approval of DCV for GT4 was mainly based on data in combination with peg-IFN and RBV; however, a therapy with IFN will be considered obsolete and seems to be necessary only in exceptional cases. The combination of DCV with ASV showed cure rates of 82-90% for GT1b in a phase III study and is available for the therapy of HCV GT1 infection in Japan [22]. The combination of low-dose DCV (30 mg instead of 60 mg once daily) with SMV showed less SVR12 rates of overall 75-85% in treatment-naïve GT1-infected patients [23]. Furthermore, a phase III study to investigate a triple therapy with DCV and ASV in combination with the non-structural polymerase inhibitor BMS-791325 (expected as BECLABUVIR®) for the therapy of treatment-naïve HCV GT1 infection is currently ongoing (UNITY 3; NCT02123654).
Further -asvirs
Since November 2014, ledipasvir (LDV) in combination with SOF is available as fixed-dose combination (HARVONI; 90/400 mg once daily) for the therapy of GT1, GT3, and GT4, and also ombitasvir (OBV) as part of the triple therapy with paritaprevir boosted with ritonavir (PTV/r) and DSV is available since January 2015 (see above). ‘Second-generation’ NS5A inhibitors like elbasvir or GS-5816 are yet to be tested in phase III studies (C-WORTHY; ASTRAL 1-3). Other NS5A inhibitors like samatasvir or ACH-3102 are pipelined for further clinical testings.
NS5B Inhibitors (-buvirs)
Non-Nucleoside Polymerase Inhibitors
Non-nucleoside polymerase inhibitors (‘Non-NUCs’) are currently only part of NUC-free combination therapies against HCV GT1 infection. DSV as part of the triple therapy regimen with paritaprevir and OBV has become available since January 2015, and BMS-791325 is currently tested in combination with DCV and ASV with or without RBV for 4-8 weeks (RHACE 1; NCT02098616).
Nucleoside and Nucleotide HCV Polymerase Inhibitors
SOF is currently the only approved nucleotide inhibitor and has been approved in January 2014. SOF can be used in combination with peg-IFN and RBV for 12 weeks, leading to SVR rates of 90% in patients with HCV GT1 infection, and with RBV alone for 12 weeks for GT2 as well as 24 weeks for GT3 [24,25,26]. IFN-free SOF-based therapies of GT1 should always include a combination with other DAA. Further substances with this pan-GT DAA activity are currently under development, e.g. MK-3682 (formerly known as IDX21437), in a phase I/IIa study.
Antiviral Resistance
With the development of the new DAA, new problems of resistance through so-called resistance-associated amino acid variants (RAVs) arise. S282T mutations are associated with resistance to SOF or other nucleos(t)ide analog NS5B polymerase inhibitors but have not been selected frequently. However, genotypic resistance analysis of treatment failure patients via next-generation sequencing showed that baseline polymorphisms at amino acid position 316 of the HCV polymerase were potentially associated with a decreased SVR in GT1b-infected HCV patients [24]. Similarly, NS5A-Y93H and/or L31M/V amino acid substitutions at baseline were negative predictors of SVR12 in various NS5 inhibitor-containing regimens [22]. Testing for HCV RAVs is currently not recommended before treatment. However, baseline RAV testing is recommended before starting a second-line DAA combination therapy.
Currently Available and Recommended Therapies
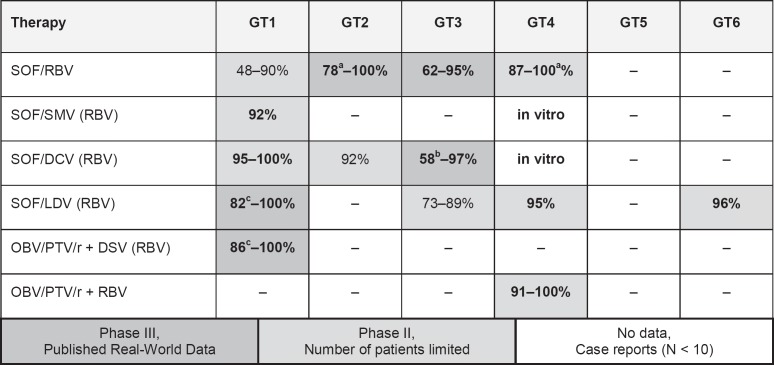
The current guidelines are freely available at www.dgvs.de and will be updated promptly after each new approval. Figure 1 gives an overview of DAA treatment regimens in different HCV GTs.
Fig. 1.
Possible interferon-free DAA treatment regimens in different HCV genotypes in 2015. Shades of grey indicate level of evidence. Bold numbers indicate preferred options in 2015. aSVR for the recommended treatment duration is indicated. bNo RBV. cDifficult-to-treat patients; SVR may be higher with optimal treatment duration (modified according to [44]).
Treatment of HCV Genotype 1
Simeprevir (OLYSIO) + Sofosbuvir (SOVALDI)
The approval of this IFN-free combination with or without RBV for 12 or 24 weeks is based on the phase II COSMOS trial. The COSMOS study showed high cure rates in GT1a/b-infected patients with prior non-response to peg-IFN/RBV and treatment-naïve patients in ≥93% (table 2) [27]. Even within the presence of the Q80K variant, which is associated with partial resistance to SMV [17], 88-93% of the patients cleared HCV. Of note, there was no major obvious benefit for additional RBV treatment or a treatment prolongation to 24 weeks. Furthermore, patients with liver cirrhosis showed high response rates. Real-world data confirmed the biological efficacy and safety of this regimen with high SVR rates (>90%). In particular patients with HCV GT1b infection almost always clear the infection [18,28].
Table 2.
Phase II and III study treatment regimens and SVR in treatment-naïve patients with HCV genotype 1 (modified according to [44])
| Study | Dosing | SVR |
|---|---|---|
| Simeprevir/sofosbuvir COSMOS [27] (N = 167 (40 naïve)) | 400 mg SOF + 150 mg SMV ± 1,000-1,200 mg RBV 12-24 weeks | 92%; RBV: 91%, no RBV 95%; naïve PP: 95% |
| Daclatasvir/sofosbuvir | ||
| AI444040 Study Group [21] (N = 126 naïve) | 400 mg SOF + 60 mg DCV ± 1,000-1,200 mg RBV 12-24 weeks | 95-100% |
| Ledipasvir/sofosbuvir | ||
| ION-1 [29] (N = 865) | 400/90 mg SOF/LDV 12 weeks; | no cirrhosis: 100%, cirrhosis: 97%; |
| 400/90 mg SOF/LDV + 1,000-1,200 mg RBV 12 weeks; | no cirrhosis: 100%, cirrhosis: 100%; | |
| 400/90 mg SOF/LDV 24 weeks | no cirrhosis: 99.5%, cirrhosis: 96.9%; | |
| 400/90 mg SOF/LDV + 1,000-1,200 mg RBV 24 weeks | no cirrhosis: 100%, cirrhosis: 100% | |
| ION-3 [31] (N = 647; no cirrhosis) | 400/90 mg SOF/LDV 8 weeks; | 94%; |
| 400/90 mg SOF/LDV + 1,000-1,200 mg RBV 8 weeks; | 93%; | |
| 400/90 mg SOF/LDV 12 weeks | 95% | |
| Paritaprevir/ombitasvir/dasabuvir | ||
| SAPPHIRE I [33] (N = 473) | 150/100 mg PTV/r + 25 mg OBV + 250 DSV BID + 1,000-1,200 mg RBV 12 weeks | 96%, G1a: 95% (96%a), G1b: 98% |
| PEARL III [34] (N = 419 genotype 1b without cirrhosis) | 150/100 mg PTV/r + 25 mg OBV + 250 DSV BID + 12 weeks; | 99% (100%a); |
| 150/100 mg PTV/r + 25 mg OBV + 250 DSV BID + 1,000-1,200 mg RBV 12 weeks | 99.5% | |
| PEARL IV [34] (N = 305 genotype 1a without cirrhosis) | 150/100 mg PTV/r + 25 mg OBV + 250 DSV BID + 12 weeks; | 90% |
| 150/100 mg PTV/r + 25 mg OBV + 250 DSV BID + 1,000-1,200 mg RBV 12 weeks | 97% | |
| TURQUOISE II [35] (N = 160 (treatment-naïve with cirrhosis)) | 150/100 mg PTV/r + 25 mg OBV + 250 DSV BID + 1,000-1,200 mg RBV 12 weeks; 150/100 mg PTV/r + 25 mg OBV + 250 DSV BID + 1,000-1,200 mg RBV 24 weeks | 94%, G1a: 92%, G1b: 100%; |
| 95% (96%a), G1a: 93% (95%a), G1b: 100% | ||
Final data given in the EMA summary and product characteristics.
SVR = Sustained virological response; PTV/r = paritaprevir/ritonavir; OBV = ombitasvir; DSV = dasabuvir; RBV = ribavirin; SOF = sofosbuvir; LDV = ledipasvir; DCV = daclatasvir.
Daclatasvir (DAKLINZA) + Sofosbuvir (SOVALDI)
The approval for this combination is based on phase II study data investigating treatment durations of 12-24 weeks with or without RBV in 211 non-cirrhotic patients [21]. 126 treatment-naïve patients were studied, including 41 patients with a treatment failure to a prior triple therapy with protease inhibitors and 44 HCV GT2/3 patients. SVR12 was reached in 95-100% of GT1 patients. Importantly, all prior TLV or BOC treatment failures achieved an SVR. Interestingly, pre-existing DCV resistance variants did not impact the response to subsequent therapy. Based on these preliminary phase II data, the EMA recommendation is to treat naïve patients without cirrhosis for 12 weeks without RBV. However, it remains unclear at present which patients can be treated for 12 weeks and which patients benefit from an additional RBV therapy or longer treatment durations.
Ledipasvir + Sofosbuvir (= HARVONI)
This fixed-dose combination called HARVONI is the first ‘single-tablet regimen’ once daily which is available since November 2014. The ION studies investigated the optimal treatment duration for treatment-naïve and previously treated patients with or without RBV [29,30,31]. 8 weeks of HARVONI are sufficient for treatment-naïve patients without cirrhosis and a HCV viral load <6 million IU/ml. Most other patients should be treated for 12 weeks, while few patients with advanced liver cirrhosis may require 24 weeks of therapy. In a retrospective analysis of >500 patients with compensated cirrhosis, additional RBV treatment showed no significant benefit even in patients with cirrhosis [32]. However, RBV may allow shortening of therapy from 24 to 12 weeks in some cirrhotic patients.
Paritaprevir/r + Ombitasvir (= VIEKIRAX) + Dasabuvir (EXVIERA)
In January 2015, the three-target triple therapy 3D consisting of the NS3 protease inhibitor paritaprevir/r boosted with ritonavir (PTV/r), co-formulated with the NS5A-inhibitor OBV (VIEKIRAX), and the non-nucleosidic polymerase inhibitor DSV (EXVIERA) has been approved for the treatment with or without RBV of chronic GT1 HCV infections. The recommendation is based on six phase III clinical trials with more than 2,300 GT1-infected HCV patients (SAPHIRE-I and -II, PEARL-II, -III, -IV, and TURQUOISE-II). Approval studies showed that almost every GT1b-infected patient can be cured with this regimen, and also HCV GT1a-infected patients experienced an SVR in more than 95% of cases [33,34,35,36]. Patients with a GT1b infection without cirrhosis can be treated for 12 weeks without RBV while all other patients should receive RBV. It should be noted that no data from phase III trials on the use of this triple therapy regimen without RBV in cirrhotic patients are available at this point in time. Nevertheless, the recommendation for GT1b-infected patients include the combination of OBV/PTV/r + DSV plus RBV for 12 weeks versus a treatment elongation to 24 weeks in GT1a-infected patients with advanced cirrhosis (thrombocytes <90,000/µl, albumin <35 g/l, or AFP >20 ng/ml).
Treatment of HCV Genotype 2
The FISSION study compared SOF/RBV for 12 weeks versus peg-IFN/RBV for 24 weeks in treatment-naïve patients and showed an overall SVR rate of 97% for SOF/RBV, whereas peg-IFN/RBV showed only an SVR of 78% in GT2 patients [37]. The POSITRON study showed an SVR >90% in cirrhotic and non-cirrhotic patients with GT2 who were treated with SOF/RBV for peg-INF intolerance, contraindication, or due to the patients' decision [25]. Based on these studies, most guidelines consider 12 weeks of SOF plus RBV as the standard of care. Treatment may be extended to 16 weeks in patients with liver cirrhosis.
The combination of the NS5A inhibitor DCV in combination with SOF and RBV for 24 weeks showed an SVR of 92% in GT2 without cirrhosis (per protocol, all ‘per protocol’ patients were cured) [21]. This combination can be recommended as a second-line therapy for SOF/RBV relapse patients.
Treatment of HCV Genotype 3
Most guidelines recommend that treatment-naïve patients with and without cirrhosis can be treated with SOF and RBV for 24 weeks – leading to SVR rates above 90% [38]. A similar rate of SVR with only 12 weeks of therapy was attained in the phase III trial ALLY-3 where patients received a combination of SOF with DCV. 120 treatment-naïve or experienced GT3 patients and 32 patients with cirrhosis were treated for 12 weeks with SOF/DCV, with an overall SVR of 90% in naïve patients but also with a lower SVR of only 58% in cirrhotic patients [39]. The EMA also approved the combination of SOF with LDV for GT3 infections. This was based on data from 51 naïve GT3 patients who were treated with SOF/LDV or SOF/LDV/RBV for 12 weeks in the ELECTRON-2 study. 64% of patients achieved an SVR without RBV while 100% did benefit from RBV [40]. The recommendation of the EMA for GT3-infected HCV patients with cirrhosis includes the combination of SOF/DCV/RBV and SOF/LDV/RBV for 24 weeks, but there are no phase II or phase III data available to strengthen this treatment. Taken together, both SOF/DCV for 12 weeks as well as SOF/RBV for 24 weeks can be considered as the first-line therapies in treatment-naïve patients with GT3 infection, while the optimal regimen for patients with liver cirrhosis and for pretreated patients remains to be determined.
Treatment of HCV Genotype 4, 5, and 6
Limited data is available for hepatitis C patients infected with HCV GTs 4, 5, or 6. The combination of peg-IFN/RBV with SOF for 12 weeks showed SVR rates of 96-100% in patients with GT4-6 infections [37]. An IFN-free therapy with SOF/RBV for 24 weeks achieved an SVR of 100% in treatment-naïve and of 87% in experienced GT4 patients, whereas the combination of SOF/LDV for 12 weeks resulted in an SVR of 95% [41,42]. The new 2D regimen with OBV/PTV/r was also evaluated in GT4-infected patients without cirrhosis and achieved an SVR of 91% without RBV as well as an SVR of 100% with RBV including treatment-experienced patients [43].
Unanswered Challenges
The new era of hepatitis C therapy represents a profound and radical change in the treatment paradigms. For the first time it is possible to cure a chronic disease with easy and extremely safe short-term oral therapies. In 2015, almost every patient with the most common GTs of a chronic HCV infection can be cured. Treatment duration will be 12 weeks in the majority of cases and can even be shortened to 8 weeks for GT1 infections if no cirrhosis exists and the viral load is below 6 million IU/ml. In contrast, treatment extension to 24 weeks may increase response rates to >90% even in pretreated patients with advanced liver cirrhosis.
Still, it is not well understood why patients with cirrhosis respond less well to these new IFN-free regimens. Moreover, the role and optimal dose of RBV in distinct IFN-free regimens requires further investigation. Furthermore, it will be important to understand the significance of resistance testing in the era of DAA therapies. It is particularly interesting to know more about resistance mechanisms against NS5A inhibitors, since almost all DAA combination regimens include one of these replication complex inhibitors. Almost all studies showed that if NS5A RAVs are present, there is a slightly reduced response rate but surprisingly the majority of HCV infections are still cured. Resistance testing should be performed before starting a second-line therapy after DAA failure.
One of the biggest challenges for the next years will be the treatment of HCV GT3-infected patients with cirrhosis. So far, no ideal therapy could be established. It remains to be seen whether new combinations of SOF/GS-5816 or MK-3682/grazoprevir/elbasvir will resolve this challenge.
The side effects profile has been excellent for all currently approved DAA. Patients treated with SMV should be informed about the need for sun blockers during therapy. Readjustment of the dosage of DCV and SMV is not dependent on kidney function; however, SOF should be administered only if the renal creatinine clearance is greater than 30 ml/min. This poses a substantial restriction for all patients with reduced kidney function, who are dialyzed, or who develop a loss of kidney function during SOF treatment.
Drug-drug interactions (DDI) are possible for all new DAA against HCV. The metabolism of the protease inhibitors as well as NS5A inhibitors involves cytochrome P450 3A4 enzymes. For SOF, DDI also have to be considered as the drug is a P-gp substrate. Concomitant medication must be evaluated for interactions before treatment is started. For example, coadministration of amiodarone with SOF in combination with another DAA may result in serious symptomatic bradycardia and is not recommended.
Furthermore, it is unknown to what extent liver function may be restored in DAA-treated patients with decompensated cirrhosis. Preliminary reports from registers and small case series show an improvement of albumin, bilirubin, and INR in some patients. Whether de-listings of patients on the transplant waiting list will be possible remains to be determined.
In conclusion, the highly effective new DAA combination therapies of hepatitis C can substantially reduce the HCV-associated disease burden. Efforts must be made to ensure access to therapy for all patients in need.
Conclusion
- IFN-free therapy with novel DAA regimens cure >90% of chronic HCV-infected patients.
- DAA regimens include NS5B nucleotide inhibitors, NS5B non-nucleoside inhibitors, NS5A replication complex inhibitors, and NS3/4A protease inhibitors.
- Depending on the stage of liver disease, HCV GT, and viral load, treatment duration varies between 8 and 24 weeks.
- RBV is no longer required for all patients,
- DAA resistance has a minor influence on cure rates in first-line treatments but resistance testing should be performed before second-line therapies are initiated.
- Current German guidelines can be found on the website of the DGVS (www.dgvs.de).
Disclosure Statement
P. Solbach has no conflict of interest. H. Wedemeyer has received honoraria from Abbott, AbbVie, Biolex, BMS, Boehringer Ingelheim, Eiger Pharmaceuticals, Falk Foundations, Gilead, IDS, JJ/Janssen-Cilag/Janssen TE, Medgenics, Merck/Schering-Plough, Novartis, Novira, Roche, Roche Diagnostics, Siemens, Transgene, and ViiV; and has received research grants from Abbott, BMS, Gilead, Merck, Novartis, Roche, Roche Diagnostics, and Siemens.
References
- 1.Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, Wang CH, Chen WJ, Chen CJ, R.E.V.E.A.L.-HCV Study Group Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 2.Maasoumy B, Wedemeyer H. Natural history of acute and chronic hepatitis C. Best Pract Res Clin Gastroenterol. 2012;26:401–412. doi: 10.1016/j.bpg.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today's treatment paradigm. J Viral Hepat. 2014;21(suppl 1):34–59. doi: 10.1111/jvh.12248. [DOI] [PubMed] [Google Scholar]
- 4.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 5.Sarrazin C, Berg T, Ross RS, et al. Prophylaxis, diagnosis and therapy of hepatitis C virus (HCV) infection: the German guidelines on the management of HCV infection (Article in German) Z Gastroenterol. 2010;48:289–351. doi: 10.1055/s-0028-1110008. [DOI] [PubMed] [Google Scholar]
- 6.Wedemeyer H, Duberg AS, Buti M, et al. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat. 2014;21(suppl 1):60–89. doi: 10.1111/jvh.12249. [DOI] [PubMed] [Google Scholar]
- 7.Heidrich B, Wiegand SB, Buggisch P, et al. Treatment of naive patients with chronic hepatitis C genotypes 2 and 3 with pegylated interferon alpha and ribavirin in a real world setting: relevance for the new era of DAA. PloS One. 2014;9:e108751. doi: 10.1371/journal.pone.0108751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 9.Hinrichsen H, Benhamou Y, Wedemeyer H, et al. Short-term antiviral efficacy of BILN 2061, a hepatitis C virus serine protease inhibitor, in hepatitis C genotype 1 patients. Gastroenterology. 2004;127:1347–1355. doi: 10.1053/j.gastro.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Tomei L, Failla C, Santolini E, De Francesco R, La Monica N. NS3 is a serine protease required for processing of hepatitis C virus polyprotein. J Virol. 1993;67:4017–4026. doi: 10.1128/jvi.67.7.4017-4026.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. New Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 12.Poordad F, McCone J, Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. New Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.www.accessdata.fda.gov/drugsatfda_docs/label/2012/201917s007lbl.pdf.
- 14.Sarrazin C, Berg T, Cornberg M, et al. Expert opinion on boceprevir- and telaprevir-based triple therapies of chronic hepatitis C (Article in German) Z Gastroenterol. 2012;50:57–72. doi: 10.1055/s-0031-1282015. [DOI] [PubMed] [Google Scholar]
- 15.Sherman KE, Flamm SL, Afdhal NH, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. New Engl J Med. 2011;365:1014–1024. doi: 10.1056/NEJMoa1014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson IM, Dore GJ, Foster GR, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403–413. doi: 10.1016/S0140-6736(14)60494-3. [DOI] [PubMed] [Google Scholar]
- 17.Manns M, Marcellin P, Poordad F, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414–426. doi: 10.1016/S0140-6736(14)60538-9. [DOI] [PubMed] [Google Scholar]
- 18.Jensen DM O'Leary J, Pockros P, for the HCV-TARGET Study Group Safety and Efficacy of Sofosbuvir-Containing Regimens for Hepatitis C: Real-World Experience in a Diverse, Longitudinal Observational Cohort. 65th Annual Meeting of the American Association for the Study of Liver Diseases. November 7-11, 2014, Boston, USA. Abstract 45. www.natap.org/2014/AASLD/AASLD_10.htm.
- 19.Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. New Engl J Med. 2012;366:216–224. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]
- 20.Gao M, Nettles RE, Belema M, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465:96–100. doi: 10.1038/nature08960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. New Engl J Med. 2014;370:211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 22.Manns M, Pol S, Jacobson IM, et al. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014;384:1597–1605. doi: 10.1016/S0140-6736(14)61059-X. [DOI] [PubMed] [Google Scholar]
- 23.Zeuzem S, Hezode C, Bronowicki J-P, et al. Daclatasvir in Combination with Simeprevir with or without Ribavirin for Hepatitis C Virus Genotype 1 Infection. 21st Conference on Retroviruses and Opportunistic Infections. March 3-6, 2014. Boston, USA. www.natap.org/2014/CROI/croi_09.htm.
- 24.Donaldson EF, Harrington PR, O'Rear JJ, Naeger LK. Clinical evidence and bioinformatics characterization of potential hepatitis C virus resistance pathways for sofosbuvir. Hepatology. 2015;61:56–65. doi: 10.1002/hep.27375. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. New Engl J Med. 2013;368:1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 26.Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir + Ribavirin for 12 or 24 Weeks for Patients with HCV Genotype 2 or 3: the VALENCE trial. 64th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD 2013). November 1-5, 2013. Washington, DC, USA. www.natap.org/2013/AASLD/AASLD_15.htm.
- 27.Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756–1765. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 28.Dieterich D, Bacon BR, Flamm S, et al. Evaluation of Sofosbuvir and Simeprevir-Based Regimens in the TRIO Network: Academic and Community Treatment of a Real-World, Heterogeneous Population. 65th Annual Meeting of the American Association for the Study of Liver Diseases. November 7-11, 2014. Boston, USA. Abstract 46. www.natap.org/2014/AASLD/AASLD_09.htm.
- 29.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. New Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 30.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. New Engl J Med. 2014;370:1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 31.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. New Engl J Med. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 32.Bourlière Sulkowski MS, Omata M, et al. Integrated Safety and Efficacy Analysis of >500 Patients with Compensated Cirrhosis Treated with Ledipasvir/ Sofosbuvir with or without Ribavirin. 65th Annual Meeting of the American Association for the Study of Liver Diseases. November 7-11, 2014. Boston, USA. Abstract 82.
- 33.Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. New Engl J Med. 2014;370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 34.Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. New Engl J Med. 2014;370:1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 35.Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. New Engl J Med. 2014;370:1973–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 36.Zeuzem S, Jacobson IM, Baykal T, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. New Engl J Med. 2014;370:1604–1614. doi: 10.1056/NEJMoa1401561. [DOI] [PubMed] [Google Scholar]
- 37.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. New Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 38.Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. New Engl J Med. 2014;370:1993–2001. doi: 10.1056/NEJMoa1316145. [DOI] [PubMed] [Google Scholar]
- 39.Nelson DR, Cooper JN, Lalezari JP, et al. All-Oral 12-Week Combination Treatment with Daclatasvir and Sofosbuvir in Patients Infected with HCV Genotype 3: ALLY-3 Phase 3 Study. 65th Annual Meeting of the American Association for the Study of Liver Diseases. November 7-11, 2014. Boston, USA. Abstract LB-3. www.natap.org/2015/EASL/EASL_73.htm.
- 40.Gane EJ, Hyland HR, An D, et al. Sofosbuvir/Ledipasvir Fixed Dose Combination Is Safe and Effective in Difficult-to-Treat Populations Including Genotype-3 Patients, Decompensated Genotype-1 Patients, and Genotype-1 Patients with Prior Sofosbuvir Treatment Experience. Program and abstracts of the 49th Annual Meeting of the European Association for the Study of the Liver. April 9-13, 2014. London, England. Abstract 06. J Hepatol. 2014;60(suppl):S3–S4. [Google Scholar]
- 41.Kapoor R, Kohli A, Sidharthan S, et al. Treatment of Hepatitis C Genotype 4 with Ledipasvir and Sofosbuvir for 12 Weeks: Results of the SYNERGY Trial. 65th Annual Meeting of the American Association for the Study of Liver Diseases. November 7-11, 2014. Boston, USA. Abstract 240.
- 42.Ruane PJ, Ain D, Meshrekey R, et al. Sofosbuvir plus Ribavirin, an Interferon-Free Regimen, in the Treatment of Treatment-Naive and Treatment-Experienced Patients with Chronic Genotype 4 HCV Infection. 49th Annual Meeting of the European Association for the Study of Liver Diseases. April 9-13, 2014. London, England. Abstract P1243.
- 43.Pol S, Reddy KR, Baykal T, et al. Interferon-Free Regimens of Ombitasvir and ABT-450/r with or without Ribavirin in Patients with HCV Genotype 4 Infection: PEARL-I Study Results. 65th Annual Meeting of the American Association for the Study of Liver Diseases. November 7-11, 2014. Boston, USA. Abstract 1928.
- 44.Cornberg M, Höner zu Siederdissen C, Maasoumy B, Solbach P, Manns MP, Wedemeyer H. Standard therapy of chronic hepatitis C virus infection; in Mauss S, Berg T, Rockstroh J, Sarrazin C, Wedemeyer H (eds): Hepatology. A Clinical Textbook, ed 6. Flying Publisher, 2015, pp 221-286.