Abstract
Background
Preoperative chemoradiotherapy (pRCT) followed by surgery has been widely practiced in locally advanced rectal cancer, esophageal cancer, gastric cancer and other cancers. However, the therapy also exerts some severe adverse effects and some of the patients show poor or no response. It is very important to develop biomarkers (e.g., gene polymorphisms) to identify patients who have a higher likelihood of responding to pRCT. Recently, a series of reports have investigated the association of the genetic polymorphisms in methylenetetrahydrofolate reductase (MTHFR) and epidermal growth factor receptor (EGFR) genes with the tumor response to pRCT; however, the results were inconsistent and inconclusive.
Material/Methods
A systematic review and meta-analysis was performed by searching relevant studies about the association of MTHFR and EGFR polymorphisms with the tumor regression grade (TRG) in response to pRCT in databases of PubMed, EMBAS, Web of science, Chinese National Knowledge Infrastructure, and Wanfang database up to March 30, 2015. The pooled odds ratios (ORs) with corresponding 95% confidence intervals (95% CIs) were calculated to assess the strength of the association under 5 genetic models.
Results
A total of 11 eligible articles were included in the present meta-analysis, of which 8 studies were performed in rectal cancer and 3 studies were performed in esophageal cancer. We finally included 8 included studies containing 839 cases for MTHFR C677T, 5 studies involving 634 cases for MTHFR A1298C, 3 studies containing 340 cases for EGFR G497A, and 4 studies containing 396 cases for EGFR CA repeat. The pooled analysis results indicated that MTHFR C677T might be correlated with the tumor response to pRCT under the recessive model (CC vs. CTTT) in overall analysis (OR=1.426(1.074–1.894), P=0.014), rectal cancer (OR=1.483(1.102–1.996), P=0.009), and TRG 1–2 vs. 3–5 group (OR=1.423(1.046–1.936), P=0.025), while other polymorphism including MTHFR A1298C, EGFR G497A, and EGFR CA repeat polymorphisms exerted significant association under all genetic models in overall analysis or subgroup analysis.
Conclusions
MTHFR C677T might be correlated with the tumor response to pRCT. Further well-designed, larger-scale epidemiological studies are needed to validate our results.
MeSH Keywords: Chemoradiotherapy, Adjuvant; Meta-Analysis; Polymorphism, Single Nucleotide
Background
Multimodality therapy clearly offers survival benefit over surgery alone, especially in high-stage cancers. In recent decades, preoperative chemoradiotherapy followed by surgery has been the standard therapy for locally advanced rectal cancer and its application is increasing in other cancers, such as esophageal cancer and gastric cancer [1]. However, the treatment also exerts some severe adverse effects and some patients are not sensitive to pRCT [2–4]. Thus, to identify patients who will benefit from the therapy strategy is very important. Therapy response is correlated not only with tumor types and tumor microenvironment, but also with patient genetics. Several biomarkers have been investigated to see if they are correlated with the response to pRCT, including the genetic polymorphisms in methylenetetrahydrofolate reductase (MTHFR) and epidermal growth factor receptor (EGFR) genes.
MTHFR can catalyze the conversion of 5,10-MTHF to 5-methylenetetrahydrofolate. There are 2 common polymorphisms in MTHFR – C677T (rs1801133) and A1298C (rs1801131) – that are widely investigated. Both of them can be used as predictors of the response to fluoropyrimidine-based chemotherapy [5]. EGFR is a member of the human epithelial receptor tyrosine kinase family and is also known as HER-1. Its kinase activity can regulate downstream gene expression, cellular proliferation, inhibition of apoptosis, and angiogenesis [6]. Its expression has been reported to be related to radiation resistance. A polymorphism in the EGFR gene has been reported to lead the substitution of an arginine (Arg) residue by a lysine (Lys) in codon 497 (G497A) [7]. Another polymorphism variant in EGFR is the CA repeats in intron 1 (rs11568315). EGFR transcription activity declines with an increasing number of CA repeats [8]. The alleles are carried as short (S) or long (L), according to the number of CA repeats. Although a series of studies have been performed to examine the association of MTHFR and EGFR polymorphisms with the tumor response to pRCT, the results were inconsistent and inconclusive. In the present study, we conducted a meta-analysis to evaluate the association of MTHFR and EGFR polymorphisms with the tumor response to pRCT.
Material and Methods
Publication search
We performed a systematic search for published articles in the database of PubMed, EMBASW, Web of Science, Chinese National Knowledge Infrastructure, and Wanfang on the association of MTHFR or EGFR polymorphisms and the response to preoperative chemoradiotherapy up to March 30, 2015. The following keywords and subject terms were used: “the methylenetetrahydrofolate reductase gene OR MTHFR”, “Epidermal growth factor receptor OR EGFR”, “polymorphism OR polymorphisms”, and “Chemoradiation OR chemoradiotherapy OR chemo-radiotherapy OR radio-chemotherapy”. The retrieved articles were screened by 2 authors independently, according to the inclusion and exclusion criteria and the review articles and reference lists of the primary studies were also screened.
Inclusion and exclusion criteria
Inclusion criteria: (1) evaluation of the association between MTHFR C677T, MTHFR A1298C, EGFR G497A, and EGFR CA repeat polymorphisms and response to preoperative chemoradiotherapy; (2) response to chemoradiotherapy was evaluated by tumor regression grade (TRG); (3) genotype frequency data could be obtained to estimate the odds ratio (OR) and 95% confidence interval (CI). Articles were excluded based on the following criteria: (1) the data of TRG were not specific to polymorphism; (2) studies with insufficient or duplicate data; (3) meeting abstracts, letters, or review articles.
Data extraction
Based on the inclusion and exclusion criteria, 2 investigators extracted the following information from all eligible studies: name of first author, year of publication, country of origin, ethnicity, age, sex ratio, cancer type, disease stage, chemotherapy drugs, radiation dose, and genotype frequency in responders and non-responders of MTHFR C677T, MTHFR A1298C, EGFR G497A, and EGFR CA repeat polymorphisms.
Quality score assessment
Two independent investigators assessed the methodological quality of every eligible article according to the Newcastle-Ottawa Scale (NOS) basing on 3 aspects: selection, comparability, and exposure, with scores ranging from 0 to 9 [9]. NOS score ≥7 was considered as high quality.
Statistical analysis
In the study, TRG grades were defined as grade 1: the absence of residual cancer; grade 2: the presence of rare residual cancer cells; grade 3: an increase in the number of residual cancer cells but with fibrosis predominating; grade 4: residual cancer outgrowing fibrosis; and grade 5: the absence of regressive changes [10,11]. Patients were subdivided into responders and non-responders (TRG 1–2 vs. 3–5 or 1 vs. 2–5). The pooled odds ratios (ORs) with corresponding 95% confidence intervals (95% CIs) were calculated to assess the strength of the association between MTHFR C677T, MTHFR A1298C, EGFR G497A, and EGFR CA repeat polymorphisms and the response to preoperative chemoradiotherapy. A p-value <0.05 was used to indicate statistically significant association. Pooled ORs were performed under 5 genetic models: allelic model, homozygote model, heterozygote model, dominant model, and recessive model. If the response was evaluated by both TRG 1–2 vs. 3–5 and 1 vs. 2–5, only the TRG 1–2 vs. 3–5 data was included for the overall analysis and subgroup analysis stratified by cancer type. However, both were included when the subgroup analyses were performed according to the responder definition. The heterogeneity among the studies was assessed by the chi-square test based on Q statistic test and I2 statistic tests. When P<0.1 or I2 >50%, the heterogeneity was considered to be significant and then the pooled OR and 95%CIs were evaluated by the random-effects model (DerSimonian-Laird method); otherwise, the fixed-effects model (Mantel-Haenszel method) was used [12]. Potential publication bias was checked by Begg’s funnel plots and Egger’s test [13,14]. Sensitivity analysis was also conducted to evaluate the stability of the final results by deleting each study in turn. Subgroup analyses according cancer type and responder definition were also performed. Stata 12.0 software (StataCorp, College Station, TX, USA) was used to perform all analyses.
Results
Study characteristics
The literature selection process is shown in Figure 1. After initial identification, a total of 147 items were obtained. Of them, 54 were duplicated papers, 45 were review or meeting abstracts, and 29 were irrelevant papers. Then, 19 articles were left for further screening according to the inclusion and exclusion criteria by reading the full text. Subsequently, 8 articles were excluded; the treatment response to pRCT in 5 of them was not evaluated by tumor regression grade (TRG) and 3 articles did not report data related to the selected SNP. Finally, 11 eligible articles were included in the present meta-analysis [15–25]. Eight studies were performed in rectal cancer [15,18–22,24,25] and 3 studies were performed in esophageal cancer [16,17,23]. The methodological quality of each eligible article was assessed by NOS scale and all studies received a high NOS score (≥7, data not shown). Table 1 shows the characteristics of each study. The articles were published from 2006 to 2015. In the present study we finally analyzed 8 included studies containing 839 cases for MTHFR C677T [15–21,24], 5 studies involving 634 cases for MTHFR A1298C [15,18,20,21,24], 3 studies containing 340 cases for EGFR G497A [21,22,25], and 4 studies containing 396 cases for EGFR CA repeat [18,21,23,24].
Figure 1.
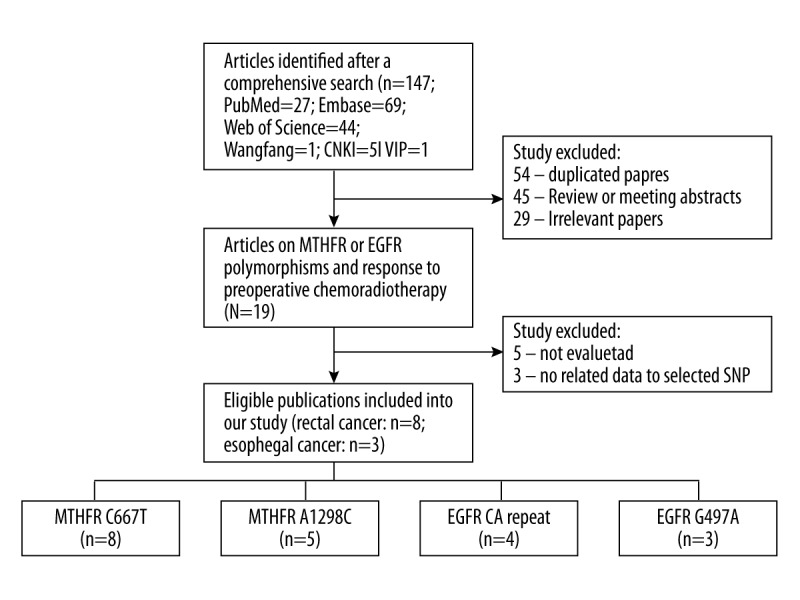
Flow chart of study selection.
Table 1.
Characteristics of the included studies.
| Reference | Country | Ethnicity | Case | Age | M/F | Cancer type | Stage | Therapy strategy | Chemotherapy drugs | Radiation dose (Gy) | TRG Evaluation | Genotype method | Sample | Polymor-phisms |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Terrazzino et al., 2006 | Italy | Caucasian | 125 | 60 (31–79) | 80/45 | Rectal cancer | T2,3,4 | RCT + surgery | 5-FU | 45.0 | 1–2 vs. 3–5 | PCR | Blood | MTHFR C677T; A1298C |
| Sarbia et al., 2006 | Germany | Caucasian | 68 | 57 (37–70) | 60/8 | Esophageal cancer | T3/T4 | RCT + surgery | 5-FU folinic acid etoposide cisplatin | 40.0 | 1 vs. 2–5 | PCR-RFLP | Tumor | MTHFR C677T |
| Warnecke-Eberz et al., 2009 | Germany | Caucasian | 52 | 59 (38–73) | NA | Esophageal cancer | T2–4 | RCT + surgery | Cisplatin/5-FU | 36 | 1–2 vs. 3–5 | TaqMan | Tumor | MTHFR C677T |
| Balboa et al., 2010 | Spain | Caucasian | 65 | 64 (37–85) | 50/15 | Rectal cancer | II/III | RCT + surgery | 5-FU/capecitabine | 50.5 | 1–2 vs. 3–5 | SNaPshot | Blood | MTHFR C677T; A1298C, EGFR CA repeat |
| Garcia-Aguilar et al., 2011 | Spain | Mixed | 132 | Responder: 56 (32–80); Nonresponder: 57 (26–87) | 77/55 | Rectal cancer | II/III | RCT + surgery | 5-FU | 50.4 | 1 vs. 2–5 | Sanger sequencing | Tumor | MTHFR C677T |
| Cecchin et al., 2011 | Italy | Caucasian | 238 | 61 (20–79) | 159/79 | Rectal cancer | T2,3,4 | RCT + surgery | 5-FU | 45.0–50.4 | 1–2 vs. 3–5 | TaqMan | Blood | MTHFR C677T; A1298C |
| Hu-Lieskovan et al., 2011 | Belgium; Slovenia; Germany | Caucasian | 130 | 61 (33–83) | 74/56 | Rectal cancer | II/III/IV | RCT + surgery | Capecitabine/cetuximab/5-FU | 45/50.4 | 1–2 vs. 3–5 | PCR-RFLP | Tumor | MTHFR C677T; A1298C, EGFR G497A; CA repeat |
| Hu-Lieskovan et al., 2011 | Belgium; Slovenia; Germany | Caucasian | 130 | 61 (33–83) | 74/56 | Rectal cancer | II/III/IV | RCT + surgery | Capecitabine/cetuximab/5-FU | 45/50.4 | 1 vs. 2–5 | PCR-RFLP | Tumor | MTHFR C677T; A1298C, EGFR G497A; CA repeat |
| Paez et al., 2011 | Spain | Caucasian | 128 | 65 (32–83) | 97/31 | Rectal cancer | II/III | RCT + surgery | 5-FU/capecitabine | 45.0 | 1–2 vs. 3–5 | Dynamic array | Blood | EGFR G497A |
| Lee et al., 2011 | Taiwan | Aisan | 132 | <60, n=80; ≥60, n=68 | NA | Esophageal cancer | IIa or less, n=55; IIb or more, n=93 | RCT + surgery | Cisplatin/5-FU | 40.0 | 1–2 vs. 3–5 | PCR | Blood | EGFR CA repeat |
| Lamas et al., 2012 | Spain | Caucasian | 93 | 67 (39–86) | 68/25 | Rectal cancer | II/III | RCT + surgery | 5-FU | 50.4 | 1–2 vs. 3–5 | SNaPshot | Blood | MTHFR C677T; A1298C, EGFR CA repeat |
| Sebio et al., 2015 | Spain | Caucasian | 84 | 67.6 (42–80) | 55/29 | Rectal cancer | II/III | RCT + surgery | Capecitabine | 45.0 | 1 vs. 2–5 | Dynamic array | Blood | EGFR G497A |
Meta-analysis results
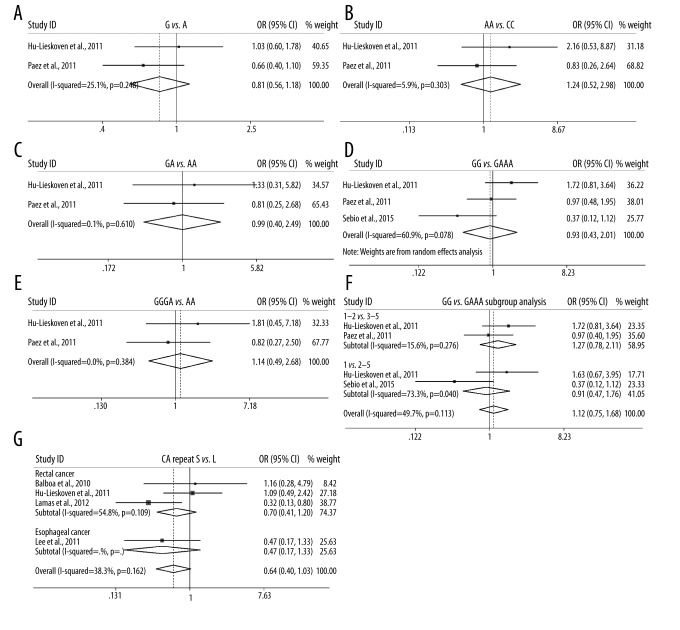
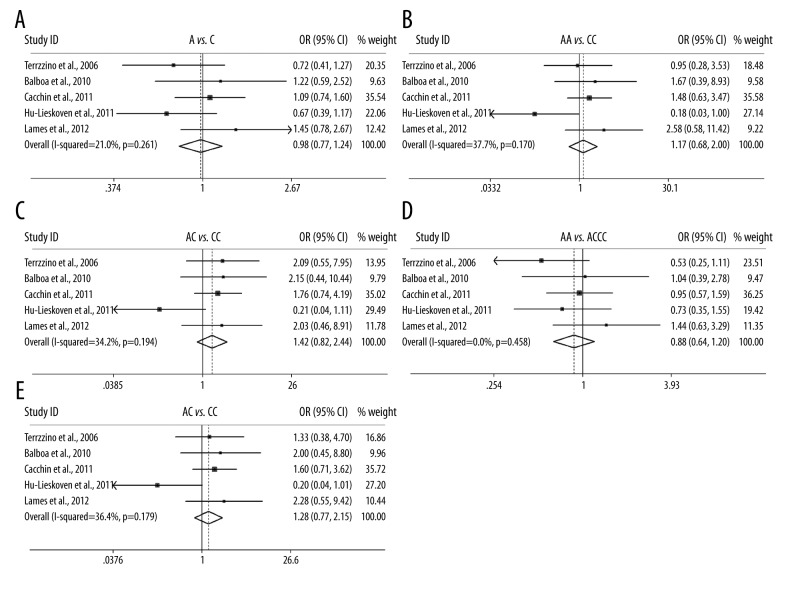
We firstly analyzed the association of MTHFR C677T with the response to pRCT under 5 genetic models. Overall, the C677T polymorphism was correlated with the response to pRCT in recessive model (CC vs. CTTT, OR=1.426(1.074–1.894), P=0.014, Table 2), but not in allele model, homozygote model, heterozygote model, or dominant model. In subgroup analysis according to cancer type, a significant association was also found in recessive model in rectal cancer (CC vs. CTTT, OR=1.483(1.102–1.996), P=0.009, Table 2), but no significant association existed in other genetic models. When the subgroup analysis was performed according to responder definition, a significant association was only found in recessive model in TRG 1–2 vs. 3–5 group (CC vs. CTTT, OR=1.423(1.046–1.936), P=0.025, Table 2, Figure 2). The results suggest that patients (especially those with rectal cancer) carrying CC genotype might benefit from pRCT compared with CT or TT carriers. The association between MTHFR A1298C, EGFR G497A, and EGFR CA repeat polymorphisms and the response to pRCT was also examined; however, no significant association was identified in overall or subgroup analyses, only a trend that EGFR short (S) CA repeat might harbor a unfavorable role in response to pRCT in overall analysis (S vs. L, OR=0.639(0.397–1.030), P=0.066, Table 2, Figure 4).
Table 2.
Summary of the meta-analysis results.
| Polymorphism | Genetic model | Cancer type | N | OR (95%CI) | POR | Effect model | I2 (%) | PHeter | PBegg | PEgger |
|---|---|---|---|---|---|---|---|---|---|---|
| MTHFR C677T | C vs. T | All | 7 | 1.178 (0.855–1.624) | 0.317 | R | 53.6 | 0.044 | 0.230 | 0.562 |
| Rectal cancer | 6 | 1.223 (0.860–1.738) | 0.263 | R | 58.7 | 0.033 | ||||
| Esophageal cancer | 1 | |||||||||
| TRG 1–2 vs. 3–5 | 6 | 1.097 (0.779–1.544) | 0.596 | R | 53.9 | 0.055 | ||||
| TRG 1 vs. 2–5 | 2 | 1.590 (0.967–2.614) | 0.068 | R | 45.9 | 0.074 | ||||
| CC vs. TT | All | 7 | 1.185 (0.603–2.328) | 0.623 | R | 51.2 | 0.056 | 0.548 | 0.835 | |
| Rectal cancer | 6 | 1.274 (0.576–2.817) | 0.550 | R | 57.8 | 0.037 | ||||
| Esophageal cancer | 1 | |||||||||
| TRG 1–2 vs. 3–5 | 6 | 1.063 (0.558–2.027) | 0.852 | R | 48.2 | 0.086 | ||||
| TRG 1 vs. 2–5 | 2 | 2.221 (0.084–58.420) | 0.632 | R | 76.0 | 0.041 | ||||
| CT vs. TT | All | 6 | 0.727 (0.378–1.401) | 0.341 | R | 44.2 | 0.096 | 0.548 | 0.746 | |
| Rectal cancer | 5 | 0.776 (0.369–1.633) | 0.504 | R | 52.0 | 0.064 | ||||
| Esophageal cancer | 1 | |||||||||
| TRG 1–2 vs. 3–5 | 6 | 0.689 (0.443–1.074) | 0.100 | F | 31.9 | 0.197 | ||||
| TRG 1 vs. 2–5 | 2 | 1.233 (0.015–98.183) | 0.925 | R | 85.5 | 0.009 | ||||
| CC vs. CTTT | All | 7 | 1.426 (1.074–1.894) | 0.014 | F | 18.6 | 0.283 | 0.386 | 0.363 | |
| Rectal cancer | 6 | 1.483 (1.102–1.996) | 0.009 | F | 35.6 | 0.170 | ||||
| Esophageal cancer | 2 | 0.953 (0.365–2.492) | 0.922 | F | 0.0 | 0.770 | ||||
| TRG 1–2 vs. 3–5 | 6 | 1.423 (1.046–1.936) | 0.025 | F | 37.8 | 0.154 | ||||
| TRG 1 vs. 2–5 | 3 | 1.619 (0.899–2.915) | 0.108 | F | 0.0 | 0.664 | ||||
| Rectal cancer | 2 | 1.766 (0.947–3.291) | 0.074 | F | 0.0 | 0.750 | ||||
| CCCT vs. TT | All | 7 | 0.913 (0.501–1.664) | 0.767 | R | 46.8 | 0.080 | 0.764 | 0.742 | |
| Rectal cancer | 6 | 0.968 (0.476–1.970) | 0.929 | R | 54.4 | 0.052 | ||||
| Esophageal cancer | 1 | |||||||||
| TRG 1–2 vs. 3–5 | 6 | 0.863 (0.580–1.283) | 0.466 | F | 39.1 | 0.145 | ||||
| TRG 1 vs. 2–5 | 2 | 1.773 (0.041–76.720) | 0.766 | R | 82.2 | 0.018 | ||||
| MTHFR A1298C | A vs. C | Rectal cancer | 5 | 0.978 (0.771–1.241) | 0.857 | F | 21.0 | 0.281 | 0.806 | 0.976 |
| AA vs. CC | Rectal cancer | 5 | 1.169 (0.683–2.002) | 0.569 | F | 37.7 | 0.170 | 0.462 | 0.550 | |
| AC vs. CC | Rectal cancer | 5 | 1.418 (0.824–2.439) | 0.207 | F | 34.2 | 0.194 | 0.221 | 0.504 | |
| AA vs. ACCC | Rectal cancer | 5 | 0.875 (0.639–1.199) | 0.406 | F | 0.0 | 0.458 | 0.806 | 0.912 | |
| AAAC vs. CC | Rectal cancer | 5 | 1.285 (0.768–2.148) | 0.340 | F | 36.4 | 0.179 | 0.462 | 0.492 | |
| EGFR G497A | G vs. A | All | 2 | 0.812 (0.561–1.176) | 0.271 | F | 25.1 | 0.248 | ||
| GG vs. AA | All | 2 | 1.244 (0.519–2.982) | 0.624 | F | 5.9 | 0.303 | |||
| GA vs. AA | All | 2 | 0.994 (0.398–2.486) | 0.990 | F | 0.0 | 0.610 | |||
| GG vs. GAAA | All | 3 | 0.930 (0.431–2.007) | 0.853 | R | 60.9 | 0.078 | 1.000 | 0.423 | |
| TRG 1–2 vs. 3–5 | 2 | 1.267 (0.762–2.106) | 0.362 | F | 15.6 | 0.276 | ||||
| TRG 1 vs. 2–5 | 2 | 0.913 (0.472–1.765) | 0.362 | R | 76.3 | 0.040 | ||||
| GGGA vs. AA | All | 2 | 1.139 (0.488–2.662) | 0.763 | F | 0.0 | 0.384 | |||
| EGFR CA repeat | S vs. L | All | 4 | 0.639 (0.397–1.030) | 0.066 | F | 38.3 | 0.182 | 1.000 | 0.938 |
| Rectal Cancer | 3 | 0.708 (0.299–1.677) | 0.433 | R | 54.8 | 0.109 |
Figure 2.
Forest plot for the association of MTHFR C677T polymorphism and the tumor response to pRCT stratified by the responder definition.
Figure 4.
Forest plot for the association of EGFR G497A and CA repeat polymorphisms and the tumor response to pRCT.
Sensitivity analysis and publication bias
Sensitivity analysis was performed to examine the influence of each single study on the estimated effects in all genetic models. For MTHFR C677T polymorphism, we arrived at almost the same results in all genetic models. For MTHFR A1298C, when the report by Hu et al. was deleted, a significant association was identified between the polymorphism and the response to pRCT under heterozygote model (AC vs. CC). For EGFR CA repeat, a significant association was also found between the polymorphism and the response when the report by Hu et al. was deleted. To evaluate the publication bias among the selected studies, Begg’s funnel plot was used for polymorphisms of MTHFR C677T, MTHFR A1298C, and EGFR CA repeat, and symmetrical funnel plots were obtained in all genetic models (and data not shown), indicating lack of publication bias. In addition, Egger’s test was also performed and the results indicated that no publication bias existed among all the studies for all the polymorphisms under 5 genetic models (Table 2).
Discussion
In the present study, we performed a meta-analysis by pooling 20 studies to investigate the association of MTHFR and EGFR polymorphisms with the tumor response to pRCT in cancers. The results suggested that MTHFR C677T might be correlated with the tumor response, while the polymorphisms of A1298C in MTHFR and G497A and CA repeat in EGFR were not associated with the response.
Preoperative chemoradiotherapy, also known as neo-adjuvant chemoradiotherapy, followed by surgery has provide an alternative choice for cancer therapy and offered survival benefit for several cancers, especially locally advanced rectal cancer, in which the treatment strategy has became a standard therapy. In high-stage esophageal cancer and gastric cancer, a series of studies proved that the patients undergoing pRCT combined with surgery had higher overall survival rate and disease-free survival rates compared with surgery alone or surgery combined with adjuvant therapy [26–28]. Nevertheless, pRCT also causes severe adverse effects and many patients show poor response to pRCT. Tumor response to treatment was correlated with tumor type and tumor microenvironment, as well as patient genetics, so it is important to discover the biomarkers to identify patients who will benefit from pRCT and advance the development of individual therapy.
In the past decade many researchers have focused on the investigation of the biomarkers in predicting the tumor response to pRCT; however, the results are not consistent and valuable biomarkers are still lacking. Because many studies have determined the association of MTHFR polymorphisms with the tumor response to pRCT, we performed a systematic search in literature databases for related studies and conducted a meta-analysis to investigate the association between MTHFR polymorphisms and the response to pRCT, also including the EGFR polymorphisms. The results suggested that MTHFR C677T might be correlated with the tumor response to pRCT under the recessive model in overall analysis, rectal cancer, and TRG 1–2 vs. 3–5 group, while other polymorphism exert significant association under all genetic models in overall analysis or subgroup analyses. In addition, only a trend of association between EGFR CA repeat and the tumor response to pRCT was found. To the best of our knowledge, this is the first meta-analysis to address the association between MTHFR and EGFR polymorphisms and the response to pRCT, in which we tried to pool all the potential related studies regardless of cancer type. However, some limitations existed in the study. Firstly, the study number and the sample size were very small, especially for EGFR polymorphisms and esophageal cancer, which also led to the lack of stability of the results for MTHFR A1298C polymorphism. Another limitation was that all the original studies were performed in white populations except for 1 that was carried out in Asians. Thus, further well-designed studies with larger sample sizes focusing on more ethnicities should be conducted to confirm the results.
Conclusions
In summary, we obtained a comprehensive result from the current meta-analysis that MTHFR C677T polymorphism was correlated with the response to pRCT in overall and in rectal cancer, while MTHFR A1298C and EGFR G497A and CA repeat polymorphisms showed no significant association with the tumor response to pRCT.
Figure 3.
Forest plot for the association of MTHFR A1298C polymorphism and the tumor response to pRCT.
Footnotes
Source of support: Departmental sources
Disclosure
The authors have not received any funding or benefits from industry in relation to this study.
Conflict of interest
None.
References
- 1.Spolverato G, Pucciarelli S, Bertorelle R, et al. Predictive factors of the response of rectal cancer to neoadjuvant radiochemotherapy. Cancers (Basel) 2011;3:2176–94. doi: 10.3390/cancers3022176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pucciarelli S, Del Bianco P, Efficace F, et al. Patient-reported outcomes after neoadjuvant chemoradiotherapy for rectal cancer: a multicenter prospective observational study. Ann Surg. 2011;253:71–77. doi: 10.1097/SLA.0b013e3181fcb856. [DOI] [PubMed] [Google Scholar]
- 3.Minsky BD, Cohen AM, Kemeny N, et al. Combined modality therapy of rectal cancer: decreased acute toxicity with the preoperative approach. J Clin Oncol. 1992;10:1218–24. doi: 10.1200/JCO.1992.10.8.1218. [DOI] [PubMed] [Google Scholar]
- 4.Mohiuddin M, Hayne M, Regine WF, et al. Prognostic significance of postchemoradiation stage following preoperative chemotherapy and radiation for advanced/recurrent rectal cancers. Int J Radiat Oncol Biol Phys. 2000;48:1075–80. doi: 10.1016/s0360-3016(00)00732-x. [DOI] [PubMed] [Google Scholar]
- 5.Etienne MC, Formento JL, Chazal M, et al. Methylenetetrahydrofolate reductase gene polymorphisms and response to fluorouracil-based treatment in advanced colorectal cancer patients. Pharmacogenetics. 2004;14:785–92. doi: 10.1097/00008571-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Laskin JJ, Sandler AB. Epidermal growth factor receptor: a promising target in solid tumours. Cancer Treat Rev. 2004;30:1–17. doi: 10.1016/j.ctrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Moriai T, Kobrin MS, Hope C, et al. A variant epidermal growth factor receptor exhibits altered type alpha transforming growth factor binding and transmembrane signaling. Proc Natl Acad Sci USA. 1994;91:10217–21. doi: 10.1073/pnas.91.21.10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amador ML, Oppenheimer D, Perea S, et al. An epidermal growth factor receptor intron 1 polymorphism mediates response to epidermal growth factor receptor inhibitors. Cancer Res. 2004;64:9139–43. doi: 10.1158/0008-5472.CAN-04-1036. [DOI] [PubMed] [Google Scholar]
- 9.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Journal. 2014 [Google Scholar]
- 10.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–86. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terrazzino S, Agostini M, Pucciarelli S, et al. A haplotype of the methylenetetrahydrofolate reductase gene predicts poor tumor response in rectal cancer patients receiving preoperative chemoradiation. Pharmacogenet Genomics. 2006;16:817–24. doi: 10.1097/01.fpc.0000230412.89973.c0. [DOI] [PubMed] [Google Scholar]
- 16.Sarbia M, Stahl M, von Weyhern C, et al. The prognostic significance of genetic polymorphisms (Methylenetetrahydrofolate Reductase C677T, Methionine Synthase A2756G, Thymidilate Synthase tandem repeat polymorphism) in multimodally treated oesophageal squamous cell carcinoma. Br J Cancer. 2006;94:203–7. doi: 10.1038/sj.bjc.6602900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warnecke-Eberz U, Vallboehmer D, Alakus H, et al. ERCC1 and XRCC1 gene polymorphisms predict response to neoadjuvant radiochemotherapy in esophageal cancer. J Gastrointest Surg. 2009;13:1411–21. doi: 10.1007/s11605-009-0881-z. [DOI] [PubMed] [Google Scholar]
- 18.Balboa E, Duran G, Lamas MJ, et al. Pharmacogenetic analysis in neoadjuvant chemoradiation for rectal cancer: high incidence of somatic mutations and their relation with response. Pharmacogenomics. 2010;11:747–61. doi: 10.2217/pgs.10.51. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Aguilar J, Chen Z, Smith DD, et al. Identification of a biomarker profile associated with resistance to neoadjuvant chemoradiation therapy in rectal cancer. Ann Surg. 2011;254:486–92. doi: 10.1097/SLA.0b013e31822b8cfa. discussion 492–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cecchin E, Agostini M, Pucciarelli S, et al. Tumor response is predicted by patient genetic profile in rectal cancer patients treated with neo-adjuvant chemo-radiotherapy. Pharmacogenomics J. 2011;11:214–26. doi: 10.1038/tpj.2010.25. [DOI] [PubMed] [Google Scholar]
- 21.Hu-Lieskovan S, Vallbohmer D, Zhang W, et al. EGF61 polymorphism predicts complete pathologic response to cetuximab-based chemoradiation independent of KRAS status in locally advanced rectal cancer patients. Clin Cancer Res. 2011;17:5161–69. doi: 10.1158/1078-0432.CCR-10-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paez D, Salazar J, Pare L, et al. Pharmacogenetic study in rectal cancer patients treated with preoperative chemoradiotherapy: polymorphisms in thymidylate synthase, epidermal growth factor receptor, GSTP1, and DNA repair genes. Int J Radiat Oncol Biol Phys. 2011;81:1319–27. doi: 10.1016/j.ijrobp.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Lee JM, Yang SY, Yang PW, et al. Polymorphism in epidermal growth factor receptor intron 1 predicts prognosis of patients with esophageal cancer after chemoradiation and surgery. Ann Surg Oncol. 2011;18:2066–73. doi: 10.1245/s10434-011-1559-9. [DOI] [PubMed] [Google Scholar]
- 24.Lamas MJ, Duran G, Gomez A, et al. X-ray cross-complementing group 1 and thymidylate synthase polymorphisms might predict response to chemoradiotherapy in rectal cancer patients. Int J Radiat Oncol Biol Phys. 2012;82:138–44. doi: 10.1016/j.ijrobp.2010.09.053. [DOI] [PubMed] [Google Scholar]
- 25.Sebio A, Salazar J, Paez D, et al. EGFR ligands and DNA repair genes: genomic predictors of complete response after capecitabine-based chemoradiotherapy in locally advanced rectal cancer. Pharmacogenomics J. 2015;15:77–83. doi: 10.1038/tpj.2014.33. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Ajani JA. Multidisciplinary management of gastric cancer. Curr Opin Gastroenterol. 2010;26:640–46. doi: 10.1097/MOG.0b013e32833efd9b. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda S, Takahashi T, Fukada J, et al. Phase I study of neoadjuvant chemoradiotherapy with S-1 plus biweekly cisplatin for advanced gastric cancer patients with lymph node metastasis: -KOGC04. Radiat Oncol. 2014;9:9. doi: 10.1186/1748-717X-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang R, Darling G, Wong RK. Multimodality approaches for the curative treatment of esophageal cancer. J Natl Compr Canc Netw. 2015;13:229–38. doi: 10.6004/jnccn.2015.0029. [DOI] [PubMed] [Google Scholar]