Summary
Background
Myocardial fibrosis is a well-known side effect of radiotherapy. Fragmented QRS (fQRS) has been shown to be a marker of myocardial fibrosis. We postulated that radiotherapy induces development of fQRS in breast cancer patients.
Patients and Methods
Breast cancer patients receiving locoregional radiotherapy were enrolled. Patients who had fQRS on electrocardiography (ECG) before radiotherapy were excluded. All patients were revaluated for the development of fQRS at 1-year follow-up. An age-matched healthy group served as controls.
Results
A total of 52 breast cancer patients receiving locoregional radiotherapy were included (median age 49 years, interquartile range (IQR) 13). Of these, 19 (37%) had developed fQRS at 1-year follow-up. Compared with the control group, prevalence of fQRS was significantly higher in the irradiated group (37 vs. 12%; p < 0.002). Median total cardiac radiation dose was significantly higher in patients who had developed fQRS (5 Gy, IQR 5.2 vs. 1.7 Gy, IQR 4.4; p = 0.003). Cardiac radiation dose, entered either as a continuous variable (odds ratio (OR) 1.35, 95% confidence interval (CI) 104-1.74) or as a dichotomized variable (≥ 2.2 Gy, OR 6.48, 95% CI 1.47-28.61), was independently associated with the development of fQRS at 1-year follow-up.
Conclusion
Radiotherapy for breast cancer induces development of fQRS on ECG. Cardiac radiation dose is independently associated with the development of fQRS.
Keywords: Fragmented QRS, Radiotherapy, Breast cancer
Introduction
Breast cancer is the most prevalent cancer subtype among women worldwide [1]. Breast cancer survival has improved with advanced treatment modalities such as chemotherapy, radiotherapy, and hormone therapy in the last decades [2,3,4]. Cardiovascular complications have become more prevalent and important with prolonged survival in these patients [5,6]. Radiotherapy may cause a broad spectrum of cardiovascular complications including pericarditis, myocardial dysfunction, heart failure, valvular abnormalities, and rhythm disturbances, largely driven by radiotherapy-induced fibrosis [5,7,8]. Radiotherapy-induced cardiovascular complications are associated with reduced quality of life, increased mortality, and risk of sudden cardiac death [9,10]. Fragmented QRS (fQRS) has been shown to be a marker of myocardial fibrosis in various clinical conditions [11,12,13,14]. To date, no study has evaluated the association of locoregional radiotherapy with the development of fQRS in patients with breast cancer. In this study, we aimed to investigate the association between locoregional radiotherapy and development of fQRS on electrocardiography (ECG).
Patients and Methods
Patients
Breast cancer patients receiving locoregional radiotherapy from January 2011 to January 2013 were prospectively enrolled in this study. Patients who had fQRS on ECG before radiotherapy were excluded from the study. Other exclusion criteria were left ventricular systolic dysfunction (left ventricular ejection fraction < 55%), history of coronary artery disease (angina pectoris, myocardial infarction, or revascularization), valvular heart disease, connective tissue disease, and sarcoidosis. All patients were revaluated for the development of fQRS at 1-year follow-up. Prevalence of fQRS in the general population was studied in an age-matched healthy control group. The study protocol was approved by the Institutional Ethics Committee of KTU Faculty of Medicine.
Assessment of fQRS
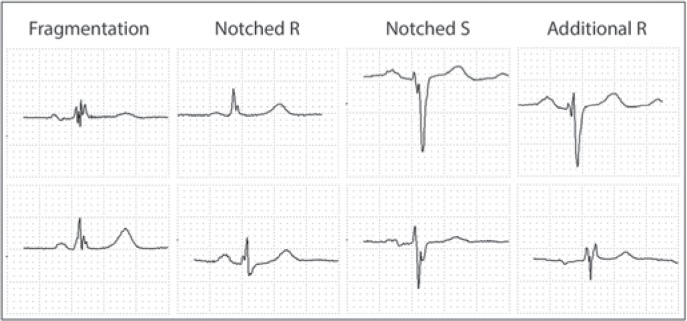
fQRS (fig. 1) was defined as the presence of an additional R wave (R') or notching of the R or S wave or the presence of fragmentation (more than 1 R') in 2 contiguous ECG leads (filter range 0.15-100 Hz, 25 mm/s, 10 mm/mV) [15]. Patients with typical bundle-branch block or incomplete right bundle-branch block were excluded from the study. 2 experienced cardiologists who were blinded to patient data evaluated all ECGs. There was an excellent interobsever agreement (95%, k = 0.96) for the presence of fQRS on ECG.
Fig. 1.
Various forms of fragmented QRS on electrocardiography.
Radiotherapy Treatment
Radiotherapy was started after the chemotherapy treatment which was administered for 4, 6, or 8 cycles. All patients had a 3-dimensional conformal treatment plan based on computed tomography (CT). CT-based supine simulation was used to assist with the field design. Patients were treated in the supine position. Respiratory control was not performed during the treatment. The region from the mandible to the base of the lung was scanned with 5-mm slice thickness. Heart, lung, and contralateral breast were outlined as organs at risk. The contouring process was guided by the Radiation Therapy Oncology Group (RTOG) Contouring Atlas. Following chemotherapy, all patients received comprehensive external beam radiotherapy to the chest wall and the regional lymphatics as a component of their treatment. Radiation doses were calculated using a dose-volume histogram. All plans were created using the Eclipse™ V.10 treatment planning system (Varian Medical Systems, Inc., Palo Alto, CA, USA), and a 6-MV photon was used as energy. Patients were treated using 3-dimensional conformal radiotherapy. The dosage was 50 Gy in 25 fractions over 5 weeks. Radiotherapy was given after breast-conserving surgery to the whole breast using unopposed tangential fields with 6-MV photon to a median dose of 50 Gy (range 44-50.4). This was followed by a supplemental tumor bed boost up to a median total dose of 60 Gy. The boost was administered by electrons or photons. In patients with risk factors for local recurrence (lymphovascular invasion or close margins), an electron or photon boost to the tumor bed via an additional dose of 10 Gy was implemented. The electron boost was the first choice to reduce radiation exposure to the heart and lungs. However, in some patients, particularly those who had deep seated tumors, we preferred to use photons in order to obtain a more homogenous dose distribution. Infra- or supraclavicular nodal irradiation was also delivered to patients with risk factors for regional recurrence (lymphovascular invasion, positive axillary lymph nodes, or extranodal extension) for a total dose of 50 Gy. Patients with positive hormone receptors received further hormonal therapy.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation or median and interquartile range (IQR) where appropriate. Categorical variables were presented as frequencies and percentages. The distributions of the continuous variables across the study groups were tested with the Shapiro-Wilks test. Continuous data were analyzed using Student's t-test or Mann-Whitney U test, and categorical data were compared using chi-square or Fisher's exact test. Receiver operating characteristic (ROC) analysis was conducted to assess the discriminative performance of cardiac radiation dose for the development of fQRS. Multivariate logistic regression analyses were conducted to assess association between cardiac radiation dose and development of fQRS. Adjusted odds ratios (ORs) and corresponding confidence intervals (CIs) were presented. A 2-tailed p value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS software (IBM SPSS Statistics for Windows, Version 21.0; IBM Corp., Armonk, NY, USA).
Results
A total of 52 breast cancer patients (44 invasive ductal, 3 mucinous, 1 papillary, 1 metaplastic, 3 lobular) were included (median age 49 years, IQR 13). Of these, 24 (46.2%) were cases of left-sided breast cancer. 45 (86%) patients received chemotherapy, and 34 (65%) received hormone therapy prior to radiotherapy. Median total and cardiac radiation dose were 50 Gy (IQR 16) and 2.4 Gy (IQR 4.4), respectively. Median cardiac radiation dose was 5.9 Gy (IQR 2.6) for left breast and 1.3 Gy (IQR 0.8) for right breast.
The breast cancer and healthy control groups had a similar baseline cardiovascular risk profile (table 1). At 1-year follow-up, 19 of 52 (37.4%) breast cancer patients had developed fQRS on ECG. The majority of patients who developed fQRS (13 of 19 (68.4%)) had left-sided breast cancer. Prevalence of fQRS in breast cancer patients was significantly higher (37.4 vs. 11.9%; p = 0.002) as compared to age-matched healthy controls.
Table 1.
Baseline characteristics of breast cancer patients and healthy controls
| Breast cancer (n = 52) | Healthy women (n = 60) | p value | |
|---|---|---|---|
| Agea, years | 49 (13) | 50 (11) | 0.622 |
| Hypertension, n (%) | 11 (21) | 17 (28) | 0.382 |
| Systolic BPa, mmHg | 120 (20) | 120 (20) | 0.963 |
| Diastolic BPa, mmHg | 65 (19) | 65 (16) | 0.798 |
| Diabetes mellitus, n (%) | 6 (12) | 9 (15) | 0.592 |
| Smoking, n (%) | 5 (10) | 11 (18) | 0.189 |
| Hyperlipidemia, n (%) | 5 (10) | 9 (15) | 0.390 |
BP = Blood pressure.
Median (interquartile range).
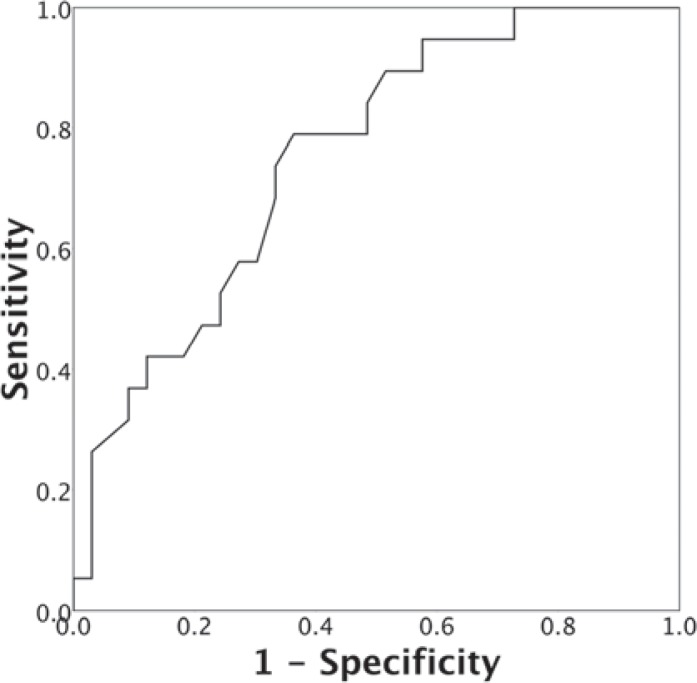
Demographic and clinical characteristics of the breast cancer patients with and without fQRS at 1-year follow-up are shown in table 2. Patients who had developed fQRS at 1-year follow-up had more often left-sided breast cancer (68.4 vs. 33.3%; p = 0.015), higher median duration of radiotherapy (30 days, IQR 8 vs. 25 days, IQR 3.8; p = 0.048), and higher median cardiac radiation dose (5 Gy, IQR 5.2 vs. 1.7 Gy IQR 4.4; p = 0.003) as compared to the patients who had not developed fQRS at 1-year follow-up. In addition, the type of chemotherapy and surgery and the number of patients who had received hormone therapy were differed between groups (table 2). Groups were similar with respect to all other baseline characteristics. ROC analysis was conducted to explore the cut-off value of cardiac radiation dose associated with the development of fQRS (fig. 2). At a cut-off value of 2.2 Gy, the area under the ROC curve was 0.753 (95% CI 0.621-0.885) with a sensitivity and specificity of 79% (95% CI 54-94) and 64% (95% CI 45-80), respectively. In multivariate logistic regression analysis, total cardiac radiation dose, entered either as a continuous variable (OR 1.35, 95% CI 1.04-1.74) or as a dichotomized variable (OR 6.48, 95% CI 1.47-28.61), was independently associated with the development of fQRS (table 3).
Table 2.
Demographic and clinical characteristics of patients with breast cancer
| fQRS (+) (n = 19) | fQRS (–) (n = 33) | p value | |
|---|---|---|---|
| Agea, years | 50 (18) | 48 (11) | 0.555 |
| Systolic BPa, mmHg | 120 ± 13 | 121 ± 17 | 0.824 |
| Diastolic BPa, mmHg | 67 ± 12 | 66 ± 11 | 0.669 |
| Hypertension, n (%) | 3 (16) | 8 (24) | 0.726 |
| Diabetes mellitus, n (%) | 1 (5) | 5 (15) | 0.397 |
| Hyperlipidemia, n (%) | 1 (5) | 4 (12) | 0.641 |
| Smoking, n (%) | 4 (21) | 1 (3) | 0.054 |
| Site, n (%) | |||
| Left breast | 13 (68) | 11 (33) | 0.015 |
| Right breast | 6 (32) | 22 (67) | |
| Duration of radiotherapy1, days | 30 (8) | 25 (5) | 0.048 |
| Total radiation dosea, Gy | 60 (16) | 50 (10) | 0.056 |
| Cardiac radiation dosea, Gy | 5.2 (4.9) | 1.5 (4.1) | 0.003 |
| Radiotherapy site, n (%) | 0.815 | ||
| Breast and lymphatic | 11 (58) | 18 (55) | |
| Breast or chest wall | 8 (42) | 15 (45) | |
| Pathological diagnosis, n (%) | |||
| Invasive ductal | 17 (89) | 27 (82) | 0.388 |
| Mucinous | 0 (0) | 3 (9) | |
| Papillary | 0 (0) | 1 (3) | |
| Metaplastic | 1 (5) | 0 (0) | |
| Lobular | 1 (5) | 2 (6) | |
| T, n (%) | |||
| 1 | 4 (21) | 11 (33) | 0.653 |
| 2 | 9 (47) | 13 (39) | |
| 3 | 6 (32) | 8 (24) | |
| 4 | 0 (0) | 1 (3) | |
| N, n (%) | |||
| 0 | 9 (47) | 13 (39) | 0.821 |
| 1 | 3 (16) | 9 (27) | |
| 2 | 5 (26) | 8 (24) | |
| 3 | 2 (11) | 3 (9) | |
| Stage, n (%) | |||
| I | 6 (32) | 9 (27) | 0.947 |
| II | 6 (32) | 11 (33) | |
| III | 7 (37) | 13 (39) | |
| Type of surgery, n (%) | |||
| None | 2 (11) | 0 (0) | 0.041 |
| Modified radical mastectomy | 7 (37) | 20 (61) | |
| Breast-conserving surgery | 8 (42) | 13 (39) | |
| Segmental mastectomy | 2 (11) | 0 (0) | |
| Chemotherapy, n (%) | 17 (89) | 28 (85) | 1 |
| Chemotherapy sessions, na | 6 (2) | 6 (4) | 0.990 |
| Type of chemotherapy, n (%) | |||
| AC | 3 (18) | 4 (14) | 0.048 |
| EC | 0 (0) | 3 (11) | |
| FEC | 9 (53) | 4 (14) | |
| EC TAX | 0 (0) | 2 (7) | |
| AC TAX | 3 (18) | 9 (32) | |
| FEC TAX | 1 (6) | 6 (21) | |
| Capecitabine | 1 (6) | 0 (0) | |
| Hormone therapy, n (%) | 9 (47) | 25 (76) | 0.038 |
| Type of hormone therapy, n (%) | |||
| Tamoxifen | 7 (78) | 12 (48) | 0.251 |
| Anastrozole | 1 (11) | 10 (40) | |
| Letrozole | 1 (11) | 3 (12) |
fQRS = Fragmented QRS; BP = blood pressure; AC = doxorubicin, cyclophosphamide; EC = epirubicin, cyclophosphamide; FEC = fluorouracil, epirubicin, cyclophosphamide; TAX = docetaxel or paclitaxel.
Continuous variables are presented as median (interquartile range) or mean
standard deviation.
Fig. 2.
Receiver operating characteristic curve for the presence of fragmented QRS on electrocardiography.
Table 3.
Association of cardiac radiation dose and development of fragmented QRS
| βa | OR | 95% CI | |
|---|---|---|---|
| As a continuous variable | |||
| Univariate | 0.301 | 1.35 | 1.08-1.69 |
| Adjustedb | 0.299 | 1.35 | 1.04-1.74 |
| Median dose of >*.2.2 Gy | |||
| Univariate | 1.881 | 6.56 | 1.77-24.35 |
| Adjustedb | 1.868 | 6.48 | 1.47-28.61 |
OR = Odds ratio; CI = confidence interval.
Regression coefficient.
For type of surgery and presence of hormone therapy.
Discussion
In this study, we found that 37% of breast cancer patients who received locoregional radiotherapy had developed new fQRS on ECG at 1-year follow-up. Moreover, cardiac radiation dose was independently associated with the development of fQRS.
The risk of cardiovascular complications is increased in breast cancer patients with the advances in adjuvant treatments [16]. As compared to chemotherapy and hormone therapy, cardiovascular complications are more frequent in patients receiving radiotherapy for breast cancer [5,17]. Fibrosis is considered to be the main pathophysiological mechanism for radiotherapy-induced cardiovascular damage [7,18,19,20]. Thus, early identification of patients with radiotherapy-induced fibrosis may help to prevent progression of the underlying disease and associated clinical conditions.
Myocardial fibrosis can be detected by histopathological evaluation, cardiac magnetic resonance imaging, and scintigraphic methods [21]. However, these imaging modalities and histopathological tests are not readily available, impractical in some cases, and expensive. Myocardial fibrosis may disrupt QRS morphology and lead to fragmentation of QRS on 12-lead ECG. Accordingly, prevalence of fQRS is relatively low among the healthy general population [22] and relatively high in patients with myocardial fibrosis or scarring [11,12,13,14,23]. These data suggest that fQRS may be a useful tool for the assessment of myocardial fibrosis.
Although ST and T wave changes have been reported after adjuvant treatments [24], no study to date has evaluated radiotherapy-associated development of fQRS in patients with breast cancer. Our results suggest that more than one-third of breast cancer patients who receive locoregional radiotherapy may develop new fQRS on ECG during 1-year follow-up. Cardiovascular complications have been reported to be associated with cardiac radiation dose [25]. Consistent with this, in the present study, development of fQRS during follow-up was independently associated with cardiac radiation dose.
In conclusion, radiotherapy for breast cancer induces development of new fQRS on ECG. Cardiac radiation dose is independently associated with the development of fQRS. Evaluation of fQRS is an inexpensive and widely available tool and can be used for noninvasive and indirect assessment of myocardial fibrosis in patients receiving radiotherapy.
Limitations
This study has several limitations. The small sample size is the main limitation. Furthermore, duration of post-radiation follow-up was relatively short, and we did not study myocardial fibrosis with advanced imaging modalities. However, recruitment and follow-up are ongoing to power the study and to investigate the long-term cardiac effect of radiotherapy by using ECG, echocardiography, and magnetic resonance imaging. Finally, a possible effect of other treatment modalities including surgery, chemotherapy, and hormone therapy on the development of fQRS could not be totally excluded.
Disclosure Statement
None of the authors have any conflict of interest to declare.
References
- 1.Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;36:237–248. doi: 10.1016/j.canep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R, Early Breast Cancer Trialists' Collaborative G Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Hage JA, van de Velde CJ, Julien JP, Floiras JL, Delozier T, Vandervelden C, Duchateau L. Improved survival after one course of perioperative chemotherapy in early breast cancer patients. long-term results from the European Organization for Research and Treatment of Cancer (EORTC) Trial 10854. Eur J Cancer. 2001;37:2184–2193. doi: 10.1016/s0959-8049(01)00294-5. [DOI] [PubMed] [Google Scholar]
- 4.Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R, Early Breast Cancer Trialists' Collaborative Group Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaworski C, Mariani JA, Wheeler G, Kaye DM. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013;61:2319–2328. doi: 10.1016/j.jacc.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 6.Tanz R, Magne N, Annede P, Mery B, Jacob J, Bauduceau O, Trone JC, Guichard JB, Meillan N, Kirova Y, Vedrine L, Chargari C. (Towards an integrated approach to cardiovascular toxicities related to the treatments of breast cancer) Bull Cancer. 2014;101:730–740. doi: 10.1684/bdc.2014.1926. [DOI] [PubMed] [Google Scholar]
- 7.Fajardo LF, Stewart JR. Pathogenesis of radiation-induced myocardial fibrosis. Lab Invest. 1973;29:244–257. [PubMed] [Google Scholar]
- 8.Duma MN, Molls M, Trott KR. From heart to heart for breast cancer patients – cardiovascular toxicities in breast cancer radiotherapy. Strahlenther Onkol. 2014;190:5–7. doi: 10.1007/s00066-013-0465-4. [DOI] [PubMed] [Google Scholar]
- 9.Paszat LF, Mackillop WJ, Groome PA, Boyd C, Schulze K, Holowaty E. Mortality from myocardial infarction after adjuvant radiotherapy for breast cancer in the surveillance, epidemiology, and end-results cancer registries. J Clin Oncol. 1998;16:2625–2631. doi: 10.1200/JCO.1998.16.8.2625. [DOI] [PubMed] [Google Scholar]
- 10.Cuzick J, Stewart H, Rutqvist L, Houghton J, Edwards R, Redmond C, Peto R, Baum M, Fisher B, Host H, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447–453. doi: 10.1200/JCO.1994.12.3.447. [DOI] [PubMed] [Google Scholar]
- 11.Basaran Y, Tigen K, Karaahmet T, Isiklar I, Cevik C, Gurel E, Dundar C, Pala S, Mahmutyazicioglu K, Basaran O. Fragmented QRS complexes are associated with cardiac fibrosis and significant intraventricular systolic dyssynchrony in nonischemic dilated cardiomyopathy patients with a narrow QRS interval. Echocardiography. 2011;28:62–68. doi: 10.1111/j.1540-8175.2010.01242.x. [DOI] [PubMed] [Google Scholar]
- 12.Das MK, Suradi H, Maskoun W, Michael MA, Shen C, Peng J, Dandamudi G, Mahenthiran J. Fragmented wide QRS on a 12-lead ECG: a sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008;1:258–268. doi: 10.1161/CIRCEP.107.763284. [DOI] [PubMed] [Google Scholar]
- 13.Park SJ, On YK, Kim JS, Park SW, Yang JH, Jun TG, Kang IS, Lee HJ, Choe YH, Huh J. Relation of fragmented QRS complex to right ventricular fibrosis detected by late gadolinium enhancement cardiac magnetic resonance in adults with repaired tetralogy of fallot. Am J Cardiol. 2012;109:110–115. doi: 10.1016/j.amjcard.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 14.Homsi M, Alsayed L, Safadi B, Mahenthiran J, Das MK. Fragmented QRS complexes on 12-lead ECG: a marker of cardiac sarcoidosis as detected by gadolinium cardiac magnetic resonance imaging. Ann Noninvasive Electrocardiol. 2009;14:319–326. doi: 10.1111/j.1542-474X.2009.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–2501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 16.Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, Mabuchi K, Marks LB, Mettler FA, Pierce LJ, Trott KR, Yeh ET, Shore RE. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–665. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 18.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 19.Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27:766–773. doi: 10.1016/s0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 20.Brand MD, Abadi CA, Aurigemma GP, Dauerman HL, Meyer TE. Radiation-associated valvular heart disease in Hodgkin's disease is associated with characteristic thickening and fibrosis of the aortic-mitral curtain. J Heart Valve Dis. 2001;10:681–685. [PubMed] [Google Scholar]
- 21.Jellis C, Martin J, Narula J, Marwick TH. Assessment of nonischemic myocardial fibrosis. J Am Coll Cardiol. 2010;56:89–97. doi: 10.1016/j.jacc.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 22.Terho HK, Tikkanen JT, Junttila JM, Anttonen O, Kenttä TV, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Prevalence and prognostic significance of fragmented QRS complex in middle-aged subjects with and without clinical or electrocardiographic evidence of cardiac disease. Am J Cardiol. 2014;114:141–147. doi: 10.1016/j.amjcard.2014.03.066. [DOI] [PubMed] [Google Scholar]
- 23.Fares H, Heist K, Lavie CJ, Kumbala D, Ventura H, Meadows R, Carter W, Deitelzweig S, Ray IB. Fragmented QRS complexes-a novel but underutilized electrocardiograhic marker of heart disease. Crit Pathw Cardiol. 2013;12:181–183. doi: 10.1097/HPC.0b013e31829e005d. [DOI] [PubMed] [Google Scholar]
- 24.Elme A, Saarto T, Totterman KJ, Utrianen M, Kautiainen H, Jarvenpaa S, Tenhuen M, Blomqvist C. Electrocardiography changes during adjuvant breast cancer therapy: incidence and risk factors. Anticancer Res. 2013;33:4933–4939. [PubMed] [Google Scholar]
- 25.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]