Abstract
Objective
To determine whether optimal human spermatogonial stem cell (SSC) cryopreservation is best achieved with testicular tissue or single cell suspension cryopreservation. This study compares the effectiveness between these two approaches by using testicular SSEA-4+ cells, a known population containing SSCs.
Design
In vitro human testicular tissues.
Setting
Academic research unit.
Patients
Adult testicular tissues (n = 4) collected from subjects with normal spermatogenesis and normal fetal testicular tissues (n = 3).
Intervention(s)
Testicular tissue vs. single cell suspension cryopreservation.
Main Outcome Measures
Cell viability, total cell recovery per milligram of tissue, as well as, viable and SSEA-4+ cell recovery.
Results
Single cell suspension cryopreservation yielded higher recovery of SSEA-4+ cells enriched in adult SSCs whereas fetal SSEA-4+ cell recovery was similar between testicular tissue and single cell suspension cryopreservation.
Conclusions
Adult and fetal human SSEA-4+ populations exhibited differential sensitivity to cryopreservation based on whether they were cryopreserved in situ as testicular tissues or as single cells. Thus, optimal preservation of human SSCs depends on the patient age, type of samples cryopreserved, and end points of therapeutic applications.
Keywords: Testicular cell cryopreservation, testicular tissue cryopreservation, spermatogonial stem cells
INTRODUCTION
Advances in oncologic medicine have provided definitive cures for many patients allowing them to live healthy productive lives. Therefore, oncologists are encouraged to refer patients to fertility specialists to discuss fertility preservation strategies before gonadotoxic cancer therapies (1). Sperm cryopreservation is an established and proven technique to restore fertility in adolescent and adult males (1). However, this approach requires the presence of mature spermatozoa, which is not possible for prepubertal boys. Currently, fertility preservation for prepubertal boys is considered to be experimental because there is a significant lack of scientific knowledge with regards to optimal cryopreservation techniques, isolation of spermatogonial stem cells (SSCs), and subsequent transplantation or in vitro differentiation (2–4). Hence, there is a lack of established standard protocol for fertility preservation for this vulnerable patient population.
The discovery of mouse SSCs and their ability to reconstitute spermatogenesis following heterotopic and orthotopic transplantations provide potential novel therapeutic applications of SSC transplantation in humans for fertility preservation and infertility treatment (5–8). Encouraging results from the murine model have garnered support from many fertility centers that view cryopreservation of prepubertal testicular tissues, presumably containing SSCs, as an acceptable strategy for fertility preservation in this patient population (9–11). Heterotopic xenografts of hamster, marmoset, and mouse testicular tissues into castrated immunodeficient nude mice resulted in limited and finite restoration of spermatogenesis (8). Specifically, heterotopic xenografts of marmoset testicular tissues did not result in successful differentiation of spermatogonia beyond the primary spermatocyte stage (8). Furthermore, autologous heterotopic transplant of fresh testicular tissues in marmoset monkeys also resulted in differentiation arrest at the primary spermatocyte stage (12). Whether cryopreserved testicular tissues exhibited similar engraftment potential to fresh testicular tissues with heterotopic transplants remained to be investigated (13, 14).
Alternatively, orthotopic SSC transplantation utilizes single cell suspensions. This allows SSCs to be positively selected and cancer cells eliminated by Fluorescence Activated Cell Sorting (FACS), which can greatly ameliorate the risk of malignant cell contamination associated with testicular grafting (15–17). Positive selection of SSCs by FACS for transplantation was shown to eliminate the risks of malignant cell contamination (15, 16). Unlike heterotopic transplantation of testicular tissues, orthotopic transplantation of SSCs resulted in long-term reconstitution of spermatogenesis, capable of fertilization, in adult rhesus macaques (18, 19).
The current clinical practice of fertility preservation for prepubertal boys involves obtaining testicular tissues by testicular sperm extraction (TESE) and then subjecting tissues to a controlled slow-freezing standard protocol with either DMSO or vitrification (9, 10,20–27). Tissue cryopreservation preserves both options for heterotopic testicular tissue and orthotopic SSC transplantations in the future. Alternatively, testicular tissues can be enzymatically digested and cryopreserved as single cell suspensions (2, 28, 29). Although, single cell cryopreservation eliminates the possibility of heterotopic tissue transplant, it may be more effective in preserving testicular cells and SSCs specifically (30).
Previous studies have investigated the effect of different cryopreservation conditions on overall post thawed cell survival using single cell suspensions (30). However, cell viability does not provide quantitative information on the efficiency of the total number of viable cells recovered following cryopreservation as freezing injuries often result (31). Additionally, fertility preservation and resumption of spermatogenesis critically rely on the survival of both SSCs and essential somatic cells after cryopreservation (32). Currently, it is unclear whether human SSCs would be best preserved in situ as testicular tissues or as single cell suspensions. We and others have demonstrated that cells expressing either SSEA-4 and THY-1 are enriched in adult human SSCs and somatic cells (Sertoli and stromal cells), essential for in vitro SSC expansion, respectively (11, 32, 33). Using SSEA-4 as a marker for testicular cell population enriched with human SSCs, Pacchiarotti and colleagues reported similar post thawed SSEA-4+cell recovery, regardless if they were cryopreserved as testicular tissues or single cell suspensions (2). However, the testicular tissues were collected from patients undergoing sexual reassignment surgery who were on extended high dose estrogen therapy. As spermatogenesis is inhibited with high dose estrogen therapy (34), it is unknown whether those results are applicable to patients with normal spermatogenesis who are not on estrogen therapy. Importantly, similar studies on human prepubertal SSC cryopreservation are not currently available due mainly to the scarcity of available tissues and the associated ethical dilemma. However, we have recently demonstrated that human fetal testicular tissues shared significant similarity in seminiferous cord morphology and primitive spermatogonia composition with prepubertal testicular tissues, thus making them viable surrogates for prepubertal tissues (11). In contrast to adult testicular tissues, fetal SSCs co-expressed both SSEA-4 and THY-1 (11).
The present study utilizes testicular tissues collected from adult men with normal spermatogenesis to investigate the effectiveness of human SSC cryopreservation following either testicular tissue or single cell suspension cryopreservation using SSEA-4 as a marker for SSCs. In parallel, human fetal testicular tissues were also used as a surrogate for human prepubertal testicular tissues. We hypothesize that the viability fraction and number of surviving SSEA-4+cells following cryopreservation depend on both the approaches of cryopreservation (tissues vs. single cells) and the age of the patients.
MATERIALS AND METHODS
Testicular tissues
Adult human testicular tissues were collected by testicular sperm extraction (TESE) from 4 subjects (34–62 years of age) with normal spermatogenesis. Each patient had normal semen parameters or prior paternity. Subject 2, 33, and 105 had TESE either for failed vasovasotomy or anejaculation as part of their fertility treatments. Subject 123 underwent a spermatocelectomy. Tissues were transported in sperm wash medium with gentamycin (Vitrolife, San Diego, CA) and processed within two hours. All subjects signed a written informed consent allowing use of their testicular specimens for research purposes as part of the UCSF LIFE study (IRB approved CHR # 10-04868). Human 22–23 weeks of gestation fetal testes (n=3) were obtained following elective termination of pregnancy with appropriate consent from subjects prior to procedure (IRB approved CHR # 12-08704). None of the terminations were for reasons of fetal abnormality, and all fetuses appeared to be morphologically normal. Gestational age was determined by last menstrual period, confirmed with ultrasound and foot length measurement. Tissues were transported at 4°C in DMEM/F12 + Glutamax (Gibco, Grand Island, NY) supplemented with 15% fetal bovine serum (FBS) (Gibco) and 1% Pencillin-Streptomycin-Fungizone (University of California San Francisco, Cell Culture Facility) and processed within 2 hours.
Tissue processing and cryopreservation
Tissues were washed in phosphate buffered saline, minced to 1mm in size, and divided into equal halves by weight for either tissue digestion prior to cryopreservation or for immediate tissue cryopreservation. Minced tissues, designated for immediate cryopreservation, were resuspended in 1.28 M dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO) DMEM/F12 with 25% FBS, equilibrated for 5 minutes at 4°C, transferred to 1.8 ml cryovials (Nunc, Rochester, NY), cryopreserved at a rate of −1°C/min, and stored in liquid nitrogen. 2–5 × 106 total cells or equivalent in tissues were cryopreserved in 1 ml of the freezing media.
The other remaining portion of fresh minced tissues was subjected to a two-step enzymatic digestion at 37°C with 1mg/ml collagenase IV in DMEM/F12 followed by 0.25% trypsin EDTA with 50 µg/ml DNase I (all from Sigma-Aldrich) for 20 min each. Single cell suspensions obtained following tissue digestion were incubated with red blood cell lysis buffer, washed with PBS, filtered through a 70 um cell strainer, resuspended with 0.4% Trypan blue, and counted with a hemocytometer. Cell suspensions were cryopreserved in the same manner described above as minced tissues. For thawing, cryovials were removed, immediately thawed in a 37°C water bath, washed, and resuspended with 15% FBS DMEM/F12. Thawed cryopreserved testicular tissues were subjected to the same two-step enzymatic digestion as fresh minced tissues.
Flow cytometry analysis
Cells were stained with anti-SSEA-4 FITC and anti-Thy-1 APC (BD Pharmingen, San Jose, CA) in PBS with 1% BSA for 30 min at 37°C, washed, and analyzed with BD FACS Aria Flow Cytometer. Only live cells were gated for analyses.
Statistical analysis
The number of cells recovered per milligram of tissue was calculated by dividing cell number by the mass of tissue it was isolated from. Cell recovery between experimental groups (cryopreserved tissues vs. single cell suspension) was reported as a percentage and was calculated by dividing the experimental cells recovered by the number of cells recovered from fresh tissue × 100. One-way with subjects ANOVA with Holm-Bonferroni correction or paired student t-test were used for analyses as appropriated (35).
RESULTS
Experimental design
The experimental design is illustrated in Figure 1A. Adult testicular tissues (n=4) were collected from fertile men with proven spermatogenesis by TESE. Fetal male gonads (n=3), at 22–23 weeks of gestation, were used as a surrogate for prepubertal testicular tissues.
Figure 1.
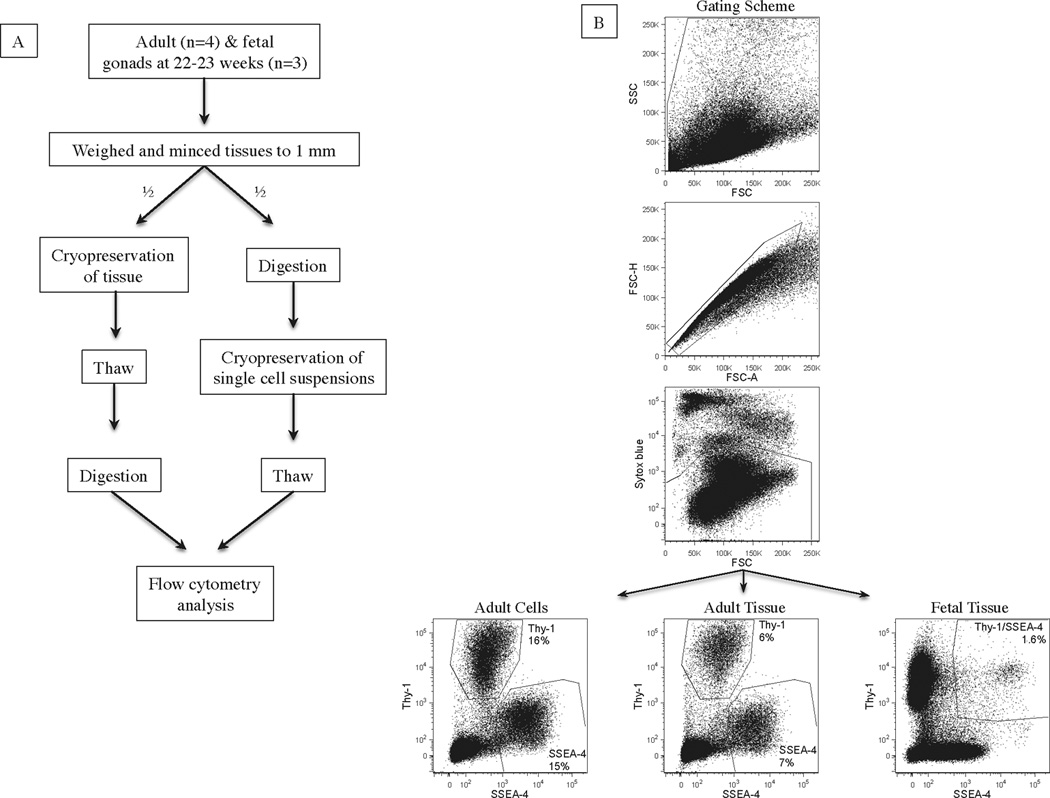
(A) Experimental design of study comparing testicular tissue and testicular single cell suspension cryopreservation. (B) FACS analyses of human adult and fetal testicular SSEA-4+ and THY-1+ cells after tissue and single cell suspension cryopreservation. Cells were initially gated based on FSC and SSC properties. Subsequently, only single and live cells were gated based on FSC-A/FSC-H properties and exclusion of Sytox blue, respectively. For adult cells, SSEA-4 and THY-1 are distinct populations enriched in SSCs and essential somatic cells. For fetal cells, only SSEA-4+/THY-1+ population is enriched in spermatogonia.
Effects of cryopreservation on testicular cell viability
Testicular cell viability was evaluated after paired samples were processed, cryopreserved, and thawed as illustrated (Fig. 1A). The average cell viability of adult and fetal testicular cells, after enzymatic digestion from freshly collected tissues, was 94% and 91%, respectively (Table 1). These single cell suspensions and their paired minced tissues were cryopreserved, thawed, enzymatically digested in cases of tissues, and assessed for cell viability. Viability of cryopreserved adult and fetal cells was compared to viability of freshly collected tissues. When adult and fetal single cell suspensions were cryopreserved and thawed, the viability of adult and fetal testicular cells was 67% and 75%, representing a statistically significant decrease of 27% and 16% in cell viability when compared to percentage of viable cells prior to cryopreservation, respectively. Similarly, when adult and fetal minced tissues were processed following cryopreservation, cell viability significantly decreased to 80% and 83% respectively (Table. 1). Furthermore, the fraction of post-thawed cell viability was significantly higher with minced tissue cryopreservation for both adult and fetal testicular samples when compared to single cell suspension cryopreservation, suggesting that tissue cryopreservation may be a superior approach to preserving testicular cell viability.
Table 1.
Cell viability before and after cryopreservation
| Fresh cells (%) | Cryo cells (%) | Cryo tissues (%) | |
|---|---|---|---|
| Adult Subjects | |||
| SID2 | 96 | 65 | 77 |
| SID33 | 94 | 68 | 79 |
| SID105 | 96 | 64 | 79 |
| SID123 | 88 | 72 | 84 |
| Ave ± SD | 94a ± 4 | 67a ± 4 | 80a ± 3 |
| Fetal Testes | |||
| Sample 1 | 89 | 79 | 84 |
| Sample 2 | 91 | 71 | 82 |
| Sample 3 | 92 | 75 | 82 |
| Ave +/− SD | 91b ± 2 | 75b ± 4 | 83b ± 1 |
Cell viability was assessed with Trypan blue exclusion and the percentage of viability was determined by dividing the number of viable cells over the total number of cells counted × 100.
p<0.001 by one-way ANOVA. Bonferroni corrections demonstrated significant differences in all three subgroups in both adult and fetal samples (p<0.02).
Effects of cryopreservation technique on testicular cell recovery
Although cell viability assessment provides the overall percentage of viable cells of each single cell suspension, it does not provide quantitative information on the efficiency of the total number of viable cells recovered following cryopreservation. Therefore, assessing the absolute number of viable cells recovered, normalized to tissue weight, provides more insight into the efficiency of cryopreservation. Due to differences in developmental stages and methods of tissue collection (TESE vs. whole testes), adult testicular tissues and fetal testes were analyzed separately and results were not directly compared. Table 2 lists the number of cells recovered per mg of tissues for all fresh samples and their experimental (cryopreserved tissues and single cell suspensions) matched pairs after cryopreservation and thaw. Cell recovery between experimental groups was compared to their matched fresh samples and reported as a percentage. In comparison to freshly digested tissues, when adult testicular single cell suspensions and tissues were cryopreserved and thawed, the number of total cells recovered per mg of tissues decreased to 39,607 (46% recovery) and 18,516 (24% recovery), respectively, (Table 2). When the number of live cells recovered per mg of tissues was evaluated by Trypan blue exclusion, the recovery fraction from single cell suspension and tissue cryopreservation decreased further to 33% and 20%, respectively (Table 2). Although the recovery rate with tissue cryopreservation was lower in comparison to single cell suspension cryopreservation, this trend was not statistically significant (p=0.09).
Table 2.
Number of total and viable cells recovered per mg of tissue
| Total cells per mg of tissue (% recovery) | Viable cells per mg of tissue (% recovery) | |||||
|---|---|---|---|---|---|---|
| Fresh cells | Cryo Cells | Cryo Tissues | Fresh Cells | Cryo Cells | Cryo Tissues | |
| Adult Subjects | ||||||
| SID2 | 124,740 | 79,002 (63%) | 26,791 (22%) | 119,751 (96%) | 51,351 (43%) | 20,768 (17%) |
| SID33 | 70,175 | 48,246 (69%) | 27,778 (40%) | 65,965 (94%) | 32,807 (50%) | 21,944 (33%) |
| SID105 | 54,276 | 13,021 (24%) | 10,284 (19%) | 52,105 (96%) | 8,463 (16%) | 7,919 (15%) |
| SID123 | 61,607 | 18,157 (29%) | 9,211 (15%) | 54,214 (88%) | 11,802 (22%) | 7,092 (13%) |
| Ave ± SD | 77,699 ± 32,026 | 39,607a ± 30,515 (46%) | 18,516a ± 10,142 (24%) | 73,009 ± 31,752 | 26,106b ±19,984(33%) | 14,431b ± 8,018(20%) |
| Fetal Testes | ||||||
| Sample 1 | 48,066 | 20,580 (43%) | 33,646 (70%) | 42,586 (89%) | 16,258 (38%) | 28,263 (66%) |
| Sample 2 | 40,441 | 19,485 (48%) | 21,547 (53%) | 36,599 (91%) | 13,834 (38%) | 17,669 (48%) |
| Sample 3 | 40,952 | 17,778 (43%) | 29,549 (72%) | 37,840 (92%) | 13,333 (35%) | 24,230 (64%) |
| Ave ± SD | 43,153 ± 4,262 | 19,281c ± 1,412 (45%) | 28,247c ± 6,154 (65%) | 39,008 ± 3,160 | 14,475d ± 1,564 (37%) | 23,387d ± 5,347 (59%) |
All tissues were weighed prior to processing into single cells or cryopreserved. The number of cells recovered after single cell or tissue cryopreservation was normalized to the original tissue weight in mg prior to processing. Percent total cell recovery was calculated by dividing the total cell number /mg from each experimental group by the total cell number/mg from its freshly digested tissues × 100. Percent viable cell recovery was calculated in similar fashion but with viability assessment as described in Table 1.
p<0.05 compared to total adult and fetal fresh testicular cells, respectively,
p<0.05 compared to viable adult and fetal fresh testicular cells, respectively, by ANOVA with Holm-Bonferroni correction. No significant differences in the recovery rates between cryo cell and cryo tissue subgroups in both adult and fetal samples.
Cell recovery was also evaluated in fetal testes in a similar fashion. The number of testicular cells per mg of fetal testes was less than the number observed in adult testicular tissues due to differences mentioned above. Similar to adult testicular cells, cryopreservation of fetal testicular cells, regardless as cell suspension or tissues, resulted in a significant decline in cell recovery after thaw. In contrast to adult testicular cells, the number of cells recovered per mg of tissue was higher when tissues were cryopreserved (28,247 total cells/mg and 23,387 viable cells/mg) in comparison to single cell cryopreservation (19,281 total cells/mg and 14,475 viable cells/mg) (Table 2); however this difference did not achieve statistical significance (p=0.07).
Effects of cryopreservation on SSEA-4+ cell recovery
Fertility preservation and spermatogenesis critically rely on the successful survival of both SSCs and essential somatic cells after cryopreservation (32). In adult, we used SSEA-4 and THY-1 as markers for cell populations enriched in SSCs and somatic cells (Sertoli and stromal cells) essential for SSC expansion, respectively (32, 33). The frequency of live SSEA-4+ and THY-1+ cells from cryopreserved tissues and cell suspensions were evaluated by FACS (Fig. 1B). Adult SSEA-4+ cell recovery was significantly higher with single cell suspension cryopreservation (19%) in comparison to tissue cryopreservation (13%) (Table 3) (p<0.03). The number of SSEA-4+ cells per mg of tissues recovered with single cell suspension and tissue cryopreservation was 5,150 and 2,233, respectively. Although there was better somatic cell recovery with single cell suspension cryopreservation, this difference did not achieve statistical significance (p=0.16).
Table 3.
Frequency and number of SSEA-4+ cells per milligram of tissue.
| Frequency of SSEA-4+ Cells | Frequency of Somatic Cells | SSEA-4+ Cells per mg | Somatic Cells per mg | |||||
|---|---|---|---|---|---|---|---|---|
| Cryo Cells | Cryo Tissues | Cryo Cells | Cryo Tissues | Cryo Cells | Cryo Tissues | Cryo Cells | Cryo Tissues | |
| Adult Subjects | ||||||||
| SID2 | 15% | 7% | 16% | 6% | 7,703 | 1,454 | 8,216 | 1,246 |
| SID33 | 30% | 28% | 9% | 5% | 9,842 | 6,144 | 2,953 | 1,097 |
| SID105 | 11% | 7% | 10% | 9% | 931 | 554 | 846 | 713 |
| SID123 | 18% | 11% | 9% | 8% | 2,124 | 780 | 1,062 | 567 |
| Ave ± SD | 19%a ± 8% | 13%a ± 10% | 11% ± 3% | 7% ± 2% | 5,150 ± 4,301 | 2,233 ± 2,635 | 3,269 ± 3,431 | 906 ± 318 |
| Fetal Testes | ||||||||
| Subject 1 | 1.2% | 1.6% | 195 | 452 | ||||
| Subject 2 | 2.5% | 1.3% | 346 | 230 | ||||
| Subject 3 | 1.2% | 1.0% | 160 | 242 | ||||
| Ave ± SD | 1.6% ± 0.8% | 1.3% ± 0.3% | 234 ± 99 | 308 ± 125 | ||||
Frequency of live SSEA-4+ and somatic cells were determined by FACS after excluding dead cells (Figure 1). Cells per mg was calculated by multiplying the frequency with the number of live cells obtained after processing divided by the original weight prior to cryopreservation.
p<0.03 by paired student t test.
In contrast to adult testicular tissues, fetal SSCs co-express both SSEA-4 and THY-1 (11). Thus, fetal SSEA-4+/THY-1+ testicular cells, used in this study, represent cell population enriched with fetal SSCs. Although adult testicular THY-1+ cells have been shown to be critical in providing an essential somatic niche for adult SSCs expansion in vitro, the precise somatic population needed to support fetal or prepubertal SSC growth has not been defined (11, 32). Thus, the frequency of fetal somatic cells will not be evaluated here. The frequency and number of fetal SSEA-4 cells recovered after cryopreservation was evaluated as described and listed in Table 3. Fetal SSEA-4+/THY-1+ cell recovery was not impacted by whether they were cryopreserved as tissues or single cell suspensions. Since cryopreserved tissues yielded higher overall number of viable fetal testicular cells (Table 2), the number of SSEA-4+/THY-1+ cells recovered per mg of tissues trended higher with tissue preservation (p=0.56) (Table 3).
DISCUSSION
Although considered experimental, testicular tissue cryopreservation prior to gonadotoxic oncotherapy is an emerging fertility preservation strategy for patients who may be rendered infertile following successful therapy (1). Testicular tissue cryopreservation had been shown to preserve the integrity of the seminiferous tubules, as well as, some in vitro and in vivo function of spermatogonia, Leydig, and Sertoli cells (21–27, 30). However, as SSC survival after cryopreservation is essential for resumption of spermatogenesis, studies assessing optimal approaches to SSC cryopreservation (tissues vs. single cell suspension) are limited (2). Currently, it is unknown whether it is best to cryopreserve SSCs within their cognate niches as frozen tissues or as a heterogeneous single cell suspension of testicular cells. This study demonstrates that optimal SSC recovery depends on both the state of the samples cryopreserved and the age of the patients. While testicular cell cryopreservation yielded a higher recovery of SSEA-4+ cells, a population enriched with SSCs in adult, similar fetal SSEA-4+ recovery rate was seen regardless whether testicular tissues or cells were cryopreserved.
Controlled slow-freezing using DMSO was used in this study as previous studies had shown that this method was suitable for human testicular tissue and cell cryopreservation for both adult and prepubertal samples (2, 21–26,28–30). These studies used various concentrations of DMSO with limited quantitative assessment of SSC survival and function as endpoints (21–26,28–30). Due to the limited availability of human testicular tissues, there are few studies comparing efficiency of cryoprotectants (22, 29). When glycerol, propanediol, and DMSO were evaluated, DMSO was superior in preserving the integrity of adult seminiferous tubules and in vitro Leydig cell function (22). In contrast, the type of cryoprotectants did not have an impact on the relative viability of adult testicular cells when cryopreserved as testicular single cell suspensions (29). However, these results must be interpreted with caution, as testicular tissues from infertile men were also included in these studies (22, 29).
Recently, vitrification has been reported as an alternative to controlled slow-freeze as it is less time consuming and has the potential to minimize freezing injuries due to ice crystal formations (31, 36). However, studies on testicular tissue vitrification are also limited. Vitrification of neonatal mouse testicular tissues resulted in similar preservation of seminiferous tubule morphology, cell structure, and overall graft survival in comparison to controlled slow-freeze (37–39). However, vitrification resulted in significantly higher levels of apoptosis (37). Vitrification of non-human primate immature testicular tissues was reported to be feasible (40). However, the relative effectiveness of vitrification remains to be investigated as it was not compared to controlled slow-freeze (40). Vitrification of human prepubertal testicular tissues from two patients was compared to controlled slow-freeze recently (21). In this case control study, vitrification resulted in similar preservation of the seminiferous tubule, spermatogonium, and Sertoli cell morphology based on histology in comparison to controlled slow-freeze (21). Using testicular biopsies from adult infertile men, vitrification of single cell suspensions was compared to different protocols of controlled slow-freeze (28). Specifically, vitrification was found to yield the highest rate of viable testicular cells recovered at 34.9% compared to 20.9% with a controlled slow-freeze protocol using DMSO (28). Although these results were encouraging, it is important to notice that the overall recovery rate was quite low for all controlled slow-freeze and samples from infertile patients were used (28). In contrast, in our study, the viability of single cell suspension cryopreservation was 33% (Table 2). Thus, although vitrification of human testicular tissues and cells is a promising approach, comparative studies are limited and more functional studies are needed.
Most studies, thus far, have focused on the feasibility of human testicular tissue or single cell suspension cryopreservation with assessments of seminiferous tubule morphology and limited short-term cellular function either with an in vitro or in vivo xenograft systems (21–26,28, 29). Specifically, the ideal cryopreservation approaches (tissues vs. single cell suspensions) that yield the highest recovery of viable SSCs and supporting cells such as Leydig and Sertoli cells, essential for resumption of spermatogenesis, at different stages of development (prepupertal vs. adult) were not evaluated. Recent studies demonstrate that cryopreservation of normal adult human testicular single cell suspensions with DMSO yields the best recovery of viable diploid testicular cells in comparison to tissue cryopreservation (30). Furthermore, in vitro culture of these cells remained viable for longer periods, suggesting that single cell suspension cryopreservation is the optimal way to preserve adult human diploid testicular cells (30). Using testicular tissues from male patients undergoing surgical sexual reassignment treatments, Pacchiarotti and colleagues reported similar rate of SSEA-4+ cells and Leydig cell recovery with both tissue and single cell suspension cryopreservation (2). In contrast, the highest recovery of viable adult SSEA-4+cells in our studies was achieved with single cell cryopreservation (19%) in agreement with Unni et. al (30). Similar to Pacchiarotti’s studies, we also used SSEA-4+ as a marker of human testicular cells enriched with SSCs and objective quantification with FACS (2, 11,32, 33). However, we used testicular samples from men with normal spermatogenesis rather than men who were taking high doses of estrogen as part of the medical treatments prior to sexual reassignment surgeries (2). The estrogen induced hypothalamic suppression was shown to alter the testicular tissue composition and inhibit spermatogenesis which may limit the application of these results to normal men (34). We typically found SSEA-4+ frequency in the range of 2–6% when we evaluated samples from men who were taking high dose estrogen (data not shown), similar to those reported by Pacchiarotti and colleagues (2). Furthermore, the enzymes used for digestion in that study were also different from our studies. Thus, these differences may explain for the discrepancies such as SSEA-4+cell frequency, recovery, and cells/mg between studies. Although we did not specifically evaluate for the recovery of Leydig cells, we did evaluate stromal and Sertoli cell recovery by FACS using THY-1 as a marker as we previously reported that adult testicular THY-1+ cells are essential in supporting SSC expansion in vitro (11, 32). Similar to Leydig cell recovery between tissue and single cell suspension cryopreservation in previous studies, we did not find significant differences in the recovery of adult testicular THY-1+ cells suggesting that adult SSCs are more sensitive to the cryopreservation process than their somatic cell counterparts.
Recent studies in prepubertal rats demonstrated that testicular single cell suspension cryopreservation with DMSO yielded the highest rate of viable spermatogonia recovered in comparison to tissue cryopreservation or with the use of other cryoprotectants (30). Unfortunately, similar studies looking at the recovery rate of viable human prepubertal SSCs are not available due to the scarcity of prepubertal tissues for research. To circumvent this problem, we used fetal testes at 22–23 weeks of gestation as a surrogate model to study the optimal freezing method for prepubertal SSCs as we have previously reported that fetal testes at this gestation showed similar seminiferous tubule morphology with similar frequency of SSCs and Sertoli cells (11). Although there was a trend in higher overall recovery rate of viable testicular cells with tissue cryopreservation (p<0.07), there was no difference in the recovery rate of fetal SSEA-4+, perhaps due to the lower frequency of SSCs in fetal testes.
Our results demonstrated that optimal preservation of adult human testicular SSEA-4+ cells, a population enriched with SSCs, is achieved with controlled slow-freeze of testicular single cell suspensions. In contrast, both testicular tissue and single cell suspension cryopreservation yielded similar recovery rate of fetal SSEA-4+ cells. These results provide much needed information for clinicians and scientists in the field of male fertility preservation (1). Although preliminary, our data support the current practice of prepubertal testicular tissue cryopreservation, as this will preserve the options for both heterotopic grafting of testicular tissues and orthotopic SSC transplantation in the future. Although sperm cryopreservation is a proven and acceptable technique of fertility preservation in adult men undergoing potential gonadotoxic treatments, future fertility treatments using the finite amount of cryopreserved sperm will require some forms of assisted reproductive technology which are often cumbersome and expensive. Autologous heterotopic or orthotopic SSC transplantations are theoretically feasible alternatives as limited results were reported in non-human primates (8, 12–14,18, 19). However, only orthotopic testicular cells transplantation had been shown to provide long-term spermatogenesis capable of conceiving in non-human primates (18, 19). Thus, cryopreservation of adult testicular cell suspensions should be considered as our data demonstrated that adult SSEA-4+ cells are best preserved with this approach. Additionally, this approach also allows for the elimination of potential contaminating malignant cells prior to transplantation with FACS (15, 16,41–43).
The strengths of our study include the use of actual TESE tissues collected from adult men with normal spermatogenesis. Furthermore, we were able to isolate these rare adult SSEA-4+cells and essential somatic cells after both tissue and single cell suspension cryopreservation by FACS and expanded them in vitro (32). Additionally, we used matched pairs of tissues for our experimental groups and objectively quantitated for the recovery of human SSCs with validated markers and FACS. While fetal tissue was used as a model of prepubertal testicular tissue, significant differences may exist between these cell populations. Further, the limited number of subjects evaluated may not accurately reflect all normal men. Additionally, we used nonclinical-grade reagents such as collagenase and fetal bovine serum in our study limiting their direct application to clinical settings.
In summary, we have shown that optimal adult human SSEA-4+ cell recovery is achieved with testicular single cell suspension cryopreservation whereas similar fetal SSC recovery was achieved with both tissue and single cell suspension cryopreservation. These results offer insight into the optimal methods of preserving SSCs from testicular tissues for future therapeutic applications.
Supplementary Material
Acknowledgments
Support: NDT is supported by ASRM new investigator award, UCSF Rap Grant, Weston Haven Foundation
Footnotes
None of the authors have any conflicts to declare
REFERENCES
- 1.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pacchiarotti J, Ramos T, Howerton K, Greilach S, Zaragoza K, Olmstead M, et al. Developing a Clinical-Grade Cryopreservation Protocol for Human Testicular Tissue and Cells. BioMed Research International. 2013;2013:10. doi: 10.1155/2013/930962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dym M, Kokkinaki M, He Z. Spermatogonial stem cells: mouse and human comparisons. Birth Defects Res C Embryo Today. 2009;87:27–34. doi: 10.1002/bdrc.20141. [DOI] [PubMed] [Google Scholar]
- 4.Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002;418:778–781. doi: 10.1038/nature00918. [DOI] [PubMed] [Google Scholar]
- 5.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biology of reproduction. 2003;69:612. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 8.Schlatt S, Kim SS, Gosden R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction (Cambridge, England) 2002;124:339–346. doi: 10.1530/rep.0.1240339. [DOI] [PubMed] [Google Scholar]
- 9.Wyns C, Curaba M, Petit S, Vanabelle B, Laurent P, Wese JF, et al. Management of fertility preservation in prepubertal patients: 5 years' experience at the Catholic University of Louvain. Human reproduction (Oxford, England) 2011;26:737–747. doi: 10.1093/humrep/deq387. [DOI] [PubMed] [Google Scholar]
- 10.Ginsberg JP, Carlson CA, Lin K, Hobbie WL, Wigo E, Wu X, et al. An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: a report of acceptability and safety. Human reproduction (Oxford, England) 2010;25:37–41. doi: 10.1093/humrep/dep371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altman E, Yango P, Moustafa R, Smith J, Klatsky PC, Tran ND. Characterization of human gonocyte, prespermatogonium, and spermatogonial stem cell markers. Reproduction (Cambridge, England) 2014 doi: 10.1530/REP-14-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wistuba J, Luetjens CM, Wesselmann R, Nieschlag E, Simoni M, Schlatt S. Meiosis in autologous ectopic transplants of immature testicular tissue grafted to Callithrix jacchus. Biol Reprod. 2006;74:706–713. doi: 10.1095/biolreprod.105.048793. [DOI] [PubMed] [Google Scholar]
- 13.Jahnukainen K, Ehmcke J, Hergenrother SD, Schlatt S. Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Human reproduction (Oxford, England) 2007;22:1060–1067. doi: 10.1093/humrep/del471. [DOI] [PubMed] [Google Scholar]
- 14.Luetjens CM, Stukenborg JB, Nieschlag E, Simoni M, Wistuba J. Complete spermatogenesis in orthotopic but not in ectopic transplants of autologously grafted marmoset testicular tissue. Endocrinology. 2008;149:1736–1747. doi: 10.1210/en.2007-1325. [DOI] [PubMed] [Google Scholar]
- 15.Dovey SL, Valli H, Hermann BP, Sukhwani M, Donohue J, Castro CA, et al. Eliminating malignant contamination from therapeutic human spermatogonial stem cells. The Journal of clinical investigation. 2013;123:1833–1843. doi: 10.1172/JCI65822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermann BP, Sukhwani M, Salati J, Sheng Y, Chu T, Orwig KE. Separating spermatogonia from cancer cells in contaminated prepubertal primate testis cell suspensions. Human reproduction (Oxford, England) 2011;26:3222–3231. doi: 10.1093/humrep/der343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahnukainen K, Hou M, Petersen C, Setchell B, Soder O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer research. 2001;61:706–710. [PubMed] [Google Scholar]
- 18.Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11:715–726. doi: 10.1016/j.stem.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shetty G, Uthamanthil RK, Zhou W, Shao SH, Weng CC, Tailor RC, et al. Hormone suppression with GnRH antagonist promotes spermatogenic recovery from transplanted spermatogonial stem cells in irradiated cynomolgus monkeys. Andrology. 2013;1:886–898. doi: 10.1111/j.2047-2927.2013.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyns C, Curaba M, Vanabelle B, Van Langendonckt A, Donnez J. Options for fertility preservation in prepubertal boys. Hum Reprod Update. 2010;16:312–328. doi: 10.1093/humupd/dmp054. [DOI] [PubMed] [Google Scholar]
- 21.Curaba M, Poels J, van Langendonckt A, Donnez J, Wyns C. Can prepubertal human testicular tissue be cryopreserved by vitrification? Fertility and sterility. 2011;95:2123.e9–2123.e12. doi: 10.1016/j.fertnstert.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Keros V, Rosenlund B, Hultenby K, Aghajanova L, Levkov L, Hovatta O. Optimizing cryopreservation of human testicular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Human Reproduction. 2005;20:1676–1687. doi: 10.1093/humrep/deh797. [DOI] [PubMed] [Google Scholar]
- 23.Keros V, Hultenby K, Borgström B, Fridström M, Jahnukainen K, Hovatta O. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Human Reproduction. 2007;22:1384–1395. doi: 10.1093/humrep/del508. [DOI] [PubMed] [Google Scholar]
- 24.Wyns C, Van Langendonckt A, Wese F-X, Donnez J, Curaba M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Human Reproduction. 2008;23:2402–2414. doi: 10.1093/humrep/den272. [DOI] [PubMed] [Google Scholar]
- 25.Wyns C, Curaba M, Martinez-Madrid B, Van Langendonckt A, Francois-Xavier W, Donnez J. Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Human reproduction (Oxford, England) 2007;22:1603–1611. doi: 10.1093/humrep/dem062. [DOI] [PubMed] [Google Scholar]
- 26.Kvist K, Thorup J, Byskov AG, Høyer PE, Møllgård K, Yding Andersen C. Cryopreservation of intact testicular tissue from boys with cryptorchidism. Human Reproduction. 2006;21:484–491. doi: 10.1093/humrep/dei331. [DOI] [PubMed] [Google Scholar]
- 27.Kvist K, Thorup J, Byskov AG, Hoyer PE, Mollgard K, Yding Andersen C. Cryopreservation of intact testicular tissue from boys with cryptorchidism. Human reproduction (Oxford, England) 2006;21:484–491. doi: 10.1093/humrep/dei331. [DOI] [PubMed] [Google Scholar]
- 28.Sa R, Cremades N, Malheiro I, Sousa M. Cryopreservation of human testicular diploid germ cell suspensions. Andrologia. 2012;44:366–372. doi: 10.1111/j.1439-0272.2012.01290.x. [DOI] [PubMed] [Google Scholar]
- 29.Brook PF, Radford JA, Shalet SM, Joyce AD, Gosden RG. Isolation of germ cells from human testicular tissue for low temperature storage and autotransplantation. Fertility and Sterility. 2001;75:269–274. doi: 10.1016/s0015-0282(00)01721-0. [DOI] [PubMed] [Google Scholar]
- 30.Unni S, Kasiviswanathan S, D’Souza S, Khavale S, Mukherjee S, Patwardhan S, et al. Efficient cryopreservation of testicular tissue: effect of age, sample state, and concentration of cryoprotectant. Fertility and Sterility. 2012;97:200–208.e1. doi: 10.1016/j.fertnstert.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Pegg DE. The relevance of ice crystal formation for the cryopreservation of tissues and organs. Cryobiology. 2010;60:S36–S44. doi: 10.1016/j.cryobiol.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Smith JF, Yango P, Altman E, Choudhry S, Poelzl A, Zamah AM, et al. Testicular Niche Required for Human Spermatogonial Stem Cell Expansion. Stem Cells Translational Medicine. 2014 doi: 10.5966/sctm.2014-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izadyar F, Wong J, Maki C, Pacchiarotti J, Ramos T, Howerton K, et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Human reproduction (Oxford, England) 2011;26:1296–1306. doi: 10.1093/humrep/der026. [DOI] [PubMed] [Google Scholar]
- 34.Schulze C. Response of the human testis to long-term estrogen treatment: morphology of Sertoli cells, Leydig cells and spermatogonial stem cells. Cell Tissue Res. 1988;251:31–43. doi: 10.1007/BF00215444. [DOI] [PubMed] [Google Scholar]
- 35.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 36.Brockbank KG, Song YC, Khirabadi BS, Lightfoot FG, Boggs JM, Taylor MJ. Storage of tissues by vitrification. Transplantation proceedings. 2000;32:3–4. doi: 10.1016/s0041-1345(99)00851-9. [DOI] [PubMed] [Google Scholar]
- 37.Curaba M, Verleysen M, Amorim CA, Dolmans M-M, Van Langendonckt A, Hovatta O, et al. Cryopreservation of prepubertal mouse testicular tissue by vitrification. Fertility and Sterility. 2011;95:1229–1234.e1. doi: 10.1016/j.fertnstert.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 38.Gouk SS, Jason Loh YF, Kumar SD, Watson PF, Kuleshova LL. Cryopreservation of mouse testicular tissue: prospect for harvesting spermatogonial stem cells for fertility preservation. Fertility and Sterility. 2011;95:2399–2403. doi: 10.1016/j.fertnstert.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 39.Baert Y, Goossens E, van Saen D, Ning L, in’t Veld P, Tournaye H. Orthotopic grafting of cryopreserved prepubertal testicular tissue: in search of a simple yet effective cryopreservation protocol. Fertility and Sterility. 2012;97:1152–1157.e2. doi: 10.1016/j.fertnstert.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Poels J, Van Langendonckt A, Dehoux JP, Donnez J, Wyns C. Vitrification of non-human primate immature testicular tissue allows maintenance of proliferating spermatogonial cells after xenografting to recipient mice. Theriogenology. 2012;77:1008–1013. doi: 10.1016/j.theriogenology.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Fujita K, Tsujimura A, Miyagawa Y, Kiuchi H, Matsuoka Y, Takao T, et al. Isolation of germ cells from leukemia and lymphoma cells in a human in vitro model: potential clinical application for restoring human fertility after anticancer therapy. Cancer research. 2006;66:11166–11171. doi: 10.1158/0008-5472.CAN-06-2326. [DOI] [PubMed] [Google Scholar]
- 42.Fujita K, Ohta H, Tsujimura A, Takao T, Miyagawa Y, Takada S, et al. Transplantation of spermatogonial stem cells isolated from leukemic mice restores fertility without inducing leukemia. J Clin Invest. 2005;115:1855–1861. doi: 10.1172/JCI24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou M, Andersson M, Zheng C, Sundblad A, Soder O, Jahnukainen K. Decontamination of leukemic cells and enrichment of germ cells from testicular samples from rats with Roser's T-cell leukemia by flow cytometric sorting. Reproduction (Cambridge, England) 2007;134:767–779. doi: 10.1530/REP-07-0240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


