Abstract
Background
Although asthma is typically characterized as a childhood disease, it can develop later in life. Older asthmatic patients may be at increased risk for corticosteroid adverse effects. We developed a novel traditional Chinese medicine to treat asthma called antiasthma simplified herbal medicine intervention (ASHMI). Herbal products may offer safer adjunctive treatment for older asthmatic patients.
Objective
To investigate the effects of ASHMI on characteristics of allergic asthma in an aged mouse model of asthma.
Methods
BALB/c mice (6 weeks old [young] and 6, 12, and 18 months old [aged]) received ASHMI treatment before and during intraperitoneal ovalbumin sensitization and intratracheal challenges. The control groups were untreated, age-matched, ovalbumin-sensitized and ovalbumin-challenged mice (ovalbumin mice) and naive mice. After the final antigen challenge, airway pressure (defined as the time-integrated change in peak airway pressure) after acetylcholine provocation was measured, representing airway hyperresponsiveness, and bronchoalveolar lavage fluid, sera, lung tissues for histologic analysis, messenger RNA, and collagen were collected.
Results
Mean time-integrated change in peak airway pressure values in 6-week-old and 6-, 12-, and 18-month-old ASHMI ovalbumin mice were significantly reduced compared with those of age-matched, nontreated ovalbumin mice. Bronchoalveolar lavage fluid eosinophil numbers were significantly lower in all ASHMI ovalbumin mice. Treatment with ASHMI of young and aged ovalbumin mice resulted in significantly decreased lung inflammation, detected via hematoxylin-eosin staining; airway mucous cell metaplasia, determined by means of periodic acid–Schiff staining; and messenger RNA copy numbers of the mucin gene MUC5AC. Levels of ovalbumin specific IgE and the TH2 cytokines interleukin 4 (IL-4), IL-5, and IL-13 in lung and splenocyte cultures were reduced. Interferon gamma secretion was increased. Treatment with ASHMI reduced collagen production.
Conclusion
Treatment with ASHMI reduces several features of asthma in aged antigen-sensitized and antigen-challenged mice.
INTRODUCTION
Although asthma is frequently characterized as a chronic inflammatory disease beginning in childhood, as many as 25% of asthmatic patients develop symptoms after the age of 40 years.1 Airway disease may be more severe in later-onset asthma compared with earlier-onset disease2,3; hospitalization and mortality rates are highest in patients older than 65 years.4,5 Despite these findings, the effect of aging on allergic airway and pulmonary inflammation in humans or murine models has not been well characterized. We6 developed a murine model of later-onset asthma that demonstrated that aged mice develop antigen specific IgE, airway hyperresponsiveness (AHR), increased airway mucous cell metaplasia, pulmonary inflammation, and eosinophilia.
Corticosteroids, the cornerstone of persistent asthma therapy, substantially reduce asthma symptoms and improve lung function through anti-inflammatory actions. Systemic corticosteroids have the potential to induce adverse effects (ie, osteoporosis, increased fracture risk, muscle weakness, glucose intolerance, and easy bruising, all particular concerns in older patients), especially when administered for prolonged periods. The potential for these effects is reduced when corticosteroids are inhaled. However, inhaled corticosteroids taken at high doses, generally greater than 1000 μg/d, have the potential for systemic absorption, producing similar effects as oral corticosteroids.7,8 Therefore, it is critical to develop corticosteroid-sparing medications for asthma. Li et al9 previously developed an herbal formula from traditional Chinese medicine (TCM) called MSSM-002, an extract of 14 herbs based on an asthma prescription used at the China-Japan Friendship Hospital in Beijing. MSSM-002 nearly eliminated AHR; significantly reduced total bronchoalveolar lavage fluid (BALF) cell numbers and eosinophil percentages and antigen specific IgE levels; and inhibited lung mucous cell hyperplasia similar to corticosteroid treatment in a murine model of allergic asthma (6-week-old mice).9 Because the formulation of 14 herbs is difficult to standardize to maintain quality, potency, and consistency, we first studied whether a simplified TCM compound could be equally effective in suppressing AHR and airway inflammation in young mice. We subsequently developed a product named antiasthma simplified herbal medicine intervention (ASHMI, original name MSSM-003d) containing 3 of the herbs found in MSSM-002. Next, we evaluated age-dependent effects of this treatment on allergic asthma in a murine model.
METHODS
Mice
Young (6-week-old) BALB/c and AKR mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Aged female BALB/c mice (6, 12, and 18 months old) were obtained from the National Institute on Aging (Bethesda, Maryland). Mice were maintained in the animal facility at Mount Sinai School of Medicine. Standard guidelines for laboratory animal care were followed,10 and institutional permission for animal handling was approved through the animal facility at Mount Sinai School of Medicine. Mice were housed under pathogenfree conditions.
Preparation of Extracts From Individual Herbs and ASHMI
Individual herbs and simplified formulas derived from MSSM-002 (Table E1, Online Repository) were prepared as previously described.9 Briefly, hot water extracts of individual herbs and formulas were prepared by boiling herbs in water twice and lyophilizing the resulting decoctions.11 The ASHMI (Sino-Lion Pharmaceutical Co, Shan Dong, China), composed of 3 herbs, has been described previously12 and is currently used in our clinical trial. Based on organoleptic and microscopic examination, the raw herbs used in ASHMI were identified as Ganoderma lucidum, radix Sophorae flavescentis, and radix Glycyrrhiza. All 3 herbs are of Chinese origin and meet the quality and safety standards of the Pharmacopoeia of the People’s Republic of China.13 Product quality was monitored by means of high-performance liquid chromatography fingerprinting according to the Food and Drug Administration’s Guidance for Industry Botanical Drug Products,14 previously described.12 Endotoxin levels in ASHMI were tested using the Pyrogent Plus assay kit (Lonza, Walkersville, Maryland). No endotoxin was detected (detection limit of <0.03 EU/mL).
Antigen Sensitization and Challenge and Treatment
AKR (n = 4-5 per group) and BALB/c (n = 5-10 per group) mice were antigen sensitized twice intraperitoneally with 200 μg of conalbumin (AKR mice) (Fig E1, Online Repository) or grade VI ovalbumin (, Sigma-Aldrich Corp, St Louis, Missouri) (BALB/c mice) (Fig 1) adsorbed with 2 mg of alum (Pierce Biotechnology Inc, Rockford, Illinois) in 0.4 mL of phosphate-buffered saline 1 week apart. Ten days after the last sensitization, mice were challenged intratracheally 3 times at 7- to 10-day intervals with 100 μg of conalbumin (AKR mice) or ovalbumin (BALB/c mice) in 0.05 mL of phosphate-buffered saline, determined to be optimal for distribution to both lobes of the lung,15 as previously described.16 (Mice sensitized to and challenged with conalbumin or ovalbumin are labeled as conalbumin or ovalbumin mice, respectively). Controls were nontreated, age-matched conalbumin mice (n=4-5 per group) and ovalbumin mice (n=5-10 per group) and age- and strain-matched naive mice (n=5 per group). In the first experiment, AKR conalbumin mice were fed individual herbs from MSSM-002 or the complete formula using the protocol previous reported (Fig E1, Online Repository).9 Based on AHR and lung inflammation results, as well as TCM formulation concept, 3 individual herbs were selected to create the simplified formula (ASHMI). The AKR conalbumin mice were then fed MSSM-002 (73.4 mg/d) or ASHMI (9.5 mg/d) using the same protocol (Fig E1), and AHR and BALF were measured. In the aging mouse protocol, BALB/c mice were fed ASHMI (10 mg/d) for 4 consecutive weeks before antigen sensitization and challenge (Fig 1). The doses of individual herbs and formulas (Fig E1) were calculated based on dry yields from raw herb decoctions according to the prescribed human dose, including a dose-escalation safety trial,12 and normalizing for mice using human to animal dose ratios.17 Treatment was delivered via intragastric feeding using a 25-gauge stainless steel blunt feeding needle (Fine Science Tool, Foster City, California). Mice were fed the ASHMI dissolved in 1 mL of water per day in 2 divided doses.
Figure 1.
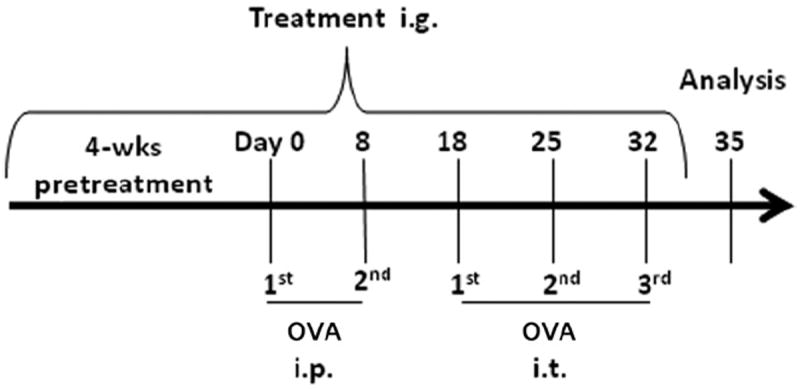
Mice (aged 6 weeks and 6, 12, and 18 months) were sensitized intraperitoneally (i.p) with ovalbumin (OVA) (200 μg per mouse) twice at weekly intervals and then were challenged intratracheally (i.t.) on days 18, 25, and 32 with OVA (100 μg per mouse). One set of mice from each age group was pretreated with intragastric (i.g.) antiasthma simplified herbal medicine intervention (ASHMI) (10 mg/d per mouse) in a twice-daily divided dose starting 4 weeks before sensitization. Treatment was continued through the sensitization and challenge periods. Seventy-two hours after the final OVA challenge, the mice were analyzed. Age-matched naive mice served as additional controls.
Airway Pressure Recording and BALF Preparation
Seventy-two hours after the last antigen challenge, airway responsiveness to acetylcholine was measured. The chest was opened and BALF was collected. Procedures are described in the “Methods” section of this article’s Online Repository.
Lung Histologic Analysis
Lung tissue was surgically removed and separated from the trachea after BALF collection (BALB/c mice) for assessment of mucous cell hyperplasia, as described in the “Methods” section of this article’s Online Repository.
RNA Extraction, Reverse Transcription and Quantitative Real-time Polymerase Chain Reaction
After BALF collection (BALB/c mice), the left upper and middle lobes were placed in reagent (TRIzol; Invitrogen, Carlsbad, California) and were frozen at −80°C. Samples were thawed at 4°C and homogenized, and RNA was extracted according to the manufacturer’s directions. Real-time reverse transcription quantitative polymerase chain reaction (qPCR) was performed as described in the “Methods” section of this article’s Online Repository.
Collagen Assay
Frozen nonlavaged right lungs (BALB/c mice) were homogenized in 1 mL of ice-cold phosphate-buffered saline with a protease inhibitor (Roche Diagnostics, Indianapolis, Indiana). The lung homogenates were gently shaken for 12 hours at 4°C and were centrifuged at 15,000g for 60 minutes to remove debris. The clear upper supernatant fluid was used for the Sircol assay (Biocolor Ltd, Carrickfergus, England) according to the manufacturer’s instructions, and optical density readings were performed at 540 nm. Results are expressed as micrograms of collagen per milligram of total protein, determined using the Bradford protein assay (Bio-Rad Laboratories, Hercules, California).
Spleen Cell Culture
Immediately after time-integrated change in peak airway pressure (APTI) measurements (BALB/c mice), pooled spleen cells from 4 or 5 mice were isolated and cultured with ovalbumin (200 μg/mL), concanavalin A (2.5 mg/mL, positive control), or medium alone for 72 hours as previously described.9 Levels of interleukin 4 (IL-4), IL-5, IL-13, and interferon gamma (IFN-γ) in spleen culture supernatants (in duplicate or triplicate) were determined by means of enzymelinked immunosorbent assay (R&D Systems, Minneapolis, Minnesota).
Statistical Analysis
Statistical analyses were performed using the t test for comparisons between 2 groups. Pearson correlation coefficients were used to determine correlation values. A P <.05 was considered statistically significant. All statistical analyses were performed using a software program (SigmaPlot/Stat; Systat Software Inc, Point Richmond, California).
RESULTS
Selection of Individual Herbs for Preparation of ASHMI
We first determined the effect of the individual herbs found in MSSM-002 on APTI and BALF eosinophil percentage in antigen-sensitized and antigen-challenged young mice. No individual herb alone had the same suppressive effect as the MSSM-002 preparation on APTI and eosinophil lung infiltration. However, some individual herbs were more effective than were others in decreasing AHR, and some were more effective in attenuating airway inflammation. Other herbs had little or no effect on either variable (Table E2, Online Repository). To prepare ASHMI, we selected 3 herbs at specific percentages of dry weight: Ling-Zhi (Ganoderma lucidum, 62.5%); Ku-Shen (radix Sophora flavescentis, 28.1%), which had higher percentage reductions of APTI and BALF eosinophilia; and Gan-Cao (radix Glycyrrhiza uralensis, 9.4%), which is included in many TCM formulas at low doses as a detoxifier. 11,18 Next, APTI and BALF eosinophilia were determined in AKR mice that were not treated or that were treated with ASHMI. Treatment with ASHMI caused a comparable reduction in APTI and BALF eosinophils as MSSM-002 use (Table E2, Online Repository).
Reduction of AHR in ASHMI-Treated Aged Ovalbumin Mice
The APTI values were significantly higher in all nontreated antigen-sensitized and antigen-challenged mice compared with age-matched naive controls, indicating induction of AHR (Fig 2). Mean (SEM) APTIs were greater in 6-week-old nontreated ovalbumin mice (725.9 [87.58] cm H20/sec) than in each group of aged nontreated ovalbumin mice (6 months old: 330.0 [36.46] cm H20/s, P=.009; 12 months old: 356.1 [50.47] cm H20/s, P=.009; and 18 months old: 318.4 [33.1] cm H20/s, P=.004). There were no significant differences in APTI between naive groups. Treatment with ASHMI significantly reduced the APTI in all aged and young ovalbumin mice compared with age-matched nontreated ovalbumin mice. The effect was most pronounced in 6-week-old mice.
Figure 2.
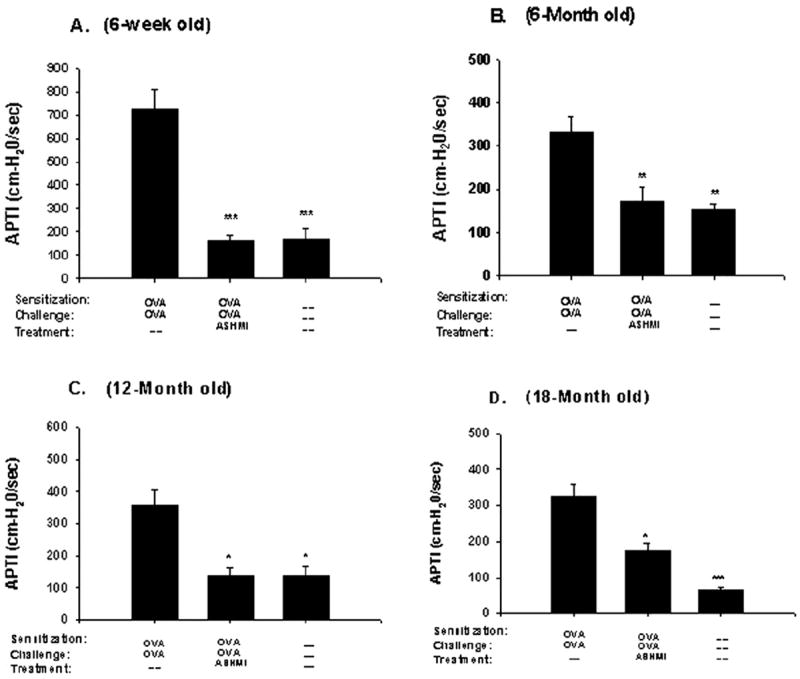
Effect of antiasthma simplified herbal medicine intervention (ASHMI) on antigen-induced airway hyperresponsiveness in mice aged 6 weeks (A) 6 months (B), 12 months (C), and 18 months (D). Three days after the last antigen challenge, the airway reactivity of mice in each group was determined by measuring the airway pressure change after acetylcholine challenge. The time-integrated changes in peak airway pressure (APTI) were calculated. Data are given as mean (SEM) for each group. *P<.05, †P<.01, ‡P<.001 compared with age-matched nontreated ovalbumin mice.
ASHMI Treatment Reduces Antigen-Induced Pulmonary Inflammation
To determine whether AHR suppression was associated with reduced airway inflammation, we measured total BALF cell numbers and percentages of eosinophils immediately after APTI. All the age groups of nontreated ovalbumin mice developed significantly (P<.001) elevated mean total BALF leukocyte numbers compared with age-matched naive mice (Fig 3A-D). Mean (SEM) BALF leukocyte numbers were increased significantly in all aged nontreated ovalbumin mice (6 months old: 110.16 [6.013] × 104, P<.01; 12 months old: 113.19 [3.46] × 104, P<.001; and 18 months old: 116.32 [5.34] × 104, P<.01) compared with 6-week-old nontreated ovalbumin mice (80.24 [4.4] × 104). Use of ASHMI significantly decreased the leukocyte count in all ovalbumin mice age groups compared with age-matched nontreated ovalbumin mice (Fig 3A-D). There were no significant differences in total BALF leukocyte counts between ASHMI-treated ovalbumin mice compared with age-matched naive mice for all ages. There were no significant differences in total cell counts among all ages of naive groups.
Figure 3.
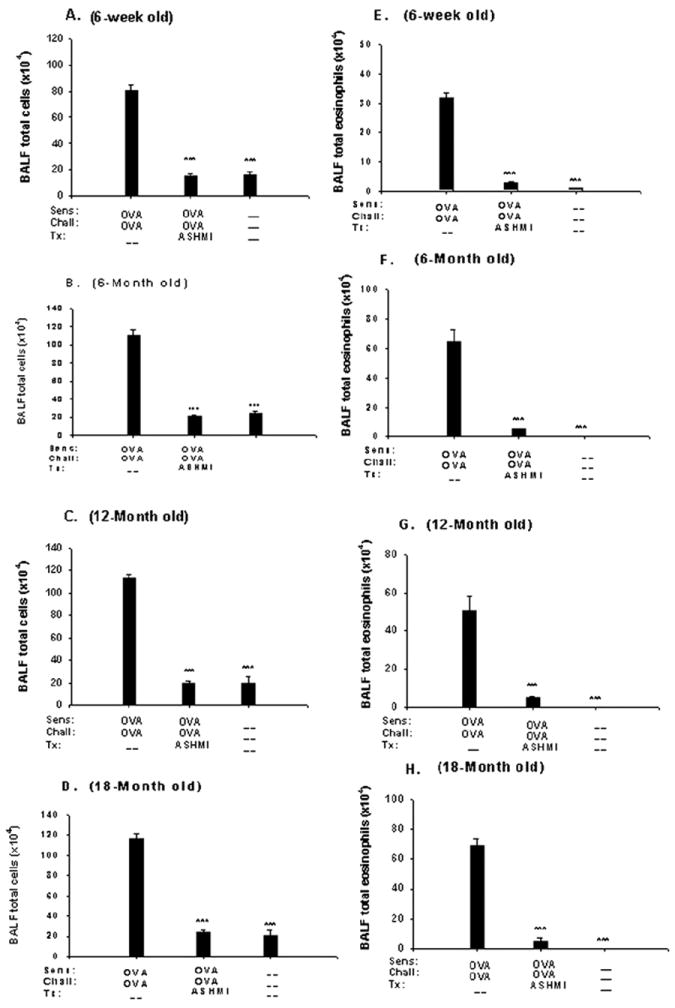
Effect of antiasthma simplified herbal medicine intervention (ASHMI) on bronchoalveolar lavage fluid (BALF) total cell counts (A-D) and eosinophil numbers (E-H). After airway response measurement, mice were humanely killed, and the lungs were lavaged. Total leukocytes were counted, and BALF was cytocentrifuged on slides, fixed, and stained for leukocyte differential. A-D, Mean BALF total cell counts in nontreated ovalbumin (OVA) mice, ASHMI-treated OVA mice, and naive mice aged 6 weeks (A), 6 months (B), 12 months (C), and 18 months (D). E-H, Mean BALF absolute eosinophil counts in the same mouse groups aged 6 weeks (E), 6 months (F), 12 months (G), and 18 months (H). Error bars represent SEM. *P<.05, **P<.01, ***P<.001 compared with age-matched nontreated OVA mice.
Mean BALF eosinophil numbers were significantly elevated in all nontreated ovalbumin mice compared with their age-matched naive controls (P<.001) (Fig 3E-H). Mean (SEM) eosinophil numbers were significantly elevated in the 6-, 12-, and 18-month-old nontreated ovalbumin mice (64.32 [8.66] × 104, P<.001; 50.63 [7.2] × 104, P<.01; and 69.38 [4.8] × 104, P<.001, respectively) compared with the nontreated 6-week-old ovalbumin mice (31.57 [1.86] × 104). Use of ASHMI in all the ovalbumin mice significantly decreased BALF eosinophil numbers compared with those in age-matched nontreated ovalbumin mice (Fig 3E-H).
To correlate BALF total cell counts with lung tissue inflammation, lung sections collected 72 hours after the final ovalbumin challenge were stained with hematoxylin-eosin. All nontreated ovalbumin mice demonstrated peribronchial and perivascular inflammation, not seen in naive control mice. Mean (SEM) perivascular and peribronchial inflammation grades, respectively, were greatest in aged ovalbumin mice (6 months old: 2.12 [0.07], P<.001, and 1.89 [0.16], P<.01; 12 months old: 1.67 [0.11], P<.01, and 1.62 [0.18], P=.10; and 18 months old: 1.98 [0.19], P<.001, and 1.87 [0.19], P<.01) compared with 6-week-old ovalbumin mice (01.18 [0.09] and 1.43 [0.09]). Treatment with ASHMI of ovalbumin mice significantly decreased perivascular lung tissue inflammation in the 6-week-old, 6-month-old, and 18- month-old ovalbumin mice (Fig 4). Perivascular inflammation was decreased with ASHMI in 12-month-old ovalbumin mice, but not significantly (Fig 4). Treatment with ASHMI significantly decreased peribronchial inflammation in all age groups of ovalbumin mice, but not to the extent in 18-monthold ovalbumin mice.
Figure 4.
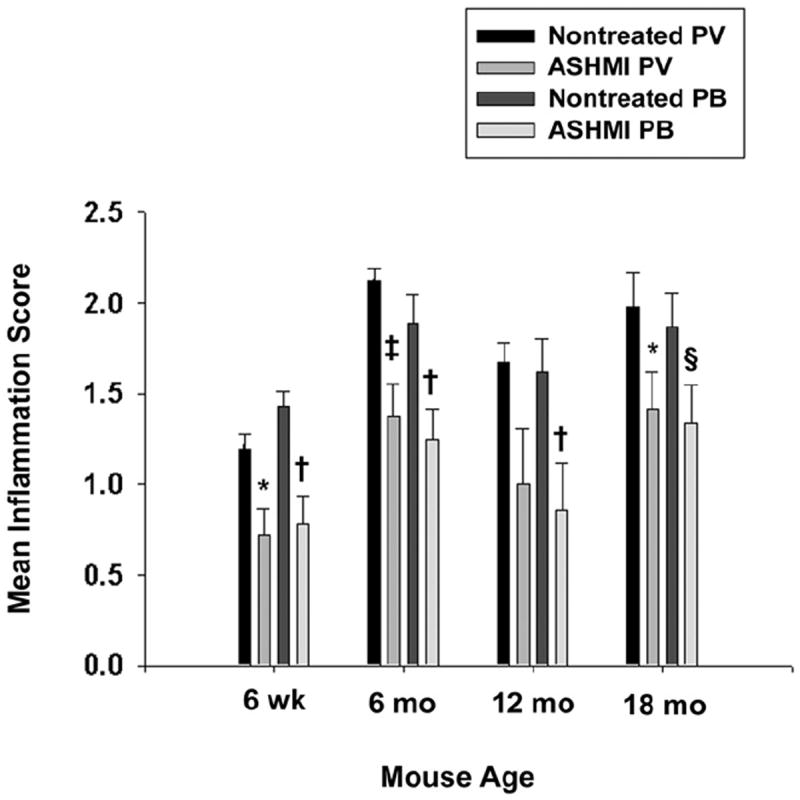
Effect of antiasthma simplified herbal medicine intervention (ASHMI) on pulmonary inflammation. Perivascular (PV) and peribronchial (PB) inflammation of lung tissues stained with hematoxylin-eosin was graded as described in the “Methods” section of the article. Error bars represent SEM. *P<0.05 (PV), ‡P<0.01 (PV), §P<0.05 (PB), †P<0.01 (PB) compared with age-matched nontreated ovalbumin mice.
Mucous Cell Metaplasia Was Suppressed by ASHMI Treatment
We previously reported that TCM treatment of young antigen-sensitized and antigen-challenged AKR/J mice decreases mucous cell metaplasia, as determined by periodic acid–Schiff (PAS) staining.9 In the present study, numbers of positively staining PAS epithelial cells in bronchioles were significantly increased in all nontreated ovalbumin mice compared with naive mice, in which there was no PAS epithelial cell staining. There was significantly increased mean (SEM) PAS staining in the airways of 12- month-old nontreated ovalbumin mice (82.05% [1.29%]) compared with 6-week-old nontreated ovalbumin mice (61.14% [3.40%]) (P<.05). There were no significant differences in PAS staining among other aged mice (6 months old: 62.64% [3.4%] (P=.87) and 18 months old: 71.33±5.18% [5.18%]) (P=.32) and 6-week-old nontreated ovalbumin mice. Treatment with ASHMI significantly decreased mucous cell numbers in all age groups (Fig 5A). We performed real-time PCR analysis of expression of the major airway mucus-producing gene MUC5AC19 in lung tissue. Results are expressed as the fold change in messenger RNA (mRNA) copy number of MUC5AC in ovalbumin mice compared with age-matched naive controls. MUC5AC mRNA expression was significantly increased in the lungs of 12- and 18-month-old nontreated ovalbumin mice (P=.003 and P=.02, respectively) compared with young nontreated ovalbumin mice. Treatment with ASHMI significantly decreased PAS mRNA expression in all age groups of ovalbumin mice (Fig 5B). Figure 5C-H shows representative histologic analyses from the different groups: naive mice, nontreated ovalbumin mice, and ASHMI-treated ovalbumin mice. The PAS staining was increased in lungs from mice of both ages in the nontreated ovalbumin groups, which was prevented by ASHM.
Figure 5.
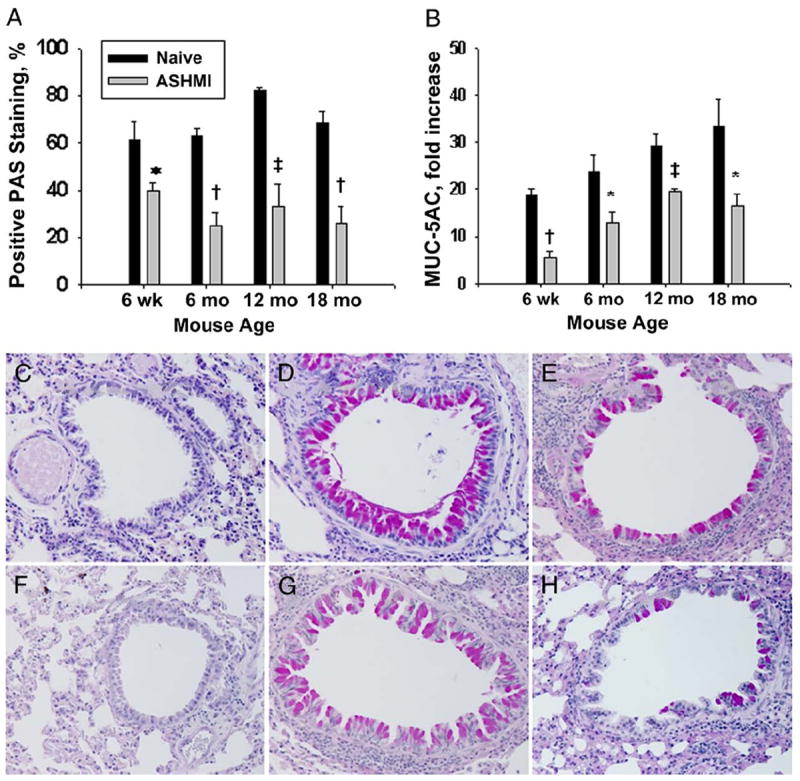
Goblet cell metaplasia. A, Mean left lower lung periodic acid–Schiff (PAS) grading for ASHMI ovalbumin mice and age-matched naive mice. B, Quantitative polymerase chain reaction for MUC5AC expressed as mean fold increase in copy number. Error bars represent SEM. *P<.05, †P<.001, ‡P<.01 compared with age-matched nontreated OVA mice. C-H, The PAS-positive (deep purple) staining in 6-week-old naïve mice (C), 6-week-old naïve mice (D), 6-week-old antiasthma simplified herbal medicine intervention (ASHMI) ovalbumin mice (E), 18-month-old naive mice (F), 18-month-old untreated OVA mice (G), and 18-month-old ASHMI ovalbumin mice (H).
ASHMI Treatment Reduced Antigen Specific IgE Levels in OVA Mice of All Ages
To assess the degree of sensitization in ovalbumin mice and to determine whether ASHMI alters sensitization, we measured ovalbumin specific serum IgE. All the mice developed significantly elevated levels of serum specific IgE to ovalbumin in response to ovalbumin sensitization and challenge (Fig 6). Aged ovalbumin mice exhibited significantly higher levels of ovalbumin specific serum IgE than did 6-week-old ovalbumin mice (Fig 6). Ovalbumin specific IgE was undetectable in all naive mice. Use of ASHMI significantly decreased ovalbumin specific IgE levels in all ages of ovalbumin mice (Fig. 6)
Figure 6.
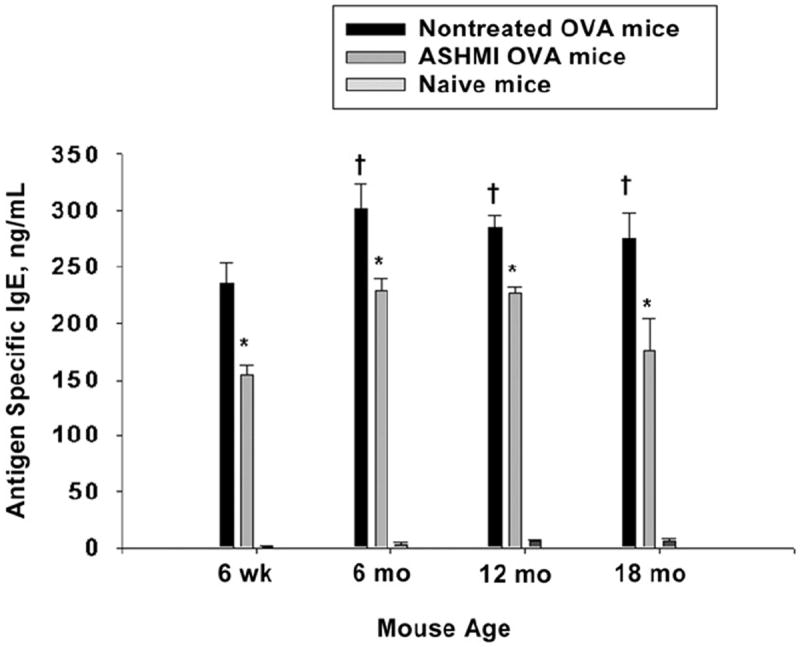
Mean serum ovalbumin (OVA) specific IgE levels determined by means of enzyme-linked immunosorbent assay from sera collected 72 hours after the final OVA challenge. Error bars represent SEM. *P<.05 compared with age-matched nontreated OVA mice. †P<.05 compared with 6-week-old nontreated OVA mice.
ASHMI Treatment Decreased Lung Collagen Formation
Several murine models of asthma and studies in patients with asthma have demonstrated increased airway collagen deposition.20,21 We determined, using a colormetric assay, the relative amounts of collagen in the experimental groups. Collagen synthesis was increased in all nontreated ovalbumin mice compared with naive age-matched mice but significantly only in 6-week-old (P=.009), 6-month-old (P<.05), and 18-month-old (P<.001) mice (Fig 7). Use of ASHMI reduced collagen synthesis in all the ovalbumin mice; however, this reduction reached statistical significance only in 6-week-old and 18-month-old mice (Fig 7).
Figure 7.
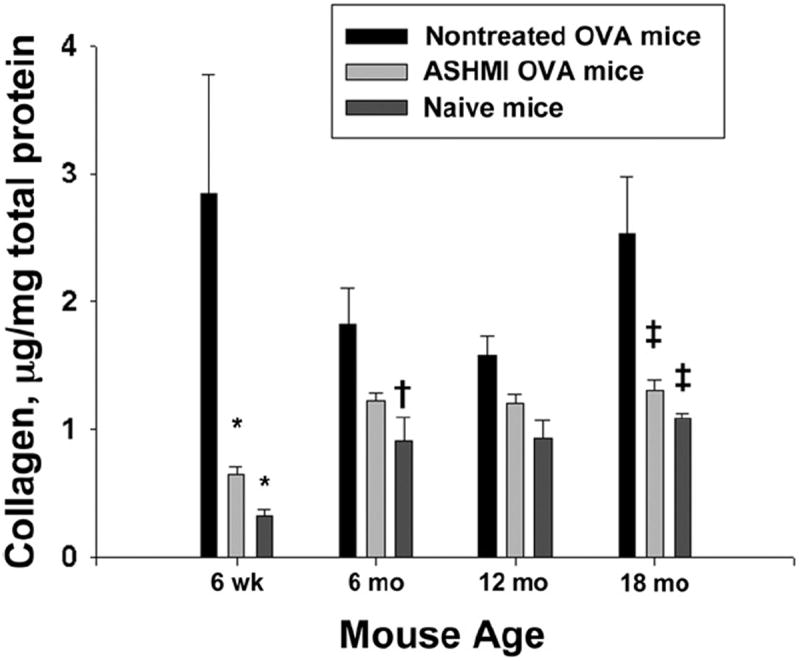
Mean collagen deposition in the lungs measured quantitatively and normalized to total protein in the lung for nontreated and antiasthma simplified herbal medicine intervention (ASHMI)-treated ovalbumin (OVA) mice and age-matched naive mice. Error bars represent SEM. †P<0.05, *P<0.01, ‡P<0.001 compared with age-matched nontreated OVA mice.
Lung and Systemic Cytokine Production
The Center for Chinese Herbal Therapy for Allergy and Asthma, Pediatric Allergy and Immunology, Mount Sinai School of Medicine previously demonstrated in young ovalbumin mice that ASHMI reduction of lung inflammation is due, at least in part, to reduced lung and systemic TH2 cytokine production and increased IFN-γ production.22a To determine whether ASHMI treatment of aged ovalbumin mice altered cytokine expression, we measured IL-4, IL-5, IL-13, and IFN-γ mRNA expression in lung tissue using qPCR. (Results are expressed in fold increase in mRNA copy number from ovalbumin mice compared with mRNA copy number from age-matched naive mice.) Treatment with ASHMI significantly decreased IL-4, IL-5, and IL-13 expression in 6-week-old ovalbumin mice compared with nontreated age-matched ovalbumin mice (Fig 8A-C). Use of ASHMI also significantly reduced the expression of IL-4 in 12-month-old (P<.01), IL-5 in all ages (P<.01), and IL-13 in 6-month-old (P<.01) ovalbumin mice (Fig 8A-C). Expression of INF-γ was increased in all ages of ovalbumin mice treated with ASHMI, but it reached statistical significance only in 6-month-old ovalbumin mice (Fig 8D).
Figure 8.
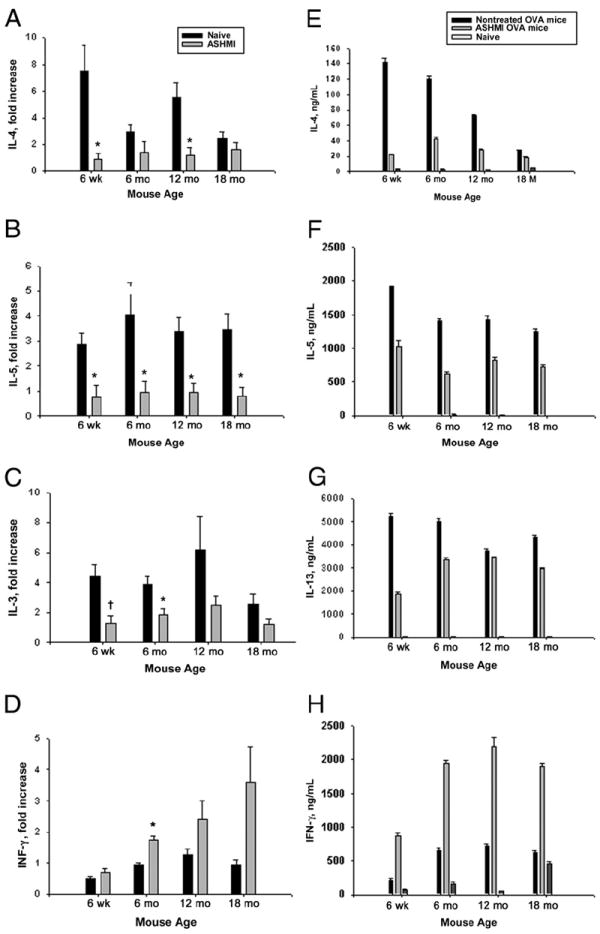
Pulmonary tissue and spleen cell culture cytokines. A-D, Quantitative polymerase chain reaction for interleukin 4 (IL-4) (A), IL-5 (B), IL-13 (C), and interferon gamma (IFN-γ) (D) from lung tissue from young and aged mice. Results are expressed as mean fold increase in copy number compared with age-matched naive mice. E-H, Pooled spleen cells from 4 mice per group were isolated and cultured in complete medium in the presence of ovalbumin, concanavalin A (data not shown), or medium alone (data not shown). Supernatants were collected after 72 hours of culture. Levels of IL-4 (E), IL-5 (F), IL-13 (G), and IFN-γ (H) were determined by means of enzyme-linked immunosorbent assay. Results are mean values for duplicate or triplicate assays. Error bars represent SEM. *P<.01, †P<.05, compared with age-matched nontreated ovalbumin mice.
To examine systemic cytokine protein levels, we stimulated pooled spleen cells with ovalbumin. Levels of cytokines IL-4, IL-5, IL-13, and IFN-γ were elevated in all nontreated ovalbumin mice compared with age-matched naive controls (Fig 8E-H). Treatment with ASHMI had the greatest effect on IL-4 protein expression in spleen cell culture. Levels of IL-4 were decreased approximately 85%, 65%, 60%, and 35% in 6-week-old and 6-, 12-, and 18-month-old ovalbumin mice, respectively (Fig 8E). The IL-5 level was reduced approximately 40% to 50% in all full-dose ovalbumin mice compared with cultures from nontreated ovalbumin mice (Fig 8F). Use of ASHMI reduced IL-13 levels in spleen cell cultures approximately 64% in 6-week-old ovalbumin mice and approximately 30% in 6- and 18-month-old ovalbumin mice (Fig 8G). The IL-13 spleen cell levels were decreased by less than 10% in 12-month-old ovalbumin mice treated with ASHMI compared with nontreated ovalbumin controls (Fig 8G). Spleen cell culture IFN-γ levels were increased in nontreated aged ovalbumin mice compared with in 6-weekold nontreated ovalbumin mice (Fig 8H). Similar to previous data, treatment with ASHMI increased IFN-γ expression by spleen cells in 6-week-old ovalbumin mice, and in this study, we noted that it also increased it in aged ovalbumin mice (Fig 8H). Concanavalin A stimulation (positive control) of splenocyte cultures produced increased expression of all the cytokines (data not shown), and culture in media alone (negative control) produced minimal cytokine expression (data not shown).
DISCUSSION
Asthma treatment of older patients is not widely investigated. Asthma is a heterogeneous disease that typically presents in the pediatric and young adult populations; however, it can affect individuals of all ages. Corticosteroids are the mainstay of treatment for persistent disease.22 When administered systemically or in high doses via inhalation, corticosteroids may produce several adverse effects. These effects may be particularly detrimental for older patients and include accelerated bone loss and osteoporosis, an increased risk of fracture, and glucose intolerance.23-25 In addition, the inhalation technique may be more challenging for older patients.26,27 Therefore, the development of corticosteroid-sparing medications, in a form that is easy to administer, for the prevention or treatment of asthma in older patients is critical, especially as the population ages.
We originally developed a novel formula for asthma, MSSM-002. This compound was an extract of 14 herbs that nearly eliminated AHR, significantly reduced BALF total cell numbers and eosinophil percentages and antigen specific IgE levels, and inhibited mucous cell hyperplasia of the lungs comparable with corticosteroid treatment in a murine model of allergic asthma using 6-week-old mice.9 To simplify this formula, we tested the effects of individual herbs in MSSM- 002 on the reduction of AHR and BALF eosinophilia and found that none alone had effects comparable with MSSM- 002. However, a combination of 3 herbs (Ling-Zhi, Ku-Shen, and Gan-Cao), named ASHMI, decreased AHR and BALF eosinophilia similar to MSSM-002.
ASHMI has an excellent safety profile; administration of 12 times the dose did not produce any abnormalities in the liver, kidneys, and complete blood cell counts of mice (Wen et al, unpublished data). In a study28 conducted in China, asthmatic adults (mean age, 44.6 years) treated with ASHMI exhibited improved asthma symptom scores and forced expiratory volume in 1 second and decreased serum IgE levels and peripheral blood eosinophil numbers similar to prednisone. A subsequent dose-escalation phase 1 trial12 in the United States in patients with asthma (aged 18-40 years) using ASHMI demonstrated an excellent safety profile. These trials, however, did not address the use of ASHMI in older asthmatic patients.
In the present study, we demonstrated that treatment of young and aged mice with 10 mg of ASHM daily before and during antigen sensitization and challenge prevented several features of allergic airway inflammation (AHR, increased total BALF cell counts and eosinophil percentages, mucous cell metaplasia, and lung tissue inflammation). In addition, treatment with ASHMI decreased the level of antigen specific IgE. We previously characterized a murine model of allergic asthma in aged mice.6 Antigen sensitization and airway challenge of aged BALB/c mice increased airway inflammation (BALF total cell number and eosinophil percentage, hematoxylin-eosin staining, and mucous cell metaplasia) compared with similarly treated 6-week-old mice. The aged antigensensitized/challenged mice also developed AHR after acetylcholine challenge. However, despite greater airway inflammation in aged mice compared with 6-week-old mice, AHR was significantly higher in the latter. The reasons for the discord between increased airway inflammation in aged antigen-treated mice and AHR are not entirely clear but may include alteration in eosinophil29 or smooth airway function with age. Use of ASHMI effectively prevented AHR in all ages of ovalbumin mice. There are several potential mechanisms by which ASHMI reduces AHR. It decreased BALF total cell numbers to the level of age-matched naive mice and significantly reduced BALF eosinophil numbers in all age groups. Furthermore, ASHMI diminished the inflammation score on airway histologic analysis. However, the potential differences in inflammatory cell function with increasing age need to be considered. Furthermore, although a causal relationship has been proposed between airway inflammation, specifically, eosinophilia, and AHR in asthma, this hypothesis is controversial.30-32
Treatment with ASHMI also prevented several other pathologic features of the asthmatic airway that may increase AHR. Use of ASHMI reduced airway mucous cell metaplasia, measured by means of PAS staining, and expression of the major mucin gene MUC5AC. The effect of mucus hypersecretion on AHR is not clearly established33,34 and may differ in an aged population. In addition, ASHMI treatment decreased lung collagen deposition to near naive levels. Increased airway collagen deposition, a component of airway remodeling, may affect AHR.35
Administration of ASHMI lowers serum antigen specific IgE levels. IgE mediates the allergic response by triggering mast cell and basophil degranulation after antigen exposure. Therefore, reduction in antigen specific IgE levels may attenuate AHR. However, a direct relationship between AHR and IgE is not straightforward. The use of anti-IgE for asthma has greater effects on the reduction of asthma symptoms and rescue medication/corticosteroid use than on airway function. 36
The mechanisms for the anti-inflammatory properties of ASHMI are not completely understood but involve TH2 suppression. We previously demonstrated that the original 14- herb TCM preparation MSSM-002 and the present simplified formula ASHMI suppress TH2 responses9,16 and the TH2 transcription factor GATA-3.37 Corticosteroid treatment also decreases the TH2 response. However, the TCM formulation, unlike corticosteroids, increases IFN-γ expression, which may play a role in its protective airway effects.9,16 In this study, ASHMI suppressed mRNA expression in the lung tissue of the TH2 cytokines IL-4, IL-5, and IL-13 and its systemic level from spleen cell culture. Similar to previous data, ASHMI treatment increased IFN-γ expression, which may attenuate AHR.
We administered ASHMI to mice before antigen exposure, suggesting that it may prevent asthma in aged people. We recently showed that ASHMI reversed the established allergic airway response in young adult mice and that this effect is long lasting (Srivastava et al manuscript submitted). Investigation of ASHMI in established asthma in aged antigensensitized mice is under way. In conclusion, ASHMI treatment of young and aged murine models of allergic asthma inhibited the development of several key features of antigeninduced airway inflammation, including antigen-induced AHR, mucous cell metaplasia, increased lung inflammation, and BALF total cell number and eosinophilia.
Supplementary Material
Acknowledgments
We thank the real-time PCR core facility at Mount Sinai Medical School for processing our samples. We thank Dr. Hugh Sampson for his support for this study and Janette Birmingham and Chi-Ken Huang for technical support.
Footnotes
SUPPLEMENTARY DATA
Additional photos and tables accompany the on-line version of this article (available at http://www.annallergy.org).
Disclosures: US Patent Application PCT/US05/08600 for ASHMI has been filed. Dr. Xiu-Min Li and Dr. Ming-Chun Wen are patentholders.
References
- 1.Broder I, Higgins MW, Mathews KP, Keller JB. Epidemiology of asthma and allergic rhinitis in a total community, Tecumseh, Michigan, 3: second survey of the community. J Allergy Clin Immunol. 1974;53:127–138. doi: 10.1016/0091-6749(74)90001-3. [DOI] [PubMed] [Google Scholar]
- 2.Hizawa N, Yamaguchi E, Konno S, Tanino Y, Jinushi E, Nishimura M. A functional polymorphism in the RANTES gene promoter is associated with the development of late-onset asthma. Am J Respir Crit Care Med. 2002;166:686–690. doi: 10.1164/rccm.200202-090OC. [DOI] [PubMed] [Google Scholar]
- 3.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113:101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 4.Ariano R, Panzani RC, Augeri G. Late onset asthma clinical and immunological data: importance of allergy. J Investig Allergol Clin Immunol. 1998;8:35–41. [PubMed] [Google Scholar]
- 5.Quadrelli SA, Roncoroni AJ. Is asthma in the elderly really different? Respiration. 1998;65:347–353. doi: 10.1159/000029294. [DOI] [PubMed] [Google Scholar]
- 6.Busse PJ, Zhang TF, Srivastava K, Schofield B, Li XM. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin Exp Allergy. 2007;37:1392–1403. doi: 10.1111/j.1365-2222.2007.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein DI, Allen DB. Evaluation of tests of hypothalamic-pituitary-adrenal axis function used to measure effects of inhaled corticosteroids. Ann Allergy Asthma Immunol. 2007;98:118–127. doi: 10.1016/S1081-1206(10)60683-7. [DOI] [PubMed] [Google Scholar]
- 8.Deeks ED, Perry CM. Ciclesonide: a review of its use in the management of asthma. Drugs. 2008;68:1741–1770. doi: 10.2165/00003495-200868120-00010. [DOI] [PubMed] [Google Scholar]
- 9.Li XM, Huang CK, Zhang TF, et al. The Chinese herbal medicine formula MSSM-002 suppresses allergic airway hyperreactivity and modulates TH1/TH2 responses in a murine model of allergic asthma. J Allergy Clin Immunol. 2000;106:660–668. doi: 10.1067/mai.2000.110102. [DOI] [PubMed] [Google Scholar]
- 10.Braman SS. Drug treatment of asthma in the elderly. Drugs. 1996;51:415–423. doi: 10.2165/00003495-199651030-00006. [DOI] [PubMed] [Google Scholar]
- 11.Bensky D, Barolet R. Chinese Herbal Medicine: Formulas and Strategies. Seattle, WA: Eastland Press; 1990. [Google Scholar]
- 12.Kelly-Pieper K, Patil SP, Busse P, et al. Safety and tolerability of an antiasthma herbal formula (ASHMI) in adult subjects with asthma: a randomized, double-blinded, placebo-controlled, dose-escalation phase I study. J Altern Complement Med. 2009;15:735–743. doi: 10.1089/acm.2008.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pharmacopoeia of the People’s Republic of China. 6. Beijing, China: People’s Medical Publishing House; 2005. State Pharmacopoeia Commission of the People’s Republic of China. [Google Scholar]
- 14.Center for Drug Evaluation and Research. Guidance for Industry Botanical Drug Products. Rockville, MD: US Food and Drug Administration; 2004. Revised ed. [Google Scholar]
- 15.Eyles JE, Spiers ID, Williamson ED, Alpar HO. Tissue distribution of radioactivity following intranasal administration of radioactive microspheres. J Pharm Pharmacol. 2001;53:601–607. doi: 10.1211/0022357011775929. [DOI] [PubMed] [Google Scholar]
- 16.Busse PJ, Zhang TF, Srivastava K, et al. Chronic exposure to TNF-α increases airway mucus gene expression in vivo. J Allergy Clin Immunol. 2005;116:1256–1263. doi: 10.1016/j.jaci.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 17.Xiu S. The Experimental Method of Pharmacology. Beijing, China: The People’s Public Health Publisher; 1986. [Google Scholar]
- 18.Wagner H, Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16:97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Zuhdi Alimam M, Piazza FM, et al. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am J Respir Cell Mol Biol. 2000;22:253–260. doi: 10.1165/ajrcmb.22.3.3768. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee DK, Lee GS, Malik SK, Daly S. Underdiagnosis of asthma in the elderly. Br J Dis Chest. 1987;81:23–29. doi: 10.1016/0007-0971(87)90104-5. [DOI] [PubMed] [Google Scholar]
- 21.Boulet LP, Turcotte H, Laviolette M, et al. Airway hyperresponsiveness, inflammation, and subepithelial collagen deposition in recently diagnosed versus long-standing mild asthma: influence of inhaled corticosteroids. Am J Respir Crit Care Med. 2000;162:1308–1313. doi: 10.1164/ajrccm.162.4.9910051. [DOI] [PubMed] [Google Scholar]
- 22.National Heart, Lung, and Blood Institute. Expert Panel Report 3: Guidelines for the Diangosis and Management of Asthma 2007. Bethesda, MD: National Institutes of Health; Aug, 2007. NIH publication 07-4051. [Google Scholar]
- 22a.Zhang T, Srivastava K, Wen MC, et al. Pharmacology and immunological actions of a herbal medicine ASHMI(TM) on allergic asthma. Phytother Res. 2009 Dec 8; doi: 10.1002/ptr.3077. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newnham DM. Asthma medications and their potential adverse effects in the elderly: recommendations for prescribing. Drug Saf. 2001;24:1065–1080. doi: 10.2165/00002018-200124140-00005. [DOI] [PubMed] [Google Scholar]
- 24.de Vries F, van Staa TP, Bracke MS, Cooper C, Leufkens HG, Lammers JW. Severity of obstructive airway disease and risk of osteoporotic fracture. Eur Respir J. 2005;25:879–884. doi: 10.1183/09031936.05.00058204. [DOI] [PubMed] [Google Scholar]
- 25.Busse PJ, Kilaru K. Complexities of diagnosis and treatment of allergic respiratory disease in the elderly. Drugs Aging. 2009;26:1–22. doi: 10.2165/0002512-200926010-00001. [DOI] [PubMed] [Google Scholar]
- 26.Allen SC, Jain M, Ragab S, Malik N. Acquisition and short-term retention of inhaler techniques require intact executive function in elderly subjects. Age Ageing. 2003;32:299–302. doi: 10.1093/ageing/32.3.299. [DOI] [PubMed] [Google Scholar]
- 27.Armitage JM, Williams SJ. Inhaler technique in the elderly. Age Ageing. 1988;17:275–278. doi: 10.1093/ageing/17.4.275. [DOI] [PubMed] [Google Scholar]
- 28.Wen MC, Wei CH, Hu ZQ, et al. Efficacy and tolerability of anti-asthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J Allergy Clin Immunol. 2005;116:517–524. doi: 10.1016/j.jaci.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 29.Mathur SK, Schwantes EA, Jarjour NN, Busse WW. Age-related changes in eosinophil function in human subjects. Chest. 2008;133:412–419. doi: 10.1378/chest.07-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brusasco V, Crimi E, Pellegrino R. Airway hyperresponsiveness in asthma: not just a matter of airway inflammation. Thorax. 1998;53:992–998. doi: 10.1136/thx.53.11.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flood-Page P, Menzies-Gow A, Phipps S, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkayya R, Lam M, Willkom M, Grunig G, Corry DB, Erle DJ. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am J Respir Cell Mol Biol. 2002;26:202–208. doi: 10.1165/ajrcmb.26.2.4600. [DOI] [PubMed] [Google Scholar]
- 33.Bai TR, Knight DA. Structural changes in the airways in asthma: observations and consequences. Clin Sci (Lond) 2005;108:463–477. doi: 10.1042/CS20040342. [DOI] [PubMed] [Google Scholar]
- 34.Wu CA, Puddington L, Whiteley HE, et al. Murine cytomegalovirus infection alters Th1/Th2 cytokine expression, decreases airway eosinophilia, and enhances mucus production in allergic airway disease. J Immunol. 2001;167:2798–807. doi: 10.4049/jimmunol.167.5.2798. [DOI] [PubMed] [Google Scholar]
- 35.Simoes DC, Xanthou G, Petrochilou K, Panoutsakopoulou V, Roussos C, Gratziou C. Osteopontin deficiency protects against airway remodeling and hyperresponsiveness in chronic asthma. Am J Respir Crit Care Med. 2009;179:894–902. doi: 10.1164/rccm.200807-1081OC. [DOI] [PubMed] [Google Scholar]
- 36.Busse WW, Massanari M, Kianifard F, Geba GP. Effect of omalizumab on the need for rescue systemic corticosteroid treatment in patients with moderate-to-severe persistent IgE-mediated allergic asthma: a pooled analysis. Curr Med Res Opin. 2007;23:2379–2386. doi: 10.1185/030079907X226258. [DOI] [PubMed] [Google Scholar]
- 37.Busse PJ, Wen MC, Huang CK, et al. Therapuetic effects of the Chinese herbal formula, MSSM-003d, on persistent airway hyperresponsiveness and airway remodeling. J Allergy Clin Immunol. 2004;113:S220. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


