Abstract
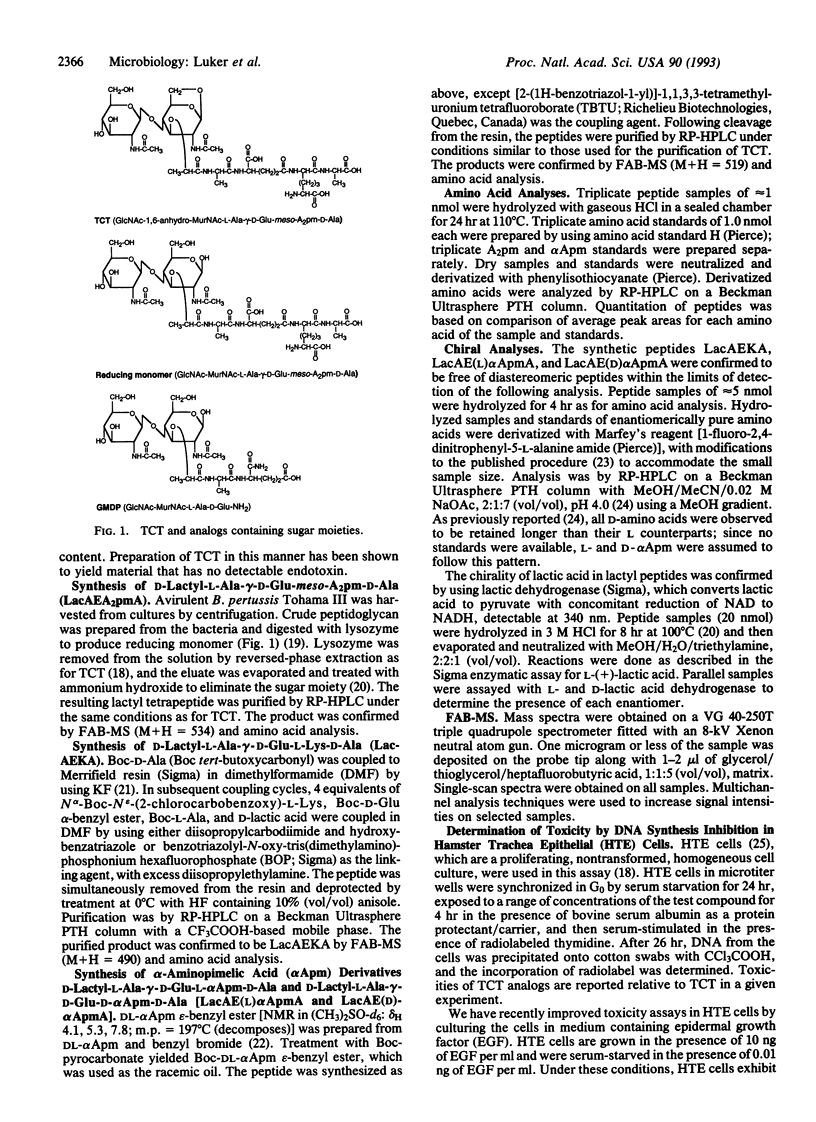
Tracheal cytotoxin (TCT) is a disaccharide-tetrapeptide released by Bordetella pertussis, the causative agent of pertussis (whooping cough). We have previously determined the structure of TCT to be GlcNAc-1,6-anhydro-MurNAc-L-Ala-gamma-D-Glu-meso-A2pm-D-Ala, where MurNAc = N-acetylmuramic acid and A2pm = diaminopimelic acid. Purified TCT reproduces the respiratory cytopathology observed during pertussis, including ciliostasis and extrusion of ciliated cells. We have tested structural analogs of TCT for their ability to reproduce native TCT toxicity in explanted hamster tracheal tissue and hamster trachea epithelial (HTE) cell cultures. Other investigators have evaluated many of these analogs, which are muramyl or desmuramyl peptides, for muramyl peptide activities such as immunopotentiation, induction of slow-wave sleep, and pyrogenicity. Four desmuramyl peptides were produced in our laboratory from B. pertussis peptidoglycan or by chemical synthesis, including unusual peptides containing alpha-aminopimelic acid in place of A2pm. Based on the relative ability of compounds to inhibit DNA synthesis in HTE cells, truncated analogs lacking A2pm entirely or lacking only the side-chain amine or carboxyl group of A2pm were less active than TCT by a factor of at least 1000. All active analogs included a native or near-native peptide moiety, independent of the presence, absence, or substitution of the sugar moiety. We conclude that the structural requirements for TCT toxicity differ considerably from those for most other muramyl peptide activities, in that the disaccharide moiety is irrelevant for toxicity and both the free amino and carboxyl groups of the A2pm side chain are required for activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Lederer E. Muramyl peptides: immunomodulators, sleep factors, and vitamins. Med Res Rev. 1984 Apr-Jun;4(2):111–152. doi: 10.1002/med.2610040202. [DOI] [PubMed] [Google Scholar]
- Ahmed K., Turk J. L. Effect of anticancer agents neothramycin, aclacinomycin, FK-565 and FK-156 on the release of interleukin-2 and interleukin-1 in vitro. Cancer Immunol Immunother. 1989;28(2):87–92. doi: 10.1007/BF00199107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr G. M., Chedid L. A., Behbehani K. Induction, in vivo and in vitro, of macrophage membrane interleukin-1 by adjuvant-active synthetic muramyl peptides. Cell Immunol. 1987 Jul;107(2):443–454. doi: 10.1016/0008-8749(87)90251-6. [DOI] [PubMed] [Google Scholar]
- Chedid L. Muramyl peptides as possible endogenous immunopharmacological mediators. Microbiol Immunol. 1983;27(9):723–732. doi: 10.1111/j.1348-0421.1983.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Cookson B. T., Cho H. L., Herwaldt L. A., Goldman W. E. Biological activities and chemical composition of purified tracheal cytotoxin of Bordetella pertussis. Infect Immun. 1989 Jul;57(7):2223–2229. doi: 10.1128/iai.57.7.2223-2229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson B. T., Tyler A. N., Goldman W. E. Primary structure of the peptidoglycan-derived tracheal cytotoxin of Bordetella pertussis. Biochemistry. 1989 Feb 21;28(4):1744–1749. doi: 10.1021/bi00430a048. [DOI] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Folkening W. J., Nogami W., Martin S. A., Rosenthal R. S. Structure of Bordetella pertussis peptidoglycan. J Bacteriol. 1987 Sep;169(9):4223–4227. doi: 10.1128/jb.169.9.4223-4227.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry-Weeks C. R., Cookson B. T., Goldman W. E., Rimler R. B., Porter S. B., Curtiss R., 3rd Dermonecrotic toxin and tracheal cytotoxin, putative virulence factors of Bordetella avium. Infect Immun. 1988 Jul;56(7):1698–1707. doi: 10.1128/iai.56.7.1698-1707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman W. E., Baseman J. B. Selective isolation and culture of a proliferating epithelial cell population from the hamster trachea. In Vitro. 1980 Apr;16(4):313–319. doi: 10.1007/BF02618337. [DOI] [PubMed] [Google Scholar]
- Goldman W. E., Klapper D. G., Baseman J. B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect Immun. 1982 May;36(2):782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen L., Wecke J., Obál F., Jr, Krueger J. M. Macrophages produce somnogenic and pyrogenic muramyl peptides during digestion of staphylococci. Am J Physiol. 1991 Jan;260(1 Pt 2):R126–R133. doi: 10.1152/ajpregu.1991.260.1.R126. [DOI] [PubMed] [Google Scholar]
- Kitaura Y., Nakaguchi O., Takeno H., Okada S., Yonishi S., Hemmi K., Mori J., Senoh H., Mine Y., Hashimoto M. N2-(gamma-D-Glutamyl)-meso-2(L),2'(D)-diaminopimelic acid as the minimal prerequisite structure of FK-156: its acyl derivatives with potent immunostimulating activity. J Med Chem. 1982 Apr;25(4):335–337. doi: 10.1021/jm00346a001. [DOI] [PubMed] [Google Scholar]
- Kotani S., Tsujimoto M., Koga T., Nagao S., Tanaka A., Kawata S. Chemical structure and biological activity relationship of bacterial cell walls and muramyl peptides. Fed Proc. 1986 Oct;45(11):2534–2540. [PubMed] [Google Scholar]
- Krueger J. M., Johannsen L. Bacterial products, cytokines and sleep. J Rheumatol Suppl. 1989 Nov;19:52–57. [PubMed] [Google Scholar]
- Krueger J. M., Pappenheimer J. R., Karnovsky M. L. The composition of sleep-promoting factor isolated from human urine. J Biol Chem. 1982 Feb 25;257(4):1664–1669. [PubMed] [Google Scholar]
- Krueger J. M., Rosenthal R. S., Martin S. A., Walter J., Davenne D., Shoham S., Kubillus S. L., Biemann K. Bacterial peptidoglycans as modulators of sleep. I. Anhydro forms of muramyl peptides enhance somnogenic potency. Brain Res. 1987 Feb 17;403(2):249–257. doi: 10.1016/0006-8993(87)90062-x. [DOI] [PubMed] [Google Scholar]
- Melly M. A., McGee Z. A., Rosenthal R. S. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J Infect Dis. 1984 Mar;149(3):378–386. doi: 10.1093/infdis/149.3.378. [DOI] [PubMed] [Google Scholar]
- Mine Y., Watanabe Y., Tawara S., Yokota Y., Nishida M., Goto S., Kuwahara S. Immunoactive peptides, FK-156 and FK-565. III. Enhancement of host defense mechanisms against infection. J Antibiot (Tokyo) 1983 Aug;36(8):1059–1066. doi: 10.7164/antibiotics.36.1059. [DOI] [PubMed] [Google Scholar]
- Olson L. C. Pertussis. Medicine (Baltimore) 1975 Nov;54(6):427–469. doi: 10.1097/00005792-197511000-00001. [DOI] [PubMed] [Google Scholar]
- Pappenheimer J. R., Koski G., Fencl V., Karnovsky M. L., Krueger J. Extraction of sleep-promoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. J Neurophysiol. 1975 Nov;38(6):1299–1311. doi: 10.1152/jn.1975.38.6.1299. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen Z., Karnovsky M. L. Qualitative detection of muramic acid in normal mammalian tissues. Infect Immun. 1984 Mar;43(3):937–941. doi: 10.1128/iai.43.3.937-941.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Rosenthal R. S. Release of soluble peptidoglycan from growing conococci: demonstration of anhydro-muramyl-containing fragments. Infect Immun. 1980 Sep;29(3):914–925. doi: 10.1128/iai.29.3.914-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szókán G., Mezö G., Hudecz F. Application of Marfey's reagent in racemization studies of amino acids and peptides. J Chromatogr. 1988 Jul 1;444:115–122. doi: 10.1016/s0021-9673(01)94014-2. [DOI] [PubMed] [Google Scholar]
- Vermeulen M. W., Gray G. R. Processing of Bacillus subtilis peptidoglycan by a mouse macrophage cell line. Infect Immun. 1984 Nov;46(2):476–483. doi: 10.1128/iai.46.2.476-483.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



