Abstract
Current biological and pharmacological evidence suggests that the melanocortin 4 and melanocortin 3 receptors which are seven transmembrane G-protein coupled receptors (GPCRs) are involved in various aspects of energy balance and feeding behaviors in animals including humans. The natural endogenous ligands for these receptors are products of the gene pro-opiomelanocortin (POMC), and include α-melanocyte stimulating hormone, γ-melanocyte stimulating hormone and perhaps other modified products of POMC. Thus well designed agonists and antagonists of these ligands might serve as drugs for the treatment of feeding disorders. However, these melanotropin peptides also can have other biological activities that involve the MC3R and MC4R, and these other biological properties will need to be modulated in ligands that are likely to be useful drugs for feeding disorders. Current progress in these areas with special emphasis on the MC3R will be discussed along with possible new directions that might be fruitful in these important aspects of contemporary biology and medicine.
Keywords: Melanocortin genes, melanotropin peptides, MC4R, MC3R, obesity, anorexia
INTRODUCTION
The Melanotropin peptides, which are processed products of the precursor protein proopiomelanocortin (POMC), include α-melanocyte stimulating hormone (α-MSH), γ-melanocyte stimulating hormone (γ-MSH), adrenal corticotropic hormone (ACTH), β-endorphin (β-Endo) and several other peptides that have been known for many years for their effects on pigmentation (α-MSH), for their effects on stress (ACTH), and for their effects on pain at opioid receptors (β-Endo) [1,2]. More recently however these peptides and their receptors (some quite recently discovered [3]), have been shown to be involved in many other critical biological activities and behaviors including feeding behavior [4,5], sexual behavior [6,7], immune responses [8,9], cardiovascular function [10,11], pain [12,13], and many other biological activities [3,14]. As a result they have become the subject of intensive investigations in many academic and industrial laboratories, and there are continuing efforts to develop novel ligands with unique biological profiles that may serve as drugs, drug leads, and important ligands for exploring the biological activities of the melanocortin receptors, especially the MC1R, MC2R, MC3R, MC4R and MC5R [14]. In this brief review, we will concentrate on examining biological activities associated with the melanocortin-3 receptor (MC3R) and the melanocortin-4 receptor (MC4R), evaluate efforts to obtain ligands (agonists and antagonists) selective for these receptors, and discuss the promise and problems associated with developing useful drugs from these ligands. Our major focus will be the melanocortin-3 receptor since the melanocortin-4 receptor has been the primary focus for ligand discovery and development in industry [15], because it was thought to offer more significant opportunities for the development of drugs, especially drugs for treatment of obesity, for treatment of anorexia, and for treatment of sexual dysfunction via a central mechanism.
The physiological function of the melanocortin-3 receptor is still not well understood. This receptor is found in several areas of the brain, especially the arcuate nucleus and the hypothalamus and in many tissues in the periphery [16]. It often is co-expressed with the MC4R, but its exact role in regulating feeding behavior in relation to obesity, cachexia, metabolic syndrome and related feeding behaviors is still controversial. There is considerable evidence that the MC3R may act as an inhibitory auto receptor on arcuate POMC neurons. For example, peripheral administration of a highly selective MC3R agonist stimulated food intake [17]. In addition there is co-expression of MC3R mRNA with NPY and POMC in the arcuate nucleus [18], and application of a highly selective MC3R agonist results in an inhibition of spontaneous firing of POMC neurons [19]. In this regard, however, while the MC3R acts to suppress the POMC neurons, paradoxically in the MC3R deficient mouse, an obesity syndrome is observed. This latter obesity syndrome however is quite different from the obesity syndrome of MC4R deficient mice. In the MC3R deficient mice deletion of the receptor produces an obesity syndrome with a loss in lean mass and increased adipose mass, whereas in MC4R deficient mice the animals demonstrate hyperphagia, increases in lean mass, increases in adipose mass, hyperlipidemia, hyperinsulinemia, steatosis, etc [20]. Body temperature and resting energy expenditure seems normal in the MC3R deficient mice. It would seem that the MC3R knockout mice may be a metabolic model of normal obesity in most humans rather than abnormal obesity as for example, obesity due to MC4R mutants [21]. Interestingly on a high fat diet the MC3R deficient and the MC4R deficient mice both develop identical % body fat (40–50%), yet the MC3R deficient mice are resistant to high fat diet induced insulin resistance and steatosis [22]. Thus MC3R deficient mice, though obese, do not appear to develop metabolic syndrome. The reasons remain unclear.
In addition to its effects related to feeding behavior and energy metabolism the melanocortin 3 receptor has been proposed to play a significant role in modulating the host inflammatory response playing a role in anti-inflammatory actions [23–25]. The MC3R and its ligands may have important anti-inflammatory effects in myocardial infarcts with a protective effect in myocardial ischemia/reperfusion-induced arrhythmias [26,27]. However, the precise role of the MC3R and the mechanism of these effects are still not clear, and thus there is a continuing need for especially highly MC3R selective agonist and antagonist ligands to elucidate this mechanism.
γ-MSH, α-MSH and other ACTH related peptides have been found to modulate blood pressure, and its effect is highly dependent on the site of injection. Interesting and possibly important bioactivity of melanotropin peptides and the melanocortin receptors, especially the MC3R, and MC4R is their participation in cardiovascular function, in particular their effects on blood pressure regulation [e.g. see 28–32 for a few early examples], and early on it was suggested using melanocortin antagonists [33] that cardiovascular control by α-MSH and γ-MSH, might occur by distinct pathways [31].
Finally there is increasing evidence that melanocortin peptides, especially γ-MSH, but also other MSH peptides, and possibly the MC3R, play an important perhaps critical role in natriuresis (salt metabolism) [e.g. 34–36]. For example, γ-MSH natriuretic activity can be blocked by the MC3R/MC4R antagonist SHU-9119 [37]. The exact mechanism of these effects and how this mechanism is involved in sodium metabolism is largely unknown, but there is increasing evidence that γ-MSH and the MC3R may be involved in salt-sensitive hypertension (reviewed in [38]). Again the availability of highly MC3R selective agonists and antagonist ligands might greatly facilitate obtaining much more specific evidence in this regard.
To briefly summarize, the MC3R and MC4R receptors are involved in important ways in cardiovascular function and regulation of blood pressure, heart rate, and in kidney function related to natriuresis and salt-sensitive hypertension. In addition there is extensive evidence of these receptors involvement in sexual function and motivation [6,7,39,40], and even in aspects of pain [12,13]. These multiple biological, physiological and pharmacological aspects of melanotropin peptide action and their interactions with melanocortin receptors 3 and 4 occur in ways that are complex and clearly not well understood. There are further observations that the melanocortin 3 and 4 receptor systems have interactions and cross talk with physiologically complex systems that involve different aspects of human behavior such as feeding, sexual and other related behaviors that are part of complex interactive systems in the human body. For example, intricate interconnections of endogenous ligands, neurotransmitters, hormones, or targets such as ion channels, second messenger systems, kinase targets, etc., all illustrate the difficulties that are presented for drug design. For instance, control of feeding behaviors related to obesity or anorexia may be possible with melanotropin analogues, but what other effects will occur? Obtaining a highly selective ligand that will affect feeding behavior primarily via the MC3R and/or MC4R receptors, without inappropriately affecting sexual behavior, cardiovascular, or kidney functions is a major challenge. In the remainder of this overview we will review some of the critical biochemical, pharmacological, and molecular biological aspects of this problem, and briefly look at efforts to create highly selective ligands to regulate specific physiological functions for the melanocortin 3 and 4 receptors with special emphasis on the melanocortin 3 receptors. Some aspects of the development of selective ligands for these receptors have been previously reviewed [15,41]. For these discussions we will be referring to a number of endogenous and designed melanotropin ligands, and to aid the reader we provide the structure of these compounds in Table 1.
Table 1.
Structures of Melanotropin Peptides
| Compound | Structure |
|---|---|
| α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 |
| β-MSH | H-Asp-Glu-Gly-Pro-Tyr-Art-Met-Glu-His-Phe-Arg-Trp-Gly-Ser-Pro-Pro-Lys-Asp-OH |
| γ1-MSH | H-Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-NH2 |
| γ2-MSH | H-Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly-OH |
| NDP-α-MSH(MT-I) | Ac-Ser-Tyr-Ser-Nle-Gly-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 |
| MT-II | (Ac-Nle7-c[Asp5,DPhe7]-α-MSH-(4-10)-NH2, Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-NH2 |
| SHU-9119 | Ac-Nle-c[Asp-His-DNal(2′)-Arg-Trp-Lys]-NH2 |
ROLE OF THE MC3R IN FEEDING BEHAVIOR AND OBESITY
As previously mentioned the melanocortin 3 receptor (MC3R) is expressed primarily in the brain in several regions including the arcuate nucleus and ventromedial nuclei of the hypothalamus and the limbic system. It also is expressed in a variety of peripheral tissues including the placenta, kidney, heart and the gut, as well as in immune cells such as macrophages. Only a few inactivating mutations of the MC3R gene have been associated with childhood obesity [42]. In gene targeting studies, the MC3R and the MC4R were shown to have non-redundant roles in regulating energy homeostasis. Thus all the current evidence points to an essential physiological role for the MC3R [43], and there is some evidence that chronic MC3R and possibly MC4R activation has important effects to enhance glucose use in obese as well as lean rats [44]. This has potentially important implications for understanding the role of the CNS, and in particular the melanocortin system, in regulating peripheral glucose homeostasis. However, the specific physiological pathways involved are still unclear and need further study.
According to Cone [43], “Evidence for a role of the MC3R as an inhibitory autoreceptor on the arcuate melanocortin circuit derives from at least four findings: 1) coexpression of the MC3R mRNA with POMC and NPY in the arcuate nucleus; 2) observation of inhibition of the spontaneous firing of POMC neurons after application of a MC3R-specific agonists; 3) stimulation of food intake by peripheral administration of a MC3R-specific agonist; and 4) demonstration of an enhanced inhibition of food intake and weight loss after IL-1β administration in the MC3R knock out (KO) mouse. Given the apparent complexity of the physiology of the MC3R, it is useful to provide some background on the pharmacology, expression, and known functions for this receptor. After the successful cloning of MC1R and MC2R cDNAs, the MC3R was the first new member of the melanocortin receptor gene family isolated using PCR and low-stringency hybridization techniques based upon MC1R and MC2R sequences” [43]. “Recombinant human AgRP (Agouti Related Protein) binds to the human MC3R and the human MC4R with high affinity (IC50 = 1.1 ± 0.5 nM and 0.5 ± 0.1 nM, respectively), and is a potent antagonist of the human MC3R and MC4R”. “Because the MC3R is thought to function as an inhibitory auto-receptor on POMC neurons, it was reasoned that peripheral injections of MC3R-specific agonists would act only within circumventricular organs, or adjacent nuclei like the arcuate to inhibit POMC neurons and thereby stimulate feeding” [43]. The development of specific agonists and antagonists of the MC3R is important to resolve the physiological roles of this receptor. More recently, additional work from our laboratory has led to the creation of peptide agonists and antagonists with specificity for the MC3R. They were able to test this hypothesis using a selective MC3R agonist [D-Trp-8]-γ-MSH and, indeed, it did stimulate feeding via the MC3R when injected peripherally [17]. Further work reported by Mayorov et al. [45] provided for the first truly useful MC3R antagonist, c[Nle-Val- Nal(2′)-Arg-Trp-Glu]-NH2, in that this compound has a 100-fold selectivity for MC3R over MC4R.
As mentioned above [43], MC3R blockade actually enhances the cachexigenic response to IL-1β, thus supporting the concept that the MC3R is an inhibitory autoreceptor in the central melanocortin system and suggesting that specific MC3R antagonists may have clinical utility in the treatment of cachexia. Northern blot hybridization experiments demonstrated that the greatest expression of the MC3R gene is in the brain with two mRNA species of approximately 2.0 and 2.5 kb detected in rat hypothalamic poly(A)RNA. However, using the more sensitive technique of in situ hybridization, a thorough examination of MC3R mRNA distribution in the rat brain demonstrated approximately 35 different nuclei expressing the receptor, with the highest expression seen in the ventromedial hypothalamus, medial habenula, ventral tegmental area, and raphe. Not surprisingly, MC3R mRNA is found primarily in areas of the brain which receive direct innervation from POMC immunoreactive neurons. However, the arcuate nucleus contains all of the forebrain POMC expressing neurons and displays moderate levels of MC3R mRNA, whereas the nucleus of the solitary tract (NTS) containing the other central POMC expressing neurons apparently does not express MC3R mRNA [43]. “MC3R expression also was detected in several human gut tissues including the stomach, duodenum, and pancreas, using a combination of RT-PCR and Southern blotting techniques. PCR analysis of human tissues similarly detected MC3R cDNA in the heart, whereas Southern blotting of amplified cDNA detected expression in the testis, ovary, mammary gland, skeletal muscle, and kidney” [43]. Again, the development of specific agonists and antagonists of the MC3R will be important to further resolve the physiological roles of this receptor under various physiological conditions.
In another study acute unilateral nephrectomy (AUN) induces an increase in both potassium and sodium excretion by the remaining kidney through an adaptive mechanism that is dependent upon intact pituitary function as well as innervation of both kidneys before AUN. Further research demonstrated that, although all of the MSH peptides have some natriuretic activity, an antibody specific to γ-MSH was able to block the experimental induction of natriuresis by AUN, thereby suggesting a specific role for γ-MSH in this experimental system. The MC3R null mouse is resistant to the induction of natriuresis by γ-MSH and is sensitive to high-salt diet-induced hypertension. Evidence suggests a role for both central and peripheral MC3R in this phenomenon [43].
Similarly there is increased evidence, both genetic or neuropharmacological, for the function of MC3R in the pathogenesis of obesity [46]. MC3R knockout mice are obese with increased fat mass and decreased lean body mass, but without hyperphagia, in contrast to MC4R knock out mice. However, mice lacking both MC3R and MC4R are more obese than MC4R KO mice alone. Also, the obesity of MC3R knock out mice is more dependent on fat intake than that of the MC4R knock out mice. Diet induced obesity in these two knockout strains affects insulin-sensitivity more adversely in the MC4R knockout mice. The MC4R knockout mice do not respond to the anorectic action of MTII [47]. MC3R gene variants are common in humans, but they often are not associated with obesity except for a few activating mutations of the MC3R gene have been associated with childhood obesity [42]. However, the MC3R may mediate different responses to leptin than the MC4R. While leptin administration reduces food intake in MC4R knockout mice, MC3R knockout mice do not show an anorexic response to leptin. This suggests that the ability of leptin to reduce food consumption depends more upon the MC3R than the MC4R. The MC3R is particularly expressed in the arcuate nucleus, including on POMC/CART neurons and AgRP/NPY neurons, and is also more widely expressed in the CNS than MC4R. α-MSH, β-MSH and γ2-MSH all activate different neuronal targets within this nucleus. However, it should be noted that MC4R is found in the arcuate nucleus too. There are intra-arcuate POMC connections suggesting that MC3R may mediate an auto feedback mechanism in the arcuate nucleus. Administration of a specific MC3R agonist reduces the frequency of action potentials in POMC-containing neurons in the arcuate nucleus, which supports this hypothesis. It is not yet known if any of these observations are important with regard to feeding behavior, but they may be important with regard to general control of POMC neural projections. Overall, the role of MC3R in feeding behavior and obesity is less clear than for MC4R. The likely model for the melanocortin regulation of feeding is that the AgRP signal in the arcuate nucleus fluctuates to modulate a more constant POMC signal, which is a function of the relatively steady hormonal level of leptin. POMC products may be released differentially in the different hypothalamic and extra-hypothalamic sites involved with feeding in the CNS, which could lead to variations in the anorexic signal being transmitted. This may or may not be independent of leptin signaling.
Furthermore, peripheral injections of the hMC3R selective agonist can stimulate food intake in mice, suggesting an important role of this receptor subtype in the regulation of feeding and energy partitioning. Finally, emerging evidence points to a potential role of the hMC3R in regulation of erectile function and sexual behavior [6]. The complex multifunctional nature of the MC3R provides further impetus for the development of highly selective orthosteric and allosteric agonists and antagonist for the hMC3R to help distinguish MC3R related functions versus MC4R functions.
STRUCTURE ACTIVITY RELATIONSHIPS (SARS) OF THE MC3R WITH POSSIBLE INSIGHTS RELATED TO FEEDING BEHAVIOR
As previously discussed MC3R-deficient (MC3R(−/−)) mice demonstrate increases fat mass, higher feeding efficiency, hyperlipidimia, and mild hyperinsulinism. At least one specific mutation of MC3R has been identified to be associated with human obesity. Functional analysis of this altered MC3R (I183N) has indicated that the mutation completely abolishes agonist-mediated receptor activation. However, the specific molecular determinants of MC3R responsible for ligand binding and receptor signaling are currently only poorly understood. A number of studies have been recently published [48–52] to determine the structural aspects of MC3R and MC4R responsible for ligand binding and receptor signaling. On the basis of a theoretical model for MC1R, using mutagenesis, Yang et al. [48], have examined 19 transmembrane domain amino acids selected for these potential roles in ligand binding and receptor signaling. Their results provided the following insights: (i) substitutions of charged amino acid residues E131 in transmembrane domain 2 (TM2), D154 and D158 in TM3, and H298 in TM6 with alanine dramatically reduced NDP-MSH binding affinity and receptor signaling; (ii) substitutions of aromatic amino acids F295 and F296 in TM6 with alanine also significantly decreased NDP-α-MSH binding and receptor activity; (iii) substitutions of D121 in the TM2 and D332 in the TM7 with alanine resulted in the complete loss of ligand binding, ligand induced receptor activation, and cell surface protein expression; and (iv) interestingly, substitution of L165 in TM3 with methionine or alanine switched antagonist SHU9119 into a receptor agonist. These results suggest that TM3 and TM6 are important for NDP-α-MSH binding, while D121 in TM2 and D332 in TM7 are crucial for receptor activity and signaling. Importantly, L165 in TM3 is critical for agonist or antagonist selectivity. These results provide important information about the molecular determinants of hMC3R responsible for ligand binding and receptor signaling. To date only one specific MC3R mutation has been specifically identified to be associated with human obesity [50], and functional analysis of this MC3R mutation (I183N) has indicated that the mutation completely abolishes agonist-mediated receptor activation. Alpha melanocyte-stimulating hormone (α-MSH) is equally potent at the MC3R and the MC4R, but γ-MSH has higher affinity at MC3R than MC4R. Agouti related protein (AGRP) is a potent antagonist at both MC3R and MC4R, but agouti is a potent antagonist only at MC4R. These differences indicate that these two receptors, though members of the same system, are likely to possess functional and structural differences in response to ligands. Determination of the molecular basis of ligand binding and receptor signaling should, therefore, provide important insights into the mechanism of MC3R action.
In studies by Wang et al. it was noted that “MC3R knockout (KO) mice exhibited increased fat mass (approximately twice that of the wildtype (WT) littemates) due to increased feeding efficiency” [51]. It is known that mice which lack both the MC3R and MC4R show exacerbated obesity compared to the single KO versions of MC3R and MC4R. This indicates that the two melanocortin receptors regulate different aspects of energy homeostasis. As mentioned earlier. Our studies with Cone showed [17] that injection of a selective MC3R agonist [DTrp8-gamma-MSH] increase food intake. In studies involving obese patients, eight naturally occurring mutations and polymorphisms have been discovered thus far, including: T6K, A70T, V81I, M134I, I183N, A293T, I335S, and X316s [50,51].
The MC3R is a typical GPCR structure with a heptahelical transmembrane structure, and an extra cellular NH2 terminus and intra cellular COOH terminus. Modeling studies which were not based on rhodopsin, appeared to show that a hydrophobic pocket of the ligand binding sites are different when comparing the MC3R to the MC4R. However, it is believed that charge-charge interactions between the ligand and the receptor are similar in both melanocortin receptor, althought this has never been proven experimentally. In addition to the D154 and D158 in TM3, eight additional acidic residues can theoretically form a salt bridge with Arg in the pharmacophore (HFRW) of the ligands [51]. These include: E73, E80, E92, D121, E131, D178, E221, and D332. The results indicate that acidic residues in TMs 1 and 3 are important for ligand binding, while those in TMs 2 and 7 are important for both binding and signaling.
The melanocortin 3 receptor (MC3R) plays a critical role in weight regulation of rodents [78], but its role in humans remains unclear, so it is critically important to identify genetic variants of the MC3R gene that may be associated with such a condition as childhood obesity. MC3R mutations may not result in autosomal dominant forms of obesity, but may contribute as a predisposing factor to childhood obesity and exert an effect on the human phenotype. Studies by Lee et al. [78] supports the role of MC3R in human weight regulation. As Lee et al. [78] have discussed “MC3R(−/−) mice homozygous for knockout mutations of MC3R gene had increased body fat not caused by increased food intake but by increased feed efficiency. The MC3R(−/−) mice were hypophagic with hyperlipidimia compared with wild-type littermates”. “Although MC3R mutations are unlikely to result in an autosomal dominant form of monogenic obesity, this study provided evidence that the MC3R can be one of the predisposing genes that contributes to increased adiposity and that the wide variation in the adiposity of the individuals with common and rare variants may be due to other modifying genetic and environmental factors”. “The MC3R (−/−) mice demonstrated mild obesity but increased body fat and leptin, whereas the phenotype of the heterozygous MC3R(−/−) mice did not appear to differ significantly from wild-type mice”. “Partially inactivating genetic variants of MC3R may likewise exert a significant effect on the phenotype even in the heterozygous state in the “obesogenic” environment. This notion was supported by the linkage of a locus encoding MC3R on human chromosome 20q13.2 to the regulation of BMI, subcutaneous fat mass, and fasting insulin”. “Common and rare novel variants at the MC3R locus, which result in partially reduced activity of the MC3R in response to MSH and demonstrate that the common variants, and possibly the rare variants, are associated with increased body fat and leptin levels (with additive effect) and perhaps decreased hunger, in human subjects, congruous with the phenotype of the MC3R(−/−) mice” [78].
SAR BASED DESIGN OF MELANOTROPIN LIGANDS WITH POSSIBLE INSIGHTS RELATED TO FEEDING BEHAVIOR
Structure-Activity relationships of the melanotropin ligands (Table 1) and numerous non-peptide ligands whose design was primarily based on the peptide pharmacophore determined many years ago for the MC1R [52,53] to be linear tetrapeptide sequence His-Phe-Arg-Trp, which also has been found to be the key pharmacophore for the MC3R, MC4R and MC5R. However, some structural differences related to the His and Arg residues are found to be the case for pharmacophore structural elements of α-MSH and γ-MSH for binding affinity and agonist efficacy. As previously discussed there have been recent reviews of the efforts to obtain selective peptide and non-peptide ligands for the MC4R and MC3R [15,17]. For the most part the previous goals of many, indeed most, of the studies were to obtain selective agonist analogues for the MC4R. A previous review [15] emphasized those aspects of structure that have lead to potent and selective orthosteric agonists for the MC4R, and those structural features that could render a ligand selective for the MC4R. Development of selective agonists for the MC3R has not received as much attention [17]. Furthermore, despite their potential usefulness for greatly aiding in sorting out the specific pharmacology, physiology and biochemistry of the MC4R vs. the MC3R in feeding behavior, energy, homeostasis, obesity, anorexia, cardiovascular function, kidney function, pain, immune and inflammatory responses, etc. selective antagonist development for the MC3R receptor has lagged behind. Thus our discussion here will emphasize the recent developments leading to selective MC3R antagonists (and agonists).
In addition there is another consideration which until recently has been largely ignored in the development of agonist and antagonists for the MC3R and MC4R, namely the development of allosteric (rather than orthosteric) agonist and antagonist ligands for these receptors. There is recent and mounting evidence that allosteric ligands for GPCRs and other membrane proteins may provide advantages in drug design and development [54–66]. Thus we will point out several cases where it appears that leads for, or actual allosteric ligands for, the melanocortin receptors may be possible.
As already mentioned there are many selective agonist ligands for the MC4R [15] and these will not be further discussed here except in regard to relationships to the MC3R ligands. In many published studies the focus was so strongly on obtaining MC4R selective agonists that other potentially useful insights that may have arisen were only briefly discussed or were ignored. In what follows we will try to take a broader perspective with regard to MC3R ligand development. Hopefully this will provide the reader with a different perspective that was discussed in our earlier reviews [17,67].
It was recognized from the earliest studies of the interactions of melanotropin peptides that γ-MSH itself had selectivity for the MC3R, but it was examination of a D-amino acid scan which led to the insight that the Trp8 residue of γ-MSH was a key to its selectivity and [D-Trp8]-γ-MSH was found to be several hundred fold selective as an MC3R agonist [68] (Table 2) and this could be retained with the α-MSH/γ-MSH hybrid peptide H-Tyr-Val-Nle-Gly-Pro-Phe-Arg-D-Nal(2′)-Asp-Arg-Phe-Gly-NH2 (3, Table 2), though the emergence of partial agonist or even antagonist activities at the hMC3R and hMC4R are evident from the low % maximum effect at these receptor for 3 (Table 2) [69]. Earlier studies by Kavarana et al. [70] had found that enhancing hydrophobic properties of a cyclic α-MSH analogues and increasing the macrocyclic ring size vs MT-II could provide some hMC3R selectivity. Balse-Srinivasan et al. [71] showed that cyclic α-MSH/β-MSH peptides such as Ac-c[Pen-Glu-His-DNal(2′)-Arg-Trp-Cyc]-Pro-Lys-Asp-NH2 (4, Table 2) could be a hMC3R highly selective antagonist vs. the hMC4R (250-fold) but not as selective against the hMC5R (31-fold). It also should be noted (data not shown) that replacement of Met3 in γ-MSH with the isosteric Nle3 residue enhances γ-MSH affinity for the MC3R. Interestingly, in this regard, when the Nle residue is replaced by a D-Phe in the cyclic MT-II analogue H-DPhe-c[Asp-Pro-DNal(2′)-Arg-Trp-Lys]-NH2 (5, Table 2) a highly potent and hMC3R selective agonist for the MC3R was discovered [72]. Here again we see the importance of the 5 position in γ-MSH analogues on determining the selectivity for the hMC3R vs the hMC4R other modification of the His6 position such as with cyclic proline analogue Acpc (Ac-Nle-c[Asp-Acpc-DNal(2′)-Arg-Trp-Lys]-NH2) (6, Table 2) which is about 100-fold selective for the hMC3R vs hMC4R receptor as an antagonist but which has potent partial agonist activity for the hMC5R.
Table 2.
Comparative Receptor Binding Affinities and cAMP Assays of Melanocortin Ligands
| Compounda | hMC1R | hMC3R | hMC4R | hMC5R | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 nM | EC50 nM | %max effect | IC50 nM | EC50 nM | %max effect | IC50 nM | EC50 nM | %max effect | IC50 nM | EC50 nM | %max effect | |
| 1. γ-MSH | 1000 | 300 | 100 | 71 | 700 | 100 | 760 | 710 | 100 | 2200 | 550 | 100 |
| 2. D-Trp8-γMSH | - | - | - | 6.7 | 0.33 | 100 | 600 | 100 | 100 | 340 | 82 | 97 |
| 3 | 20 | 55 | 120 | 32 | 13 | 28 | 360 | 2.6 | 14 | 650 | 250 | 46 |
| 4 | - | - | - | 3 | >10000 | 0 | 750 | >10000 | 0 | 94 | >10000 | 0 |
| 5 | - | - | - | 3.6 | 4.9 | 100 | 660 | >104 | 0 | 1020 | >104 | 0 |
| 6 | - | - | - | 25 | >105 | 0 | 270 | >105 | 0 | 4.1 | 390 | 50 |
| 7 | - | - | - | 1.7 | >104 | 0 | 180 | 16 | 13 | 3.8 | 200 | 10 |
| 8 | - | - | - | 1.4 | >104 | 0 | 65 | 130 | 50 | 1.4 | >104 | 0 |
| 9 | >10000 | >104 | 0 | 50 | >104 | 0 | >10000 | >104 | 0 | 2900 | >104 | 0 |
| 10 | - | - | - | 34 | 48 | 13 | 2700 | 26 | 0.2 | 54 | 5 | 30 |
| 11 | - | - | - | NB | >104 | 0 | 50 | 43 | 3.9 | 590 | 2500 | 24 |
| 12 | 4.0 | >104 | 50 | 1.2 | >104 | 50 | NB | 0 | 0 | NB | 0 | 0 |
| 13 | 1.3 | >104 | 90 | 5 | >104 | 30 | 1.2 | >104 | 30 | NB | 0 | 0 |
see text for structures
NB- no binding at >10−4 M
An interesting N-terminal backbone to side chain cyclization from Nle4 to Glu10 residues with replacement of His5 with a wide variety of hydrophobic and basic residues in α-MSH lead to some peptidomimetic analogues with high potency and selectivity for the hMC3R vs the hMC4R and the hMC1R, but generally not against the hMC5R [73]. The most selective analogue was c[Nle-Val-DNal(2′)-Arg-Trp-Glu]-NH2 (7, Table 2) which was 100-fold selective vs. the hMC4R. An equally interesting analogue is 8, Table 2 c[Nle-Gln-DNal(2′)-Arg-Trp-Glu]-NH2 which is a potent and hMC3R selective antagonist at the hMC3R (50-fold vs the hMC4R) but which is a partial agonist at the hMC4R, which possibly also was the case for 7. What this would translate into in in vivo activity in animals has not been examined.
The importance of position 6 for modulating selectivity for hMC3R vs other melanocortin receptors also is demonstrated when a constrained aromatic amino acid 4-amino-1,2,4,5-tetrahydro-2-benzazepine-3-one (Aba) (Fig. 1) is substituted for His in the MT-II scaffold [75]. The analogue of MT-II (9, Table 2), Ac-Nle-c[Asp-Aba-D-Phe-Arg-Trp-Lys]-NH2 was found to be a potent hMC3R antagonist with greater than 200-fold selectivity vs the hMC4R and hMC1R and about 60-fold selectivity vs the hMC3R.
Fig. 1.

The 4-amino-1,2,4,5-tetrahydro-2-benzazepin-3-one (Aba) scaffold.
As briefly discussed earlier allosteric effectors of the melanocortin receptors may offer advantages in terms of drug design and specific biological effects as a result of differentially affecting second messenger and other signaling mechanisms. In the case of ligands targeted for the hMC3R and hMC4R there are possible lead compounds that have been reported in this regard.
For example in melanotropin ligands related to MT-II, a series of analogues were prepared in which the His6 or D-Phe7 positions were replaced with p-substituted 3-phenyl-proline derivatives [76]. Most of these compounds were selective for the hMC3R vs the MC4R (10–20 fold) but could only partially displace the radiolabeled NDP-α-MSH in the ligand binding assays and appeared to partial agonists as example 10, Table 2 (Ac-Nle-c[Asp-His-D-(o-Ph)Phe-Arg-Trp-Lys]-NH2).
When the Arg residue in MT-II was replaced by a Pro residue or by trans- or cis-4-guanidinyl-Pro, the compounds obtained such as 11 (Table 2), Ac-Nle-c[Asp-trans-gPro-DPhe-Arg-Trp-Lys]-NH2, were shown to be allosteric binders [77] to the MC4R.
Finally another very interesting example of likely allosteric ligands for the hMC3R and other melanocortin receptors are the peptide mimetic pyrrolopiperazine compounds shown in Figs. 2 and 3 [74]. These very minimal structures related to the melanocortin peptide pharmacophore have very interesting biological properties. For example compound HMC010 (12, Table 2) interacts only with the hMC3R and hMC1R and is an allosteric antagonist at these receptors. HMC002 (13, Table 2) on the other hand has potent nM binding affinity at the hMC1R, hMC3R and hMC4R receptor only and are allosteric antagonists at these receptors.
Fig. 2.
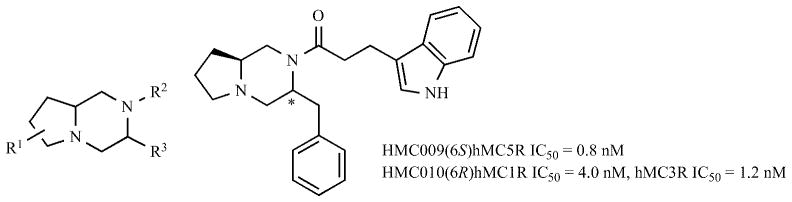
Structure of peptide mimetics, pyrrolopiperazine analogues.
Fig. 3.
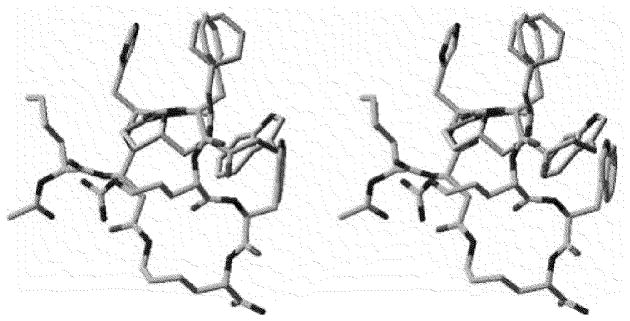
Superimposition of computational model of Pyrrolopiperazine Compound (orange) and NMR-derived structure of MT-II (cyan).
These examples and others suggest that a more careful evaluation of the SAR results of ligand design for the hMC3R, hMC4R and other melanocortin receptors where both binding affinities and second messenger activities are more carefully evaluated, may provide a useful starting point for the development of potent and selective allosteric agonists and antagonists for the hMC3R and hMC4R.
CONCLUSIONS
There has been a very large effort in academic laboratories, but especially in pharmaceutical companies, to develop selective agonists for the MC4R as possible compounds for the treatment of obesity and other feeding disorders. Yet despite these efforts and the tremendous needs for such drugs, there does not appear to be any compounds that are in advanced human clinical evaluation for treatment of obesity. There are many possible explanations for this considering the complexities of biological systems that control and modulate feeding behavior, obesity, and related syndromes such as cachexia and metabolic syndrome. At the most fundamental level it is clear that both the human MC4R and the human MC3R are both involved in as yet unclear ways in the modulation and control of feeding behavior and related metabolic effects. From a physiological and pharmacological point of view it would seem reasonable to make every effort to establish the specific roles and interplay of the MC3R and MC4R in feeding behavior, obesity and related behaviors. The MC3R, MC4R, and double knockout animals have been examined, but they have not been able to sort out these relationships and how modulation of the melanocortin receptors and their corresponding ligands may best be utilized to treat obesity and related diseases.
As we have discussed, the MC3R and MC4R and their ligands have additional biological activities that can lead to unrelated biological effects that are either undesirable as treatments for feeding behavior, or can actually result in toxicities. The involvement of the MC4R and likely the MC3R on centrally mediated erectile function and sexual motivation is a concern, and ligands that can separate feeding behavior and sexual function certainly would be desirable. Furthermore as we have seen, the MC4R and MC3R also appear to be involved in blood pressure regulation and heart rate, though the exact effect appears to depend on the site of action (central vs. peripheral). This aspect clearly needs further study and careful examination of both local and system wide global activities. In addition the natriuretic effects of melanotropins, as well as their effects on the immune system, also need further examination and evaluation relative to effects on feeding behavior. Clearly there is still much we do not know.
So where do we go from here if we wish to sort out the complexities of these ligands and receptors in animals including human so that the potential of these ligands to become useful drugs can be realized. There is much that one can suggest depending on ones perspective, but it seems to us that we clearly need much more functionally selective agonist and antagonist ligands for both the MC3R and MC4R. The development and availability of such compounds is a critical need for the careful pharmacological and physiological studies that will be required to clearly delineate the roles and interactions of the MC3R and MC4R in feeding behavior and energy utilization. Of course, the central issue here is the fact that the melanotropins have effects on so many different aspects of human behavior. How do we modulate one facet of the melanocortin system without affecting its other components? What small differences in the structures of the system can be exploited to enable selective biological effects. In addition labeled receptors, labeled G-proteins and labeled melanotropin ligands will be needed so that actions of the receptors in response to these ligands can be followed using the powerful new imaging and spectroscopic technologies that are or soon will be available to examine directly the changes in receptor, ligand, G-protein, adenylcyclase, ion channel, β-arrestin and other proteins that occur on ligand-receptor interaction and signaling. These studies coupled with the likely large differences in phenotypes of human in terms of feeding behavior, tendencies to obesity or anorexia, and related energy utilization is a huge challenge, but it would seem a clear path is available to reach the goal of understanding and treating obesity and related disorders with novel melanotropin ligands.
Acknowledgments
The partial support of the U.S. Public Health Services, National Institutes of Health, especially Grant DK-17420 is gratefully acknowledged. We also wish to thank Margie Colie for her great help in preparing this manuscript.
References
- 1.Hadley ME, editor. The Melanotropic Peptides. I, II & III. CRC Press, Inc; Boca Raton, FL: 1988. [Google Scholar]
- 2.Vaudry H, Eberle AN, editors. The Melanotropic Peptides. Ann N Y Acad Sci. 1993;680:1–687. [Google Scholar]
- 3.Cone RD, editor. The Melanocortin Receptors. Humana Press; Totowa, NJ: 2000. [Google Scholar]
- 4.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of the melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 5.Yang YK, Harmon CM. Recent developments in our understanding of melanocortin system in the regulation of food intake. Obes Rev. 2003;4:239–248. doi: 10.1046/j.1467-789x.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 6.Wessells H, Gralnek D, Dorr R, Hruby VJ, Hadley ME, Levine N. Effect of an α-melanocyte stimulating hormone analogue on penile erection and sexual desire in men with organic erectile dysfunction. Urology. 2000;56:641–646. doi: 10.1016/s0090-4295(00)00680-4. [DOI] [PubMed] [Google Scholar]
- 7.Wessells H, Fuciarelli K, Hansen J, Hadley ME, Hruby VJ, Dorr R, Levine N. Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: double-blind placebo controlled crossover study. J Urology. 1998;160:389–393. [PubMed] [Google Scholar]
- 8.Hughes S, Smith ME, Bailey CJ. Beta-endorphin and corticotropin immunoreactivity and specific binding in the neuromuscular system of obese-diabetic mice. Neuroscience. 1992;48(2):463–468. doi: 10.1016/0306-4522(92)90505-v. [DOI] [PubMed] [Google Scholar]
- 9.Tatro JB. Receptor biology of the melanocortins, a family of neuroimmunomodulatory peptides. Neuroimmunomodulation. 1996;3(5):259–284. doi: 10.1159/000097281. [DOI] [PubMed] [Google Scholar]
- 10.Li SJ, Varga K, Archer P, Hruby VJ, Sharma SD, Kesterson RA, Cone RD, Kunos G. Melanocortin antagonists define two distinct pathways of cardiovascular control by α- and γ-melanocyte stimulating hormones. J Neurosci. 1996;16:5182–5188. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni XP, Butler AA, Cone RD, Humphreys MH. Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. J Hypertens. 2006;24:2239–2246. doi: 10.1097/01.hjh.0000249702.49854.fa. [DOI] [PubMed] [Google Scholar]
- 12.Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KVS, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, Ness TJ, Glover TL, Stankova M, Mayorov AV, Hruby VJ, Grisel JE, Fillingim RB. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci USA. 2003;100:4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vrinten DH, Kalkman CJ, Adan RAH, Gispen WH. Neuropathic pain: a possible role for the melanocortin system? Eur J Pharmacol. 2001;429:61–69. doi: 10.1016/s0014-2999(01)01306-1. [DOI] [PubMed] [Google Scholar]
- 14.Cone RD, editor. The Melanocortin System. Ann NY Acad Sci. 2003;994:1–387. [Google Scholar]
- 15.Emerson PJ, Fisher MJ, Yan LZ, Mayer JP. Melanocortin-4 receptor agonists for treatment of obesity. Curr Topics Med Chem. 2007:1121–1130. doi: 10.2174/156802607780906636. [DOI] [PubMed] [Google Scholar]
- 16.Kesterson RA. The melanocortin 3-receptor. In: Cone RD, editor. The Melanocortin Receptors. Chapter 13. Humana Press Inc; Totowa, NJ: 2000. pp. 385–403. [Google Scholar]
- 17.Marks DL, Hruby V, Brookhart G, Cone RD. The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R) Peptides. 2006;27:259–264. doi: 10.1016/j.peptides.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagnot T, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ. The anatomy of an endogenous antagonist: relationship between agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 20.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Stack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, Maclntyre DE, Chen HY, Van der Ploeg LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 21.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 22.Sutton GM, Trevaskis JL, Hulver MW, McMillan RP, Markward NJ, Babin MJ, Meyer EA, Butler AA. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology. 2006;147:2183–2196. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Getting SJ, Allcock GH, Flower R, Perretti M. Natural and synthetic agonists of the melanocortin receptor type 3 possess antiinflammatory properties. J Leukoc Biol. 2001;69:98–104. [PubMed] [Google Scholar]
- 24.Getting SJ, Christian HC, Lam CW, Gavins FN, Flower RJ, Schiöth HB, Perretti M. Redundancy of a functional melanocortin 1 receptor in the anti-inflammatory actions of melanocortin peptides: studies in the recessive yellow (e/e) mouse suggest an important role for melanocortin 3 receptor. J Immunol. 2003;170(6):3323–3330. doi: 10.4049/jimmunol.170.6.3323. [DOI] [PubMed] [Google Scholar]
- 25.Getting SJ, Christian HC, Flower RJ, Perretti M. Activation of melanocortin type 3 receptor as a molecular mechanism for adrenocorticotropic hormone efficacy in gouty arthritis. Arthritis Rheum. 2002;46:2765–75. doi: 10.1002/art.10526. [DOI] [PubMed] [Google Scholar]
- 26.Getting SJ, Di Filippo C, Christian HC, Lam CW, Rossi F, D’Amico M, Perretti M. MC-3 receptor and the inflammatory mechanisms activated in acute myocardial infarct. J Leukoc Biol. 2004;76:845–53. doi: 10.1189/jlb.0306175. [DOI] [PubMed] [Google Scholar]
- 27.Guarini S, Schiöth HB, Mioni C, Cainazzo M, Ferrazza G, Giuliani D, Wikberg JES, Bertolini A, Bazzani C. MC(3) receptors are involved in the protective effect of melanocortins in myocardial ischemia/reperfusion-induced arrhythmias. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:177–182. doi: 10.1007/s00210-002-0572-8. [DOI] [PubMed] [Google Scholar]
- 28.Gruber KA, Eskridge SI. Central vasopressin system mediation of acute pressor effect of γ-MSH. Am J Physiol Endocrinol Metab. 1986;251:E134–E137. doi: 10.1152/ajpendo.1986.251.1.E134. [DOI] [PubMed] [Google Scholar]
- 29.Van Bergen P, Janssen PML, Hoogerhout P, De Wildt DJ, Versteeg DHG. Cardiovascular Effects of γ-MSH/ACTH-like peptides: structure-activity relationship. Eur J Pharmacol. 1995;294:795–803. doi: 10.1016/0014-2999(95)00657-5. [DOI] [PubMed] [Google Scholar]
- 30.Van Bergen P, Kleijne JA, De Wildt DJ, Versteeg DHG. Different cardiovascular profiles of three melanocortins in conscious rats; evidence for antagonist between γ2-MSH and ACTH-(l-24) Br J Pharmacol. 1997;120:1561–1567. doi: 10.1038/sj.bjp.0701065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li SJ, Varga K, Archer P, Hruby VJ, Sharma SD, Kesterson RA, Cone RD, Kunos G. Melanocortin antagonists define two distinct pathways of cardiovascular control by α- and γ-melanocyte-stimulating hormones. J Neurosci. 1996;16:5182–5188. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunos G, Li S-J, Varga K, Archer P, Kesterson RA, Cone RD, Hruby VJ, Sharma SD. Novel neural pathways of cardiovascular control by α- and γ-MSH. Fundam Clin Pharmacol. 1997;11(Suppl 1):44s–48s. [Google Scholar]
- 33.Hruby VJ, Lu D, Sharma SD, de L Castrucci A, Kesterson RA, Al-Obeidi FA, Hadley ME, Cone RD. Cyclic lactam α-melanotropin analogues of Ac-Nle4-c[Asp3,D-Phe7,Lys10]α-MSH(4-10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem. 1995;38:3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- 34.Orias R, McCann SM. Natriuresis induced by alpha and beta melanocyte stimulating hormone (MSH) in rats. Endocrinol. 1972;90:700–706. doi: 10.1210/endo-90-3-700. [DOI] [PubMed] [Google Scholar]
- 35.Chen XW, Ying WZ, Valentin JP, Ling KT, Lin SY, Wiedemann E, Humphreys MH. Mechanism of the natriuretic action of γ-melanocyte-stimulating hormone. Am J Physiol Regul Integr Comp Physiol. 1997;272:R1946–R1953. doi: 10.1152/ajpregu.1997.272.6.R1946. [DOI] [PubMed] [Google Scholar]
- 36.Lin SY, Chaves C, Wiedemann E, Humphreys MH. A γ-MSH like peptide causes reflex natriuresis after acute unilateral nephrectomy. Hypertension. 1987;10:619–627. doi: 10.1161/01.hyp.10.6.619. [DOI] [PubMed] [Google Scholar]
- 37.Ni X-P, Kesterson RA, Sharma SD, Hruby VJ, Cone RD, Wiedemann E, Humphreys MH. Prevention of reflex natriuresis after acute unilateral nephrectomy by melanocortin receptor antagonists. Amer J Physiol. 1998;274(43):R931–R938. doi: 10.1152/ajpregu.1998.274.4.R931. [DOI] [PubMed] [Google Scholar]
- 38.Humphreys MH. γ-MSH, sodium metabolism and salt-sensitive hypertension. Am J Physiol Intgr Comp Physiol. 2004;286:R417–R430. doi: 10.1152/ajpregu.00365.2003. [DOI] [PubMed] [Google Scholar]
- 39.King SH, Mayorov AV, Balse-Srinivasan P, Hruby VJ, Vanderah TW, Wessells H. Melanocortin receptors, melanotropic peptides and penile erection. Curr Top Med Chem. 2007;7:1111–1119. [PMC free article] [PubMed] [Google Scholar]
- 40.Martin WJ, McGowan E, Cashen DE, Gautert LT, Drisko JE, Hom GJ, Nargund R, Sebhat I, Howard AD, Vander Ploeg L, Maclntyre PE. Activation of MC4 receptors increase erectile activity in rats E copula. Eur J Pharmacol. 2002;451:71–79. doi: 10.1016/s0014-2999(02)02479-2. [DOI] [PubMed] [Google Scholar]
- 41.Hruby VJ, Cai M, Cain JP, Mayorov AV, Dedek MM, Trivedi D. Design, synthesis and biological evaluation of ligands selective for the melanocortin-3 receptor. Curr Top Med Chem. 2007;7:1085–1097. doi: 10.2174/156802607780906645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang SX, Fan Z-C, Tao Y-X. Functions of acidic transmembrane residues in human melanocortin-3 receptor binding and activation. Biochem Pharmacol. 2008;76:520–530. doi: 10.1016/j.bcp.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cone RD. Studies on the Physiological Functions of the Melanocortin System. Endocrine Rev. 2006;27(7):736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 44.Sutton GM, Perez-Tilve D, Nogueiras R, Fang J, Kim JK, Cone RD, Gimble JM, Tschöp MH, Butler AA. The melanocortin-3 receptor is required for entrainment to meal intake. J Neurosci. 2008;28(48):12946–12955. doi: 10.1523/JNEUROSCI.3615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayorov AV, Cai M, Palmer ES, Dedek MM, Cain JP, Van Scoy AR, Tan B, Vagner J, Trivedi D, Hruby VJ. Structure-Activity Relationships of Cyclic Lactam Analogues of α-Melanocyte-Stimulating Hormone (α-MSH) Targeting the Human Melanocortin-3 Receptor. J Med Chem. 2008;51(2):187–195. doi: 10.1021/jm070461w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen AS, Marsh J, Trumbauer ME, Frazier EG, Guan X-M, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Alison MM, Strack JM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, Maclntyre DE, Chen HY, Van der Ploeg LHT. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Gene. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 47.Lee M, Kim A, Conwell IM, Hruby V, Mayorov A, Cai M, Wardlaw SL. Effects of selective modulation of the central melanocortin-3-receptor on food intake and hypothalamic POMC expression. Peptides. 2008;29(3):440–447. doi: 10.1016/j.peptides.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen M, Aprahamian CJ, Celik A, Georgeson KE, Garvey WT, Harmon CM, Yang Y. Molecular characterization of human melanocortin-3 receptor ligand-receptor interaction. Biochem. 2006;45:1128–1137. doi: 10.1021/bi0521792. [DOI] [PubMed] [Google Scholar]
- 49.Chen M, Cai M, Aprahamian CJ, Georgeson KE, Hruby V, Harmon CM, Yang Y. Contribution of the conserved amino acids of the melanocortin-4 receptor in [Nle4, D-Phe7]-α-melanocyte-stimulating hormone binding and signaling. J Biol Chem. 2007;282:21712–21719. doi: 10.1074/jbc.M702285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tao X, Segaloff DL. Functional characterization of melanocortin-3 receptor variants identify a loss-of-function mutation involving an amino acid critical for G protein-coupled receptor activation. J Clin Endocrinol Metab. 2004;89:3936–3942. doi: 10.1210/jc.2004-0367. [DOI] [PubMed] [Google Scholar]
- 51.Wang SX, Fan Z-C, Tao YX. Functions of acidic transmembrane residues in human melanocortin-3 receptor binding and activation. Biochem Pharmacol. 2008;76:520–530. doi: 10.1016/j.bcp.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hruby VJ, Wilkes BC, Hadley ME, Al-Obeidi F, Sawyer TK, Staples DJ, de Vaux AE, Dym O, de Lauro Castrucci A-M, Hintz MF, Riehm JR, Rao RR. α-Melanotropin: the minimum active sequence in the frog skin bioassay. J Med Chem. 1987;30:2126–2130. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- 53.de Lauro Castrucci AM, Hadley ME, Sawyer TK, Wilkes B, Al-Obeidi F, Staples DJ, de Vaux AE, Dym O, Hintz MF, Riehm JP, Rao RR, Hruby VJ. α-Melanotropin: the minimal active sequence in the lizard skin bioassay. Gen Comp Endocrinol. 1989;73:157–163. doi: 10.1016/0016-6480(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 54.Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8(1):41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bridges TM, Lindsley CW. G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem Biol. 2008;3(9):530–541. doi: 10.1021/cb800116f. [DOI] [PubMed] [Google Scholar]
- 56.Lewis JA, Lebois EP, Lindsley CW. Allosteric modulation of kinases and GPCRs: design principles and structural diversity. Curr Opin Chem Biol. 2008;12(3):269–280. doi: 10.1016/j.cbpa.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Avlani VA, McLoughlin DJ, Sexton PM, Christopoulos A. The impact of orthosteric radioligand depletion on the quantification of allosteric modulator interactions. J Pharmacol Exp Therap. 2008;325(3):927–934. doi: 10.1124/jpet.108.136978. [DOI] [PubMed] [Google Scholar]
- 58.Rees S, Morrow D, Kenakin T. GPCR drug discovery through the exploitation of allosteric drug binding sites. Receptors Channels. 2002;8(5/6):261–268. [PubMed] [Google Scholar]
- 59.Christopoulos A, Kenakin T. G-protein coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54(2):323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 60.Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov. 2002;1(3):198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- 61.Milligan G, Smith NJ. Allosteric modulation of heterodimeric G-protein coupled receptors. Trends Pharm Sci. 2007;28(12):615–620. doi: 10.1016/j.tips.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 62.May LT, Lin Y, Sexton PM, Christopoulos A. Regulation of M2 muscarinic acetylcholine receptor expression and signaling by prolonged exposure to allosteric modulators. J Pharmacol Exp Therap. 2005;312(1):382–390. doi: 10.1124/jpet.104.073767. [DOI] [PubMed] [Google Scholar]
- 63.Raddatz R, Schaffhauser H, Marino MJ. Allosteric approaches to the targeting of G-Protein-Coupled Receptors for novel drug discovery: a critical assessment. Biochem Pharmacol. 2007;74:383–391. doi: 10.1016/j.bcp.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Kenakin T. Allosteric theory: taking therapeutic advantage of the malleable nature of GPCRs. Curr Neuropharmacol. 2007;5:149–156. doi: 10.2174/157015907781695973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.May LT, Avlani VA, Sexton PM, Christopoulos A. Allosteric modulation of G Protein-Coupled Receptors. Curr Pharm Des. 2004;10:2003–2013. doi: 10.2174/1381612043384303. [DOI] [PubMed] [Google Scholar]; May LT, Christopoulos A. Allosteric modulators of G-Protein Coupled Receptors. Curr Opin Pharmacol. 2003;3:551–556. doi: 10.1016/s1471-4892(03)00107-3. [DOI] [PubMed] [Google Scholar]
- 66.Soudijn W, van Wijngaarden I, Ijzerman AP. Allosteric modulation of G Protein Coupled Receptors: perspectives and recent developments. Drug Discov Today. 2004;9:17. doi: 10.1016/S1359-6446(04)03220-9. [DOI] [PubMed] [Google Scholar]
- 67.Hruby VJ, Cai M, Grieco P, Han G, Kavarana M, Trivedi D. Exploring the stereostructural requirements of peptide ligands for the melanocortin receptors. Ann NY Acad Sci. 2003;994:12–20. doi: 10.1111/j.1749-6632.2003.tb03157.x. [DOI] [PubMed] [Google Scholar]
- 68.Grieco P, Balse PM, Weinberg D, MacNeil T, Hruby VJ. D-amino acid scan of γ-melanocyte-stimulating hormone: importance of Trp8 on human MC3 receptor selectivity. J Med Chem. 2002;43:4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- 69.Cai M, Mayorov AV, Cabello C, Stankova M, Trivedi D, Hruby VJ. Novel 3D pharmacophore of α-MSH/γ-MSH hybrids leads to selective human MC1R and MC3R analogues. J Med Chem. 2005;48:1839–1848. doi: 10.1021/jm049579s. [DOI] [PubMed] [Google Scholar]
- 70.Kavarana MJ, Trivedi D, Cai M, Ying J, Hammer M, Cabello C, Grieco P, Han G, Hruby VJ. Novel cyclic templates of alpha-MSH give highly selective and potent antagonists/agonists for human melanocortin-3/4 receptors. J Med Chem. 2002;45:2644–2650. doi: 10.1021/jm020021z. [DOI] [PubMed] [Google Scholar]
- 71.Balse-Srinivasan PM, Grieco P, Cai M, Trivedi D, Hruby VJ. Structure-activity relationships of novel cyclic α-MSH/(β-MSH hybrid analogues that lead to potent and selective ligands for the human MC3R and human MC5R. J Med Chem. 2003;46:3728–3733. doi: 10.1021/jm030111j. [DOI] [PubMed] [Google Scholar]
- 72.Cai M, Mayorov AV, Ying J, Stankova M, Trivedi D, Cabello C, Hruby VJ. Design of novel melanotropin agonists and antagonists with high potency and selectivity for human melanocortin receptors. Peptides. 2005;26:1481–1485. doi: 10.1016/j.peptides.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 73.Mayorov AV, Cai M, Chandler KB, Petrov RR, Van Scoy AR, Yu Z, Tanaka DK, Trivedi D, Hruby VJ. Development of cyclic γ-MSH analogues with selective hMC3R agonist and hMC3R/hMC5R antagonist activities. J Med Chem. 2006;49:1946–1952. doi: 10.1021/jm0510326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cain JP, Mayorov AV, Cai M, Wang H, Tan B, Chandler K, Lee YS, Petrov RR, Trivedi D, Hruby VJ. Design, synthesis, and biological evaluation of a new class of small molecule peptide mimetics targeting the melanocortin receptors. Bioorg Med Chem Letts. 2006;16(20):5462–5467. doi: 10.1016/j.bmcl.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ballet S, Mayorov AV, Cai M, Tymecka D, Chandler KB, Palmer ES, Van Rompaey K, Misicka A, Tourwe D, Hruby VJ. Novel selective human melanocortin-3 receptor ligands: Use of the 4-amino-1,2,4,5-tetrahydro-2-benzazepin-3-one (Aba) scaffold. Bioorg Med Chem Letts. 2007;17:2492–2498. doi: 10.1016/j.bmcl.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cai M, Cai C, Mayorov AV, Xiong C, Cabello CM, Soloshonok VA, Swift JR, Trivedi D, Hruby VJ. Biological and conformational study of β-substituted prolines in MT-II template: steric effects leading to human MC5 receptor selectivity. J Peptide Res. 2004;63:116–131. doi: 10.1111/j.1399-3011.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 77.Qu H, Cai M, Mayorov AV, Grieco P, Zingsheim M, Trivedi D, Hruby VJ. Substitution of arginine with proline and proline derivatives in melanocyte-stimulating hormones leads to selectivity for human melanocortin 4 receptor. J Med Chem. 2009;52(12):3627–3635. doi: 10.1021/jm801300c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee YS, Poh LKS, Kek BLK, Loke KY. The role of melanocortin 3 receptor gene in childhood obesity. Diabetes. 2007;56(10):2622–2630. doi: 10.2337/db07-0225. [DOI] [PubMed] [Google Scholar]


