Abstract
The presence of drugs and their metabolites in surface waters and municipal effluents has been reported in several studies, but its impacts on aquatic organisms are not yet well understood. This study investigated the effects of acute exposure to the antipsychotic risperidone on the stress and behavioral responses in zebrafish. It became clear that intermediate concentration of risperidone inhibited the hypothalamic-pituitary-interrenal axis and displayed anxiolytic-like effects in zebrafish. The data presented here suggest that the presence of this antipsychotic in aquatic environments can alter neuroendocrine and behavior profiles in zebrafish.
Introduction
The contamination of water resources like natural water bodies or urban effluents by pharmaceutical drugs and/or its metabolites has been reported since the 1970’s, consequently increasing the concern with health consequences for human population as well as for the aquatic life [1–9]. Risperidone, an atypical antipsychotic, has high affinity for serotonin type 2 (5-HT2) and dopamine type 2 (D2) receptors. This drug is widely used for the treatment of schizophrenia and bipolar disorder. In people with schizophrenia in maintenance treatment, risperidone induces relatively few extrapyramidal syndromes (EPS), especially less akathisia and tremor, as compared to typical antipsychotic haloperidol. As expected, due to its high prescription rates and off-label uses [10–13], risperidone has been detected in urban water wastes [3].
The activation of the hypothalamus-pituitary-interrenal (HPI) axis and the consequent cortisol elevation is an important reaction of the organism against physical stressors, predator encounters, and chemical stressors such as drug contaminations, aiming to restore the homeostasis [14–18] with generalized effects in metabolism, growth, immune system and osmoregulation processes [19–21]. Thus, pollutants that interfere with the fish stress response can adversely affect their survival [22–23].
We hypothesized that risperidone residues, due its central effects, might alter the functioning of the stress neuroendocrine axis, impairing the general response of the fish residing in contaminated water bodies. We tested our hypothesis using zebrafish (Danio rerio) as the experimental model, since this species presents many advantages such as easy handling and maintenance as well as shows high genetic homology with humans [24–26]. Several recent studies have reinforced its use as an organism model for stress research [26–32].
Materials and Methods
Ethical note
This study followed the guidelines of Conselho Nacional de Controle de Experimentação Animal (CONCEA) and was approved by the Ethics Commission for Animal Use (CEUA) at Universidade de Passo Fundo, UPF, Passo Fundo, RS, Brazil (Protocol # 7/2013 –CEUA). The use of this method was justified to avoid potential confounding factors while interpreting data regarding the effects of risperidone on the stress response axis.
Animals
A population of approximately 500 mixed-sex, adult wild-type zebrafish (Danio rerio) short-fin (SF) strain, weighing 0.7 to 0.9 g were held in glass aquaria with constant aeration and equipped with biological filtering under a natural photoperiod (approximately 14 h light: 10 h dark). Water was maintained at 26 ± 2°C and pH 7.0, with dissolved oxygen levels at 6 ± 0.5 mg/L, total ammonia levels at 0.01 mg/L, total hardness at 6 mg/L, and alkalinity at 22 mg/L CaCO3. Behavioral testing was performed during the afternoon.
Experiment 1 – acute stress challenge
Zebrafish were distributed in 36 glass aquaria (30 x 30 x 30 cm, six fish per tank), acclimatized for seven days and fed with commercial food flakes (Tetra Min, Tetra, Melle, Germany). Twenty-four hours later, fish were exposed to risperidone for 15 minutes. Fish were then stressed by chasing them with a net for two minutes [28], and sampled after 0, 15, 60 and 240 minutes for whole-body cortisol analysis. Similarly, groups of fish were exposed to risperidone without stress (sampled at the same time points), aiming to evaluate an eventual stress effect of the risperidone per se. A basal situation, i.e. without drug exposure and stress test was performed as control. We used five risperidone concentrations (with or without stress) plus the controls (with or without stress). Thus, we have 12 glass aquaria with six fish each. We repeated this setup 3 times, using two fish from each aquaria in each replication, totalizing a final sample of 6 fish (n = 6).
Risperidone (Risperidon, 1 mg/mL, Laboratório EMS, Hortolândia, SP) was used in five concentrations: 0.00034 μg/L, 85 μg/L, 170 μg/L, 340 μg/L and 680 μg/L. The lower concentration was already detected in the environment [3] while the highest concentration was calculated based on the concentration that produced effect zebrafish behavior [33]. The environmental concentration was then multiplied by 250,000, 500,000, 1,000,000 and 2,000,000 to reach the intermediate concentrations of 85, 170, 340 and 680 μg/L, respectively.
Experiment 2 – novel tank test
We performed a behavioral test (novel tank test [26, 27]) using risperidone at the concentration of 170 μg/L, which impaired cortisol response in experiment 1 (see results section, Fig 1). For this purpose, 24 zebrafish not exposed in the experiment 1, were distributed in four groups: (1) control (no exposure to risperidone, no stress), (2) risperidone exposed, (3) stress and (4) risperidone exposed + stress. After 15 min of exposure, fish were individually transferred to the novel tank (4 x 20 x 20 cm) and video recorded for 5 minutes, 15 min after stressor application. The videos were then analyzed using AnyMaze® video tracking system (Stoelting, CO, USA) and the following parameters were analyzed: total distance (m), mean speed (m/s), absolute turn angle, number of crossings between different compartments of the tank (upper, middle and bottom) and the time spent in upper, middle and bottom compartments.
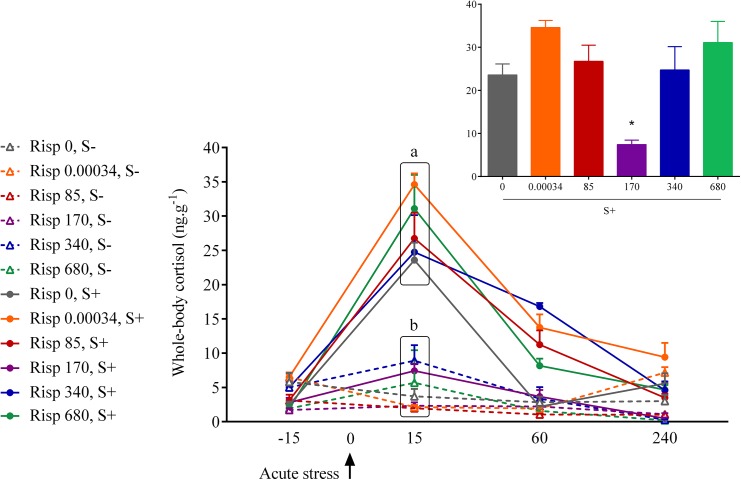
Fig 1. Whole-body cortisol concentrations in zebrafish exposed to risperidone followed by an acute stress test and respective controls.
The values are expressed as the mean ± standard error of mean of 5–6 fish. Different small letters indicate significant group differences in each sampling time. The insert shows graphical demonstration of U-shaped dose response curve at the time of cortisol peak. S- and S+ refer to non-stressed and stressed fish, respectively.
Procedures–whole-body cortisol extraction and analysis
Fish were captured and immediately frozen in liquid nitrogen for 10–30 s, followed by storage at -20°C until cortisol extraction. Whole-body cortisol was extracted using the method described by Oliveira et al. [28]. Tissue extracts were resuspended in 1mL PBS, and whole-body cortisol levels were measured in duplicate for each extraction using a commercially available enzyme-linked immune sorbent as say kit (EIAgen CORTISOL test, Bio Chem Immuno Systems). This kit was fully validated for zebrafish tissue extracts using the methodology described by Sink et al. [34].
Statistics
Whole-body cortisol levels were analyzed by three-way Analysis of Variance (ANOVA) with treatment, stress and sampling time as the independent variables, followed by two-way ANOVAs restricted to each stress condition. Differences at each time point were analyzed by Bonferroni post-hoc test. Behavioral parameters were analyzed by two-way ANOVAs with treatment and stress as the independent variables, followed by Tukey’s multiple range post-hoc test. For both data sets, the homogeneity of variance was determined using Hartley’s test, and normality was determined using the Kolmogorov-Smirnov test. Data were expressed as means ± standard error of mean (S.E.M). Significance level was set at p<0.05.
Results
Experiment 1 – acute stress challenge
The acute stress protocol increased cortisol levels in zebrafish. There was a significant interaction among treatment, stress and time. Zebrafish exposed to 170 mg/L of risperidone and submitted to the acute stress test had a reduced cortisol response to an acute stressor at 15, 60 and 240 minutes after stress (Fig 1). Significant effects were observed in the analysis restricted to non-stressed animals, but cortisol levels in these groups are within the normal range reported in the literature. Table 1 summarizes the results yielded by the statistical analysis. The cortisol raw data and the complete statistical analysis are presented in supporting information (S1 File and S2 File).
Table 1. Results of analysis of variance (ANOVA) for cortisol levels.
| Analysis | Effects | F-value | DF | P-value |
|---|---|---|---|---|
| 3-way ANOVA | Treatment | 29.650 | 5,233 | 0.0001 |
| Stress | 421.812 | 1,233 | 0.0001 | |
| Time | 165.849 | 3,233 | 0.0001 | |
| Treatment × stress | 16.413 | 5,233 | 0.0001 | |
| Treatment × time | 5.598 | 15,233 | 0.0001 | |
| Stress × time | 169.065 | 3,233 | 0.0001 | |
| Treatment × stress × time | 6.667 | 15,233 | 0.0001 | |
| 2-way ANOVA: S- | Treatment | 16.687 | 5,116 | 0.0001 |
| Time | 10.144 | 3,116 | 0.0001 | |
| Treatment × time | 6.355 | 15,116 | 0.0001 | |
| 2-way ANOVA: S+ | Treatment | 23.401 | 5,117 | 0.0001 |
| Time | 188.867 | 3,117 | 0.0001 | |
| Treatment × time | 5.792 | 15,117 | 0.0001 |
The table summarizes the main effects of and the interaction between treatment, stress and time, as well as the restricted ANOVAs to each stress condition. DF refers to degrees of freedom. Significant effects (p<0.05) are given in bold font.
Experiment 2 – novel tank test
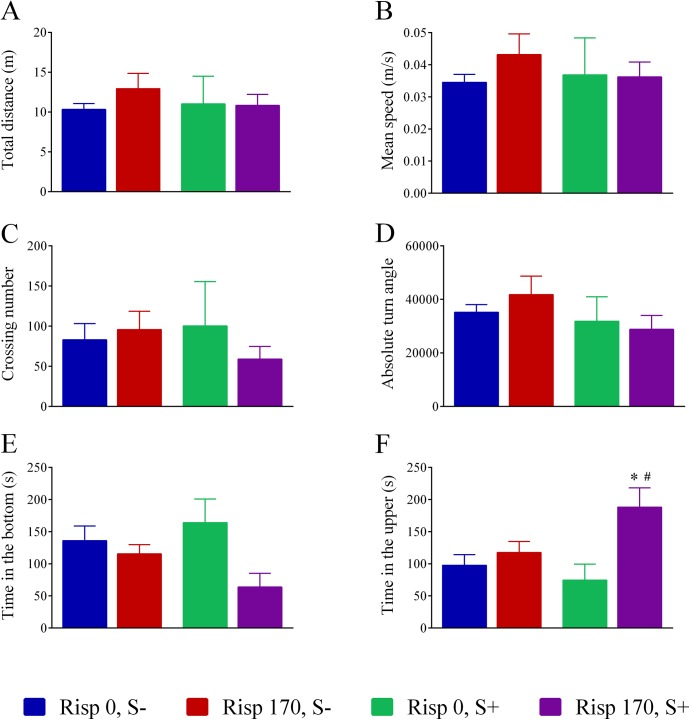
The Fig 2 shows the effects of risperidone (170 μg/L) in the novel tank test. A significant main effect of treatment was observed for time in the bottom and upper, and a significant interaction between treatment and stress was observed for time in the upper. Table 2 summarizes the results yielded by the statistical analysis for each behavioral parameter evaluated. Post hoc analysis revealed that risperidone increased time spent in the upper in stressed fish. Total distance, mean speed, absolute turn angle, and number of crossings did not differ among experimental groups. The behavioral raw data and the complete statistical analysis are presented in supporting information (S3 File and S4 File).
Fig 2. Behavioral parameters of zebrafish in the novel tank test followed by an acute stress protocol.
Total distance (A), mean speed (B), crossings between compartments (C), absolute turn angle (D), time spent in the bottom (E), and time in the upper (F). The data are expressed as the mean ± standard error of mean of 5–6 fish. * = p<0.05 compared to Risp 0, S- group; # = p<0.05 compared to Rips 0, S+ group. S- and S+ refer to non-stressed and stressed fish, respectively.
Table 2. Results of analysis of variance (ANOVA) for the behavioral tests.
| Dependent variable | Effects | F-value | DF | P-value |
|---|---|---|---|---|
| Total distance | Treatment | 0.302 | 1,19 | 0.589 |
| Stress | 0.103 | 1,19 | 0.751 | |
| Treatment × stress | 0.405 | 1,19 | 0.532 | |
| Crossings | Treatment | 0.175 | 1,19 | 0.680 |
| Stress | 0.084 | 1,19 | 0.775 | |
| Treatment × stress | 0.637 | 1,19 | 0.435 | |
| Mean speed | Treatment | 0.300 | 1,19 | 0.591 |
| Stress | 0.100 | 1,19 | 0.756 | |
| Treatment × stress | 0.401 | 1,19 | 0.534 | |
| Absolute turn angle | Treatment | 0.074 | 1,19 | 0.788 |
| Stress | 1.536 | 1,19 | 0.230 | |
| Treatment × stress | 0.530 | 1,19 | 0.476 | |
| Time in the bottom | Treatment | 5.559 | 1,19 | 0.029 |
| Stress | 0.212 | 1,19 | 0.650 | |
| Treatment × stress | 2.432 | 1,19 | 0.135 | |
| Time in the middle | Treatment | 0.189 | 1,19 | 0.668 |
| Stress | 0.661 | 1,19 | 0.426 | |
| Treatment × stress | 0.230 | 1,19 | 0.637 | |
| Time in the upper | Treatment | 9.029 | 1,19 | 0.007 |
| Stress | 1.145 | 1,19 | 0.298 | |
| Treatment × stress | 4.469 | 1,19 | 0.048 |
The table summarizes the main effects of and the interaction between treatment and stress. DF refers to degrees of freedom. Significant effects (p<0.05) are given in bold font.
Discussion
Here we showed that acute exposure to an intermediate concentration of risperidone of 170 μg/L impaired the stress axis response, since the exposed zebrafish had lower cortisol levels than control fish, when exposed to an acute stress challenge. We also showed that zebrafish exposed to this concentration of risperidone decreased the time spent in the bottom compartment of the tank when compared with stressed fish, reinforcing the anxiolytic effect of this drug [35–38]. To our knowledge, this is the first report about risperidone effects on the stress neuroendocrine axis in zebrafish.
The risperidone mechanism is yet unclear, but it acts directly on the central nervous system, more specifically in dopaminergic and serotoninergic receptors, probably without any direct effect on interrenal tissue [13, 39]. The serotoninergic system plays an essential role in the fish stress response [40].
Considering the concentrations used, the HPI impairing effect was verified only in the intermediate concentration of 170 μg/L. The lowest (0.00034 μg/L and 85 μg/L) and highest (340 μg/L and 680 μg/L) concentrations did not block the cortisol elevation after the acute stress challenge. Thus, the dose-response curve presented a U-shaped response (see the insert in the Fig 1). This type of response was verified previously for the effects of diazepam [41] and ethanol [28] on stress response and behavior [42]. Similar U-shaped curve was also found for the cortisol effects on human memory [43].
The biological meaning of the minor cortisol fluctuations induced by risperidone in non-stressed animals remains to be elucidated. Nevertheless, the risperidone concentration that blunted the cortisol increase in stressed animals did not alter cortisol in non-stressed, control animals, suggesting that risperidone does not influence basal hormonal levels per se, but rather it modulates the response elicited by stress.
Regarding the novel tank test, zebrafish exposed to risperidone showed a decrease in the time spent in the tank bottom and stressed fish exposed to risperidone spent more time in the upper part of the tank, demonstrating its anxiolytic effects. This anxiolytic-like effect of atypical antipsychotics was also verified in mammals [44–45]. The risperidone effect on zebrafish behavior was previously verified using a light-dark test, where the drug decreased the number of crossing between light and dark compartments [33]. In this study, we analyzed the zebrafish behavior at 15 minutes after stress. Thus, we cannot discard that more clear behavioral effects of risperidone exposure could be detected if analyzed earlier than 15 minutes after stress exposure.
The anxiolytic effect of risperidone is not well understood since the exact mechanisms by which it blocks the HPI functioning in response to a stress challenge are still unclear. Despite the unclear mechanism, a fish with an impaired capacity to respond and cope with stress lost its ability to maintain homeostasis against stressors by reducing the ability to promote the necessary adjustments [22–23, 46–47]. Also, the behavioral changes produced by risperidone exposure may reduce the caution in predator inspection and consequently increase the risk of predation [48]. Combining the neuroendocrine and behavioral results and considering that these effects were detected in a concentration much higher than the one found in the environment, the consequences of an environmental contamination with risperidone are difficult to predict.
The concentration of 170 μg/L of risperidone is unexpected in natural environments. However, aquatic organisms may be exposed to accidental spills of pollutants, incorrect discharges of substances or contaminants already present in the water. Such contamination may cause biomagnification, in which concentrations much higher than those found in the environment may be observed. This phenomenon has been reported for the presence of pesticides [49–51].
Our results highlight that the presence of risperidone residues in aquatic ecosystems may blunt the cortisol response to stress as well the fish behavior with consequences on fish survival and welfare.
Supporting Information
Raw data of cortisol determination.
(PDF)
Statistics of cortisol data.
(PDF)
Raw data of behavioral tests.
(PDF)
Statistics of behavioral data.
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico—research fellowships for LJGB and ALP have a CNPq (301992/2014-2 and 472715/2012-7, respectively). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hignite C, Azarnoff DL (1977) Drugs and drug metabolites as environmental contaminants: chloro phenoxyisobutyrate and salicylic acid in sewage water effluent. Life Sci 20: 337–341. [DOI] [PubMed] [Google Scholar]
- 2. Brooks BW, Chambliss CK, Stanley JK, Ramirez A, Banks KE, Johnson RD et al. (2005) Determinations of Select Antidepressant in Fish From An Effuent–Dominated Stream. Environm Toxicol Chem 24: 464–469. [DOI] [PubMed] [Google Scholar]
- 3. Calisto V, Esteves VI (2009) Psychiatric pharmaceuticals in the environment. Chemosphere 77: 1257–1274. 10.1016/j.chemosphere.2009.09.021 [DOI] [PubMed] [Google Scholar]
- 4. Alonso AG, Catala M, Maroto RR, Gil JL, de Miguel AG, Valcarcel Y. (2010) Pollution by psychoactive pharmaceuticals in the Rivers of Madrid metropolitan area (Spain). EnvironmInternat 36:195–201. [DOI] [PubMed] [Google Scholar]
- 5. Jones OA, Lester JN, Voulvoulis N (2005) Pharmaceuticals: a threat to drinking water? Trends Biotechnol 23: 163–167. [DOI] [PubMed] [Google Scholar]
- 6. Calisto V, Domingues MRM, Esteves VI (2011) Photodegradation of Psychiatric pharmaceuticals in aquatic environments–Kinetics and photodegradation products. Water Res 45: 6097–6106. 10.1016/j.watres.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 7. Heberer T (2002) Traching persistent pharmaceutical residues from municipal sewage to drinking water. J Hydrol 266: 175–189. [PubMed] [Google Scholar]
- 8. Calamari D, Zuccato E, Castiglioni S, Bagnati R, Fanelli R. (2003). Strategic Survey of therapeutic Drugs in the Rivers PO and Lambro in Northern Italy. Environm Sci Technol 37: 1241–1248. [Google Scholar]
- 9. Brodin T, Fick J, Jonsson M, Klaminder J. (2013) Dilute Concentration of a Psychiatric Drug Alter Behavior of Fish from Natural Populations. Science 339: 814–815. 10.1126/science.1226850 [DOI] [PubMed] [Google Scholar]
- 10. Baldessarini RJ (2000) A plea for integrity of the bipolar disorder concept. Bipolar Dis 2: 3–7. [DOI] [PubMed] [Google Scholar]
- 11. Bascunana H, Villarreal I, Alfonso S, Bernabeu M, Terr’e R. (2000) Agitation in head injury. I. Definition and treatment with anxiolytic neuroleptics and antiepileptic drugs. Rev Neurol 30: 850–854. [PubMed] [Google Scholar]
- 12. Daniel DG (2000) Antipsychotic treatment of psychosis and agitation in the elderly. J Clin Psychiatry 61: 49–52. [PubMed] [Google Scholar]
- 13. Oliveira IR (2000) Antipsicóticos atípicos: farmacologia e uso clínico. Rev Bras Psiquiatr 22: 38–40. [Google Scholar]
- 14. Blanchard DC, Blanchard RJ (1988) Ethoexperimental approaches to the biology of emotion. Ann Rev Psychol 39: 43–68. [DOI] [PubMed] [Google Scholar]
- 15. Blanchard RJ, Hori KM, Mayer SI, Rodgers RJ, Blanchard DC. (1990) Anethopharmacological approach to the biology of anxiety In: Morato S, Carobrez AP, Lima TCM. Neurosciences & Behavaior – 2 Ribeirão Preto: Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, p. 125–141, 1990. [Google Scholar]
- 16. Graeff FG, Zangrossi H Jr (2010) The hypothalamic-pituitary-adrenal axil in anxiety and panic. Psychol Neurosci 3: 3–8. [Google Scholar]
- 17. Mariano WS, Oba ET, Santos LRB, Fernandes MN. Physiological responses in jeju (Hoplerythrinus unitaeniatus) to atmospheric air exposure. Rev. Bras. Saúde Prod. An., v.10, n.1, p.210–223, 2009. [Google Scholar]
- 18. Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42: 517–525. 10.1093/icb/42.3.517 [DOI] [PubMed] [Google Scholar]
- 19. Chrousos GP, Gold PW (1992) The Concepts of Stress and stress System Disorders. Overview of Physical and Behavioral Homeostasis. JAMA 4; 267(9):1244–52. [PubMed] [Google Scholar]
- 20. Wendelaar Bonga SE (1997) The stress response in fish. Phisiol Rev 77:591–625. [DOI] [PubMed] [Google Scholar]
- 21. Mancera MJ, Carrión RL, Martin DP, Rio DM (2002) Osmorresgulatory action of PRL, GH and cortisol in the gilthead seabrem (Sparus aurata L). Gen Comp Endocrinol 129: 95–103. [DOI] [PubMed] [Google Scholar]
- 22. Cericato L, Neto JGM, Fagundes M, Kreutz LC, Quevedo RM, Finco J et al. (2008) Cortisol response to acute stress in jundia Rhamdia quelen acutely exposed to sub-lethal concentrations of agrichemicals. Comp Biochem Physiol C 148: 281–286. [DOI] [PubMed] [Google Scholar]
- 23. Cericato L, Neto JGM, Kreutz LC, Quevedo RM, Rosa JGS, Koakoski G et al. (2009) Responsiveness of the interrenal tissue of Jundia (Rhamdia quelen) to an in vivo ACTH test following acute exposure to sublethal concentrations of agrichemicals. Comp Biochem Physiol C 149: 363–367. [DOI] [PubMed] [Google Scholar]
- 24. Barbazuk WB, Korf I, Kadavi C, Heyen J, Tate S, Wun E et al. (2000) The synthetic relationship of the zebrafish and human genomes. Genome Res 10: 1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldsmith P (2004) Zebrafish as a pharmacological tool: the how, why and when. Curr Opin Pharmacol 4: 504–12. [DOI] [PubMed] [Google Scholar]
- 26. Egan R, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF et al. (2009) Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res 205: 38–44 10.1016/j.bbr.2009.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cachat J, Stewart A, Grossman L, Gaikwad S, Kadri F, Chung KM et al. (2010) Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nature Prot 5(11): 1786–1799. [DOI] [PubMed] [Google Scholar]
- 28. Oliveira TA, Koakoski G, Kreutz LC, Ferreira D, Rosa JGS, Abreu MS et al. (2013) Alcohol Impairs Predation Risk Response and Communication in Zebrafish. PLOSONE 8(10):e75780 10.1371/journal.pone.0075780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barcellos LJG, Ritter F, Kreutz LC, Quevedo RM, Silva LB, Bedin AC et al. (2007) Whole body cortisol increases after direct and visual contact with the predator in zebrafish, Danio rerio . Aquaculture 272: 774–778. [Google Scholar]
- 30. Barcellos LJG, Ritter F, Kreutz LC, Cericato L (2010) Can Zebrafish Danio rerio learn about predation risk? The effect of a previous experience on the cortisol response in subsequent encounters with a predator. J Fish Biol 76: 1032–1038. [Google Scholar]
- 31. Piato AL, Capiotti KM, Tamborski A, Oses JP, Barcellos LJG, Bogo MR et al. (2011) Unpredictable chronic stress model in zebrafish (Danio rerio): Behavioral and physiological responses. Progr Neuro-Psychopharm Biol Psychiatry 35: 561–567. [DOI] [PubMed] [Google Scholar]
- 32. Dal Santo G, Conterato GMM, Barcellos LJG, Rosemberg DB, Piato AL (2013)Acute restraint stress induces an imbalance in the oxidative status of the zebrafish brain. Neurosci Lett 558: 103–108. 10.1016/j.neulet.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 33. Magno LD, Fontes A, Gonçalves BM, Gouveia A Jr. (2015) Pharmacological study of the light/dark preference test in zebrafish (Danio rerio): Waterborne administration. Pharmacol Biochem Behav 135:169–176. 10.1016/j.pbb.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 34. Sink TD, Lochmann RT, Fecteau KA (2007) Validation, use, and disadvantages of enzyme-linked immunosorbent assay kits for detection of cortisol in channel catfish, largemouth bass, red pacu and golden shiners. Fish Physiol Biochem 75:165–171. [DOI] [PubMed] [Google Scholar]
- 35. Blin O, Azorin JM, Bouhours P (1996) Antipsychotic and anxiolytic properties of risperidone, haloperidol, and methotrimeprazine in schizophrenic patients. J Clin Psychopharmacol 16: 38–44. [DOI] [PubMed] [Google Scholar]
- 36. Rogóż Z, Skuza G (2011) Anxiolytic-like effects of olanzapine, risperidone and fluoxetine in the elevated plus-maze test in rats. Pharmacol Rep 63: 1547–52. [DOI] [PubMed] [Google Scholar]
- 37. Sun T, He W, Hu G, Li M (2010) Anxiolytic-like property of risperidone and olanzapine as examined in multiple measures of fear in rats. Pharmacol Biochem Behav 95: 298–307. 10.1016/j.pbb.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krishnamurthy S, Garabadu D, Joy KP (2013) Risperidone ameliorates post-traumatic stress disorder-like symptoms in modified stress re-stress model. Neuropharmacol 75: 62–77. [DOI] [PubMed] [Google Scholar]
- 39. Cuesta MJ, Peralta V, Zarzuela A (2001) Effects of olanzapine and other antipsychotics on cognitive function in chronic schizophrenia: a longitudinal study. Schizophr Res 48: 17–28. [DOI] [PubMed] [Google Scholar]
- 40. Winberg S, Nilsson A, Hylland P, Södesrtöm V, Nilsson GE (1997) Serotonin as a regulator of hypothalamic-pituitary-interrenal activity in teleost fish. Neurosci Lett 230: 113–116. [DOI] [PubMed] [Google Scholar]
- 41. Abreu MSd, Koakoski G, Ferreira D, Oliveira TA, Rosa JGSd, Gusso D et al. (2014) Diazepam and Fluoxetine Decrease the Stress Response in Zebrafish. PLoSONE 9(7): e103232 10.1371/journal.pone.0103232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gerlai R, Lahav M, Guo S, Rosenthal A (2000) Drinks like a fish: zebrafish (Danio rerio) as abehavior genetic model to study alcohol effects. Pharmacol Biochem Behav 67: 773–782. [DOI] [PubMed] [Google Scholar]
- 43. Schilling TM, Kölsch M, Larra MF, Zech CM, Blumenthal TD. (2013) For whom the bell (curve) tolls: Cortisol rapidly affects memory retrieval by an inverted U-shaped dose-response relationship. Psychoneuroendocrinol 38: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 44. Nowakowskaet E, Chodera A, Kusk K, Rybakowski J. (1999) Some behavioral of risperidone in rats: comparison with haloperidol. Euro Neuropsychopharmacol 9: 421–426. [DOI] [PubMed] [Google Scholar]
- 45. Sun T, He W, Hu G, Li M. (2010) Anxiolytic-like property of risperidone and olanzapine as examined in multiple measures of fear in rats. PharmacolBiochemBehav 95:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hontela A (1998) Interrenal dysfunction in fish from contaminated sites: In Vivo and In Vitro Assessment. Environ Toxicol Chem 17: 44–48. [Google Scholar]
- 47. Pacheco M, Santos MA (2001) Biotransformation, endocrine and genetic responses of Anguilla Anguilla L. to petroleum distillate products and environmentally contaminated waters. Ecotox Environm Saf 49: 64–75. [DOI] [PubMed] [Google Scholar]
- 48. Dugatkin LA (1992) Tendency to inspect predators predicts mortality risk in the guppy (Poecilia reticulata). Behav Ecol 3: 124–127. [Google Scholar]
- 49. Niethammer KR, White DH, Baskett TS, Sayre MW (1984) Presence and biomagnification of organochlorine chemical residues in oxbow lakes of Northeastern Louisiana. Arc Environm Contam Toxicol 13: 63–74. [DOI] [PubMed] [Google Scholar]
- 50. Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FAPC (2007) Food web–specific biomagnification of persistent organic pollutants. Science 317: 236–239. [DOI] [PubMed] [Google Scholar]
- 51. Goerke H, Weber K, Bornemann H, Ramdohr S, Plötz J (2004). Increasing levels and biomagnification of persistent organic pollutants (POPs) in Antarctic biota. Mar Pollut Bull 48: 295–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data of cortisol determination.
(PDF)
Statistics of cortisol data.
(PDF)
Raw data of behavioral tests.
(PDF)
Statistics of behavioral data.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.