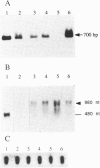
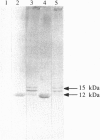
Abstract
Cytoplasmic male sterility in plants is associated with mitochondrial dysfunction. We have proposed that a nuclear-encoded chimeric peptide formed by mitochondrial sequences when imported into the mitochondria may impair organelle function and induce male sterility in plants. A model developed to test this hypothesis is reported here. Assuming that the editing process in higher plant mitochondria reflects a requirement for producing active proteins, we have used edited and unedited coding sequences of wheat ATP synthase subunit 9 (atp9) fused to the coding sequence of a yeast coxIV transit peptide. Transgenic plants containing unedited atp9 exhibited either fertile, semifertile, or male-sterile phenotypes; controls containing edited atp9 or only the selectable marker gave fertile plants. Pollen fertility ranged from 31% to 75% in fertile plants, 10% to 20% in semifertile plants, and < 2% in male-sterile plants. Genetic and molecular data showed that the chimeric plasmid containing the transgene is inherited as a Mendelian trait. The transgenic protein is imported into the mitochondria. The production and frequency of semifertile or male-sterile transgenic plants conform to the proposed hypothesis.
Full text
PDF
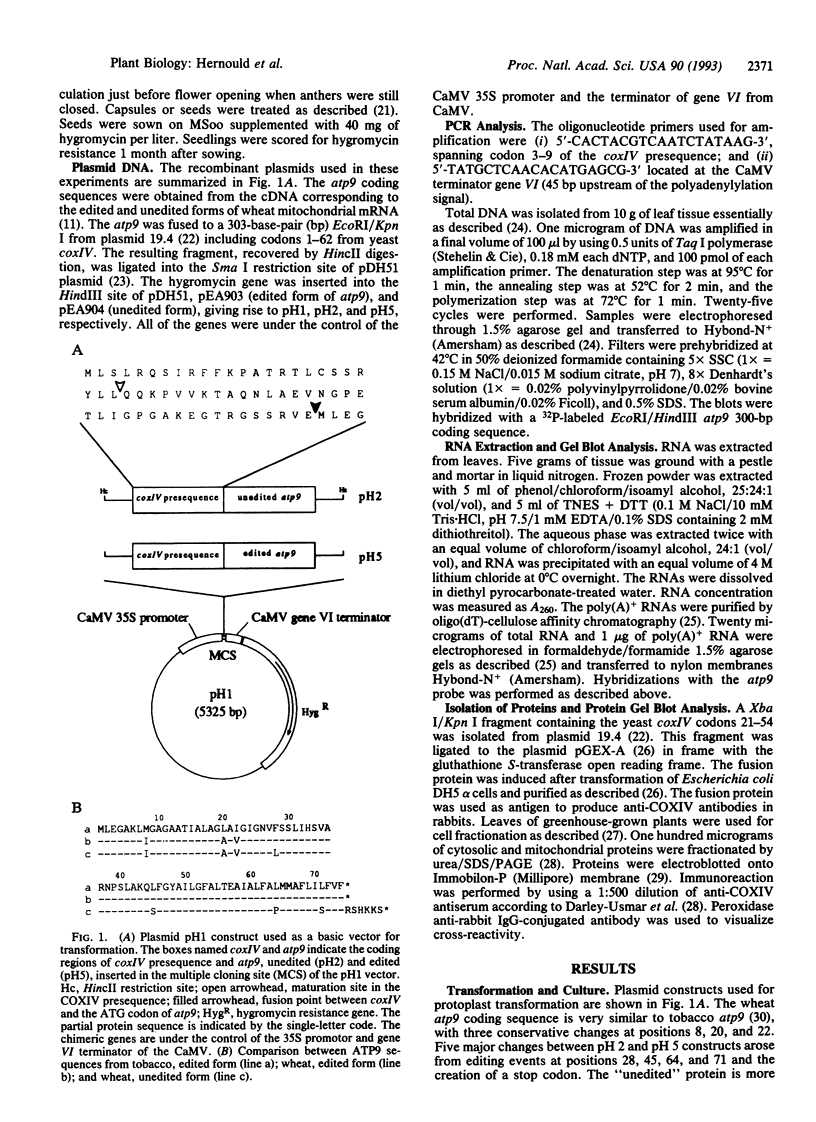
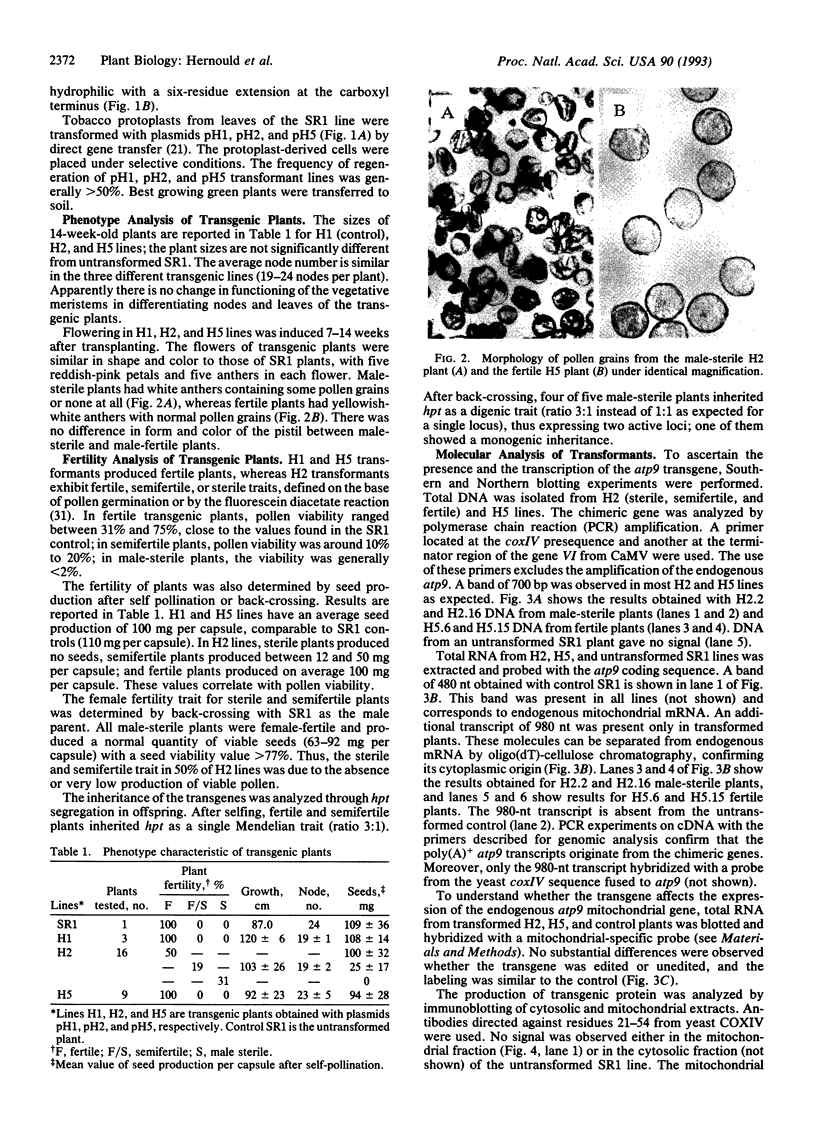


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bégu D., Graves P. V., Domec C., Arselin G., Litvak S., Araya A. RNA editing of wheat mitochondrial ATP synthase subunit 9: direct protein and cDNA sequencing. Plant Cell. 1990 Dec;2(12):1283–1290. doi: 10.1105/tpc.2.12.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. Y., Bogorad L., Van Montagu M., Schell J. Relocating a gene for herbicide tolerance: A chloroplast gene is converted into a nuclear gene. Proc Natl Acad Sci U S A. 1988 Jan;85(2):391–395. doi: 10.1073/pnas.85.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., 3rd, Timothy D. H. Novel recombinations in the maize mitochondrial genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell. 1986 Feb 14;44(3):439–449. doi: 10.1016/0092-8674(86)90465-4. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. Two genes encoding the bovine mitochondrial ATP synthase proteolipid specify precursors with different import sequences and are expressed in a tissue-specific manner. EMBO J. 1985 Dec 16;4(13A):3519–3524. doi: 10.1002/j.1460-2075.1985.tb04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing D. P., Nagley P. Yeast mitochondrial ATPase subunit 8, normally a mitochondrial gene product, expressed in vitro and imported back into the organelle. EMBO J. 1986 Dec 20;5(13):3651–3655. doi: 10.1002/j.1460-2075.1986.tb04695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves P. V., Bégu D., Velours J., Neau E., Belloc F., Litvak S., Araya A. Direct protein sequencing of wheat mitochondrial ATP synthase subunit 9 confirms RNA editing in plants. J Mol Biol. 1990 Jul 5;214(1):1–6. doi: 10.1016/0022-2836(90)90138-c. [DOI] [PubMed] [Google Scholar]
- Hanson M. R. Plant mitochondrial mutations and male sterility. Annu Rev Genet. 1991;25:461–486. doi: 10.1146/annurev.ge.25.120191.002333. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Hernould M., Mouras A., Litvak S., Araya A. RNA editing of the mitochondrial atp9 transcript from tobacco. Nucleic Acids Res. 1992 Apr 11;20(7):1809–1809. doi: 10.1093/nar/20.7.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop-Harrison J., Heslop-Harrison Y. Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technol. 1970 May;45(3):115–120. doi: 10.3109/10520297009085351. [DOI] [PubMed] [Google Scholar]
- Holden M. J., Sze H. Dissipation of the Membrane Potential in Susceptible Corn Mitochondria by the Toxin of Helminthosporium maydis, Race T, and Toxin Analogs. Plant Physiol. 1987 Jul;84(3):670–676. doi: 10.1104/pp.84.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J., Igloi G. L., Metzlaff M., Hagemann R., Kössel H. RNA editing in tobacco chloroplasts leads to the formation of a translatable psbL mRNA by a C to U substitution within the initiation codon. EMBO J. 1992 Mar;11(3):1099–1103. doi: 10.1002/j.1460-2075.1992.tb05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarse A. C., Van Loon A. P., Riezman H., Gregor I., Schatz G., Grivell L. A. Subunit IV of yeast cytochrome c oxidase: cloning and nucleotide sequencing of the gene and partial amino acid sequencing of the mature protein. EMBO J. 1984 Dec 1;3(12):2831–2837. doi: 10.1002/j.1460-2075.1984.tb02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliga P., Sz-Breznovits A., Márton L. Streptomycin-resistant plants from callus culture of haploid tobacco. Nat New Biol. 1973 Jul 4;244(131):29–30. doi: 10.1038/newbio244029a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Nitsch J. P., Nitsch C. Haploid plants from pollen grains. Science. 1969 Jan 3;163(3862):85–87. doi: 10.1126/science.163.3862.85. [DOI] [PubMed] [Google Scholar]
- Paszkowski J., Shillito R. D., Saul M., Mandák V., Hohn T., Hohn B., Potrykus I. Direct gene transfer to plants. EMBO J. 1984 Dec 1;3(12):2717–2722. doi: 10.1002/j.1460-2075.1984.tb02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak M., Shillito R. D., Hohn T., Potrykus I. Expression in plants of two bacterial antibiotic resistance genes after protoplast transformation with a new plant expression vector. Nucleic Acids Res. 1986 Jul 25;14(14):5857–5868. doi: 10.1093/nar/14.14.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghai-Maroof M. A., Soliman K. M., Jorgensen R. A., Allard R. W. Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci U S A. 1984 Dec;81(24):8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz U. K., Lonsdale D. M. A yeast mitochondrial presequence functions as a signal for targeting to plant mitochondria in vivo. Plant Cell. 1989 Aug;1(8):783–791. doi: 10.1105/tpc.1.8.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitzguebel J. P., Siegenthaler P. A. Purification of peroxisomes and mitochondria from spinach leaf by percoll gradient centrifugation. Plant Physiol. 1984 Jul;75(3):670–674. doi: 10.1104/pp.75.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science. 1992 May 1;256(5057):628–632. doi: 10.1126/science.1533953. [DOI] [PubMed] [Google Scholar]
- Young E. G., Hanson M. R. A fused mitochondrial gene associated with cytoplasmic male sterility is developmentally regulated. Cell. 1987 Jul 3;50(1):41–49. doi: 10.1016/0092-8674(87)90660-x. [DOI] [PubMed] [Google Scholar]
- van den Boogaart P., Samallo J., Agsteribbe E. Similar genes for a mitochondrial ATPase subunit in the nuclear and mitochondrial genomes of Neurospora crassa. Nature. 1982 Jul 8;298(5870):187–189. doi: 10.1038/298187a0. [DOI] [PubMed] [Google Scholar]