Abstract
Purpose of Review
This review aims to describe the benefits and limitations of using the DuchenneConnect patient registry to provide information particularly in regard to active treatment choices in Duchenne muscular dystrophy and their impact on disease progression.
Recent findings
Clinical trials and natural history studies are difficult for rare diseases like Duchenne muscular dystrophy. Using an online patient self-report survey model, DuchenneConnect provides relevant data that are difficult to gather in other ways. Validation of the overall dataset is supported by comparable mutational spectrum relative to other cohorts and demonstrated beneficial effect of corticosteroid use in prolonging ambulation. These types of analyses are provocative and allow multivariate analyses across the breadth of patient and physician medication and supplement practices. Because the data is self-reported and online, the barrier to participation is low and great potential exists for novel directions of further research in a highly participatory forum.
Summary
Patient registries for Duchenne and Becker muscular dystrophy are powerful tools for monitoring patient outcomes, comparing treatments options, and relating information between patients, researchers and clinicians. DuchenneConnect is an online patient self-report registry for individuals with DBMD that facilitates aggregation of treatment modalities, outcomes and genotype data and has played a vital role in furthering DBMD research, particularly in the US, in a highly participatory and low cost manner.
Keywords: Duchenne muscular dystrophy, online registries, patient reported outcomes, Becker muscular dystrophy
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked progressive muscle wasting disease with an incidence of approximately 1 in 3500 to 5000 live male births. While rare, it is one of the most common pediatric diseases with a well-established genetic basis. It is noteworthy, that most families have no prior evidence of DMD in the family and thus linking families to informational resources is essential. Boys are often diagnosed due to delay in independent walking, perceived clumsiness, or speech and language delays accompanied by muscle weakness. The disease process invariably leads to loss of the ability to independently ambulate and a requirement for full time wheelchair use around the age of 10. This is a key milestone of the disease process and allows accurate self-reporting by families. Death occurs most often in the mid-twenties due to cardiac complications or respiratory failure and pneumonia. Broader implementationof published standards of care are improving the quality and length of life with DMD. Becker muscular dystrophy (BMD) is an allelic disorder with a milder and more variable phenotype.
Both DMD and BMD are caused by mutations in DMD, which spans 2.2 Mb and encodes dystrophin, a large sub-sarcolemmal structural protein. In DMD, most affected individuals possess exonic or multiexonic frameshifting deletion or duplication type mutations that render the transcribed messenger RNA out of frame leading to the expression of no functional dystrophin protein. BMD, in general, is caused by large exonic deletions and duplications that retain reading frame and thus result in partially functional dystrophin of abnormal size.
DuchenneConnect: an online self-report registry
DuchenneConnect is a patient self-report registry established by Parent Project Muscular Dystrophy in 2007 to educate individuals and families affected by Duchenne and Becker muscular dystrophies (DBMD), facilitate pre-recruitment and feasibility studies for industry, and collect information about the progression and natural history of the disease. Participants are encouraged to update their results every 6–12 months through electronic notification. It is the largest registry of Duchenne and Becker muscular dystrophy in the United States and includes 2,591 participants from 78 other countries [1].
Rare disease registries have played a key role in aiding the diagnosis and description of rare Mendelian conditions [2]. Two recent examples are N-glycanase deficiency [3] and KAT6A Syndrome [4] both discovered through the help of heavily invested family members in identifying other patients with similar features and providing phenotypic information to researchers.
DuchenneConnect, like other online registries, increases participation by lowering the barrier of entry by eliminating requirements for in-person visits or real-time communication. Registration requires access to a device with internet access. In DuchenneConnect, parents and guardians provide information such as age, age at loss of ambulation, current ambulatory status, drug and supplement usage or quality of life items. The entire registry survey contains questions covering different aspects of the disease: diagnosis, quality of life, ambulation, cardiorespiratory parameters, behavior, therapy, genetic testing and family history, and participation in research or clinical trials (Table 1). The inclusion of some outcomes and phenotypic descriptive data is immensely useful as it allows a survey of real-life practices in the community, comparison of therapies, commentary on implementation of standards of care [5] and in silico analysis of multiple parameter treatments.
Table 1.
DuchenneConnect Survey Questionnaire Topics
| Area | Examples |
|---|---|
| Diagnosis | “What diagnosis was given by your doctor?” (checklist) “Have you had muscle pain with increased activity or exercise?” |
| Mobility, Walking & Sitting | “How do you usually get around when you are at home?” (checklist) “How old were you when you started to use a wheelchair all the time?” |
| Steroids | “Have you ever used corticosteroids?” “How old were you when you started corticosteroids?” |
| Breathing | “Do you use any breathing devices?” “Have you had a lung function test (sometimes called the Forced Vital Capacity or FVC)?” |
| Cardiac | “Have you been diagnosed with decreased heart function by echocardiogram (heart ultrasound) and/or cardiac MRI?” “Have you ever taken any heart medications?” |
| Back, Bone & Tendon | “Has your doctor diagnosed scoliosis/curvature of the spine (back)?” “Have you broken a bone following minor trauma (like a simple fall)?” |
| Behavior & Learning | “Do you have concerns about your behavior or emotions that are more than typical for someone your age?” “Have you had problems with learning in school or a learning disability?” (checklist) |
| Therapies | “Do you use any of the following vitamins, supplements or other medications?” (checklist) |
| Genetic Testing, Insurance and Family History | “Have you had genetic testing?” “Have any of your blood relatives (living or deceased) had a similar muscle disease?” |
| Clinical Trials, Research & Registry Participation | “Are you currently participating in a clinical trial?” “Are you currently participating in a research study other than a clinical trial (such as an observational/non-treatment study)?” |
Truly centralized treatment centers for DBMD are impractical due to its geographical dispersion and low population prevalence. Current paradigms used in clinical trials or natural history studies are costly because patients must visit a research institution for in-person assessments often at great sacrifice. DuchenneConnect enables the aggregation of information in larger numbers, with bias in who can participate potentially, but also with less bias by clinic of attendance. These data offer a unique insight and can yield meaningful interpretations. Traditional descriptions of DMD have been largely anecdotal: a PubMed search for “case report” and “Duchenne muscular dystrophy” turned up 854 results spanning nearly 50 years. In contrast, DuchenneConnect has amassed phenotypic and genotypic data on over 2,500 DBMD individuals in 7 years, and all of the data are available in a systematic format allowing investigation.
Patient centered research has come to the forefront of medicine. Surveys using the DuchenneConnect platform have, for example, allowed patients, researchers and clinicians to quantify the financial burden of DBMD on families and government [6], assess caregiver preferences for potential risks and benefits of emerging treatments for DMD [7] and determine the spectrum of mutations among DBMD patients [8].
Care should be taken when using online resources for analysis since inaccuracies in response, participant bias, language barriers, unmeasured variables and other systematic biases can be confounding. Also, DuchenneConnect reaches a global audience and different interpretations of questions may affect responses. Incomplete responses or inconsistent answers can also be problematic and must be censored or adjusted with some diligence. It is reasonable to hypothesize that DuchenneConnect registrants are biased toward individuals with greater knowledge of treatment options, financial resources, and understanding of advocacy guidelines than the general population, but this is unlikely to be different than recruitments in other large natural history studies of DBMD which require even more personal resources to enable participation. Further, all of the within-registry comparisons remain unbiased as all participants were subjected to the same participation bias.
Because it relies on online self-reporting, DuchenneConnect has tremendous opportunity for expansion. Over 10,000 individuals with DMD are expected to exist in the United States [9,10] and less than 20% of that population has registered with DuchenneConnect or participated in a clinical trial or research study [11]. This is an outstanding sampling of the US DMD population nonetheless. Moreover, the registry is constantly evolving and growing as new survey topics and responses are added making this a dynamic resource for future studies.
Mutational spectrum
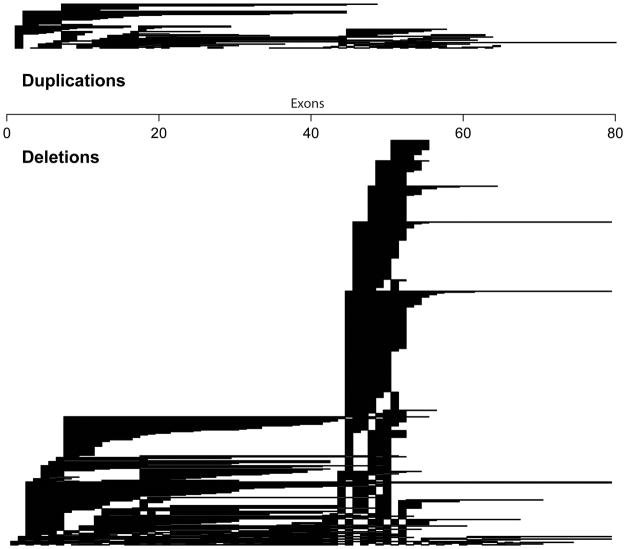
Among DuchenneConnect registrants, 72% possess large (≥1) exonic deletions, 10% large intronic duplications, 5% insertion deletion mutations, 3% splice site mutations and 9% point mutations in the DMD gene. As expected, this mutation distribution is similar to the TREAT-NMD DMD Global database of 7000 individuals from 31 countries [8]. In TREAT-NMD, mutations are 68% large deletions, 11% large duplications, 7% small indels, 3% splice site and 11% point mutations. The Dutch Leiden database records 72% of their 4,704 mutations to be large deletions, 7% large duplications [12] while the French UMD-DMD database had slightly fewer large exonic deletions (61%), slightly more large duplications (13%), comparable indels (8%) and splice site mutations (5%) but a larger number of point mutations (26%) [13]. Some of these differences are due to technologies used in molecular diagnosis while some registries did not consider cases that could not be molecularly diagnosed. Like all other studies, DuchenneConnect observed that the single most common mutation was deletion of exon 45 and most deletions and duplications occurred between exons 2–20 or exons 45–55 (Figure 1). All registries employ genetic counselors to interpret and enter mutation information in the database, a vital step for ensuring accuracy.
Figure 1.
Exonic mutations in DuchenneConnect. Regions of exonic and multiexonic duplications (top) and deletions (bottom) within the DuchenneConnect registry. Genetic reports are assessed by a clinical genetic counselor prior to entry into the database.
Following the “reading frame hypothesis” [14], mutations leading to in-frame transcripts and internally deleted dystrophin proteins strongly dispose affected individuals towards the milder Becker muscular dystrophy whereas out-of-frame mutations overwhelming lead to Duchenne muscular dystrophy.
A census of mutations in the DMD gene provides epidemiological insight on the predicted therapeutic benefit of exciting new treatments such as exon skipping therapies and stop codon read-through drugs. In the case of exon-skipping strategies, the use of antisense oligonucleotides can be used to hide exons adjacent to out-of-frame deletions to produce in-frame transcripts resulting in internally deleted but functional dystrophin protein. In effect, this mimics a Becker phenotype in patients with Duchenne muscular dystrophy. Based on the Leiden database, targeted exon skipping of exons 44, 45, 50, 51, and 53 are expected to benefit 8%, 13%, 5%, 15%, and 9% of DMD affected individuals [15] respectively. In DuchenneConnect, the respective percentages are comparable: 11%, 12%, 7%, 18% and 15%.
Beneficial effect of corticosteroids
The previously observed benefit of corticosteroid treatment on preservation of muscle strength in Duchenne patients required numerous studies to demonstrate a convincing effect. Initial studies were stymied by small samples sizes, inadequate controls and a lack of standardized outcome measures. Not until 1989 was a well-controlled, sufficiently powered study initiated to test the effect of prednisone on preserving pulmonary and skeletal muscle function [16,17]. A group of 103 boys randomized into placebo, 0.75mg/kg and 1.5 mg/kg groups and followed for 6 months whereupon prednisone usage was correlated with significant improvements in muscle strength, timed walking tasks and pulmonary function. Results of these studies coalesced into clinical guidelines issued by the American Academy of Neurology [18] and European Neuromuscular Centre [19]. Even now, however, optimal dosing regimens are not completely clear as evidenced by 29 different regimens in use across 105 different Duchenne treatment centers [20] for individuals with Duchenne muscular dystrophy. Further, in the DuchenneConnect Registry, we can observe practice in the US across treatment centers. Of patients ages 4–12, 74% were prescribed steroids indicating that steroid treatment is not yet universal in the US, despite strong evidence of short and long-term benefit [21].
Using data from 1057 DuchenneConnect registrants, a recent study replicated the beneficial effects of corticosteroids by using a simple online survey questionnaire. Prolonged age at loss of ambulation, defined as the age at which a boy requires full time wheelchair use, was highly correlated with use of either deflazacort or prednisone (N=633) by a median of three years as compared to steroid naïve boys (N=280) with P<0.0001 [11]. The hazard ratio of 0.35 for corticosteroid use as a predictor of age at loss of ambulation agrees well with other studies using the 6 minute walk test illustrating the robustness of the DuchenneConnect data despite its patient entered information. What is most remarkable is that the sample size studied in the DuchenneConnect data was substantially larger than prior studies allowing additional insights regarding efficacy of different steroid regimens.
The two most commonly prescribed steroids in the United States are prednisone and deflazacort, typically dosed at 0.75mg/kg/day and 0.9mg/kg/day respectively. Both are shown to be effective in slowing disease progression modestly [22]. Interestingly, Cox analysis of DuchenneConnect participants found hazard ratios (HR) for deflazacort more protective than prednisone (HR 0.2 and 0.4 respectively) [11]. This finding differs from an earlier but underpowered study of 18 boys [23] and agrees with a subsequently and recently published study of 340 participants [24]. While other factors may be correlated with choice of steroid, deflazacort is often selected by physicians, even in the US, because of some observations that there are fewer side effects with its chronic administration than prednisone including weight gain, osteoporosis and behavioral problems. There was also a trend of less-than-daily deflazacort performing better than daily dosing although the sample size was limited in that analysis. These data from DuchenneConnect are available substantially earlier than FOR-DMD, which is a randomized trial studying dosing of prednisone and deflazacort in DMD [25].
Other Medications
Numerous mouse studies suggest therapeutic benefit of FDA-approved medications in DMD, but none has been firmly established to be clinically beneficial other than steroid usage. Literature reports using mdx mouse models have suggested that ACEI/ARB may protect skeletal muscle function. Since these drugs are largely safe and well-tolerated and often prescribed for potential cardioprotective effect, there are some data to explore for potential impact of ACEI/ARB on slowing skeletal muscle function. Among boys reporting chronic steroids use, there were 415 boys reporting use of ACEI and 94 boys reporting use of ARB. Age at loss of ambulation can be assessed and adjusted for corticosteroids. In these exploratory analyses, there was a small but not significant increase in age at loss of ambulation among those using ACEI/ARB by 0.9 years. However, additional data collection strategies being implemented in DuchenneConnect may allow a more refined assessment of this and other medications in DMD. DuchenneConnect can thus serve as a low-cost, multivariate platform to observe off-label use of many drugs and potential benefits of specific combinations. Further, reports of commonly used medications in DMD can also potentially serve as a proxy for overlooked symptoms in DMD and provoke future investigations to better understand the full spectrum of the disease.
Effect of supplements
Several small clinical trials suggested some supplements may improve skeletal muscle function relative to the natural history or as placebo controls of DMD. One of the great benefits of DuchenneConnect is that we have the opportunity to explore patient/physician practices across areas of medicine that are not yet well supported in the medical literature. In total, 28 different supplements were reportedly being used, often by only a small fraction of the DMD population (Table 2).
Table 2.
Supplement and vitamin use among DuchenneConnect participants
| Supplements and Vitamins | Percentage of users in DC |
|---|---|
| Alpha Lipoic Acid | 1.0 |
| B-50 Complex | 1.8 |
| Bisphosphonates (Fosamax; Actonel) | 1.1 |
| Calcium | 2.8 |
| Coenzyme Q10 | 1.9 |
| Creatine Monohydrate | 4.0 |
| Grape Seed Extract | 0.4 |
| Green Tea Extract | 2.1 |
| Human Growth Hormone (HGH) | 1.3 |
| Haelan 951 | 0.2 |
| Idebenone (Catena) | 1.3 |
| Inositol | 0.3 |
| Juven | 0.7 |
| L-Arginine | 0.2 |
| L-Carnitine | 3.0 |
| Magnesium | 3.8 |
| Melatonin | 3.0 |
| Phosphatidyl Choline | 0.2 |
| Protandim | 3.7 |
| Resveratrol | 0.4 |
| Selenomax | 0.3 |
| Taurine | 0.9 |
| Viagra (sildenafil citrate) | 0.0 |
| Vitamin A (Beta-Carotene) | 4.1 |
| Vitamin C | 10.7 |
| Vitamin D | 33.4 |
| Vitamin E | 7.8 |
In a recent publication, supplements and alternative therapies were assessed for their potential effect on age at loss of ambulation. Interestingly, even controlling for the large effect of steroids, boys using Coenzyme Q10 or Vitamin D were observed to have a delay in age at loss of ambulation suggesting some small therapeutic effect. Coenzyme Q10 benefit was noted by a recent CINRG study [26], but the effect of Vitamin D is novel and the benefit appeared unrelated to a decrease in bone fractures [11]. This illustrates the benefits of aggregating individual data for seeding ideas for future clinical trials. No other supplements were statistically significantly associated with delay in loss of ambulation, potentially due to relatively small numbers of individuals taking each of these supplements.
Conclusions
The value of patient participatory self-report data has clearly been demonstrated simply by the fact that the substantial therapeutic benefit of corticosteroids is apparent and that subtle differences between deflazacort and prednisone are observable and consistent with other now reported data. Further, variations in patient/physician practices are a benefit in observing the many different possible combinations and can be meaningfully controlled using multivariate analysis to more rapidly explore the very large number of possible combinations. Combination therapies will grow ever more complex as drug approvals for various Duchenne treatment strategies near. We anticipate that the low barrier to participation of DuchenneConnect will remain a major advantage allowing facile clinical data exploration into the future. Improvements in the type and quality of data will also make the resource more valuable over time. For instance, medication start and stop dates, precise dosing information, and additional phenotype information (such as a clinic provided 10 meter timed walk/run) would all improve interpretations from the relative static data. Further, ongoing participation by the patient community and continually updated information would provide better longitudinal data and serve as an important resource for the research community. Geographical location and socioeconomic status remain important areas to broaden inclusion within DuchenneConnect such that underrepresented minorities and non-English speakers (important even in the largely English speaking US) are included in these clinical inferences.
Finally, we note that because DuchenneConnect provides a mechanism to empower the patient community to directly gather data outside of clinic, wearable electronics capable of reporting motion, activity, and work may become a mechanism to gather complementary datasets to clinical trial data. A recent commentary on precision medicine argued that individual diagnostic measurements can be compared to one’s historical values rather than a population-level cohort to determine whether a disease is progressing in a typical or atypical fashion [27]. In DMD, the 6 minute walk test has been validated as an effective early clinical trial measure to assess comparative differences in treatment. The 6MWT can be approximated and correlated with the shorter 10M walk/run test or correlated with at-home measurement of disease progression. With continuous home-monitoring a very different aspect of disease that cannot be captured in clinic based assessments may become apparent including total distance traveled in a day, total work output, fatigue, etc. These data types may help guide a more rapid assessment of therapeutics. With these types of tools, the effect of corticosteroids, in the context of other new medications, off label prescribing practices, precise dosing regimens and combinations with other supplements at different stages of the disease may be more feasible to explore. Placing these types of assessments within DuchenneConnect would be a valuable addition to the data available for exploration.
Key Points.
DuchenneConnect is a powerful platform for collecting informative self-reported data directly from patients with DBMD
Registry data can be used to investigate efficacies of treatment, therapies and conduct in silico clinical trial research
Over 2500 patients have registered with DuchenneConnect making it the largest registry of DBMD in the United States with both genotypic and phenotypic data to power patient centered outcomes research
Acknowledgments
We thank the DuchenneConnect Registry Operations Team, particularly, Holly Peay, Ann Martin, and Ann Lucas for their dedication to creating and improving the DuchenneConnect Registry, and we thank the many families with Duchenne and Becker muscular dystrophy who provide critical input. DuchenneConnect is a program of Parent Project Muscular Dystrophy.
Financial support and sponsorship
This work was supported by the Center for Duchenne Muscular Dystrophy at UCLA through funding by the National Institute of Arthritis, Musculoskeletal, and Skin Disorders (P30 AR057230) and the NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI UL1TR000124.
Footnotes
Conflicts of interest
None
References
- 1.Rangel V, Martin AS, Peay HL. DuchenneConnect Registry Report. PLoS Curr. 2012;4:RRN1309. doi: 10.1371/currents.RRN1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2**.Johnson KJ, Mueller NL, Williams K, Gutmann DH. Evaluation of participant recruitment methods to a rare disease online registry. Am J Med Genet A. 2014;164A:1686–1694. doi: 10.1002/ajmg.a.36530. This article addresses highlights issues associated with recruiting individuals to rare disease registries particularly through the use of internet based methods. As online and social media use becomes more prevalent targeted online recruitment tools will become increasingly essential. [DOI] [PubMed] [Google Scholar]
- 3.Need AC, Shashi V, Hitomi Y, Schoch K, Shianna KV, et al. Clinical application of exome sequencing in undiagnosed genetic conditions. Journal of Medical Genetics. 2012 doi: 10.1136/jmedgenet-2012-100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arboleda VA, Lee H, Dorrani N, Zadeh N, Willis M, et al. De novo nonsense mutations in KAT6A, a lysine acetyl-transferase gene, cause a syndrome including microcephaly and global developmental delay. Am J Hum Genet. 2015;96:498–506. doi: 10.1016/j.ajhg.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scully MA, Cwik VA, Marshall BC, Ciafaloni E, Wolff JM, et al. Can outcomes in Duchenne muscular dystrophy be improved by public reporting of data? Neurology. 2013;80:583–589. doi: 10.1212/WNL.0b013e318282334e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Landfeldt E, Lindgren P, Bell CF, Schmitt C, Guglieri M, et al. The burden of Duchenne muscular dystrophy: an international, cross-sectional study. Neurology. 2014;83:529–536. doi: 10.1212/WNL.0000000000000669. This study gathered information about direct cost of DMD care using an online patient/family-reported survey questionnaire. It is first study of the direct and indirect cost of DMD on caregivers, families and institutions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peay HL, Hollin I, Fischer R, Bridges JFP. A Community-Engaged Approach to Quantifying Caregiver Preferences for the Benefits and Risks of Emerging Therapies for Duchenne Muscular Dystrophy. Clinical Therapeutics. 36:624–637. doi: 10.1016/j.clinthera.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 8*.Bladen CL, Salgado D, Monges S, Foncuberta ME, Kekou K, et al. The TREAT-NMD DMD Global database: Analysis of More Than 7000 Duchenne Muscular Dystrophy Mutations. Hum Mutat. 2015 doi: 10.1002/humu.22758. This paper describes the mutation spectrum from the largest aggregation of DMD patients to date using 7000 genotypes from 31 countries. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Mah JK, Korngut L, Dykeman J, Day L, Pringsheim T, et al. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 2014;24:482–491. doi: 10.1016/j.nmd.2014.03.008. This study fills a critical gap in the epidemiology of DMD. Using a meta-analysis of multiple studies worldwide, they provide global and national prevalence statistics for Duchenne and Becker muscular dystrophies. [DOI] [PubMed] [Google Scholar]
- 10.Romitti PA, Zhu Y, Puzhankara S, James KA, Nabukera SK, et al. Prevalence of Duchenne and Becker muscular dystrophies in the United States. Pediatrics. 2015;135:513–521. doi: 10.1542/peds.2014-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Wang RT, Silverstein Fadlon CA, Ulm JW, Jankovic I, Eskin A, et al. Online Self-Report Data for Duchenne Muscular Dystrophy Confirms Natural History and Can Be Used to Assess for Therapeutic Benefits. PLoS Currents. 2014;6 doi: 10.1371/currents.md.e1e8f2be7c949f9ffe81ec6fca1cce6a. currents.md.e1e8f2be7c949f949ffe981ec946fca941cce946a. This study is the first analysis of self-report online registry data based on DuchenneConnect. It replicates the known benefit of corticosteroids on skeletal muscle function, finds novel correlation between supplement use and prolonged ambulation, and serves as a paradigm for future patient outcomes centered research in DBMD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aartsma-Rus A, Van Deutekom JC, Fokkema IF, Van Ommen GJ, Den Dunnen JT. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 2006;34:135–144. doi: 10.1002/mus.20586. [DOI] [PubMed] [Google Scholar]
- 13.Tuffery-Giraud S, Beroud C, Leturcq F, Yaou RB, Hamroun D, et al. Genotype-phenotype analysis in 2,405 patients with a dystrophinopathy using the UMD-DMD database: a model of nationwide knowledgebase. Hum Mutat. 2009;30:934–945. doi: 10.1002/humu.20976. [DOI] [PubMed] [Google Scholar]
- 14.Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2:90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- 15.van Deutekom JC, Bremmer-Bout M, Janson AA, Ginjaar IB, Baas F, et al. Antisense-induced exon skipping restores dystrophin expression in DMD patient derived muscle cells. Hum Mol Genet. 2001;10:1547–1554. doi: 10.1093/hmg/10.15.1547. [DOI] [PubMed] [Google Scholar]
- 16.Mendell J, Moxley R, Griggs R, Brooke M, Fenichel G, et al. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. The New England journal of medicine. 1989;320:1592. doi: 10.1056/NEJM198906153202405. [DOI] [PubMed] [Google Scholar]
- 17.Griggs RC, Moxley RT, III, Mendell JR, Fenichel GM, Brooke MH, et al. Prednisone in Duchenne dystrophy: a randomized, controlled trial defining the time course and dose response. Archives of neurology. 1991;48:383. doi: 10.1001/archneur.1991.00530160047012. [DOI] [PubMed] [Google Scholar]
- 18.Moxley RT, 3rd, Ashwal S, Pandya S, Connolly A, Florence J, et al. Practice parameter: corticosteroid treatment of Duchenne dystrophy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2005;64:13–20. doi: 10.1212/01.WNL.0000148485.00049.B7. [DOI] [PubMed] [Google Scholar]
- 19.Bushby K, Muntoni F, Urtizberea A, Hughes R, Griggs R. Report on the 124th ENMC International Workshop. Treatment of Duchenne muscular dystrophy; defining the gold standards of management in the use of corticosteroids 2–4 April 2004, Naarden, The Netherlands. Neuromuscular Disorders. 2004;14:526–534. doi: 10.1016/j.nmd.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Griggs RC, Herr BE, Reha A, Elfring G, Atkinson L, et al. Corticosteroids in Duchenne muscular dystrophy: Major variations in practice. Muscle & Nerve. 2013;48:27–31. doi: 10.1002/mus.23831. [DOI] [PubMed] [Google Scholar]
- 21.Merlini L, Gennari M, Malaspina E, Cecconi I, Armaroli A, et al. Early corticosteroid treatment in 4 Duchenne muscular dystrophy patients: 14-year follow-up. Muscle Nerve. 2012;45:796–802. doi: 10.1002/mus.23272. [DOI] [PubMed] [Google Scholar]
- 22.Biggar WD, Gingras M, Fehlings DL, Harris VA, Steele CA. Deflazacort treatment of Duchenne muscular dystrophy. J Pediatr. 2001;138:45–50. doi: 10.1067/mpd.2001.109601. [DOI] [PubMed] [Google Scholar]
- 23.Bonifati MD, Ruzza G, Bonometto P, Berardinelli A, Gorni K, et al. A multicenter, double-blind, randomized trial of deflazacort versus prednisone in Duchenne muscular dystrophy. Muscle Nerve. 2000;23:1344–1347. doi: 10.1002/1097-4598(200009)23:9<1344::aid-mus4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 24.Bello L, Gordish Dressman HA, Morgenroth L, Henricson E, Duong T, et al. Prednisone/prednisolone and deflazacort differ in long term outcomes on ambulation and side effects in the CINRG Duchenne Natural History Study. Neurology. 2015:84. doi: 10.1212/WNL.0000000000001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escolar DM, Hache LP, Clemens PR, Cnaan A, McDonald CM, et al. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology. 2011;77:444–452. doi: 10.1212/WNL.0b013e318227b164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spurney CF, Rocha CT, Henricson E, Florence J, Mayhew J, et al. CINRG pilot trial of coenzyme Q10 in steroid-treated Duchenne muscular dystrophy. Muscle Nerve. 2011;44:174–178. doi: 10.1002/mus.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schork NJ. Personalized medicine: Time for one-person trials. Nature. 2015;520:609–611. doi: 10.1038/520609a. [DOI] [PubMed] [Google Scholar]