Abstract
Background
Radiotherapy is known to be detrimental to bone and soft-tissue repair. Bone marrow stromal cells have been shown to enhance bone regeneration during distraction osteogenesis following radiation therapy. The authors posit that transplanted bone marrow stromal cells will significantly augment the mandibular vascularity devastated by radiation therapy.
Methods
Nineteen male Lewis rats were split randomly into three groups: distraction osteogenesis only (n = 5), radiation therapy plus distraction osteogenesis (n = 7), and radiation therapy plus distraction osteogenesis with intraoperative placement of 2 million bone marrow stromal cells (n = 7). A mandibular osteotomy was performed, and an external fixator device was installed. From postoperative days 4 through 12, rats underwent a gradual 5.1-mm distraction followed by a 28-day consolidation period. On postoperative day 40, Microfil was perfused into the vasculature and imaging commenced. Vascular radiomorphometric values were calculated for regions of interest. An analysis of variance with post hoc Tukey or Games-Howell tests was used, dependent on data homogeneity.
Results
Stereologic analysis indicated significant remediation in vasculature in the bone marrow stromal cell group compared with the radiation therapy/distraction osteogenesis group. Each of five metrics idicated significant improvements from radiation therapy/distraction osteogenesis to the bone marrow stromal cell group, with no difference between the bone marrow stromal cell group and the distraction osteogenesis group.
Conclusions
Bone marrow stromal cells used together with distraction osteogenesis can rejuvenate radiation-impaired vasculogenesis in the mandible, reversing radiation therapy–induced isotropy and creating a robust vascular network. Bone marrow stromal cells may offer clinicians an alternative reconstructive modality that could improve the lifestyle of patients with hypovascular bone.
Head and neck cancer affects an estimated 53,000 people each year in the United States, of whom approximately 12,000 eventually die as a result of the disease.1–3 For many head and neck cancer patients, high-dose radiotherapy is used as an important treatment modality. Although simultaneously promoting survival, the many adverse consequences of radiation on bone and soft-tissue repair can severely diminish a patient’s quality of life, damage his or her physical appearance, and ultimately necessitate invasive corrective surgery to repair and rebuild the affected area.
Distraction osteogenesis, the stimulation of new bone formation by the gradual separation of two osteogenic fronts, has become a powerful tool for reconstructing mandibular defects. The primary current application of distraction osteogenesis has been in the setting of congenital mandibular deformities.4,5 However, the use of distraction osteogenesis as a reconstructive option for tissue replacement after oncologic resection and irradiation could be immensely beneficial for the head and neck cancer population. This valuable reconstructive technique provides advantages over current methods, including use of endogenous tissue, avoidance of donor-site morbidity, and concurrent generation of bone and soft tissue. In addition, the recovery from distraction osteogenesis is primarily in the outpatient setting, lowering the overall cost of treatment. Unfortunately, radiation damage to bone has heretofore precluded the use of distraction osteogenesis in reconstructive efforts after head and neck cancer.
Bone marrow stromal cells inherently express both osteogenic and vasculogenic progenitor cells that stimulate bone regeneration and vascularization.6,7 Bone marrow stromal cells also activate endogenous vasculogenic and osteogenic growth factors8 but have not yet been used as a therapeutic modality for increasing vascularization and facilitating bony tissue repair in coordination with distraction osteogenesis in a clinical setting.
We hypothesize that the use of intraoperatively placed bone marrow stromal cells preceding distraction osteogenesis will rehabilitate the hypocellularity and hypovascularity induced by radiation in bone according to Marx’s famous 3-H principle,9 thus allowing for the successful reconstruction of irradiated bony tissue. We further posit that bone marrow stromal cells will restore the anisotropy normally generated by distraction osteogenesis but impaired by radiation therapy. To assay these hypotheses, we examined the stereologic metrics of vascularity of the regenerate region.
MATERIALS AND METHODS
Animals
Male Lewis rats (400 g) were obtained through the University of Michigan’s Unit for Laboratory Animal Medicine department in compliance with the Committee for the Utilization and Care of Animals. Rats were weighed and provided water and regular chow ad libitum on arrival. Animals were acclimated for 7 days before irradiation and randomly assigned to three groups: (1) distraction osteogenesis only (n = 5), (2) radiation therapy plus distraction osteogenesis (n = 7), and (3) radiation therapy plus distraction osteogenesis with intraoperative placement of 2 million bone marrow stromal cells (n = 7) (bone marrow stromal cell group).
Radiation Therapy
Rat hemimandibles were irradiated using a Philips RT250 orthovoltage unit (250 kV, 15 mA) (Kimtron, Inc., Oxford, Conn.), fractionating the dose at 3.72 Gy/minute over 5 days, for a total of 35 Gy. This radiation protocol has been performed for several years in the Department of Radiation Oncology at the University of Michigan under Unit for Laboratory Animal Medicine/Committee for the Utilization and Care of Animals– approved protocols and is optimal for Lewis rats. Radiographs were used because they taper off quickly, affecting only one side of the mandible, thus obviating the need for any intraoral shield. They provided the same physiologic effect on bone and soft tissue as gamma radiation. Rats were anesthetized using isoflurane/oxygen (2% and 1 liter/minute, respectively) and placed right side down, so that only the left hemimandible was irradiated. A lead shield with a rectangular window protected the pharynx, brain, and remainder of the animal. Dosimetry was carried out using an ionization chamber connected to an electrometer system, which is directly traceable to a National Institute of Standards and Technology calibration. The rats were maintained on regular chow and water and observed for 2 weeks before surgery. The diet was changed to moist chow 48 hours preoperatively along with Hill’s high-calorie diet (Hill’s Pet Nutrition, Inc., Topeka, Kan.).
Perioperative Preparation
Gentamicin (30 mg/kg subcutaneously) was given prophylactically preoperatively and once postoperatively. Rats were given buprenorphine (0.15 mg/kg subcutaneously) along with 15 cc/kg subcutaneous lactated Ringer solution, and then anesthetized using isoflurane/oxygen throughout the surgical procedure. Animals were placed supine on a warming blanket with a protective ocular lubricant and monitored with a pulse oximeter connected to an oxygen saturation monitor.
Surgical Procedure
The surgical procedure was previously described and published10 for the distraction osteogenesis and radiation therapy/distraction osteogenesis groups. In the bone marrow stromal cell group, a Surgifoam (Ethicon, Inc., Somerville, N.J.) scaffold loaded with 2 million bone marrow stromal cells was placed within the osteotomy. The external fixator device was adjusted to ensure reduction and hemostasis of the osteotomy edges. The wounds were irrigated, hemostasis was verified, and the incision was closed with staples.
Postoperative Care
The animals were caged under a heat lamp and monitored with additional hydration by means of subcutaneous lactated Ringer solution (15 cc/kg) and observed for 1 hour. Two doses of gentamicin were given every 12 hours after surgery. Buprenorphine was continued (0.15 to 0.5 mg/kg) with 10 cc of 5% dextrose in lactated Ringer solution subcutaneously every 12 hours through postoperative day 4 and as needed thereafter, along with moist chow, a high-calorie Hill’s a/d diet, and water ad libitum. Bactrim (Roche, Nutley, N.J.) was given daily to prevent infection. Pin sites were cleaned with Novalsan (Zoetis, Florham Park, N.J.) twice daily, teeth were clipped weekly, and staples were removed by postoperative day 14. All use and care was in compliance with Committee for the Utilization and Care of Animals and the National Institutes of Health Guide for the Care and Use of Laboratory Animals according to our protocol no. PRO00001267.
Distraction Protocol
The distraction screw was half-turned, corresponding to a 0.3-mm separation of the osteotomy fronts, beginning in the pm of postoperative day 4 and continued through the pm of postoperative 12. We performed a total of 17 half-turns, each on an every-12-hour interval, for a total of 5.1-mm osteotomy distraction. After distraction osteogenesis, rats underwent 28 days of consolidation and were killed on postoperative day 40. Previous studies have validated our mandibular distraction protocol.10,11
Bone Marrow Stromal Cell Cultures
Bone marrow from the femoral and humeral medullary cavities of isogenic siblings was flushed with a modified Minimum Essential Medium Alpha (Life Technologies, Carlsbad, Calif.). Cells were cultured in a growth medium [Minimum Essential Medium Alpha, 2 mM glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin sulfate, and 20% fetal bovine serum from preselected lots (Life Technologies)]. Cells were cultured at 37°C in an atmosphere of 100% humidity and 5% carbon dioxide. Confluent layers of adherent cells were formed by 11 to 14 days. The adherent layers were harvested using the following protocol: (1) two washes with the Hanks’ Balanced Salt Solution (Life Technologies), (2) incubation with chondroitinase ABC (20 mU/ml; Seidaguku Corp., Tokyo, Japan) in Minimum Essential Medium Alpha for 25 to 35 minutes at 37°C, (3) one wash with the Hanks’ Balanced Salt Solution, (4) incubation with 1× trypsin-ethylenediaminetetraacetic acid (Life Technologies) for 25 to 35 minutes at room temperature, (5) a second incubation with trypsin-ethylenediaminetetraacetic acid for 25 to 35 minutes at 37°C, and (6) a final wash in the growth medium. The subcultured cells were plated at 2 × 106 cells/75-cm2 flask. Steps 2 and 3 were omitted after the second subculture of cells. Marrow cells were centrifuged at 1000 rpm for 10 minutes, and the cell pellet was resuspended in fresh Minimum Essential Medium Alpha containing 10% fetal bovine serum (HyClone, Logan, Utah), 100 U/ml penicillin, and 50 µg/ml streptomycin (Life Technologies) at 37°C and 5% carbon dioxide.7
Loading Gelatin Scaffolds with Cells
Each gelatin sponge was designed to be a 5 × 9 × 2-mm rectangle to match the maximum critical size defect of our distraction gap. The sponges were prewetted in the complete medium, and air bubbles were removed by applying gentle pressure on the sponge between two pieces of sterile filter paper. Two million bone marrow stromal cells were collected and suspended in 50 µl of collagen (2.5 mg/ml; rat tail collagen, type I; BD Biosciences, Franklin Lakes, N.J.), as described previously.7
Transplantation of Bone Marrow Stromal Cells
The gelatin sponges previously loaded with bone marrow stromal cells were placed over the lateral mandible, centered within the reduced osteotomy and medial to the dissected masseter muscle. The sponges covered the entire subperiosteal surgical mandibular region and touched the osteotomy edges throughout their inferior to superior margins.
Tissue Harvest
Animals were killed on postoperative day 40 by means of an isoflurane overdose followed by thoracotomy. Mandibles were dissected out immediately following perfusion and euthanasia.
Perfusion Protocol
The perfusion protocol for this murine mandible model has been published previously.11,12 Briefly, all rats were anesthetized before a thoracotomy and left cardioventricular catheterization were performed. Perfusion with heparinized normal saline followed by pressure fixation with normal buffered formalin solution ensured that the animals were killed. After fixation, the vasculature was injected with Microfil (MV-122; Flow Tech, Carver, Mass.). Perfusion was verified by coloration of the dissected mandible and by subsequent micro–computed tomographic maximal intensity projection. Mineral leaching was confirmed with serial radiographs to ensure adequate demineralization before scanning.
Imaging
Specimens were scanned at 18-µm voxel size with micro–computed tomography. We previously used this voxel size and found it optimal to adequately resolve small, murine mandibular vessel networks.13 The region of interest was defined as a distance measuring 5.1 mm after the third molar of the left hemimandible. At an 18-µm resolution, this is equivalent to 283 slices. Analysis of the region of interest was then performed with MicroView 2.2 software (GE Healthcare, Milwaukee, Wis.). Contours were defined, highlighting the regions of interest using the spline function. Analysis of vascularity in this region is accomplished by setting a global grayscale threshold of 1000 to differentiate vessels from surrounding tissue, as described previously.14 The methodologies for measuring vessel volume fraction, vessel number, vessel thickness, and vessel separation were described previously by our laboratory.13 Furthermore, we quantified the degree of anisotropy, a measure of the directional orientation of the trabecular architecture. Degree of anisotropy is calculated using the mean intercept length method, which measures the intersections of a test grid with the trabecular structure and calculates the fabric ellipsoid, a three-dimensional ellipse. MicroView uses an algorithm to determine the length of the axes of the ellipsoid and their corresponding directions. Degree of anisotropy is defined as the ratio of the length of the maximum and minimum axes.
Statistical Analysis
A power analysis was performed using SPSS software (SPSS, Inc., Chicago, Ill.) in consultation with the Center for Statistical Consultation and Research at the University of Michigan. Further analysis of the five metrics under study was performed with analysis of variance. Post hoc Tukey honestly significant difference or Games-Howell tests were run depending on homogeneity of variances as for the Levene test.
RESULTS
The mandibular vascular networks in the bone marrow stromal cell group revealed notably enhanced vascularity, filling the region of interest and aligning along the axis of distraction. Vascular density in the bone marrow stromal cell group increased substantially from radiation therapy/distraction osteogenesis, with the dominant medullary vessels appearing to receive plentiful contributions from the surrounding coronoid, condylar, and angular vascular networks. The inferior alveolar artery was distinct and robust in the bone marrow stromal cell group, and anisotropy was restored within the region of interest (Fig. 1).
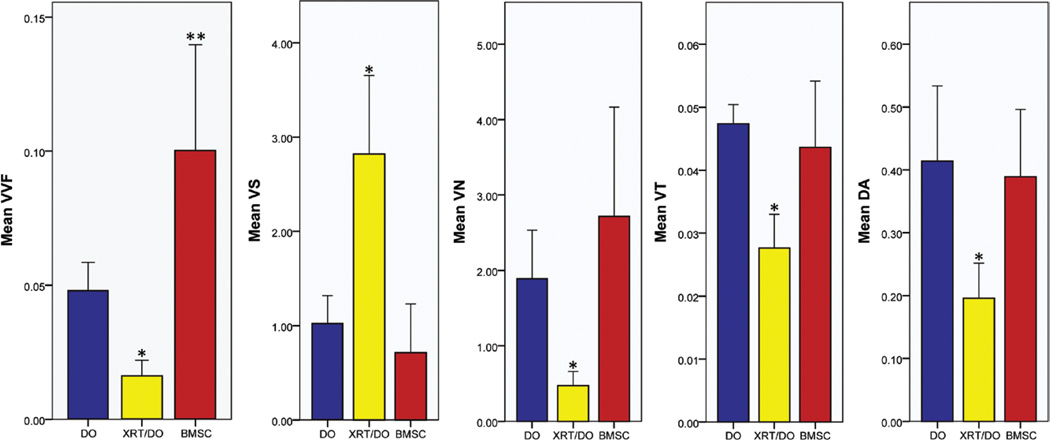
Fig. 1.
Mean values of vessel volume fraction (VVF; unitless), vessel separation (VS; in millimeters), vessel number (VN; 1/mm), vessel thickness (VT; in millimeters), and degree of anisotropy (DA; unitless) showing significant remediation of radiation therapy–induced hypovascularity. *p < 0.05 with comparison to both distraction osteogenesis and bone marrow stromal cell groups; **p < 0.05 with comparison to radiation therapy/distraction osteogenesis and distraction osteogenesis groups.
All five of the metrics measured showed significant improvement (p < 0.05) from radiation therapy/distraction osteogenesis to the bone marrow stromal cell group. In three of the five metrics, as illustrated in Figure 1, the bone marrow stromal cell group exceeded the distraction osteogenesis control group. Vessel volume fraction showed a significant increase from the radiation therapy/distraction osteogenesis group to the bone marrow stromal cell group (radiation therapy/distraction osteogenesis, 0.0159 ± 0.00613; bone marrow stromal cell group, 0.1003 ± 0.03946; p = 0.003), and a significant increase from the distraction osteogenesis control to the bone marrow stromal cell group (distraction osteogenesis, 0.0479 ± 0.01056; bone marrow stromal cell group, 0.1003 ± 0.03946; p = 0.028). Vessel separation decreased significantly from radiation therapy/distraction osteogenesis to the bone marrow stromal cell group (radiation therapy/distraction osteogenesis, 2.8206 ± 0.83275; bone marrow stromal cell group, 0.7129 ± 0.51745; p = 0.000). However, there was no significant difference between distraction osteogenesis and the bone marrow stromal cell group (distraction osteogenesis, 1.0232 ± 0.29515; bone marrow stromal cell group, 0.7129 ± 0.51745; p = 0.674). Vessel number increased significantly from radiation therapy/distraction osteogenesis to the bone marrow stromal cell group (radiation therapy/distraction osteogenesis, 0.4716 ± 0.18821; bone marrow stromal cell group, 2.7155 ± 1.45054; p = 0.015). Although the mean vessel number of the bone marrow stromal cell group surpassed that of the distraction osteogenesis group, this difference was not significant (distraction osteogenesis, 1.8928 ± 0.64075; bone marrow stromal cell group, 2.7155 ± 1.45054; p = 0.416). Vessel thickness also increased significantly from radiation therapy/distraction osteogenesis to the bone marrow stromal cell group (radiation therapy/distraction osteogenesis, 0.0276 ± 0.00536; bone marrow stromal cell group, 0.0436 ± 0.01056; p = 0.003). Once more, there was no significant difference between the distraction osteogenesis group and the bone marrow stromal cell group (distraction osteogenesis, 0.0474 ± 0.00306; bone marrow stromal cell group, 0.0436 ± 0.01056; p = 0.668). Finally, degree of anisotropy was restored, becoming more ordered from the radiation therapy/distraction osteogenesis to the bone marrow stromal cell group (radiation therapy/distraction osteogenesis, 0.1956 ± 0.05602; bone marrow stromal cell group, 0.3889 ± 0.10721; p = 0.004). This restoration approximated the degree of anisotropy of distraction osteogenesis, with no significant difference between the two groups (distraction osteogenesis, 0.4138 ± 0.11970; bone marrow stromal cell group, 0.3889 ± 0.10721; p = 0.896) (Fig. 2).
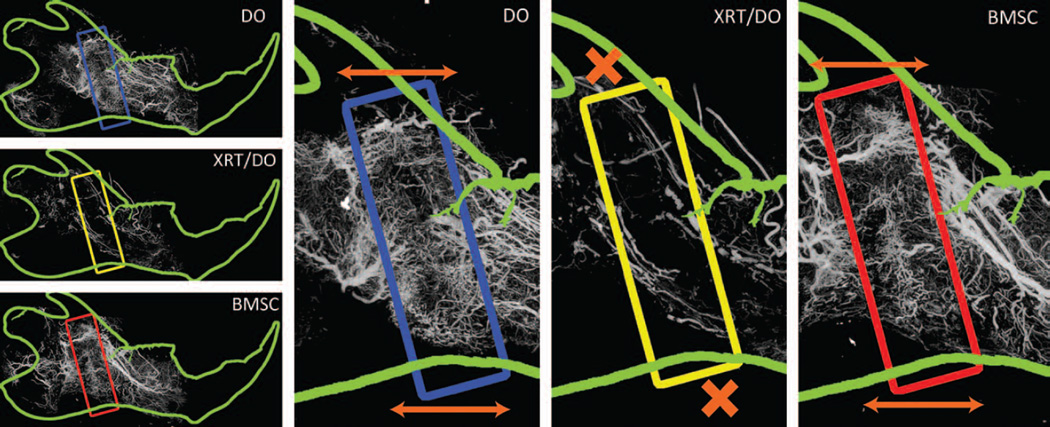
Fig. 2.
Arrows indicate the axis of distraction, with regions of interest highlighted in corresponding colors. Orange arrows demarcate the axis of distraction and corresponding directionality of vascularity. (Blue) Distraction osteogenesis shows an ordered, anisotropic vascular network within the region of interest. (Yellow) Radiation therapy/distraction osteogenesis demonstrates a severely diminished vascular regenerate. The vascularity that did exist was highly stochastic and isotropic. Orange Xs indicate the interruption of anisotropy. (Red) The bone marrow stromal cell group portrays a restoration of microvasculature within the region of interest and a remediation of anisotropy of the vascularity, as indicated by the orange arrows.
The data are illustrated in Figure 1. Maximal intensity projection images from a left hemimandible of the distraction osteogenesis, radiation therapy/distraction osteogenesis, and bone marrow stromal cell groups are shown in Figure 2.
DISCUSSION
Currently, many late-stage cancers require radiotherapy as an important treatment modality. However, radiotherapy is detrimental to bone and soft-tissue repair, resulting in a high incidence of devastating wound healing complications and the associated morbidity of late pathologic fractures and osteoradionecrosis.15–21
Radiotherapy disrupts the bone microvasculature by decreasing vascular density and intraluminal vessel diameter, exacerbating the complications of wound healing and impairing oxygen and nutrient delivery.13 These established findings were validated in our experiment, as exhibited graphically in Figure 1 and displayed in maximal intensity projection images in Figure 2. Sufficient vascularity is vital for wound healing, and the rate and range of vascular growth determine the efficiency of osteogenesis.22 However, the maladies resulting from radiation damage can subject head and neck cancer survivors to a lifelong disability that is severely limiting and exceedingly painful.23 Thus, our laboratory sought a therapeutic strategy aimed at mitigating the adverse consequences of radiation therapy on bone regeneration.
Bone marrow stromal cells offer a viable method of countering the adverse effects of radiation. Previous work demonstrated that bone marrow stromal cells greatly aided in remediating metrics of bony union, radiomorphometry, and biomechanical response parameters.24 We hypothesized that conjunctive treatment of bone marrow stromal cells with distraction osteogenesis would induce vasculogenesis and facilitate bony regeneration in the setting of radiotherapy. As bone marrow stromal cells are a heterogeneous cell population, we elected to use the full spectrum of cells to evaluate their ability, in total, to remediate the irradiated vasculature. As illustrated in Figure 1, the bone marrow stromal cell group showed significant improvements in all of the metrics tested, eliciting a robust vasculogenic response and restoring the vascular network observed in the distraction osteogenesis control.25
Studies have shown that bone marrow stromal cells increase vascularity by facilitating the anchoring, maintenance, organization, and differentiation of endothelial cells into stable vascular structures that comprise the vascular bed through growth factors such as vascular endothelial growth factor (VEGF), bone morphogenetic protein 2, and basic fibroblast growth factor (bFGF).26 VEGF is a strong promoter of neovascularization and has been shown to promote the growth of vascular endothelial cells.27 Bone morphogenetic protein 2 can engage endothelial cells by chemotaxis,28 and bFGF can recruit endothelial cells and induce vessel formation.29 In all three instances, the growth factors expressed by bone marrow stromal cells promote vasculogenesis at the healing site by recruiting vascular progenitor cells to the area. Thus, it is possible that the expression of these growth factors by bone marrow stromal cells is responsible for mitigating the radiation-induced hypovascularity in the region of interest.
As bone marrow stromal cells also possess a population of vascular progenitor cells,8 it is possible that these cells are directly responsible for the striking remediation of vasculogenesis. Moreover, bone marrow stromal cells can innately differentiate into pericytes, which are known to play a major role in vasculogenesis. The distraction osteogenesis process intrinsically up-regulates bFGF, transforming growth factor-β, and insulin-like growth factor 1 in endogenous tissues,30,31 encouraging bone marrow stromal cells to act in a vasculogenic manner. bFGF stimulates the vasculogenic properties of bone marrow stromal cells, causing bone marrow stromal cells to generate capillary-like networks.8 Transforming growth factor-β and insulin-like growth factor 1 influence cellular network formation and induce vascular tubule formation, further leading to wound healing.8 Therefore, bone marrow stromal cells and distraction osteogenesis could function synergistically to express these vasculogenic factors, leading to robust neovascularization by the stem cells themselves.
Degree of anisotropy quantifies the directionality of the regenerate vascularity. Distraction osteogenesis is an innately highly angiogenic process wherein mechanical stretching of the fracture callus elicits a potent mechanotransductive response, in accordance with focal adhesion kinase expression demonstrated by Tong et al.32 As illustrated in Figure 2, the overall directionality of the regenerate mirrors the axis of distraction, whereas the vascularity outside the regenerate is more stochastic, illustrating the normal orientation of vascularity.33 Although distraction has been shown to produce an anisotropic organization of the bone within the distraction regenerate, it has only recently been conclusively demonstrated that the vasculature within the distracted region maintains a similar organization.26 The exact mechanism of mechanotransduction is still an area of active research, although studies have indicated potential activation of VEGF receptor 2 during mechanical stretching, and cytoplasmic remodeling along the axis of distraction in response to mechanical stimuli.34,35
Our laboratory has recently demonstrated that radiotherapy hinders this anisotropic response (in press), wherein we postulated that radiation therapy impairs the anisotropy of the regenerate vascularity in distraction osteogenesis. Bone marrow stromal cells are able to remediate an anisotropic response intrinsic in the distraction osteogenesis process and impaired by radiation therapy, as demonstrated in Figure 2, by robust vascular horizontal orientation along the axis of distraction. These data coincide with previous research with bone marrow stromal cells in anisotropic environments.36,37 Lastly, the innate VEGF expression of bone marrow stromal cells may repotentiate the VEGF-related pathway responsible for this anisotropy, as described by Liu.34
This technique for vascular repopulation in an irradiated bed has several barriers that limit its clinical translation. In its current form, it would require a potentially painful bone marrow aspiration from, for example, the pelvis or femur. Previously, large-volume aspirations would be required to obtain the necessary number of cells. However, recently published protocols have greatly improved the efficacy of isolation protocols, with yields of up to 80 to 100 million high-viability (>90 percent) mesenchymal stem cells from 1 to 3 ml of bone marrow aspirate.38 However, these protocols still require ex vivo expansion of cells, which is subject to strict U.S. Food and Drug Administration guidelines and regulations. Secondarily, as individuals age, resident populations of bone marrow stromal cells accelerate in their senescence,39 and their osteogenic potential decreases.40 As those stricken with head and neck cancer tend to be of advancing or advanced age, this technique may be limited in the elderly by the characteristic of their bone marrow stromal cells. Lastly, a limitation with all animal research is that results may not be mirrored in human tissues, and must be validated in human studies before routine clinical use.
CONCLUSIONS
Our findings illustrate the effectiveness of transplanted bone marrow stromal cells and support our contention that the induced vasculogenic response can directly precede osteogenesis and subsequent bony regeneration in the irradiated mandible. Bone marrow stromal cells stimulate endothelial progenitor cell differentiation, promote tubule formation at the site of radiation-induced vascular damage,24,25 activate endogenous vasculogenic growth factors,25–29 and inherently possess a population of vascular progenitor cells.8,29,30 Bone marrow stromal cells further facilitate the anisotropic organization of vascularization into an ordered network along the axis of distraction.31–35 The use of bone marrow stromal cells conjunctively with distraction osteogenesis presents a viable, less invasive means of rehabilitating the mandibular vascular network following radiotherapy for head and neck cancer. Future studies will focus on characterizing the cells and implanting selected populations to assay vasculogenic potential. By rejuvenating vasculogenesis and facilitating bone and soft-tissue repair, bone marrow stromal cells can function as a potential therapeutic modality that could drastically improve a patient’s reconstructive outcome.
Acknowledgments
This work was supported by National Institutes of Health grants NIH-R01 CA 125187-06 (to S.R.B.) and NIH-T32 GM 008616 (to C.L.M.), both of the Department of Surgery at the University of Michigan. The authors would like to thank Charles Roehm for fabrication of fixator devices. The authors would also like to thank the members of the Craniofacial Research Laboratory for assistance with operations and animal care, and Dr. Mary Davis and the Department of Radiation Oncology. Finally, the authors would like to thank Dr. Catherine Tchanque-Fossuo, Dr. Douglas Chepeha, and Salman Ahsan for their contributions to this study.
Footnotes
Presented in part at the 59th Annual Meeting of the Plastic Surgery Research Council, in New York, New York, March 7 through 9, 2013.
Disclosure: The authors have no financial interest in any of the products or devices mentioned in this article.
REFERENCES
- 1.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer facts and figures 2013. [Accessed August 1, 2013]; Available at: http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2013. [Google Scholar]
- 3.U.S. Department of Health and Human Services. Health care cost and utilization project (UCUP): Outcomes by cancer of head and neck. [Accessed August 1, 2013]; Available at: http://www.ahrq.gov/research/data/hcup/index.html.
- 4.Block MS, Otten J, McLaurin D, Zoldos J. Bifocal distraction osteogenesis for mandibular defect healing: Case reports. J Oral Maxillofac Surg. 1996;54:1365–1370. doi: 10.1016/s0278-2391(96)90499-1. [DOI] [PubMed] [Google Scholar]
- 5.Sun J, Wang X, Li YL, Chen LQ, Yu JY, Huo JF. Reconstruction of segmental mandibular defects using trifocal distraction osteogenesis. Int J Oral Maxillofac Surg. 2009;38:459–460. [Google Scholar]
- 6.Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20:848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 7.Krebsbach PH, Mankani MH, Satomura K, Kuznetsov SA, Robey PG. Repair of craniotomy defects using bone marrow stromal cells. Transplantation. 1998;66:1272–1278. doi: 10.1097/00007890-199811270-00002. [DOI] [PubMed] [Google Scholar]
- 8.Annabi B, Naud E, Lee YT, Eliopoulos N, Galipeau J. Vascular progenitors derived from murine bone marrow stromal cells are regulated by fibroblast growth factor and are avidly recruited by vascularizing tumors. J Cell Biochem. 2004;91:1146–1158. doi: 10.1002/jcb.10763. [DOI] [PubMed] [Google Scholar]
- 9.Marx RE. Osteoradionecrosis: A new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41:283–288. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 10.Buchman SR, Ignelzi MA, Jr, Radu C, et al. Unique rodent model of distraction osteogenesis of the mandible. Ann Plast Surg. 2002;49:511–519. doi: 10.1097/00000637-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Jing XL, Farberg AS, Monson LA, et al. A quantitative analysis of angiogenesis in mandibular distraction osteogenesis. Plast Reconstr Surg. 2010;125:133. [Google Scholar]
- 12.Donneys A, Tchanque-Fossuo CN, Farberg AS, et al. Quantitative analysis of vascular response after mandibular fracture repair using microcomputed tomography with vessel perfusion. Plast Reconstr Surg. 2011;127:1487–1493. doi: 10.1097/PRS.0b013e318208f3c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshpande SS, Donneys A, Farberg AS, Tchanque-Fossuo CN, Felice PA, Buchman SR. Quantification and characterization of radiation-induced changes to mandibular vascularity using micro-computed tomography. Ann Plast Surg. 2014;72:100–103. doi: 10.1097/SAP.0b013e318255a57d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jing XL, Farberg AS, Monson LA, Donneys A, Tchanque-Fossuo CN, Buchman SR. Radiomorphometric quantitative analysis of vasculature utilizing micro-computed tomography and vessel perfusion in the murine mandible. Craniomaxillofac Trauma Reconstr. 2012;5:223–230. doi: 10.1055/s-0032-1329540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobsson M, Albrektsson T, Turesson I. Dynamics of irradiation injury to bone tissue: A vital microscopic investigation. Acta Radiol Oncol. 1985;24:343–350. doi: 10.3109/02841868509136063. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsson MG, Jönsson AK, Albrektsson TO, Turesson IE. Short- and long-term effects of irradiation on bone regeneration. Plast Reconstr Surg. 1985;76:841–850. doi: 10.1097/00006534-198512000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Markbreiter LA, Pelker RR, Friedlaender GE, Peschel R, Panjabi MM. The effect of radiation on the fracture repair process: A biomechanical evaluation of a closed fracture in a rat model. J Orthop Res. 1989;7:178–183. doi: 10.1002/jor.1100070204. [DOI] [PubMed] [Google Scholar]
- 18.Aitasalo K. Bone tissue response to irradiation and treatment model of mandibular irradiation injury: An experimental and clinical study. Acta Otolaryngol Suppl. 1986;428:1–54. [PubMed] [Google Scholar]
- 19.Stinson SF, DeLaney TF, Greenberg J, et al. Acute and long-term effects on limb function of combined modality limb sparing therapy for extremity soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 1991;21:1493–1499. doi: 10.1016/0360-3016(91)90324-w. [DOI] [PubMed] [Google Scholar]
- 20.Marx RE, Johnson RP. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg Oral Med Oral Pathol. 1987;64:379–390. doi: 10.1016/0030-4220(87)90136-8. [DOI] [PubMed] [Google Scholar]
- 21.Bagan JV, Scully C, Zapater E, Basterra J, Bagan L. Osteoradionecrosis of the jaws. Clin Rev Bone Miner Metab. 2011;9:47–53. [Google Scholar]
- 22.Yu H, VandeVord PJ, Mao L, Matthew HW, Wooley PH, Yang SY. Improved tissue-engineered bone regeneration by endothelial cell mediated vascularization. Biomaterials. 2009;30:508–517. doi: 10.1016/j.biomaterials.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 23.Donneys A, Weiss DM, Deshpande SS, et al. Localized deferoxamine injection augments vascularity and improves bony union in pathologic fracture healing after radiotherapy. Bone. 2013;52:318–325. doi: 10.1016/j.bone.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshpande SS, Gallagher KK, Donneys A, et al. Stem cell therapy remediates reconstruction of the craniofacial skeleton after radiation therapy. Stem Cells Dev. 2013;22:1625–1632. doi: 10.1089/scd.2012.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Khaldi A, Eliopoulos N, Martineau D, Lejeune L, Lachapelle K, Galipeau J. Postnatal bone marrow stromal cells elicit a potent VEGF-dependent neoangiogenic response in vivo. Gene Ther. 2003;10:621–629. doi: 10.1038/sj.gt.3301934. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Wan C, Ramaswamy G, Clemens TL, Ponnazhagan S. Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther. 2010;18:1026–1034. doi: 10.1038/mt.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindhorst D, Tavassol F, von See C, et al. Effects of VEGF loading on scaffold-confined vascularization. J Biomed Mater Res A. 2010;95:783–792. doi: 10.1002/jbm.a.32902. [DOI] [PubMed] [Google Scholar]
- 28.Raida M, Heymann AC, Günther C, Niederwieser D. Role of bone morphogenetic protein 2 in the crosstalk between endothelial progenitor cells and mesenchymal stem cells. Int J Mol Med. 2006;18:735–739. doi: 10.3892/ijmm.18.4.735. [DOI] [PubMed] [Google Scholar]
- 29.Fierro FA, Kalomoiris S, Sondergaard CS, Nolta JA. Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells. 2011;29:1727–1737. doi: 10.1002/stem.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yates KE, Kaban LB, Glowacki J. Molecular evidence that TGF-b, IGF-1, and BMP4 are expressed during distraction osteogenesis. J Bone Miner Res. 1999;14(suppl 1):S426. [Google Scholar]
- 31.Pacicca DM, Patel N, Lee C, et al. Expression of angiogenic factors during distraction osteogenesis. Bone. 2003;33:889–898. doi: 10.1016/j.bone.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Tong L, Buchman SR, Ignelzi MA, Jr, Rhee S, Goldstein SA. Focal adhesion kinase expression during mandibular distraction osteogenesis: Evidence for mechanotransduction. Plast Reconstr Surg. 2003;111:211–222. doi: 10.1097/01.PRS.0000033180.01581.9A. discussion 223. [DOI] [PubMed] [Google Scholar]
- 33.Donneys A, Tchanque-Fossuo CN, Farberg AS, Deshpande SS, Buchman SR. Bone regeneration in distraction osteogenesis demonstrates significantly increased vascularity in comparison to fracture repair in the mandible. J Craniofac Surg. 2012;23:328–332. doi: 10.1097/SCS.0b013e318241db26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J. Mechanotransduction in Endothelial Cells: Cell Growth, Angiogenesis and Wound Healing (Dissertation) Columbus, Ohio: The Ohio State University; 2010. [Google Scholar]
- 35.del Alamo JC, Norwich GN, Li YS, Lasheras JC, Chien S. Anisotropic rheology and directional mechanotransduction in vascular endothelial cells. Proc Natl Acad Sci USA. 2008;105:15411–15416. doi: 10.1073/pnas.0804573105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bashur CA, Shaffer RD, Dahlgren LA, Guelcher SA, Goldstein AS. Effect of fiber diameter and alignment of electrospun polyurethane meshes on mesenchymal progenitor cells. Tissue Eng Part A. 2009;15:2435–2445. doi: 10.1089/ten.tea.2008.0295. [DOI] [PubMed] [Google Scholar]
- 37.Li WJ, Mauck RL, Cooper JA, Yuan X, Tuan RS. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech. 2007;40:1686–1693. doi: 10.1016/j.jbiomech.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penfornis P, Pochampally R. Isolation and expansion of mesenchymal stem cells/multipotential stromal cells from human bone marrow. Methods Mol Biol. 2011;698:11–21. doi: 10.1007/978-1-60761-999-4_2. [DOI] [PubMed] [Google Scholar]
- 39.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Simonsen JL, Rosada C, Serakinci N, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20:592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]