Abstract
In 1975, at the start of my junior year in college, I took a course on experimental methods in psychology from Dr. James C. Smith, when he was a Visiting Professor at Penn State University. That experience set me on the professional path of studying the neural bases of taste function and ingestion on which I remain to this day. Along the way, I did my graduate work at Florida State University under the tutelage of Jim, I did my postdoctoral training at the University of Pennsylvania under the supervision of Harvey Grill, and I also worked closely with Ralph Norgren, who was at the Penn State Medical College. This article briefly summarizes some of the lessons I learned from my mentors and highlights a few key research findings arising from my privilege of working with gifted students and postdocs. After close to 40 years of being a student of the gustatory system and ingestive behavior, it is still with the greatest conviction that I believe rigorous analysis of behavior is indispensable to any effort seeking to understand brain function.
Keywords: taste psychophysics, feeding patterns, licking microstructure, brief-access taste test, gustometer
Introduction
It is a great privilege to have this opportunity to reflect upon my last 40 years of academic research on taste and ingestive behavior as a student, postdoc, and faculty member. Let me start by expressing my deepest gratitude to the Society for the Study of Ingestive Behavior for recognizing my work with the 2014 Hoebel Award for Creativity in honor of the scientific contributions of Bart Hoebel to our field. In the following pages, I have been given license to reminisce about some key experiences and insights that have influenced the direction of my scientific path to this day.
The Beginning of the Path
I did my graduate work under the tutelage of Dr. James C. Smith at the Florida State University (FSU). We met at Penn State where I did my undergraduate training. He was in residence for the 1975–76 academic year as a visiting Professor and taught a course on experimental methods in Psychology that I took as a junior. I knew nothing about neuroscience, or taste, or ingestive behavior, etc. But that class, that had an enrollment of 11 students, changed my life. Jim convinced 5 of the 11 to attend graduate school in the Psychobiology Program at FSU. I joined his lab as a first year graduate student in 1979 and worked for several years on the mechanisms of radiation-induced conditioned taste aversion (e.g., [1, 2]). This eventually led to my interest in the controls of feeding and drinking and ultimately to my work involving the analysis of meal patterns. If I had to point to a single event that led me on my professional path, without doubt, it would be that propitious day I decided to enroll in the methods course that Jim taught at Penn State. Through the years, Jim Smith has been my mentor, colleague, and friend. I would not be writing this article today, if it were not for him.
The “Rat Hotel”
Jim has always been a diehard empiricist with a passion for rigorous and objective analysis of behavior. He taught me to always let the data guide the way. Since I can remember, the Psychology Department at FSU has always had a terrific machine and electronic shop that designs and fabricates rather sophisticated equipment. Accordingly, I learned the power of instrumentation in the precise and detailed measurement of behavior and for my dissertation I used the first version of, what Jim affectionately called, the “rat hotel”, to analyze the feeding and drinking patterns of rats given various concentrations of different sugar solutions [3]. The first model of the hotel was connected to a new state-of-the-art computer, called a microcomputer, possessing a mind-numbing 64KB of memory. It was able to read the signals arising from the electronic sensors in the cage to accurately reconstruct patterns of feeding and drinking and then parcel the behavior into criterion-defined bouts of activity. It was quickly apparent that the day-night distribution of bouts became more uniform as sucrose concentration was raised and the number of bouts taken radically changed in a nonmonotonic fashion [3].
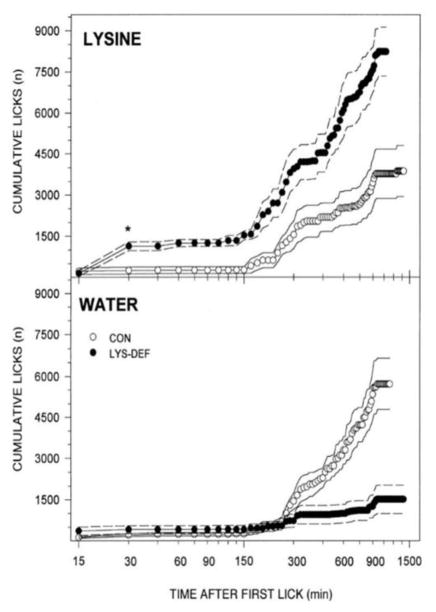
The rat hotel went through various incarnations and Jim continued to apply it in many different theoretical contexts to study the controls of ingestive behavior. Indeed, 15 years later my student Stacy Markison and I collaborated with Jim and used the renovated hotel to study how soon L-lysine-deficient rats start to consume lysine when made available [4]. She found that when given a two-bottle choice (against water), deficient rats started differentially increasing their lysine intake between 15 – 30 min after the start of the test relative to controls. However, when lysine was pitted against another amino acid, threonine, the elevated lysine intake by the deficient rats took 90 min (see Figure 1). In combination with results from other experiments [5], Stacy interpreted this as a rapid learning process in which the animal pairs the taste and/or bottle position of the lysine with its positive postingestive consequences. This was opposed to the well-established and seemingly innate specific appetite toward sodium, induced by depletion of that essential electrolyte [6–9]. Over the decades my students and I have collaborated with Jim using the various versions of his hotel and applying it to other research questions [10, 11].
Figure 1.
Mean cumulative licks as a function of time after first lick. Dashed lines represent ±se. Left: Rats maintained on lysine-deficient diet and given 0.1 M L-lysine for the first time first water on the home cage. Although in other experiments such rats did not demonstrate enhanced licking in a brief-access taste test in contrast to what is seen in in sodium-deficient rats presented with sodium solutions, they did significantly increase their intake of lysine after just 30 min. When 0.1 M L-lysine was pitted against 0.2 M L-threonine, noticeable preferential intake took longer. Reprinted with permission from Markison S, Thomson BL, Smith JC, Spector AC. (2000) Time course and pattern of compensatory ingestive behavioral adjustments to lysine deficiency in rats. Journal of Nutrition 130: 1320–1328.
From Meal Patterns to Microstructure
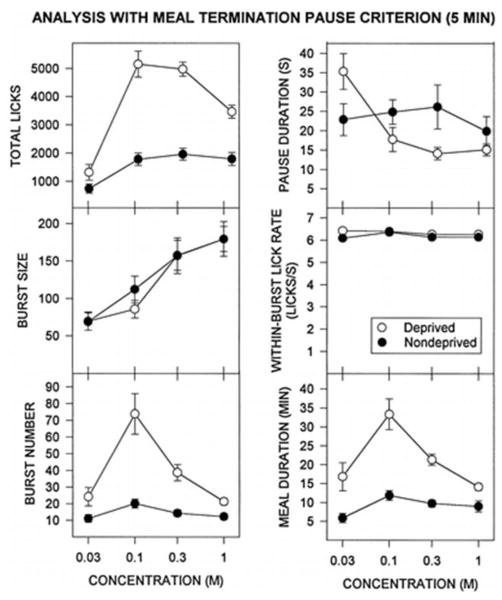
My experience with meal pattern analysis led naturally to my interest in feeding on a more reduced time scale. My colleague and friend Joel Kaplan and I used a scaled down version of the hotel to follow the pioneering work of Jack Davis (e.g., [12–14]) to explore the microstructure of ingestive behavior within a single meal [15]. In our first application of this approach, we found that food deprivation increased lick burst number, but not lick burst size. In contrast, burst size was monotonically increased with sucrose concentration. In other words, deprivation tends to turn the central pattern generator for licking on and the orosensory signals tend to keep in on (see Figure 2). We also analyzed the data employing several criteria defining a pause to see if and how such a key analytical feature fundamentally changed the nature of the concentration-response functions under food-deprived and food-replete conditions for the various microstructural parameters such a burst size and burst number. As expected, there were key differences. The curves for a 0.3 s pause criterion were quite different from those for a 1 s criterion. Yet, the nature of the curves for 1 s, 3 s, and 10 s criteria were quite similar with the exception that as would be anticipated the number of bursts went down and the burst size went up as the pause criterion was increased. For pause criteria ≥30s, the shape of the functions started to resemble the character of those describing the entire meal. Thus, there appeared to be several layers of organization to the temporal structure of feeding. Although all are potentially meaningful, we argued that for most applications, a 1 s pause criterion would be suitable, if not even optimal. But the real lesson from this type of study is that intake is the outcome of behavior, not the behavior itself, and measuring the latter better positions one to learn about the neural mechanisms leading to the former.
Figure 2.
Mean ±se for various microstructural licking parameters, with a pause criterion of 1 s and a meal termination criterion of 5 min. Copyright © 1998 by the American Psychological Association. Reproduced with permission. Spector AC, Klumpp PA, Kaplan JM. (1998) Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behavioral Neuroscience 112: 678–694. The use of APA information does not imply endorsement by APA.
The University of Pennsylvania
After graduate school, I moved to the University of Pennsylvania where I did my postdoctoral training with Harvey Grill. I had first met Harvey when Jim invited him to FSU to give a colloquium. I was bewitched by his talk. When I visited Penn for an interview with him, he greeted me with his full beard, clad in Hawaiian shirt and Birkenstocks, pipe in hand, in an anteroom to his office (referred to as the inner sanctum) that was appointed with Persian rugs and bean bag chairs. My search for a postdoc position ended that day. It was Harvey who introduced me to the neural analysis of ingestive behavior and who taught me many things, both professional and otherwise.
My time in Harvey’s lab was exceptionally stimulating. At one point, Bill Flynn and Joel Kaplan were postdocs, and Gary Schwartz, Paul Breslin, and eventually Randy Seeley were students. Lab meetings were intellectually brutal and it taught me that if I could survive a lab meeting, I could handle anything else, including the famed Penn feeding seminar which was always attended by some of the great minds of the field including Alan Epstein, Eliot Stellar, and Randy Gallistel, as well as many others during those years.
Serial Taste Reactivity Test to Measure the Conditioning of a Taste Aversion During a Single Trial
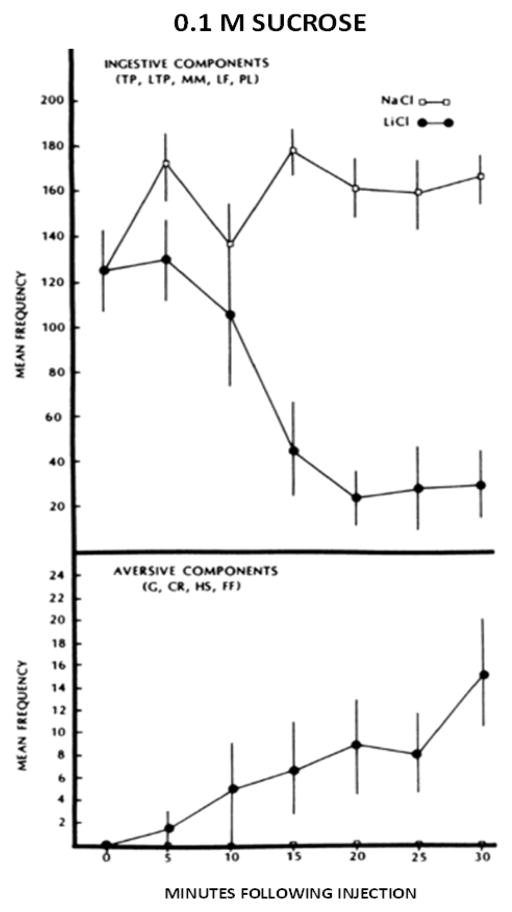
It was from Harvey that I learned about taste-elicited oromotor responses referred to as taste reactivity [16, 17]. Harvey, Paul Breslin and I, used the taste reactivity paradigm to assess the rapid conditioning of a taste aversion within a single session [18]. Rats that were injected with LiCl initially displayed exclusive ingestive responses to 0.1 M sucrose infused during 30 s sample epochs starting at time 0 and every 5 min thereafter. However, whereas the NaCl-injected rats maintained this response profile for the entire 30 min session, the LiCl-injected animals began to decrease their ingestive behavior and increase their aversive behavior starting at ~15 min postinjection (Figure 3). At first, we thought that these effects were due to the unconditioned effects of the LiCl on taste reactivity. However, other experiments confirmed that the change in taste reactivity was due to an associative process. In other words, the paradigm was a means to assess the rapid formation of a taste aversion within the conditioning trial. We argued that this paradigm would be an effective tool for researchers interested in studying the formation of taste-visceral associations. Paul was actually an undergraduate at the time; this was his first scientific paper that he co-authored.
Figure 3.
Mean ±se ingestive responses (top panel) and aversive responses (bottom panel) to 30 s intraoral infusions of 0.5 ml of 0.1 M sucrose as a function of time. The rats were injected (ip) with 2.0 mEq/kg of 0.15 M LiCl or NaCl at time 0. Copyright © 1988 by the American Psychological Association. Reproduced with permission. Spector AC, Breslin P, Grill HJ. (1988) Taste reactivity as dependent measure of the rapid formation of conditioned taste aversion: A tool for the neural analysis of taste-visceral interactions. Behavioral Neuroscience 102: 942–952. The use of APA information does not imply endorsement by APA.
The Effect of PBN Lesions on Taste Function
While I was in Harvey’s Lab at Penn, I had the great privilege to collaborate with Ralph Norgren, who this past year was honored by SSIB as the recipient of the Distinguished Career Award. Ralph taught me how to find my way around the gustatory system. His technical acumen, scholarship, and clarity of thought are unsurpassed. Although perhaps not as formal as with Jim Smith and Harvey Grill, I credit Ralph as a mentor. Ralph, Harvey and I used the serial taste reactivity task described above to demonstrate that rats with PBN lesions were not able to express a CTA on subsequent tests, because they were unable to acquire it in the first place. In other words, it was not that the rats with PBN lesions forgot the association when tested the next day [19]. We went onto perform other experiments assessing the competence of rats with PBN lesions on a variety of taste-related behavioral tasks, some of which were conducted after I moved to the University of Florida [20–23].
The Gustometer
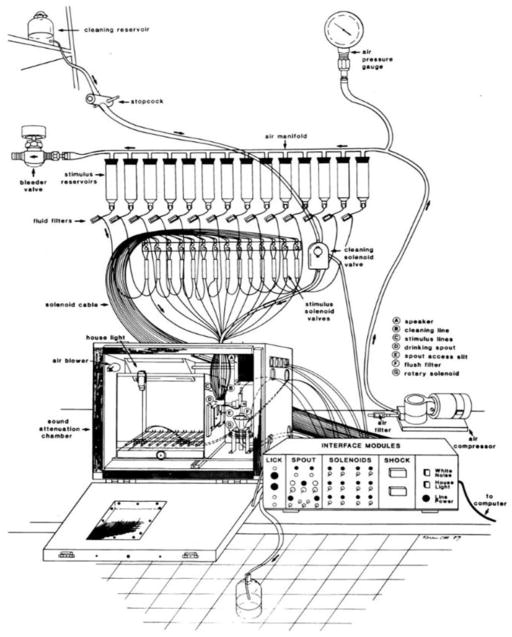
In his prime, Jim Smith was an accomplished animal psychophysicist and we were always planning to design a gustometer and conduct some experiments in rats. However, while I learned a lot about psychophysics from Jim as a graduate student, we never actually did any experiments. So the gustometer was developed when I was in Harvey’s lab [24]. When I told Harvey about the idea, he said go for it and so I did. To this day, I don’t think Harvey realizes how much of his resources were devoted to prototypes, but we finally settled on a design which is still basically in use today (see Figure 4).
Figure 4.
Schematic of the gustometer. Reprinted from Spector AC, Andrews-Labenski J, and Letterio FC. (1990) A new gustometer for psychophysical taste testing in the rat. Physiology and Behavior 47: 795–803 with permission.
The Effects of Chorda Tympani Transection on NaCl and Sucrose Taste Sensitivity
In one of the first applications of the gustometer, Harvey Grill, Gary Schwartz and I demonstrated that transection of chorda tympani nerve, which innervates taste buds on the anterior tongue, significantly shifts the NaCl detection threshold by about 1.5 log10 units, while having no effect on sucrose thresholds [25]. This was significant because it was thought that the chorda tympani nerve did not have a privileged role in sodium taste coding because its transection did not affect NaCl preferences in two-bottle intake tests.
The University of Florida
In 1990, I had the good fortune of receiving an offer to join the Behavioral Neuroscience Program in the Department of Psychology at the University of Florida (UF) as an Assistant Professor. I am indebted to Neil Rowland, Phil Teitelbaum, and Betty Capaldi for their support during that time. My time at UF was formative. Especially noteworthy was the fact the Department of Psychology had one of the leading programs in Behavior Analysis. I developed close intellectual and social ties with two faculty members in this group, Marc Branch and Tim Hackenberg, and later Clive Wynne and David W. Smith (although David was a psychophysicist in the Behavioral Neuroscience area) when they joined the faculty. In the tradition of the field of Behavior Analysis, the faculty, students, and postdocs in the program at UF had an exceptionally rigorous and strictly objective way of viewing behavior. I learned much from these colleagues as did my students, and their influence is still evident in my thinking to this day. A few years after joining the faculty at UF, I began interacting with Barry Ache, an internationally renowned olfactory researcher, who eventually asked me to help him create the University of Florida Center for Smell and Taste (UFCST) for which I served as Assistant Director from 1998 until I left UF in 2007. The UFCST is still thriving today under Barry’s leadership.
The Functional Consequences of Gustatory Nerve Transection, Regeneration, and Cross-Regeneration
The experiment I conducted in Harvey Grill’s lab on the effects of chorda tympani nerve transection on taste sensitivity in rats set the stage for a large part of my research program when I left Penn. My students, postdocs, and I used the gustometer to study the effects of transections, regeneration, and cross-regeneration of various taste nerves on taste function. In fact, Ginger Blonde, formerly a student in my lab, and Mircea Garcea, a technician with gifted surgical talents, went on to show that while transection of the gustatory branches of the seventh nerve, including the chorda tympani, has a large effect on NaCl taste sensitivity, transection of the glossopharyngeal nerve has no effect [26] (Figure 5).
Figure 5.
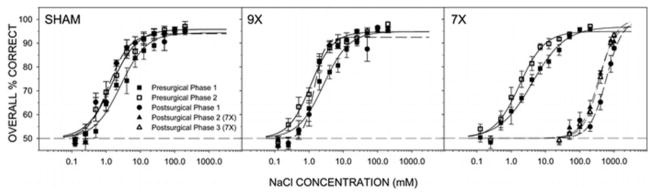
The effect of transection of the glossopharyngeal nerve (9X), which innervates taste buds in the posterior tongue, and the combined gustatory braches of the facial nerve (7X), which innervate taste buds on the anterior tongue and palate, taste detection of NaCl in a two-response operant task. Copyright © 1988 by the American Psychological Association. Reproduced with permission. Blonde GD, Garcea M, Spector AC. (2006). The relative effects of transection of the gustatory branches of the 7th and 9th cranial nerves on NaCl taste detection in rats. Behavioral Neuroscience 120: 580–589. The use of APA information does not imply endorsement by APA.
One interesting principle that came out of our nerve transection work was that the taste signals in the seventh cranial nerve seem to be more critical in qualitative discrimination among taste stimuli, at least in rats, whereas the signals in the glossopharyngeal nerve, which innervates the posterior tongue and the majority of taste buds in the oral cavity, is unnecessary for this aspect of taste function. In his dissertation, Steven St. John, my first graduate student, trained rats to discriminate quinine from KCl across a range of concentrations before various taste nerve transections [27]. Surprisingly, after transection of the glossopharyngeal nerve there was no change in discrimination performance but after transection of the taste branches of the seventh nerve, performance dropped to near chance levels. This basic finding was obtained in other taste discriminations that we conducted through the years [28–31].
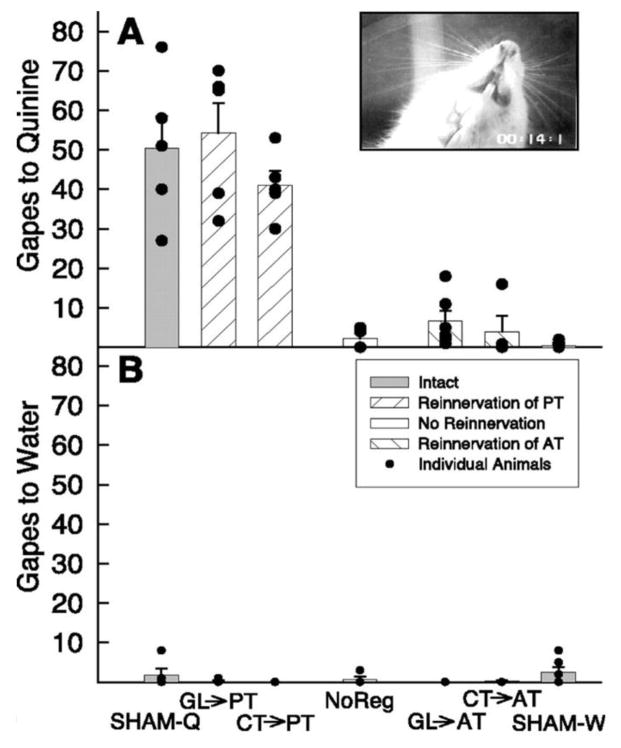
The glossopharyngeal nerve, as initially shown by Joe Travers when he was in Ralph Norgren’s lab, is critical for the maintenance of normal gaping to bitter substances such as quinine in rats [32, 33]. Camille King, when she was a postdoc in my lab, and Mircea Garcea, extended Joe’s work and showed that, in the absence of the glossopharyngeal nerve, the chorda tympani nerve could maintain normal gaping to quinine, if it was cross-regenerated into the posterior tongue (Figure 6) [34]. In fact, this profile of responding emulated the profile seen for Fos activation throughout the central gustatory system [34, 35].
Figure 6.
A: Mean ±se number of gapes to 3 mM quinine in first minute of infusion (0.223 ml/min) in rats that had: sham surgery (SHAM-Q), regenerated glossopharynegeal nerves (GL->PT), the glossopharyngeal nerve cross regenerated into the anterior tongue in the absence of the chorda tympani (GL->AT), regenerated chorda tymapni nerves (CT->AT), the chorda tympani nerve cross-regenerated into the posterior tongue in the absence of the glossopharyngeal nerve (CT->PT), or both the chorda tympani and glossopharyngeal nerve permanently transected. Rats in the SHAM-W group has water instead of quinine. Inset in A; Stll frame of a gape. B: Mean ±se number of gapes to water on the session prior to quinine testing in all groups. Filled circles represent individual responses of individual rats. Reprinted from King CT, Garcea M, Stolzenberg DS, Spector AC. (2008) Experimentally cross-wired lingual taste nerves can restore normal unconditioned gaping behavior in response to quinine stimulation. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 294: R738–R747 with permission.
Thus, it was the receptor field that was critical in the maintenance of the behavior not the way the signals were neurally channeled. In contrast, for salt taste discrimination to be maintained, the signals arising from the anterior tongue taste receptors had to be channeled through their normal route of transmission via the chorda tympani nerve [36].
Amiloride and the Transcellular Sodium Taste Transduction Pathway
Through a series of experiments involving the drug amiloride, which is tasteless to rats [37], we found that the major effect of the CT nerve transection on NaCl sensitivity and salt taste discrimination was due to the loss of epithelial sodium channels (ENaCs). Functional ENaCs in the rat, are expressed primarily in the anterior tongue and palate, and not the posterior tongue, and serve as selective transcellular mechanism of sodium taste transduction through direct depolarization of the receptor cell (Figure 7) [38–43]. Many of these studies were conducted by Laurie Geran when she was a graduate student in my lab,
Figure 7.
Mean ±se corrected hit rate for NaCl detection in a two-response taste detection task for groups of rats fed different dietary levels of NaCl. Open symbols represent performance when all solutions even water were adulterated with tasteless amiloride hydrochloride (100 μM), an epithelial sodium channel blocker. Closed symbols are without amiloride. Although dietary NaCl had no effect on performance, amiloride shifted the psychometric taste sensitivity function by about one order of magnitude to the right. Copyright © 2000 by the American Psychological Association. Reproduced with permission. Geran LC, Spector AC. (2000) Sodium taste detectability in rats is dependent of anion size: The psychophysical characteristics of the transcellular sodium taste transduction pathway. Behavioral Neuroscience, 114(1229–1238. The use of APA information does not imply endorsement by APA.
A Novel Operant Technique for Assessing Taste Quality Generalization
One of the challenges in working with nonverbal animals is trying to construct perceptual quality profiles of taste compounds. The most common practice to achieve this is through the use of the conditioned taste aversion procedure in which an animal is trained to avoid a particular taste compound serving as the conditioned stimulus as a result of pairing its ingestion with experimenter-induced visceral malaise. An array of other test compounds is subsequently presented and the degree of avoidance relative to both noncondtioned control rats and the conditioned stimulus is taken as a measure of similarity. Although this technique has been effective, investigators are always fighting the possibility of extinction of the aversion if multiple tests are involved. Moreover, the procedure loses sensitivity when naturally avoided compounds are used as either conditioned or test stimuli. Consequently, Connie Grobe, when she was a graduate student in my lab, adapted the two-response taste discrimination procedure that we use with our gustometer and trained one group of rats to discriminate sucrose (respond left) from, NaCl, quinine, and citric acid (respond right). Concentration was varied and the position of the correct response was counterbalanced across rats. She trained a second group of rats to discriminate NaCl from the other taste compounds, and a third group citric acid from the other taste compounds, and a fourth group quinine from the others. She subsequently presented unreinforced/non-punished test stimuli on a portion of the trials and quantified the extent to which they responded on one side vs. others. By doing this for all 4 groups, she received reports about how much the test stimulus tasted like sucrose, NaCl, citric acid, and quinine. It does take a lot of effort and at least a total of 24 rats (6/group), but once trained the animals represent an expert taste panel and they can provide valuable information on novel or controversial compounds regarding taste quality [44].
Taste Psychophysics in the Mouse Model
About 15 years ago, we decided to “mousify” the gustometer. It was sort of like “honey I shrunk the gustometer”. At the time, I did not yet know that mice are some of the dumbest creatures on earth. I say this in part in jest, because natural selection specialized mice to excel in their environment, but it is true that they are challenging animals with which to work behaviorally. Nevertheless, my postdoc at the time, Shachar Eylam, spearheaded the effort to use this gustometer and adapt our protocols for performing psychophysical taste tests in mice. In one such study, she replicated earlier work that we conducted in rats showing how the ability of various inbred strains of mice to discriminate detectable concentrations of sodium from potassium is quantitatively related to the degree of epithelial sodium channel blockade by the drug amiloride [45] (Figure 8). Shachar also conducted a variety of psychophysical studies in mice involving salt and sugar taste [46–49].
Figure 8.
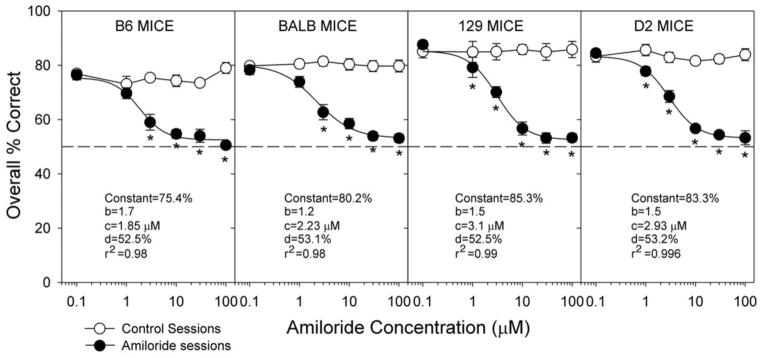
Mean ±se overall percentage correct collapsed across concentration in NaCl vs. KCl taste discrimination as a function of the dose of amiloride hydrochloride adulterating all of the solutions in various strains of inbred mice. Amiloride sessions are represented by closed circles and control sessions (no amiloride) are represented by open circles. Reprinted from Eylam S, Spector AC. (2005). Taste discrimination between NaCl and KCl is disrupted by amiloride in inbred mice with amiloride-insensitive chorda tympani nerves. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 288: R1361–R1368 with permission.
Later, Shawn Dotson, a former graduate student, used the gustometer to show that C57B6/6J mice cannot discriminate sucrose from glucose or fructose suggesting that sugars produce a unitary perceptual quality in rodents, in the spirit of the metameric matching experiments that were so critical for understanding color vision [50].
I also had the good fortune of collaborating with John Glendinning. John and I had an NIDCD-funded grant to develop a behavioral screening test for mouse taste function. Following the pioneering work of P.T. Young [51, 52], we developed a mouse version of the brief-access taste test ([53], see also [54]) which is still used by researchers today.
Neural Representation of Taste Quality
As a result of many of the psychophysical findings we were compiling in the rodent model in my laboratory and the electrophysiological findings that my friend and colleague, Susan Travers, was compiling in her lab, she and I collaborated on a major review article on the neural representation of taste quality that we published in 2005 [55]. At the time, as is true today, it was hotly debated whether specific taste qualities are represented by activity in dedicated classes of neurons (labeled-line or sparse coding) or by the pattern of activity in large groups of heterogeneous neurons (across-neuron pattern or ensemble coding) or whether even temporal features of the spike trains contribute. We argued that, in the periphery, there was substantial evidence that coding was primarily labeled-line, but in the brain, there were insufficient data to dissociate among the possible coding theories. One of the reviews of the manuscript was 11 pages long with 53 points. Later, the late David V. Smith, a highly acclaimed taste researcher that possessed incredible experimental breadth and who was also Susan’s prior graduate mentor, confessed to being the reviewer. In reality, Dave was a tremendous supporter of my work throughout my professional life and I miss him dearly as does the taste field.
Returning to the Florida State University
New Gustometer
In 2007, I returned to FSU and joined the Department of Psychology and the Program in Neuroscience as a Professor. Around that time, along with the creative inspirations of Ross Henderson, we designed an entirely new gustometer that was built by the great instrumentation shop at FSU that I mentioned earlier. We now have a new rat version and a new mouse version (Figure 9) [56].
Figure 9.
Photographs of the mouse version of the new gustometer. Reprinted from Spector AC, Blonde GD, Henderson RP, Treesukosol Y, Hendrick P, Newsome R, Fletcher FH, Tang T, Donaldson JA. (2015) A new gustometer for taste testing in rodents. Chem Senses, 40: 187–96 with permission.
Taste Psychophysics in T1R Knock-Out Mice
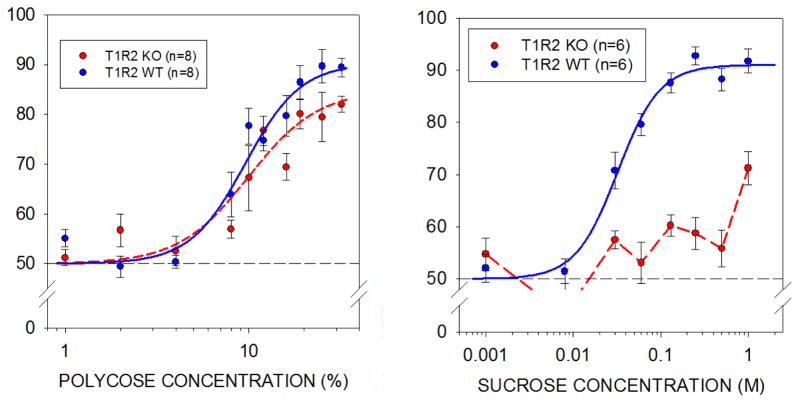
When Yada Treesukosol was a student in my lab, she used the new gustometer to test the psychophysical capacities of mice lacking subunits of the T1R2+3 heterodimeric sweet taste receptor (Figure 10) [57]. Her work [57–59] has helped support the hypothesis, first proposed by Tony Sclafani 30 years ago (see [60–62]), that glucose polymers activate a receptor different from the ones that sweeteners activate. My current student, Kimberly Smith, has been similarly using the gustometer to test the psychophysical capacities of mice lacking subunits of the T1R1+3 heterodimeric amino acid taste receptor. Her work has suggested that there may be T1R- and mGluR–independent receptors that contribute to L-amino acid detection and the so-called “umami” taste [63].
Figure 10.
Mean ±se overall percentage correct by rats as a function of concentration for Polycose (left) and for sucrose (right) in T1R2 knock-out (KO) and wild type (WT) mice in a two-response taste detection task. Replotted from Treesukosol Y, Spector AC. (2012). Orosensory detection of sucrose, maltose, and glucose is severely impaired in mice lacking T1R2 or T1R3, but Polycose sensitivity remains relatively normal. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 303: R218–R235 with permission.
The Effects of Gastric Bypass on Taste-Related Motivation
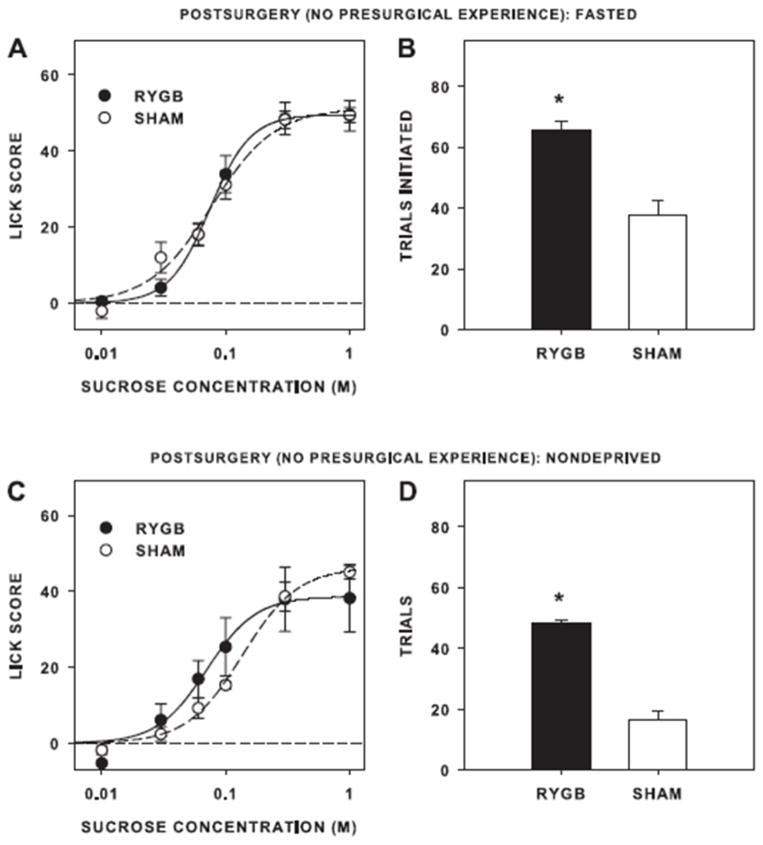
As a postdoc in my lab, Clare Mathes, played a large role in my work with Carel le Roux, Marco Bueter, Thomas Lutz, and Torsten Olbers studying the effects of Roux-en-Y gastric bypass (RYGB) on taste function. It is clear that RYGB substantially blunts the preference for sucrose and Intralipid, a fat emulsion, across a range of concentrations in two-bottle tests while not affecting preference-avoidance functions for prototypical compounds representing other basic taste qualities such as NaCl (salty), citric acid (sour), and quinine (bitter) [64, 65]. But does this finding represent a fundamental RYGB-induced change in the “palatability” or motivational potency of sucrose and Intralipid? Clare used a brief-access test to show that there was no decrease in the effectiveness of sucrose to generate concentration-dependent licking in rats after RYGB regardless of whether the rats were tested in a fasted or nondeprived state. In fact, when tested in a nondeprived state, the rats with RYGB actually were more responsive to the sucrose, and under all physiological states they initiated ~2–3 times more trials compared with controls [66] (Figure 11). Although others have found decreases in responsiveness to sucrose in this test [67–69], our results hardly reflect an animal displaying decreased taste-motivated behavior. Carel le Roux and Thomas Lutz and our colleagues have found a similar lack of decreased responsiveness to Intralipid in a brief access test as a function of RYGB [65].
Figure 11.
A and C: mean ±se licks, with water licks subtracted (Lick Score), as a function of of sucrose concentration in randomized blocks of 10 s trials after Roux-en-Y gastric bypass surgery (RYGB) or sham operation (SHAM). B and D: the mean+se number of trials initiated in the 30 min session. Animals were tested in a fasted (A and B) or nondeprived state (C and D). Reprinted from Mathes CM, Bueter M, Smith KR, Lutz TA, le Roux CW, Spector AC. (2012). Roux-en-Y gastric bypass in rats increases sucrose taste-related motivated behavior independent of pharmacological GLP-1-receptor modulation. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 302: R751–R767 with permission.
The Effects of Gustatory Cortex Lesions on Taste Function
For the last several years, we have been exploring the consequences of extensive bilateral lesions in the conventionally defined gustatory cortex of the rat [70–73]. Lindsey Schier, a current postdoc and Koji Hashimoto, a prior postdoc, along with Ginger Blonde and my graduate student Michelle Bales helped develop a new lesion mapping system to quantify the size, position, and bilateral symmetry of insular cortex lesions (Figure 12) [71]. We have been using this method to study the role of the gustatory zone of insular cortex in taste function.
Figure 12.
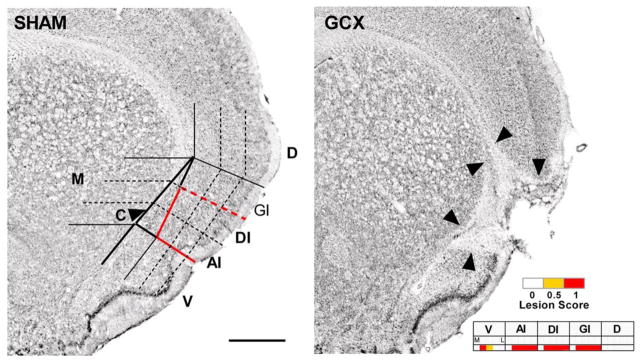
Example of the lesion analysis system applied to the study of the functional consequences of gustatory cortex lesions. Left: a Nissl-stained cross section of an intact rat brain at the level of +1.2 mm relative to bregma. A grid is placed over the region of interest and an experimenter blinded to the behavioral outcome of the animal scores each grid cell for the presence of a complete (1.0), partial, (0.5), or no lesion (0). Abbreviations: AI, agranular insular cortex (dorsal to the rhinal fissure); C, claustrum (outlined column just medial to AI, GI, and DI); D, dorsal to insular cortex; DI, dysgranular insular cortex; GI, granular insular cortex; M, medial to claustrum; V, ventral to rhinal fissure; (Scale bar: 1.0 mm.). Right: an example of how a lesion would be scored. Reprinted from Schier LA, Hashimoto K, Bales MB, Blonde GD, Spector AC. (2014) High-resolution lesion-mapping strategy links a hot spot in rat insular cortex with impaired expression of taste aversion learning. Proceedings of the National Academy of Sciences of the United States of America. 111: 1162–1167 with permission.
FINAL REMARKS
With respect to the various devices that are used in my lab to study taste function and feeding such as the gustometers, the Davis Rig, the microstructure rig, the rat hotel, etc., these are merely pieces of equipment. What counts is the design of proper experiments to optimize the information these devices have the potential to provide. And it is with the greatest conviction that I believe that the rigorous experimental analysis of behavior is indispensable to achieving any comprehensive understanding of brain function.
In closing, it should be entirely apparent, that the Hoebel Award is really not mine alone. The body of my scientific work is the collective product of my mentors, my students, my postdocs, and my collaborators. So it was a privilege to accept this award on their behalf, even though I’m the one who got the check. Moreover, as we all know, a biomedical researcher never rests. Even on holiday, the mind always manages to wander into the lab. Families have to adapt to that reality and thus personal sacrifices are made. In this context, I would like to express my deepest appreciation to my wife, Mary Jo, who has endured through my trials and tribulations as a scientist and all the other mishegas (technical term). I am also indebted, in numerous ways, to my two sons, Aaron and Eliot, especially for their seemingly limitless tolerance. Finally, I would be remiss if I did not acknowledge how hard my parents worked to be sure that I received a college education, a privilege they themselves did not enjoy. Thanks once again to SSIB for this honor and for creating a fertile environment where new and inventive scientific ideas on the controls of ingestive behavior can be exchanged in an intellectually rigorous yet supportive way.
HIGHLIGHTS.
My scientific work is the collective product of my mentors, students, and postdocs.
Instrumentation, while invaluable, can never supplant strong experimental design.
The rigorous analysis of behavior is indispensable for understanding the brain.
Acknowledgments
I am grateful for the numerous undergraduate students and laboratory technicians that have helped with many experiments in my laboratory of the years. I am also grateful for the support I have received from the National Institutes of Health, including grants: F32-NS07915, P01-NS16365, K04-DC00104, P01-DC02641, R01-DC04475, R01-DC04574, R21DC012751, and R01-DC009821.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spector AC, Smith JC, Hollander GR. A comparison of dependent measures used to quantify radiation-induced taste aversion. Physiol Behav. 1981;27:887–901. doi: 10.1016/0031-9384(81)90059-7. [DOI] [PubMed] [Google Scholar]
- 2.Spector AC, Smith JC, Hollander GR. The effects of postconditioning CS experience on recovery from radiation-induced taste aversion. Physiol Behav. 1983;30:647–649. doi: 10.1016/0031-9384(83)90236-6. [DOI] [PubMed] [Google Scholar]
- 3.Spector AC, Smith JC. A detailed analysis of sucrose drinking in the rat. Physiol Behav. 1984;33:127–136. doi: 10.1016/0031-9384(84)90023-4. [DOI] [PubMed] [Google Scholar]
- 4.Markison S, Thompson BL, Smith JC, Spector AC. Time course and pattern of compensatory ingestive behavioral adjustments to lysine deficiency in rats [In Process Citation] J Nutr. 2000;130:1320–1328. doi: 10.1093/jn/130.5.1320. [DOI] [PubMed] [Google Scholar]
- 5.Markison S, Gietzen DW, Spector AC. Essential amino acid deficiency enhances long-term intake but not short- term licking of the required nutrient. J Nutr. 1999;129:1604–1612. doi: 10.1093/jn/129.8.1604. [DOI] [PubMed] [Google Scholar]
- 6.Wolf G. Innate mechanisms for regulation of sodium intake. Olfaction and Taste, III. 1969:548–553. [Google Scholar]
- 7.Krieckhaus EE. “Innate recognition” aids rats in sodium regulation. J Comp Physiol” Psychol. 1970;73:117–122. doi: 10.1037/h0030020. [DOI] [PubMed] [Google Scholar]
- 8.Breslin PAS, Spector AC, Grill HJ. Chorda tympani section decreases the cation specificity of depletion-induced sodium appetite in rats. Am J Physiol (Regulatory Integrative Comp Physiol) 1993;264:R319–R323. doi: 10.1152/ajpregu.1993.264.2.R319. [DOI] [PubMed] [Google Scholar]
- 9.Markison S, St John SJ, Spector AC. Glossopharyngeal nerve transection does not compromise the specificity of taste-guided sodium appetite in rats. Am J Physiol. 1995;269:R215–R221. doi: 10.1152/ajpregu.1995.269.1.R215. [DOI] [PubMed] [Google Scholar]
- 10.Treesukosol Y, Blonde GD, Jiang E, Gonzalez D, Smith JC, Spector AC. Necessity of the glossopharyngeal nerve in the maintenance of normal intake and ingestive bout size of corn oil by rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1050–R1058. doi: 10.1152/ajpregu.00763.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dotson CD, Colbert CL, Garcea M, Smith JC, Spector AC. The consequences of gustatory deafferentation on body mass and feeding patterns in the rat. Am J Physiol Regul Integr Comp Physiol. 2012;303:R611–R623. doi: 10.1152/ajpregu.00633.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis JD. The microstructure of ingestive behavior. In: Schneider LH, Cooper SJ, Halmi KA, editors. The psychobiology of human eating disorders. Vol. 575. New York: Annals of the New York Academy of Sciences; 1989. pp. 106–121. [DOI] [PubMed] [Google Scholar]
- 13.Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci. 1992;106:217–228. [PubMed] [Google Scholar]
- 14.Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol (Regulatory Integrative Comp Physiol) 1993;264:R97–R103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- 15.Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci. 1998;112:678–694. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- 16.Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978;143:281–297. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- 17.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 18.Spector AC, Breslin P, Grill HJ. Taste reactivity as a dependent measure of the rapid formation of conditioned taste aversion: a tool for the neural analysis of taste-visceral associations. Behav Neurosci. 1988;102:942–952. doi: 10.1037//0735-7044.102.6.942. [DOI] [PubMed] [Google Scholar]
- 19.Spector AC, Norgren R, Grill HJ. Parabrachial gustatory lesions impair taste aversion learning in rats. Behav Neurosci. 1992;106:147–161. doi: 10.1037//0735-7044.106.1.147. [DOI] [PubMed] [Google Scholar]
- 20.Spector AC, Grill HJ, Norgren R. Concentration-dependent licking of sucrose and sodium chloride in rats with parabrachial gustatory lesions. Physiol Behav. 1993;53:277–283. doi: 10.1016/0031-9384(93)90205-t. [DOI] [PubMed] [Google Scholar]
- 21.Grigson PS, Spector AC, Norgren R. Lesions of the pontine parabrachial nuclei eliminate successive negative contrast effects in rats. Behav Neurosci. 1994;108:714–723. doi: 10.1037//0735-7044.108.4.714. [DOI] [PubMed] [Google Scholar]
- 22.Scalera G, Spector AC, Norgren R. Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav Neurosci. 1995;109:997–1008. [PubMed] [Google Scholar]
- 23.Spector AC, Scalera G, Grill HJ, Norgren R. Gustatory detection thresholds after parabrachial nuclei lesions in rats. Behav Neurosci. 1995;109:939–954. [PubMed] [Google Scholar]
- 24.Spector AC, Andrews-Labenski J, Letterio FC. A new gustometer for psychophysical taste testing in the rat. Physiol Behav. 1990;47:795–803. doi: 10.1016/0031-9384(90)90099-p. [DOI] [PubMed] [Google Scholar]
- 25.Spector AC, Schwartz GJ, Grill HJ. Chemospecific deficits in taste detection after selective gustatory deafferentation in rats. Am J Physiol (Regulatory Integrative Comp Physiol) 1990;258:R820–R826. doi: 10.1152/ajpregu.1990.258.3.R820. [DOI] [PubMed] [Google Scholar]
- 26.Blonde GD, Garcea M, Spector AC. The relative effects of transection of the gustatory branches of the seventh and ninth cranial nerves on nacl taste detection in rats. Behav Neurosci. 2006;120:580–589. doi: 10.1037/0735-7044.120.3.580. [DOI] [PubMed] [Google Scholar]
- 27.St John SJ, Spector AC. Behavioral discrimination between quinine and KCl is dependent on input from the seventh cranial nerve: implications for the functional roles of the gustatory nerves in rats. J Neurosci. 1998;18:4353–4362. doi: 10.1523/JNEUROSCI.18-11-04353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spector AC, Grill HJ. Salt taste discrimination after bilateral section of the chorda tympani nerve or the glossopharyngeal nerves. Am J Physiol (Regulatory Integrative Comp Physiol) 1992;263:R169–R176. doi: 10.1152/ajpregu.1992.263.1.R169. [DOI] [PubMed] [Google Scholar]
- 29.Spector AC, Markison S, St John SJ, Garcea M. Sucrose vs. maltose taste discrimination by rats depends on the input of the seventh cranial nerve. Am J Physiol (Regulatory Integrative Comp Physiol) 1997;41:R1210–R1228. doi: 10.1152/ajpregu.1997.272.4.R1210. [DOI] [PubMed] [Google Scholar]
- 30.Geran LC, Garcea M, Spector AC. Transecting the gustatory branches of the facial nerve impairs NH(4)Cl vs. KCl discrimination in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R739–R747. doi: 10.1152/ajpregu.00103.2002. [DOI] [PubMed] [Google Scholar]
- 31.Spector AC. The functional organization of the peripheral gustatory system: lessons from behavior. In: Fluharty SJ, Grill HJ, editors. Progress in psychobiology and physiological psychology. Vol. 18. Academic Press; San Diego: 2003. pp. 101–161. [Google Scholar]
- 32.Travers JB, Grill HJ, Norgren R. The effects of glossopharyngeal and chorda tympani nerve cuts on the ingestion and rejection of sapid stimuli: an electromyographic analysis in the rat. Behav Brain Res. 1987;25:233–246. doi: 10.1016/0166-4328(87)90071-4. [DOI] [PubMed] [Google Scholar]
- 33.Grill HJ, Schwartz GJ, Travers J. The contribution of gustatory nerve input to oral motor behavior and fluid intake-based preference: I. Effects of chorda tympani or glossopharyngeal nerve section in the rat. Brain Res. 1991 doi: 10.1016/0006-8993(92)90117-r. [DOI] [PubMed] [Google Scholar]
- 34.King CT, Garcea M, Stolzenberg DS, Spector AC. Experimentally cross-wired lingual taste nerves can restore normal unconditioned gaping behavior in response to quinine stimulation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R738–R747. doi: 10.1152/ajpregu.00668.2007. [DOI] [PubMed] [Google Scholar]
- 35.King CT, Garcea M, Spector AC. Restoration of quinine-stimulated Fos-immunoreactive neurons in the central nucleus of the amygdala and gustatory cortex following reinnervation or cross-reinnervation of the lingual taste nerves in rats. J Comp Neurol. 2014 doi: 10.1002/cne.23546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spector AC, Blonde G, Garcea M, Jiang E. Rewiring the gustatory system: specificity between nerve and taste bud field is critical for normal salt discrimination. Brain Res. 2010;1310:46–57. doi: 10.1016/j.brainres.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markison S, Spector AC. Amiloride is a poor conditioned stimulus in taste aversion. 20. 1996. p. 785. [DOI] [PubMed] [Google Scholar]
- 38.Spector AC, Guagliardo NA, St John SJ. Amiloride disrupts NaCl versus KCl discrimination performance: implications for salt taste coding in rats. J Neurosci. 1996;16:8115–8122. doi: 10.1523/JNEUROSCI.16-24-08115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geran LC, Guagliardo NA, Spector AC. Chorda tympani nerve transection, but not amiloride, increases the KCl taste detection threshold in rats. Behav Neurosci. 1999;113:185–195. doi: 10.1037//0735-7044.113.1.185. [DOI] [PubMed] [Google Scholar]
- 40.Geran LC, Spector AC. Amiloride increases sodium chloride taste detection threshold in rats. Behav Neurosci. 2000;114:623–634. doi: 10.1037/0735-7044.114.3.623. [DOI] [PubMed] [Google Scholar]
- 41.Geran LC, Spector AC. Sodium taste detectability in rats is independent of anion size: the psychophysical characteristics of the transcellular sodium taste transduction pathway. Behav Neurosci. 2000;114:1229–1238. [PubMed] [Google Scholar]
- 42.Geran LC, Spector AC. Anion size does not compromise sodium recognition by rats after acute sodium depletion. Behav Neurosci. 2004;118:178–183. doi: 10.1037/0735-7044.118.1.178. [DOI] [PubMed] [Google Scholar]
- 43.Geran LC, Spector AC. Amiloride-insensitive units of the chorda tympani nerve are necessary for normal ammonium chloride detectability in the rat. Behav Neurosci. 2007;121:779–785. doi: 10.1037/0735-7044.121.4.779. [DOI] [PubMed] [Google Scholar]
- 44.Grobe CL, Spector AC. Constructing quality profiles for taste compounds in rats: a novel paradigm. Physiol Behav. 2008;95:413–424. doi: 10.1016/j.physbeh.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Eylam S, Spector AC. Taste discrimination between NaCl and KCl is disrupted by amiloride in inbred mice with amiloride-insensitive chorda tympani nerves. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1361–R1368. doi: 10.1152/ajpregu.00796.2004. [DOI] [PubMed] [Google Scholar]
- 46.Eylam S, Spector AC. The effect of amiloride on operantly conditioned performance in an NaCl taste detection task and NaCl preference in C57BL/6J mice. Behav Neurosci. 2002;116:149–159. [PubMed] [Google Scholar]
- 47.Eylam S, Spector AC. Oral amiloride treatment decreases taste sensitivity to sodium salts in C57BL/6J and DBA/2J mice. Chem Senses. 2003;28:447–458. doi: 10.1093/chemse/28.5.447. [DOI] [PubMed] [Google Scholar]
- 48.Eylam S, Spector AC. Stimulus processing of glycine is dissociable from that of sucrose and glucose based on behaviorally measured taste signal detection in Sac ‘taster’ and ‘non-taster’ mice. Chem Senses. 2004;29:639–649. doi: 10.1093/chemse/bjh068. [DOI] [PubMed] [Google Scholar]
- 49.Eylam S, Moore M, Haskell-Luevano C, Spector AC. Melanocortin-4 receptor-null mice display normal affective licking responses to prototypical taste stimuli in a brief-access test. Peptides. 2005;26:1712–1719. doi: 10.1016/j.peptides.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 50.Dotson CD, Spector AC. Behavioral discrimination between sucrose and other natural sweeteners in mice: implications for the neural coding of T1R ligands. J Neurosci. 2007;27:11242–11253. doi: 10.1523/JNEUROSCI.1227-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young PT, Madsen CH., Jr Individual isohedons in sucrose-sodium chloride and sucrose-saccharin gustatory areas. J Comp Physiol Psychol. 1963;56:903–909. doi: 10.1037/h0047364. [DOI] [PubMed] [Google Scholar]
- 52.Young PT, Trafton CL. Activity contour maps as realted to preference in four gustatory stimulus areas of the rat. J Comp Physiol Psychol. 1964;58:68–75. doi: 10.1037/h0044823. [DOI] [PubMed] [Google Scholar]
- 53.Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses. 2002;27:461–474. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- 54.Boughter JD, Jr, St John SJ, Noel DT, Ndubuizu O, Smith DV. A brief-access test for bitter taste in mice. Chem Senses. 2002;27:133–142. doi: 10.1093/chemse/27.2.133. [DOI] [PubMed] [Google Scholar]
- 55.Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev. 2005;4:143–191. doi: 10.1177/1534582305280031. [DOI] [PubMed] [Google Scholar]
- 56.Spector AC, Blonde GD, Henderson RP, Treesukosol Y, Hendrick P, Newsome R, Fletcher FH, Tang T, Donaldson JA. A new gustometer for taste testing in rodents. Chem Senses. 2015;40:187–196. doi: 10.1093/chemse/bju072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Treesukosol Y, Spector AC. Orosensory detection of sucrose, maltose, and glucose is severely impaired in mice lacking T1R2 or T1R3, but Polycose sensitivity remains relatively normal. Am J Physiol Regul Integr Comp Physiol. 2012;303:R218–R235. doi: 10.1152/ajpregu.00089.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Treesukosol Y, Blonde GD, Spector AC. The T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: Implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R855–R865. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Treesukosol Y, Smith KR, Spector AC. Behavioral evidence for a glucose polymer taste receptor that Is Independent of the T1R2+3 heterodimer in a mouse model. J Neurosci. 2011;31:13527–13534. doi: 10.1523/JNEUROSCI.2179-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sclafani A. The sixth taste? Appetite. 2004;43:1–3. doi: 10.1016/j.appet.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Sclafani A, Nissenbaum JW. Oral versus postingestive origin of polysaccharide appetite in the rat. Neurosci Biobehav Rev. 1987;11:169–172. doi: 10.1016/s0149-7634(87)80022-2. [DOI] [PubMed] [Google Scholar]
- 62.Sclafani A, Clyne AE. Hedonic response of rats to polysaccharide and sugar solutions. Neurosci Biobehav Rev. 1987;11:173–180. doi: 10.1016/s0149-7634(87)80023-4. [DOI] [PubMed] [Google Scholar]
- 63.Smith KR, Spector AC. The importance of the presence of a 5′-ribonucleotide and the contribution of the T1R1 + T1R3 heterodimer and an additional low-affinity receptor in the taste detection of L-glutamate as assessed psychophysically. J Neurosci. 2014;34:13234–13245. doi: 10.1523/JNEUROSCI.0417-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR, Unwin RJ, Lutz TA, Spector AC, le Roux CW. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav. 2011;104:709–721. doi: 10.1016/j.physbeh.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 65.le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Lowenstein C, Athanasiou T, Bloom SR, Spector AC, Olbers T, Lutz TA. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1057–R1066. doi: 10.1152/ajpregu.00139.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mathes CM, Bueter M, Smith KR, Lutz TA, le Roux CW, Spector AC. Roux-en-Y gastric bypass in rats increases sucrose taste-related motivated behavior independent of pharmacological GLP-1-receptor modulation. Am J Physiol Regul Integr Comp Physiol. 2012;302:R751–R767. doi: 10.1152/ajpregu.00214.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes. 2011;35:642–651. doi: 10.1038/ijo.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tichansky DS, Glatt AR, Madan AK, Harper J, Tokita K, Boughter JD. Decrease in sweet taste in rats after gastric bypass surgery. Surg Endosc. 2011;25:1176–1181. doi: 10.1007/s00464-010-1335-0. [DOI] [PubMed] [Google Scholar]
- 69.Hajnal A, Kovacs P, Ahmed T, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G967–G979. doi: 10.1152/ajpgi.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hashimoto K, Spector AC. Extensive lesions in the gustatory cortex in the rat do not disrupt the retention of a presurgically conditioned taste aversion and do not impair unconditioned concentration-dependent licking of sucrose and quinine. Chem Senses. 2014;39:57–71. doi: 10.1093/chemse/bjt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schier LA, Hashimoto K, Bales MB, Blonde GD, Spector AC. High-resolution lesion-mapping strategy links a hot spot in rat insular cortex with impaired expression of taste aversion learning. Proc Natl Acad Sci U S A. 2014;111:1162–1167. doi: 10.1073/pnas.1315624111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blonde GD, Bales MB, Spector AC. Extensive Lesions in Rat Insular Cortex Significantly Disrupt Taste Sensitivity to NaCl and KCl and Slow Salt Discrimination Learning. PLoS ONE. 2015;10:e0117515. doi: 10.1371/journal.pone.0117515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.King CT, Hashimoto K, Blonde GD, Spector AC. Unconditioned oromotor taste reactivity elicited by sucrose and quinine is unaffected by extensive bilateral damage to the gustatory zone of the insular cortex in rats. Brain Res. 2015;1599:9–19. doi: 10.1016/j.brainres.2014.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]