Abstract
ABO-incompatible kidney transplantation (ABOi KT) was introduced to expand the donor pool and minimize shortage of kidneys for transplantation. Because improved outcomes of ABOi KT were reported in Japan in the early 2000s, the number of ABOi KTs has been increasing worldwide. In addition, a better understanding of immune pathogenesis and subsequent aggressive immunosuppression has helped to make effective desensitization protocols. Current strategies of ABOi KT consist of pretransplant antibody removal using plasmapheresis or immunoadsorption to prevent hyperacute rejection and potent maintenance immunosuppression, such as tacrolimus and mycophenolate mofetil, to inhibit antibody-mediated rejection. Recent outcomes of ABOi KT are comparable with ABO-compatible KT. However, there are still many problems to be resolved. Very high anti-ABO antibody producers are difficult to desensitize. In addition, ABOi KT is associated with an increased risk of infection and possibly malignancy due to aggressive immunosuppression. Optimization of desensitization and patient-tailored immunosuppression protocols are needed to achieve better outcomes of ABOi KT. This review provides an overview of the history, immune mechanism, immunosuppressive protocol, outcomes, current obstacles, and future perspectives in ABOi KT.
Keywords: Blood group incompatibility, Desensitization, Kidney transplantation, Outcomes
Introduction
Kidney transplantation (KT) is the treatment of choice for patients with end-stage renal disease. Unfortunately, the number of end-stage renal disease patients on waiting lists is increasing while the number of available kidneys is static. As a result, the waiting time for KT continues to be very long. To overcome the shortage of organs, various strategies have been tried. Although xenotransplantation using pig organs and differentiation to new organs using stem cell technology have been studied, their clinical application is far from being realized. Various desensitization strategies have been attempted to overcome immunologic barriers such as anti-human leukocyte antigen (HLA) donor-specific antibody (DSA) and ABO blood group incompatibility.
ABO-incompatible living-donor KT (ABOi KT) is another strategy to expand the organ pool and decrease the long waiting times for deceased-donor KT [1,2]. After Alexandre et al [3] introduced an effective desensitization protocol based on plasmapheresis and splenectomy to prevent hyperacute rejection across the ABO antibody barrier in 1987, ABOi KT has been actively studied in Japan because of the lack of deceased donors [4]. Although outcomes were not satisfactory before 2000, they improved significantly after the introduction of tacrolimus and mycophenolate mofetil (MMF) around 2000 [5]. When 5-year graft survival rates of ABOi KT in Japan were analyzed according to the three eras (1989–1994, 1995–2000, and 2001–2006), they were 68%, 76%, and 90%, respectively [6]. Outcomes of ABOi KT between 2002 and 2008 in Japan were further improved after the introduction of rituximab, based on the successful outcomes of a Swedish group [7]. Five-year graft survival rates of ABO-compatible (ABOc) KT, ABOi KT using splenectomy, and ABOi KT using rituximab were 88.4%, 90.3%, and 100%, respectively [8]. Currently, ABOi KT reached approximately 30% of all living-donor KTs in Japan [4].
Based on the successful outcomes of ABOi KTs in Japan, this technique has been increasingly performed in the United States and Europe since the late 1990s [9,10]. Although only 738 cases (0.94%) of ABOi KTs were performed between 1995 and 2010 in the United States, this number has been increasing annually [11]. The same trend was observed in the United Kingdom where, over the last decade, there has been an increase of ABOi KT from<10 to 73 per year, now representing 6.8% of all living-donor KTs [12]. After Tyden et al [7,10] reported successful antibody reduction using ABO antigen-specific immunoadsorption (IA), greater numbers of ABOi KTs were performed in Europe. Although ABOi KT was introduced relatively late in Korea, the numbers of patients and participating centers have expanded rapidly, and ABOi KT accounted for 10% and>20% of all living-donor KTs in Korea in 2010 and 2014, respectively [13,14].
To understand the current status and future perspectives of ABOi KT, the present review focuses on immune mechanisms including accommodation and antibody-mediated rejection (AMR), immunosuppressive regimens, pretransplant and posttransplant immune monitoring, outcomes, and unsolved problems of ABOi KT.
Blood group antigens and antibodies
ABO blood group antigens
Blood group antigens are polysaccharides, do not require T-cell sensitization for antibody induction, and induce poor T cell-specific responses [15]. The most important blood antigens consist of A, B, and O group antigens. These antigens are found in many cells, including erythrocytes, platelets, and endothelial cells of all vascular organs. ABO blood group incompatibility is a significant immunologic barrier because humans have natural antibodies against the A and/or B antigens that they do not have.
Blood group A carries A1 or A2 antigen, with expression of A2 antigen weaker than that of A1 antigen [16]. The A2 subtype represents approximately 20% of blood type A in Caucasians, whereas it is only 0.15% in the Japanese population [17]. A2 kidneys may be less likely to undergo AMR in the presence of anti-A antibodies. Non-A recipients receiving kidneys from A2 donors can safely accept transplantation without preconditioning.
Anti-ABO antibodies
Antibodies against the blood group antigens A and B are isohemagglutinins because they react with A antigen and B antigen in red blood cells and induce agglutination. Anti-ABO antibodies are either immunoglobulin (Ig) M (IgM) or IgG type. These antibodies are made during the development of the immune system against cross-reactive epitopes on the cell wall of gut bacteria, and their titer increases with age [18,19].
Anti-ABO antibodies are produced mainly by extrafollicular B-1 cells [20], in contrast to antipeptide antibodies that are produced by follicular B-2 cells. However, the main anti-ABO-specific B-cell populations are not yet established. On the other hand, the anti-ABO response is classically regarded as a T cell-independent IgM antibody response; however, recent studies suggested a role for natural killer cells or T cells in the anti-ABO antibody response [21] and demonstrated that anti-ABO IgG response was more important than the IgM response in AMR after ABOi KT [22]. Therefore, the role of helper T cells in the anti-ABO antibody response should be defined.
Monitoring techniques
Monitoring of anti-ABO antibody levels is important for determining the effectiveness of desensitization and the optimal time to perform ABOi KT. In addition, the titer of anti-ABO antibodies should be monitored after transplantation to detect any rebound in antibody production, which may indicate or induce AMR.
There are various methods to measure the anti-ABO antibody titer. The most common is the saline tube technique, although there is significant intercenter variation in the titer determined by this method [23]. New techniques, such as gel card technique and flow cytometry, may be better than the saline tube test because both show improved reproducibility [23–27]. Flow cytometry would be suitable for an accurate measurement but is not available at all centers because of its high cost [27].
Rejection and accommodation in ABOi KT
Antibody-mediated rejection
AMR is clinically suspected when the serum creatinine level is elevated relative to the baseline value. Natural and induced anti-ABO antibodies might cause AMR in ABOi KT and can manifest as hyperacute rejection, acute AMR, or delayed AMR [28]. After a silent period during the first 2 days, most AMRs occur between 1 week and 3 weeks after ABOi KT. Anti-ABO antibodies usually do not induce AMR after 3 weeks despite a titer rebound due to accommodation [28].
AMR after ABOi KT is diagnosed on the basis of morphologic, immunohistologic, and serologic evidences, with at least one finding in each of the three categories [29–31]. Morphologic features include leukocyte infiltration into peritubular capillaries and/or glomeruli, arterial fibrinoid necrosis, glomerular and arterial thrombi, and acute tubular injury. Immunohistologic features involve peritubular capillary C4d deposition and deposition of immunoglobulin and/or complement in arterial fibrinoid necrosis. Circulating DSAs at the time of biopsy should be found. However, deposition of C4d, without other evidence of AMR in biopsy, was observed in 60–80% of ABOi KT patients [32–35]. A protocol biopsy study demonstrated no differences in the incidence of either AMR or transplant glomerulopathy according to C4d positivity [34]. Moreover, graft function was stable despite the presence of anti-ABO antibodies [31]. Therefore, the presence of anti-ABO antibodies or the presence of peritubular capillary C4d deposition alone is not diagnostic of AMR with ABOi KT [36,37]. C4d deposition without other evidence of AMR might indicate accommodation instead of AMR because the presence of this feature in 3-month protocol biopsies was associated with fewer chronic injuries than in 1-year protocol biopsies [32].
Accommodation
The resistance of an allograft to AMR, despite the presence of significant levels of anti-ABO antibodies and C4d deposition, is known as accommodation. Accommodation differs from tolerance which lacks DSA or anti-ABO antibodies. Accommodation in xenotransplantation is transient and accompanied by acute vascular or cellular rejection [38]. However, durable accommodation is observed in many ABOi KT patients. Several preliminary studies have examined the mechanisms leading to accommodation. These include changes in the function of DSA, changes in the antigen, acquired resistance of the allograft through the expression of antiapoptotic genes such as heme oxygenase-1, A20, bcl-2, and bcl-xL; expression of complement regulatory proteins such as CD45, CD55, and CD59; and inactivation of extracellular-signal-regulated kinase-1/2 [39–43]. A small microarray study suggested roles for SMAD, protein tyrosine kinase, tumor necrosis factor-α, and mucin 1 in accommodation [1]. Overall, further studies are needed to elucidate the mechanisms of accommodation and to develop noninvasive diagnostic tools of accommodation for patient-tailored immunosuppressive therapy.
Desensitization for ABOi KT
Current immunosuppressive strategies for ABOi KT have two main principles: (1) pretransplant antibody removal and (2) induction and maintenance of immunosuppression to inhibit the reappearance of anti-ABO antibodies.
Antibody depletion
Antibody-depleting treatment is the basis of desensitization for ABOi KT. Current methods for the removal of anti-ABO antibodies involve classical plasmapheresis, double-filtration plasmapheresis (DFPP), and antigen-specific or antigen-nonspecific IA.
Classical plasmapheresis completely removes all plasma proteins from the circulation, and the recipient plasma is replaced by either albumin, fresh-frozen plasma (FFP), or a combination of both. Owing to the removal of coagulation factors and useful immunoglobulins, the risk of bleeding and infections is increased after plasmapheresis. To avoid these complications, many centers use FFP for the final sessions before transplantation. Other complications were hypocalcemia, hypotension, and nausea or vomiting [44].
For DFPP, plasma is separated by filtration and passed through a second filter where immunoglobulins are selectively filtered out and discarded. DFPP can minimize hemodynamic instability and the amount of replacement volume needed. DFPP also removes 60–70% of the antibodies per session. This is greater efficiency than classical plasmapheresis that removes only 40–50% [45]. Although DFPP avoids the loss of coagulation factors and albumin, unlike classical plasmapheresis, albumin is almost always needed in the replacement fluid.
IA can remove antigen-specific antibodies, such as anti-ABO antibodies, or antigen-nonspecific immunoglobulins. Between the specific and nonspecific IA techniques, antigen-specific IA is used more commonly in ABOi KT, whereas antigen-nonspecific IA is suitable for the depletion of anti-HLA antibodies. In ABO-specific IA, the plasma is processed through an ABO immunoadsorbent column that is coated with either blood type A or B antigens. This allows the selective removal of anti-A or anti-B antibodies, and the processed plasma is then reinfused into the recipients. Antigen-specific IA removes a twofold to fourfold titer per session. At least four preoperative IAs are usually needed to obtain an acceptable titer [46]. IA is normally preferred because of its safety and efficacy. However, the application of IA outside Europe and Australia is limited because of its high cost that is often not covered by health insurance.
Posttransplant antibody depletion is used routinely in some centers with a variable number of sessions, depending on the pretransplant titer [47]. Several investigators suggested that the decision to perform posttransplant plasmapheresis should be based on the pretransplant anti-ABO antibody titer before desensitization [48,49]. Others suggested that posttransplant plasmapheresis depended on an elevation of the posttransplant antibody titer [50]. Although there are no established criteria for performing posttransplant antibody depletion, many centers apply it to patients with a high risk for AMR, e.g., patients with a high initial titer (>1:256), a rapidly increasing posttransplant titer (≥8-fold), or a high posttransplant titer (≥1:64).
B-cell depletion
To avoid the reappearance of anti-ABO antibodies and the associated risk of AMR, B-cell depletion is important. Old protocols for ABOi KT included splenectomy to eliminate B-cell pools. The principle of splenectomy was to remove a major reservoir of lymphocytes, including antibody-secreting B cells, B-cell precursors, and plasma cells. However, the effect of splenectomy on the immune system is permanent and increases the risk of infection in ABOi KT.
After some studies reported that treating ABOi KT patients with rituximab had a long-acting B cell-depleting effect without any serious side effects, splenectomy was largely replaced by rituximab [7,10,51]. Rituximab is an anti-CD20 monoclonal antibody that binds to CD20 on immature and mature B cells, inducing apoptosis through antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, or direct apoptosis mechanisms. However, rituximab cannot induce apoptosis of some memory B or plasma cells. B-cell depletion with rituximab may prevent the development of mature B cells into antibody-producing plasma cells, reduce alloreactive antibody production, and attenuate T-cell responses by reducing B-cell antigen presentation and costimulation [52]. Although bortezomib, a proteasome inhibitor, is effective in controlling plasma cells, it does not suppress either B-1 or B-2 cells [20]. Rituximab may affect macrophages and natural killer cells through binding to the Fc receptor. B-cell depletion by rituximab occurs at 1–3 days after administration and persists for up to 2 years [53]. The timing and dosage of rituximab remain variable. Because an interval is needed to deplete antibody-producing cells, it is better to administer rituximab at least 1 week before KT [54]. Recently, some investigators introduced an ABOi KT protocol without rituximab, or with only a low dose (<375 mg/m2), to avoid overimmunosuppression [55–58].
Intravenous immunoglobulin
Intravenous Ig (IVIG) is widely used in KT to suppress both cell-mediated rejection and AMR, although the mechanism of action remains unclear. Potential modes of action include suppression of plasma cells, neutralization of alloantibodies, inhibition of complement activation, neutralization of proinflammatory cytokines, and induction of anti-inflammatory cytokines [59]. IVIG is usually administered after plasmapheresis to reconstitute the natural levels of IgG. However, there is no uniform dose of IVIG used in the desensitization protocol of ABOi KT.
Maintenance immunosuppression
Maintenance immunosuppressive regimens included calcineurin inhibitors (CNI), antimetabolites (i.e., MMF), and steroids. In ABOi KT protocols, tacrolimus is the CNI of choice. In addition, stronger induction agents, such as antithymocyte globulin (ATG), are often used for induction to suppress T cells׳ help to B cells. B-1 cells are susceptible to CNI and antimetabolites, whereas B-2 cells are resistant to CNI. A comparative study showed that a 7-day regimen was more effective than a 2-day regimen as pretransplant immunosuppression [60]. Thus, maintenance immunosuppression should start 7–14 days before KT to adequately inhibit antibody production. However, a 2-day regimen of tacrolimus (0.1 mg/kg) in ABOi KT achieved similar outcomes to ABOc KT [56]. The effects of CNI-sparing strategies or long-term steroids in ABOi KT remain unclear [61,62].
Current desensitization protocol of ABOi KT
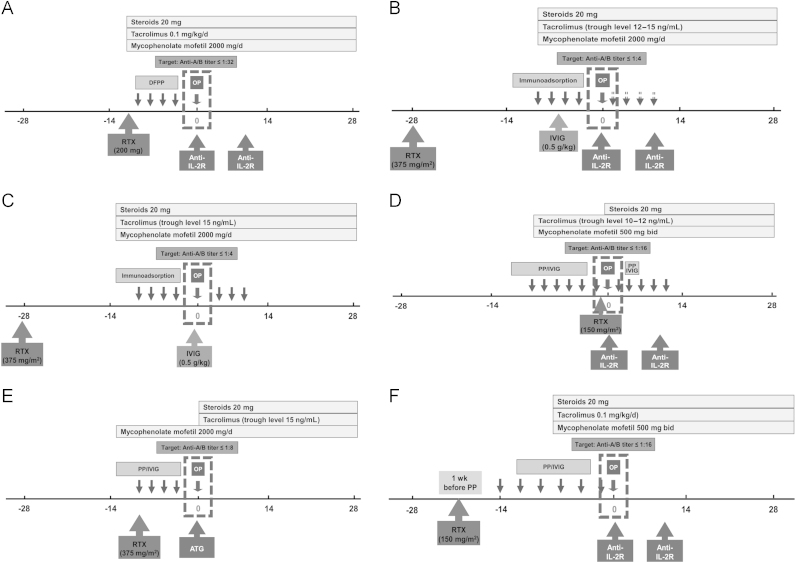
With increasing experience over the last decade, desensitization protocols were modified and continue to evolve (Fig. 1) [22,30,53,63–65]. However, the lack of controlled studies makes it difficult to compare various protocols and determine which is best.
Figure 1.
Desensitization protocols for ABOi KT.* This figure shows representative desensitization protocols for ABOi KT used at several transplant centers. Most centers have modified the original successful protocol. Desensitization protocols of Tokyo Women’s Medial University, Japan (A), Freiburg University Hospital, Germany (B), Stockholm group, Sweden (C), Johns Hopkins Hospital, USA (D), Mayo Clinic, USA (E), and Seoul National University Hospital, Korea (F), are shown. Details of the desensitization protocols of several centers can be found in the references [22,30,53,63–65].
ABOi KT, ABO incompatible kidney transplantation; Anti-IL-2R, anti-interleukin-2 antibody; ATG, antithymocyte globulin; DFPP, double filtration plasmapheresis; IVIG, intravenous immunoglobulin; OP, transplantation operation; PP, plasmapheresis; RTX, rituximab.
*These figures are based on published data of current protocols for an individual center. These protocols may have changed since publication.
Most transplants in Japan are desensitized by DFPP, and splenectomy has been replaced by rituximab. In addition, most centers in Japan do not use IVIG. The target titers of anti-ABO antibodies immediately before KT in Japan are usually 1:16 to 1:32 or less [22,64], even though high target titers up to 1:32 were associated with a high rate of acute AMR (33%) in the prerituximab era [22].
In Europe, IA is widely practiced in desensitization of ABOi KT. After Alexandre et al [3] began using ABOi KT in the 1980s, it took 20 years until this procedure was widely used throughout Europe. In 2003, it was reported that successful ABOi KT could be achieved using antigen-specific IA and rituximab without splenectomy [7]. To date, this protocol has led to successful ABOi KTs with more than 1,000 transplants in Europe. The basis of the North European protocol is IA followed by high-dose IVIG. However, posttransplant IA is not performed routinely, and its use is determined by antibody titer [50]. The target titer of anti-ABO antibodies immediately before KT was 1:4 or less in the Stockholm and Freiburg groups [53,63]. Although this strict target titer can be helpful for good posttransplant outcomes, 14–21% of patients failed to satisfy this criterion [63]. In the United States, desensitization protocols consist of classical plasmapheresis, low-dose IVIG, and rituximab [37,65]. Some centers use a rituximab-free protocol [65]. Target titers are 1:8 to 1:16 [30,65]. A recent report from Australia suggests that ABOi KT can be performed without B-cell depletion procedures such as splenectomy or rituximab [56].
In Korea, the desensitization protocols of ABOi KT are highly uniform with routine plasmapheresis, rituximab, and tacrolimus-based maintenance immunosuppression [13]. The timing and dosage of rituximab vary depending on the center. Most centers use a single dose of 375 mg/m2 at the initiation of the protocol, although the dose tends to be reduced to 200 mg/person or 100 mg/m2. Pretransplant conventional plasmapheresis is used in most centers, although DFPP is also used at times. Posttransplant plasmapheresis is performed in selected recipients with a high risk for AMR, and IVIG (100 mg/kg or 200 mg/kg) is administered after plasmapheresis in most centers.
The desensitization protocol in Seoul National University Hospital consists of rituximab, plasmapheresis, low-dose IVIG, and tacrolimus-based maintenance immunosuppressants (Fig. 1F). Recipients with anti-ABO antibody titers less than or equal to 1:512 are acceptable for ABOi KT, although patients with a higher titer can be eligible according to the individual situation. Rituximab administration without plasmapheresis is applied to patients with a very low titer (≤1:8). Other recipients received DFPP and conventional plasmapheresis at a ratio of 2:1, for three to 12 sessions before KT, with a target titer of 1:16 or less, because a target titer higher than 1:16 is associated with an increased risk for acute AMR [22]. Low-dose IVIG (100 mg/kg) is administered after plasmapheresis. A single dose of rituximab (150 mg/m2) was administered 1 week before plasmapheresis to minimize removal of rituximab. Plasmapheresis with FFP is performed on the last session before KT. Tacrolimus-based immunosuppression is initiated 1 week or 2 weeks before KT and combined with two doses of basiliximab. The target trough level of tacrolimus was 8–10 ng/mL, and MMF was administered at a dose of 1.0 g/d in most patients. In the presence of high anti-ABO antibody levels (≥1:256 before KT, ≥1:64 after KT), plasmapheresis and low-dose IVIG are recommended during the posttransplant period.
Treatment of AMR
Standard treatment for AMR consists of plasmapheresis or IA and IVIG [66]. Most centers treat AMR with a series of plasma exchanges followed by low-dose IVIG in addition to methylprednisolone, until clinical improvement or histologic resolution of AMR is achieved [30,67,68]. Although reversal rates for AMR using this protocol were better than reversal rates with traditional immunosuppression alone [31], data about reversal rates for AMR in ABOi KT are rare and complicated by the concomitant presence of DSA. Rituximab is an efficient agent to treat AMR by depleting B cells and thereby controlling DSA production [69]. Some studies suggest that rituximab is the treatment of choice in AMR [70,71]. ATG, at the time of plasmapheresis, is also often administered to treat AMR [22,66].
New drugs have been introduced for the treatment of AMR because plasmapheresis, IVIG, ATG, and rituximab can have suboptimal results because of poor direct effects on mature plasma cells [72]. Bortezomib is effective in the treatment of refractory AMR due to anti-HLA DSA by inducing plasma cell apoptosis [72,73]. However, the ability of bortezomib to affect anti-ABO antibodies is controversial [74,75]. Eculizumab, a humanized monoclonal antibody against complement protein C5, prevents the cleavage of C5 to C5a and C5b, subsequently preventing the generation of the C5b-C9 membrane attack complex. Recent reports indicate that eculizumab is useful in the treatment of AMR [66,76,77].
Outcomes of ABOi KT
Patient and graft survival
Short-term patient and graft survival in ABOi KT are notable in most published data [3,4,11,22,53,56,57,67,78–84]. Long-term outcomes of ABOi KT reported by most centers are comparable with ABOc KT [11,64].
Outcomes of a large number of Japanese ABOi KTs, with follow-up periods of more than 20 years, are reported [4]. In addition, outcomes for earlier (1989–2000) and recent eras (2001–2010) were compared. The patient and graft survival rates during the earlier era were 84% and 58% at 9 years, respectively. During the recent era, the patient and graft survival rates were 91% and 83% for 9 years, respectively. These data showed that both patient and graft survival significantly improved in the recent era. This is due to maintenance immunosuppression based on tacrolimus and MMF and the use of rituximab. Takahashi et al [64] investigated the comparison of graft survival between 441 ABOi KT patients and >1,000 ABOc KT patients. The graft survival tended to be lower in ABOi KT group after 1 year, but this difference was not significant after 5 years and 10 years.
Data from the Scientific Registry of Transplant Recipients on the outcomes of 738 ABOi KTs that were performed between 1995 and 2010 have been reported [11]. Comparing ABOi KT cases with >3,000 ABOc KT cases, the 1-year graft survival was 94.1% in ABOi KT versus a slightly higher 97.1% in ABOc KT. However, there was no difference in the 10-year graft survival [11]. The Collaborative Transplant Study reported outcomes from 1,420 ABOi KTs performed at 101 transplants centers from 2005 to 2012 [84]. Three-year graft survival rates of ABOi KT were not different from those of the matched ABOc KT (89.9% vs. 90.1%). Although patient survival rates in the early period were lower in ABOi KT because of a higher rate of death from infection, 3-year patient survival rates were similar (95.6% vs. ABOc, 96.3%). Antibody reduction with IA resulted in a similar rate of graft survival compared with reduction with plasmapheresis. Kong et al [13] analyzed 125 ABOi KTs that were performed from 2007 to 2010 in Korea. Two-year graft and patient survival rates were 97.5% and 99.2%, respectively. These data indicate similar survival outcomes after ABOi and ABOc KT.
Acute AMR and T cell-mediated rejection in ABOi KT
Protocol biopsy specimens at 3 months after ABOi KT showed a significantly higher incidence of AMR compared with ABOc KT (18% vs. 1%) [85], indicating an increased risk for acute AMR in ABOi KT due to anti-ABO antibodies. However, there was no significant difference in the incidence of acute T cell-mediated rejection between ABOi KT and ABOc KT (48.4% vs. 35.7%). Gloor et al [86] analyzed 1-year protocol biopsy specimens and found that there was a significant difference in the rate of AMR between ABOi KT and ABOc KT without HLA antibodies. Setoguchi et al [33] also showed that ABOi allografts had a higher overall incidence of AMR compared with ABOc allografts (27% vs. 5.3%). AMR was detected in 15% of ABOi KT at 1 month and 30% at 6–12 months, compared with 35% at 1 month and 11% at 6–12 months in ABOc KT. However, recent studies using rituximab reported improved outcomes of ABOi KT and demonstrated that acute AMR rates were similar between ABOi KT and ABOc KT [8,87]. Acute AMR rates during the first 5 years were 4.0% and 2.5% in ABOi and ABOc KT, respectively. Five-year rates of acute T cell-mediated rejection were 4.0% and 14.3% in ABOi and ABOc KT, respectively [8]. Acute AMR rates in 6-month and 2-year biopsy samples were 3.5% and 0.0% in ABOi KT and 10.8% and 2.4% in ABOc KT, respectively [87]. We should consider that anti-HLA DSA could be a main pathogenic factor, rather than anti-ABO antibodies, in acute AMR after ABOi KT [22].
Chronic rejection
The National Institutes of Health suggested diagnostic criteria for chronic AMR in ABOi KT [31]. Specifically, at least three of the following four lesions could be present: arterial intimal fibrosis, interstitial fibrosis/tubular atrophy, duplication of the glomerular basement membrane, or lamination of the peritubular capillary basement membrane.
AMR in the early posttransplant period has adverse impacts on long-term graft survival and contributes to chronic rejection [34]. Several studies show that a history of AMR is significantly associated with the development of transplant glomerulopathy [22,37]. When chronic AMR rates were compared in the rituximab era, they were significantly lower in the 2-year biopsy sample but not the 6-month biopsy sample from ABOi KT compared with ABOc KT (1.8% and 3.5% in ABOi KT and 0.0% and 28.9% in ABOc KT, at 6 months and 2 years, respectively) [87]. Furthermore, formation of de novo DSA and DSA-related chronic AMR occurred less in ABOi KT because of desensitization effects [87].
Adverse effects of ABOi KT
The literature on infectious complications after ABOi KT is controversial. Genberg et al [53] reported no statistical difference in infectious complications between ABOi KT and ABOc KT. Later, Habicht et al [81] reported that the infection rate in ABOi KT was significantly higher than that in ABOc KT (60% vs. 30%). Viral infections, including with cytomegalovirus, herpes simplex virus, varicella-zoster virus, and BK virus, showed a higher incidence in ABOi KT compared with ABOc KT.
B-cell depletion by rituximab may be associated with an increased risk of infection in ABOi KT. Grim et al [88] reported that the incidence of posttransplant infection in HLA-sensitized KT or ABOi KT treated with rituximab (48%) was greater than in HLA-sensitized KT without rituximab (11%). Kamar et al [89] showed that the infection rate in KT was similar with and without rituximab (45.5% vs. 53.9%). However, infection-associated mortality was significantly higher in the rituximab group. Therefore, treatment with sulfamethoxazole/trimethoprim or acyclovir is recommended to prevent relatively common Pneumocystis jiroveci or viral infections in ABOi KT.
Although immunosuppression in KT is associated with an increased incidence of malignancy compared with the general population [90], when Yamamoto et al [91] retrospectively analyzed the malignancy risk of ABOi KT compared with ABOc KT, there was no significant difference (4.8% vs. 4.2%). Similarly, Hall et al [92] showed that the incidence of malignancy in ABOi KT was similar to that in matched ABOc KT. Further analyses with long-term follow-up are needed to adequately assess the risk of malignancy in ABOi KT.
Cost of ABO incompatible KT
Although KT is a cost-effective modality over dialysis [93,94], ABOi KT is more expensive than ABOc KT because of desensitization procedures. The cost of ABOi KT in the first 90 days after transplantation is $90,300 compared to $52,500 for ABOc KT in the U.S.A. However, ABOi KT is cost-effective compared to maintenance dialysis while waiting for ABOc KT, because ABOi KT saves $130,000 for 5 years compared to dialysis [95]. Cost of ABOi KT based on immunoadsorption is higher than that based on plasmapheresis, while it is still cost-effective compared to dialysis [96].
Unresolved issues in ABOi KT
Acceptable titer of anti-ABO antibodies before and after KT
The prognostic value of a baseline anti-ABO titer is controversial. A high baseline titer is associated with higher failure rate to reach the target titer immediately before KT [97,98] and was also associated with AMR, severity of AMR, and graft failure [2,9,99,100]. However, several studies reported that a high baseline titer is not a predictor of poor allograft outcomes in recipients treated with tacrolimus or MMF immunosuppressive regimens [22,37]. The lack of well-controlled comparative studies and variable study designs make it difficult to resolve this issue. Nevertheless, a high baseline titer itself is not an absolute contraindication to ABOi KT but should be managed very carefully as a risk factor for failing to reach the target titer and development of acute AMR.
The anti-ABO antibody titer before KT should be low, but the acceptable upper limit is based on empirical evidence. Acceptable titers of anti-ABO antibodies at the time of transplantation have varied between 1:4 and 1:32 according to the protocol of individual centers [53,55–57,65,67,78,79,81–83]. Therefore, the optimal titer should be determined according to the pretransplant and posttransplant immunosuppressive protocols.
Some centers recommend that the anti-ABO antibody titer should be low (1:8 to 1:16) during the early posttransplant period [9,30,101]. Other studies demonstrated that the clinical significance of an increased anti-ABO antibody titer during the posttransplant period is variable and that there was no significant correlation with AMR [1,2,102]. A high antibody titer may be necessary but is not sufficient for AMR. Overall, these findings indicate that the titer should be monitored for at least 3 weeks after ABOi KT, and high posttransplant titers (≥1:64) that are associated with a high risk for acute AMR should be treated [2,22].
Necessity of rituximab and IVIG
The necessity and optimal dosage of rituximab remain unclear, although most centers use low doses for desensitization in ABOi KT. Despite good short-term effects of low-dose rituximab, long-term controlled studies are needed to determine their value. The empirical use of postpheresis replacement of IVIG is common, but its necessity has also not been confirmed experimentally.
Minimizing immunosuppression
Various groups have attempted to minimize immunosuppression to reduce the long-term risk of overimmunosuppression in ABOi KT [36,103]. Magee et al [36] suggested avoiding lymphocyte-depleting antibodies because they were not effective for preventing AMR and were associated with a higher incidence of infection. Tacrolimus has contributed to successful outcomes of ABOi KT in the modern era. However, Chuang et al [103] reported that there was no significant difference in the isoagglutinin titer between tacrolimus and cyclosporine groups. Two studies analyzed the effect of steroid withdrawal 1–2 weeks after ABOi KT [67,104]. One study reported successful withdrawal in 44% of recipients, but with 30% biopsy-proven acute rejection within 1 year [104]. Another study analyzed 10 recipients, 30% with biopsy-proven acute rejection [67]. Oettl et al [78] analyzed 11 ABOi KT recipients with late steroid withdrawal. Biopsy-proven acute rejection occurred in 55% during or soon after steroid cessation [62]. Based on these data, steroid withdrawal in ABOi KT is not recommended or should only be performed after a protocol biopsy showing normal histologic findings. There should also be clinical, and in doubtful cases, a histologic follow-up [105]. Overall, the available evidence is not sufficient to minimize maintenance immunosuppression in ABOi KT.
Conclusion
Thanks to advances in our understanding of immunopathogenesis and immune-modulating techniques during the last 10 years, ABOi KT now has outcomes comparable with ABOc KT in both efficacy and safety. ABOi KT is thus an important step forward in expanding the kidney donor pool. Additional studies are needed to clarify optimal pretransplant desensitization regimens, mechanisms of accommodation, and the best treatment protocol for acute and chronic AMR after ABOi KT.
Conflicts of interest
All authors declare no conflict of interest.
References
- 1.Park WD, Grande JP, Ninova D, Nath KA, Platt JL, Gloor JM, Stegall MD. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant. 2003;3:952–960. doi: 10.1034/j.1600-6143.2003.00179.x. [DOI] [PubMed] [Google Scholar]
- 2.Tobian AA, Shirey RS, Montgomery RA, Cai W, Haas M, Ness PM, King KE. ABO antibody titer and risk of antibody-mediated rejection in ABO-incompatible renal transplantation. Am J Transplant. 2010;10:1247–1253. doi: 10.1111/j.1600-6143.2010.03103.x. [DOI] [PubMed] [Google Scholar]
- 3.Alexandre GP, Squifflet JP, De Bruyere M, Latinne D, Reding R, Gianello P, Carlier M, Pirson Y. Present experiences in a series of 26 ABO-incompatible living donor renal allografts. Transplant Proc. 1987;19:4538–4542. [PubMed] [Google Scholar]
- 4.Takahashi K, Saito K. ABO-incompatible kidney transplantation. Transplant Rev. 2013;27:1–8. doi: 10.1016/j.trre.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Ishida H, Miyamoto N, Shirakawa H, Shimizu T, Tokumoto T, Ishikawa N, Shimmura H, Setoguchi K, Toki D, Iida S, Teraoka S, Takahashi K, Toma H, Yamaguchi Y, Tanabe K. Evaluation of immunosuppressive regimens in ABO-incompatible living kidney transplantation-single center analysis. Am J Transplant. 2007;7:825–831. doi: 10.1111/j.1600-6143.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- 6.Ichimaru N, Takahara S. Japan׳s experience with living-donor kidney transplantation across ABO barriers. Nat Clin Pract Nephrol. 2008;4:682–692. doi: 10.1038/ncpneph0967. [DOI] [PubMed] [Google Scholar]
- 7.Tyden G, Kumlien G, Fehrman I. Successful ABO-incompatible kidney transplantations without splenectomy using antigen-specific immunoadsorption and rituximab. Transplantation. 2003;76:730–731. doi: 10.1097/01.TP.0000078622.43689.D4. [DOI] [PubMed] [Google Scholar]
- 8.Fuchinoue S, Ishii Y, Sawada T, Murakami T, Iwadoh K, Sannomiya A, Koyama I, Kubota K, Tojimbara T, Nakajima I, Teraoka S. The 5-year outcome of ABO-incompatible kidney transplantation with rituximab induction. Transplantation. 2011;91:853–857. doi: 10.1097/TP.0b013e31820f08e8. [DOI] [PubMed] [Google Scholar]
- 9.Stegall MD, Dean PG, Gloor JM. ABO-incompatible kidney transplantation. Transplantation. 2004;78:635–640. doi: 10.1097/01.tp.0000136263.46262.0d. [DOI] [PubMed] [Google Scholar]
- 10.Tyden G, Kumlien G, Genberg H, Sandberg J, Lundgren T, Fehrman I. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005;5:145–148. doi: 10.1111/j.1600-6143.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery JR, Berger JC, Warren DS, James NT, Montgomery RA, Segev DL. Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation. 2012;93:603–609. doi: 10.1097/TP.0b013e318245b2af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Organ Donation and Transplantation Activity Report 2014/15. Available at: http://nhsbtmediaservices.blob.core.windows.net/organ-donation-assets/pdfs/activity_report_2014_15.pdf [Date accessed: 31 July 2015]
- 13.Kong JM, Ahn J, Park JB, Chung BH, Yang J, Kim JK, Huh KH, Kim JM. ABO incompatible living donor kidney transplantation in Korea: highly uniform protocols and good medium-term outcome. Clin Transplant. 2013;27:875–881. doi: 10.1111/ctr.12249. [DOI] [PubMed] [Google Scholar]
- 14.Korean Organ Transplantation Registry_1st year analyzed data_Kidney. Available at: http://www.kotry.org/ko [Date accessed: 31 July 2015]
- 15.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 16.Economidou J, Hughes-Jones NC, Gardner B. Quantitative measurements concerning A and B antigen sites. Vox Sang. 1967;12:321–328. doi: 10.1111/j.1423-0410.1967.tb03362.x. [DOI] [PubMed] [Google Scholar]
- 17.Thielke J, Kaplan B, Benedetti E. The role of ABO-incompatible living donors in kidney transplantation: state of the art. Semin Nephrol. 2007;27:408–413. doi: 10.1016/j.semnephrol.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Fehr T, Stussi G. ABO-incompatible kidney transplantation. Curr Opin Organ Transplant. 2012;17:376–385. doi: 10.1097/MOT.0b013e328355f013. [DOI] [PubMed] [Google Scholar]
- 19.Conway J, Manlhiot C, Allain-Rooney T, McCrindle BW, Lau W, Dipchand AI. Development of donor-specific isohemagglutinins following pediatric ABO-incompatible heart transplantation. Am J Transplant. 2012;12:888–895. doi: 10.1111/j.1600-6143.2011.03910.x. [DOI] [PubMed] [Google Scholar]
- 20.Irei T, Ohdan H, Zhou W, Ishiyama K, Tanaka Y, Ide K, Asahara T. The persistent elimination of B cells responding to blood group A carbohydrates by synthetic group A carbohydrates and B-1 cell differentiation blockade: novel concept in preventing antibody-mediated rejection in ABO-incompatible transplantation. Blood. 2007;110:4567–4575. doi: 10.1182/blood-2007-04-082719. [DOI] [PubMed] [Google Scholar]
- 21.Tazawa H, Irei T, Tanaka Y, Igarashi Y, Tashiro H, Ohdan H. Blockade of invariant TCR-CD1d interaction specifically inhibits antibody production against blood group A carbohydrates. Blood. 2013;122:2582–2590. doi: 10.1182/blood-2012-02-407452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toki D, Ishida H, Setoguchi K, Shimizu T, Omoto K, Shirakawa H, Iida S, Horita S, Furusawa M, Ishizuka T, Yamaguchi Y, Tanabe K. Acute antibody-mediated rejection in living ABO-incompatible kidney transplantation: long-term impact and risk factors. Am J Transplant. 2009;9:567–577. doi: 10.1111/j.1600-6143.2008.02538.x. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi T, Saito K. A series of surveys on assay for anti-A/B antibody by Japanese ABO-incompatible Transplantation Committee. Xenotransplantation. 2006;13:136–140. doi: 10.1111/j.1399-3089.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- 24.Stussi G, Huggel K, Lutz HU, Schanz U, Rieben R, Seebach JD. Isotype-specific detection of ABO blood group antibodies using a novel flow cytometric method. Br J Haematol. 2005;130:954–963. doi: 10.1111/j.1365-2141.2005.05705.x. [DOI] [PubMed] [Google Scholar]
- 25.Lindberg L, Johansson SM, Liu J, Grufman P, Holgersson J. Is there a clinical need for a diagnostic test allowing detection of chain type-specific anti-A and anti-B? Transfusion. 2011;51:494–503. doi: 10.1111/j.1537-2995.2010.02870.x. [DOI] [PubMed] [Google Scholar]
- 26.Kumlien G, Wilpert J, Safwenberg J, Tyden G. Comparing the tube and gel techniques for ABO antibody titration, as performed in three European centers. Transplantation. 2007;84:S17–S19. doi: 10.1097/01.tp.0000296019.85986.af. [DOI] [PubMed] [Google Scholar]
- 27.Tanabe K. Interinstitutional variation in the measurement of anti-A/B antibodies: the Japanese ABO-Incompatible Transplantation Committee survey. Transplantation. 2007;84:S13–S16. doi: 10.1097/01.tp.0000296018.82857.ef. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K. Recent findings in ABO-incompatible kidney transplantation: classification and therapeutic strategy for acute antibody-mediated rejection due to ABO-blood-group-related antigens during the critical period preceding the establishment of accommodation. Clin Exp Nephrol. 2007;11:128–141. doi: 10.1007/s10157-007-0461-z. [DOI] [PubMed] [Google Scholar]
- 29.Fidler ME, Gloor JM, Lager DJ, Larson TS, Griffin MD, Textor SC, Schwab TR, Prieto M, Nyberg SL, Ishitani MB, Grande JP, Kay PA, Stegall MD. Histologic findings of antibody-mediated rejection in ABO blood-group-incompatible living-donor kidney transplantation. Am J Transplant. 2004;4:101–107. doi: 10.1046/j.1600-6135.2003.00278.x. [DOI] [PubMed] [Google Scholar]
- 30.Gloor JM, Lager DJ, Fidler ME, Grande JP, Moore SB, Winters JL, Kremers WK, Stegall MD. A comparison of splenectomy versus intensive posttransplant antidonor blood group antibody monitoring without splenectomy in ABO-incompatible kidney transplantation. Transplantation. 2005;80:1572–1577. doi: 10.1097/01.tp.0000184622.69708.c1. [DOI] [PubMed] [Google Scholar]
- 31.Racusen LC, Haas M. Antibody-mediated rejection in renal allografts: lessons from pathology. Clin J Am Soc Nephrol. 2006;1:415–420. doi: 10.2215/CJN.01881105. [DOI] [PubMed] [Google Scholar]
- 32.Haas M, Segev DL, Racusen LC, Bagnasco SM, Locke JE, Warren DS, Simpkins CE, Lepley D, King KE, Kraus ES, Montgomery RA. C4d deposition without rejection correlates with reduced early scarring in ABO-incompatible renal allografts. J Am Soc Nephrol. 2009;20:197–204. doi: 10.1681/ASN.2008030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Setoguchi K, Ishida H, Shimmura H, Shimizu T, Shirakawa H, Omoto K, Toki D, Iida S, Setoguchi S, Tokumoto T, Horita S, Nakayama H, Yamaguchi Y, Tanabe K. Analysis of renal transplant protocol biopsies in ABO-incompatible kidney transplantation. Am J Transplant. 2008;8:86–94. doi: 10.1111/j.1600-6143.2007.02036.x. [DOI] [PubMed] [Google Scholar]
- 34.Haas M, Rahman MH, Racusen LC, Kraus ES, Bagnasco SM, Segev DL, Simpkins CE, Warren DS, King KE, Zachary AA, Montgomery RA. C4d and C3d staining in biopsies of ABO- and HLA-incompatible renal allografts: correlation with histologic findings. Am J Transplant. 2006;6:1829–1840. doi: 10.1111/j.1600-6143.2006.01356.x. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Saito K, Nakagawa Y, Tasaki M, Hara N, Imai N. Mechanism of acute antibody-mediated rejection in ABO-incompatible kidney transplantation: which anti-A/anti-B antibodies are responsible, natural or de novo? Transplantation. 2010;89:635–637. doi: 10.1097/TP.0b013e3181c89307. [DOI] [PubMed] [Google Scholar]
- 36.Magee CC. Transplantation across previously incompatible immunological barriers. Transpl Int. 2006;19:87–97. doi: 10.1111/j.1432-2277.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 37.Gloor JM, Stegall MD. ABO incompatible kidney transplantation. Curr Opin Nephrol Hypertens. 2007;16:529–534. doi: 10.1097/MNH.0b013e3282f02218. [DOI] [PubMed] [Google Scholar]
- 38.Williams JM, Holzknecht ZE, Plummer TB, Lin SS, Brunn GJ, Platt JL. Acute vascular rejection and accommodation: divergent outcomes of the humoral response to organ transplantation. Transplantation. 2004;78:1471–1478. doi: 10.1097/01.tp.0000140770.81537.64. [DOI] [PubMed] [Google Scholar]
- 39.Iwasaki K, Miwa Y, Haneda M, Uchida K, Nakao A, Kobayashi T. Significance of HLA class I antibody-induced antioxidant gene expression for endothelial cell protection against complement attack. Biochem Biophys Res Commun. 2010;391:1210–1215. doi: 10.1016/j.bbrc.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 40.Chen Song S, Zhong S, Xiang Y, Li JH, Guo H, Wang WY, Xiong YL, Li XC, Chen Shi S, Chen XP, Chen G. Complement inhibition enables renal allograft accommodation and long-term engraftment in presensitized nonhuman primates. Am J Transplant. 2011;11:2057–2066. doi: 10.1111/j.1600-6143.2011.03646.x. [DOI] [PubMed] [Google Scholar]
- 41.Iwasaki K, Miwa Y, Ogawa H, Yazaki S, Iwamoto M, Furusawa T, Onishi A, Kuzuya T, Haneda M, Watarai Y, Uchida K, Kobayashi T. Comparative study on signal transduction in endothelial cells after anti-a/b and human leukocyte antigen antibody reaction: implication of accommodation. Transplantation. 2012;93:390–397. doi: 10.1097/TP.0b013e3182424df3. [DOI] [PubMed] [Google Scholar]
- 42.West LJ. Targeting antibody-mediated rejection in the setting of ABO-incompatible infant heart transplantation: graft accommodation vs. B cell tolerance. Curr Drug Targets Cardiovas Haematol Disord. 2005;5:223–232. doi: 10.2174/1568006054064762. [DOI] [PubMed] [Google Scholar]
- 43.Smith RN, Kawai T, Boskovic S, Nadazdin O, Sachs DH, Cosimi AB, Colvin RB. Four stages and lack of stable accommodation in chronic alloantibody-mediated renal allograft rejection in Cynomolgus monkeys. Am J Transplant. 2008;8:1662–1672. doi: 10.1111/j.1600-6143.2008.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tobian AA, Shirey RS, Montgomery RA, Tisch DJ, Ness PM, King KE. Therapeutic plasma exchange reduces ABO titers to permit ABO-incompatible renal transplantation. Transfusion. 2009;49:1248–1254. doi: 10.1111/j.1537-2995.2008.02085.x. [DOI] [PubMed] [Google Scholar]
- 45.Higgins R, Lowe D, Hathaway M, Lam FT, Kashi H, Tan LC, Imray C, Fletcher S, Chen K, Krishnan N, Hamer R, Zehnder D, Briggs D. Double filtration plasmapheresis in antibody-incompatible kidney transplantation. Ther Apher Dial. 2010;14:392–399. doi: 10.1111/j.1744-9987.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 46.Tyden G, Kumlien G, Efvergren M. Present techniques for antibody removal. Transplantation. 2007;84:S27–S29. doi: 10.1097/01.tp.0000296102.94695.c0. [DOI] [PubMed] [Google Scholar]
- 47.Tobian AA, Shirey RS, Montgomery RA, Ness PM, King KE. The critical role of plasmapheresis in ABO-incompatible renal transplantation. Transfusion. 2008;48:2453–2460. doi: 10.1111/j.1537-2995.2008.01857.x. [DOI] [PubMed] [Google Scholar]
- 48.Montgomery RA. Renal transplantation across HLA and ABO antibody barriers: integrating paired donation into desensitization protocols. Am J Transplant. 2010;10:449–457. doi: 10.1111/j.1600-6143.2009.03001.x. [DOI] [PubMed] [Google Scholar]
- 49.Segev DL, Simpkins CE, Warren DS, King KE, Shirey RS, Maley WR, Melancon JK, Cooper M, Kozlowski T, Montgomery RA. ABO incompatible high-titer renal transplantation without splenectomy or anti-CD20 treatment. Am J Transplant. 2005;5:2570–2575. doi: 10.1111/j.1600-6143.2005.01031.x. [DOI] [PubMed] [Google Scholar]
- 50.Geyer M, Donauer J, Pisarski P, Drognitz O, Schulz-Huotari C, Wisniewski U, Gropp A, Gobel H, Gerke P, Teschner S, Walz G, Wilpert J. Preemptive postoperative antigen-specific immunoadsorption in ABO-incompatible kidney transplantation: necessary or not? Transplantation. 2007;84:S40–S43. doi: 10.1097/01.tp.0000296021.72977.3b. [DOI] [PubMed] [Google Scholar]
- 51.Sawada T, Fuchinoue S, Teraoka S. Successful A1-to-O ABO-incompatible kidney transplantation after a preconditioning regimen consisting of anti-CD20 monoclonal antibody infusions, splenectomy, and double-filtration plasmapheresis. Transplantation. 2002;74:1207–1210. doi: 10.1097/00007890-200211150-00001. [DOI] [PubMed] [Google Scholar]
- 52.Clatworthy MR. Targeting B cells and antibody in transplantation. Am J Transplant. 2011;11:1359–1367. doi: 10.1111/j.1600-6143.2011.03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Genberg H, Kumlien G, Wennberg L, Berg U, Tyden G. ABO-incompatible kidney transplantation using antigen-specific immunoadsorption and rituximab: a 3-year follow-up. Transplantation. 2008;85:1745–1754. doi: 10.1097/TP.0b013e3181726849. [DOI] [PubMed] [Google Scholar]
- 54.Egawa H, Ohmori K, Haga H, Tsuji H, Yurugi K, Miyagawa-Hayashino A, Oike F, Fukuda A, Yoshizawa J, Takada Y, Tanaka K, Maekawa T, Ozawa K, Uemoto S. B-cell surface marker analysis for improvement of rituximab prophylaxis in ABO-incompatible adult living donor liver transplantation. Liver Transpl. 2007;13:579–588. doi: 10.1002/lt.21092. [DOI] [PubMed] [Google Scholar]
- 55.Chikaraishi T, Sasaki H, Tsutsumi H, Miyano S, Nakazawa R, Nakano T, Kitajima K, Kudo H, Takahashi T, Sato Y, Kimura K. ABO blood type incompatible kidney transplantation without splenectomy prepared with plasma exchange and rituximab. Transplant Proc. 2008;40:3445–3447. doi: 10.1016/j.transproceed.2008.06.110. [DOI] [PubMed] [Google Scholar]
- 56.Flint SM, Walker RG, Hogan C, Haeusler MN, Robertson A, Francis DM, Millar R, Finlay M, Landgren A, Cohney SJ. Successful ABO-incompatible kidney transplantation with antibody removal and standard immunosuppression. Am J Transplant. 2011;11:1016–1024. doi: 10.1111/j.1600-6143.2011.03464.x. [DOI] [PubMed] [Google Scholar]
- 57.Ashimine S, Watarai Y, Yamamoto T, Hiramitsu T, Tsujita M, Nanmoku K, Goto N, Takeda A, Katayama A, Uchida K, Kobayashi T. Neither pretransplant rituximab nor splenectomy affects de novo HLA antibody production after renal transplantation. Kidney Int. 2014;85:425–430. doi: 10.1038/ki.2013.291. [DOI] [PubMed] [Google Scholar]
- 58.Toki D, Ishida H, Horita S, Setoguchi K, Yamaguchi Y, Tanabe K. Impact of low-dose rituximab on splenic B cells in ABO-incompatible renal transplant recipients. Transpl Int. 2009;22:447–454. doi: 10.1111/j.1432-2277.2008.00821.x. [DOI] [PubMed] [Google Scholar]
- 59.Jordan SC, Toyoda M, Kahwaji J, Vo AA. Clinical aspects of intravenous immunoglobulin use in solid organ transplant recipients. Am J Transplant. 2011;11:196–202. doi: 10.1111/j.1600-6143.2010.03400.x. [DOI] [PubMed] [Google Scholar]
- 60.Tanabe K. Japanese experience of ABO-incompatible living kidney transplantation. Transplantation. 2007;84:S4–S7. doi: 10.1097/01.tp.0000296008.08452.4c. [DOI] [PubMed] [Google Scholar]
- 61.Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant. 2008;22:1–15. doi: 10.1111/j.1399-0012.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 62.Oettl T, Zuliani E, Gaspert A, Hopfer H, Dickenmann M, Fehr T. Late steroid withdrawal after ABO blood group-incompatible living donor kidney transplantation: high rate of mild cellular rejection. Transplantation. 2010;89:702–706. doi: 10.1097/TP.0b013e3181c9cc67. [DOI] [PubMed] [Google Scholar]
- 63.Geyer M, Fischer KG, Drognitz O, Walz G, Pisarski P, Wilpert J. ABO-incompatible kidney transplantation with antigen-specific immunoadsorption and rituximab – insights and uncertainties. Contrib Nephrol. 2009;162:47–60. doi: 10.1159/000170812. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi K, Saito K, Takahara S, Okuyama A, Tanabe K, Toma H, Uchida K, Hasegawa A, Yoshimura N, Kamiryo Y. Excellent long-term outcome of ABO-incompatible living donor kidney transplantation in Japan. Am J Transplant. 2004;4:1089–1096. doi: 10.1111/j.1600-6143.2004.00464.x. [DOI] [PubMed] [Google Scholar]
- 65.Montgomery RA, Locke JE, King KE, Segev DL, Warren DS, Kraus ES, Cooper M, Simpkins CE, Singer AL, Stewart ZA, Melancon JK, Ratner L, Zachary AA, Haas M. ABO incompatible renal transplantation: a paradigm ready for broad implementation. Transplantation. 2009;87:1246–1255. doi: 10.1097/TP.0b013e31819f2024. [DOI] [PubMed] [Google Scholar]
- 66.Venetz JP, Pascual M. New treatments for acute humoral rejection of kidney allografts. Expert Opin Investig Drugs. 2007;16:625–633. doi: 10.1517/13543784.16.5.625. [DOI] [PubMed] [Google Scholar]
- 67.Galliford J, Charif R, Chan KK, Loucaidou M, Cairns T, Cook HT, Dorling A, Hakim N, McLean A, Papalois V, Malde R, Regan F, Redman M, Warrens A.N., Taube D. ABO incompatible living renal transplantation with a steroid sparing protocol. Transplantation. 2008;86:901–906. doi: 10.1097/TP.0b013e3181880c0f. [DOI] [PubMed] [Google Scholar]
- 68.Montgomery RA, Zachary AA, Racusen LC, Leffell MS, King KE, Burdick J, Maley WR, Ratner LE. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation. 2000;70:887–895. doi: 10.1097/00007890-200009270-00006. [DOI] [PubMed] [Google Scholar]
- 69.Kaplan B, Gangemi A, Thielke J, Oberholzer J, Sankary H, Benedetti E. Successful rescue of refractory, severe antibody mediated rejection with splenectomy. Transplantation. 2007;83:99–100. doi: 10.1097/01.tp.0000243739.31440.2b. [DOI] [PubMed] [Google Scholar]
- 70.Garrett HE, Jr, Groshart K, Duvall-Seaman D, Combs D, Suggs R. Treatment of humoral rejection with rituximab. Ann Thorac Surg. 2002;74:1240–1242. doi: 10.1016/s0003-4975(02)03824-9. [DOI] [PubMed] [Google Scholar]
- 71.Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M, Salvatierra O., Jr Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med. 2003;349:125–138. doi: 10.1056/NEJMoa035588. [DOI] [PubMed] [Google Scholar]
- 72.Everly JJ, Walsh RC, Alloway RR, Woodle ES. Proteasome inhibition for antibody-mediated rejection. Curr Opin Organ Transplant. 2009;14:662–666. doi: 10.1097/MOT.0b013e328330f304. [DOI] [PubMed] [Google Scholar]
- 73.Wahrmann M, Haidinger M, Kormoczi GF, Weichhart T, Saemann MD, Geyeregger R, Kikic Z, Prikoszovich T, Drach J, Bohmig GA. Effect of the proteasome inhibitor bortezomib on humoral immunity in two presensitized renal transplant candidates. Transplantation. 2010;89:1385–1390. doi: 10.1097/TP.0b013e3181d9e1c0. [DOI] [PubMed] [Google Scholar]
- 74.Westphal S, Hansson S, Stelin G, Holgersson J, Mjornstedt L, Friman S. Successful treatment of severe ABO antibody-mediated rejection using bortezomib: a case report. Transplant Proc. 2013;45:1213–1215. doi: 10.1016/j.transproceed.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 75.Boothpur R, Torrence S, Brennan DC. Bortezomib does not reduce ABO-isoagglutinin titers and may not be useful for ABO-incompatible transplant desensitization. Clin Transplant. 2009;23:491–493. [PubMed] [Google Scholar]
- 76.Crew RJ, Ratner LE. ABO-incompatible kidney transplantation: current practice and the decade ahead. Curr Opin Organ Transplant. 2010;15:526–530. doi: 10.1097/MOT.0b013e32833bfbba. [DOI] [PubMed] [Google Scholar]
- 77.Stewart ZA, Collins TE, Schlueter AJ, Raife TI, Holanda DG, Nair R, Reed AI, Thomas CP. Case report: eculizumab rescue of severe accelerated antibody-mediated rejection after ABO-incompatible kidney transplant. Transplant Proc. 2012;44:3033–3036. doi: 10.1016/j.transproceed.2012.03.053. [DOI] [PubMed] [Google Scholar]
- 78.Oettl T, Halter J, Bachmann A, Guerke L, Infanti L, Oertli D, Mihatsch M, Gratwohl A, Steiger J, Dickenmann M. ABO blood group-incompatible living donor kidney transplantation: a prospective, single-centre analysis including serial protocol biopsies. Nephrol Dial Transplant. 2009;24:298–303. doi: 10.1093/ndt/gfn478. [DOI] [PubMed] [Google Scholar]
- 79.Wilpert J, Fischer KG, Pisarski P, Wiech T, Daskalakis M, Ziegler A, Neumann-Haefelin E, Drognitz O, Emmerich F, Walz G, Geyer M. Long-term outcome of ABO-incompatible living donor kidney transplantation based on antigen-specific desensitization. An observational comparative analysis. Nephrol Dial Transplant. 2010;25:3778–3786. doi: 10.1093/ndt/gfq229. [DOI] [PubMed] [Google Scholar]
- 80.Tyden G, Kumlien G, Berg UB. ABO-incompatible kidney transplantation in children. Pediatr Transplant. 2011;15:502–504. doi: 10.1111/j.1399-3046.2011.01480.x. [DOI] [PubMed] [Google Scholar]
- 81.Habicht A, Broker V, Blume C, Lorenzen J, Schiffer M, Richter N, Klempnauer J, Haller H, Lehner F, Schwarz A. Increase of infectious complications in ABO-incompatible kidney transplant recipients – a single centre experience. Nephrol Dial Transplant. 2011;26:4124–4131. doi: 10.1093/ndt/gfr215. [DOI] [PubMed] [Google Scholar]
- 82.Lipshutz GS, McGuire S, Zhu Q, Ziman A, Davis R, Goldfinger D, Reed EF, Wilkinson AH, Danovitch GM, Pham PT. ABO blood type-incompatible kidney transplantation and access to organs. Arch Surg. 2011;146:453–458. doi: 10.1001/archsurg.2011.40. [DOI] [PubMed] [Google Scholar]
- 83.Morath C, Becker LE, Leo A, Beimler J, Klein K, Seckinger J, Kihm LP, Schemmer P, Macher-Goeppinger S, Wahrmann M, Bohmig GA, Opelz G, Susal C, Zeier M, Schwenger V. ABO-incompatible kidney transplantation enabled by non-antigen-specific immunoadsorption. Transplantation. 2012;93:827–834. doi: 10.1097/TP.0b013e31824836ae. [DOI] [PubMed] [Google Scholar]
- 84.Opelz G, Morath C, Susal C, Tran TH, Zeier M, Dohler B. Three-year outcomes following 1420 ABO-incompatible living-donor kidney transplants performed after ABO antibody reduction: results from 101 centers. Transplantation. 2015;99:400–404. doi: 10.1097/TP.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 85.Ushigome H, Okamoto M, Koshino K, Nobori S, Okajima H, Masuzawa N, Urasaki K, Yoshimura N. Findings of graft biopsy specimens within 90 days after ABO blood group incompatible living donor kidney transplantation compared with ABO-identical and non-identical transplantation. Clin Transplant. 2010;24(Suppl 22):S16–S21. doi: 10.1111/j.1399-0012.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- 86.Gloor JM, Cosio FG, Rea DJ, Wadei HM, Winters JL, Moore SB, DeGoey SR, Lager DJ, Grande JP, Stegall MD. Histologic findings one year after positive crossmatch or ABO blood group incompatible living donor kidney transplantation. Am J Transplant. 2006;6:1841–1847. doi: 10.1111/j.1600-6143.2006.01416.x. [DOI] [PubMed] [Google Scholar]
- 87.Kohei N, Hirai T, Omoto K, Ishida H, Tanabe K. Chronic antibody-mediated rejection is reduced by targeting B-cell immunity during an introductory period. Am J Transplant. 2012;12:469–476. doi: 10.1111/j.1600-6143.2011.03830.x. [DOI] [PubMed] [Google Scholar]
- 88.Grim SA, Pham T, Thielke J, Sankary H, Oberholzer J, Benedetti E, Clark NM. Infectious complications associated with the use of rituximab for ABO-incompatible and positive cross-match renal transplant recipients. Clin Transplant. 2007;21:628–632. doi: 10.1111/j.1399-0012.2007.00700.x. [DOI] [PubMed] [Google Scholar]
- 89.Kamar N, Milioto O, Puissant-Lubrano B, Esposito L, Pierre MC, Mohamed AO, Lavayssiere L, Cointault O, Ribes D, Cardeau I, Nogier MB, Durand D, Abbal M, Blancher A, Rostaing L. Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am J Transplant. 2010;10:89–98. doi: 10.1111/j.1600-6143.2009.02785.x. [DOI] [PubMed] [Google Scholar]
- 90.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905–913. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 91.Yamamoto T, Kawaguchi T, Watarai Y, Tujita M, Hiramitsu T, Nanmoku K, Goto N, Katayama A, Kobayashi T, Uchida K. Potent immunosuppression for ABO-incompatible renal transplantation may not be a risk factor for malignancy. Transplant Proc. 2012;44:210–213. doi: 10.1016/j.transproceed.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 92.Hall EC, Engels EA, Montgomery RA, Segev DL. Cancer risk after ABO-incompatible living-donor kidney transplantation. Transplantation. 2013;96:476–479. doi: 10.1097/TP.0b013e318299dc0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Loubeau PR, Loubeau JM, Jantzen R. The economics of kidney transplantation versus hemodialysis. Prog Transplant. 2001;11:291–297. doi: 10.1177/152692480101100411. [DOI] [PubMed] [Google Scholar]
- 94.Haller M, Gutjahr G, Kramar R, Harnoncourt F, Oberbauer R. Cost-effectiveness analysis of renal replacement therapy in Austria. Nephrol Dial Transplant. 2011;26:2988–2995. doi: 10.1093/ndt/gfq780. [DOI] [PubMed] [Google Scholar]
- 95.Schwartz J, Stegall MD, Kremers WK, Gloor J. Complications, resource utilization, and cost of ABO-incompatible living donor kidney transplantation. Transplantation. 2006;82:155–163. doi: 10.1097/01.tp.0000226152.13584.ae. [DOI] [PubMed] [Google Scholar]
- 96.Tyden G. Cost effectiveness of ABO-incompatible kidney transplantations. Transplantation. 2006;82:166–167. doi: 10.1097/01.tp.0000226104.73438.5e. [DOI] [PubMed] [Google Scholar]
- 97.Lawrence C, Galliford JW, Willicombe MK, McLean AG, Lesabe M, Rowan F, Papalois V, Regan F, Taube D. Antibody removal before ABO-incompatible renal transplantation: how much plasma exchange is therapeutic? Transplantation. 2011;92:1129–1133. doi: 10.1097/TP.0b013e31823360cf. [DOI] [PubMed] [Google Scholar]
- 98.Chung BH, Lee JY, Kang SH, Sun IO, Choi SR, Park HS, Kim JI, Moon IS, Choi BS, Park CW, Kim YS, Yang CW. Comparison of clinical outcome between high and low baseline anti-ABO antibody titers in ABO-incompatible kidney transplantation. Ren Fail. 2011;33:150–158. doi: 10.3109/0886022X.2011.552149. [DOI] [PubMed] [Google Scholar]
- 99.Shimmura H, Tanabe K, Ishikawa N, Tokumoto T, Takahashi K, Toma H. Role of anti-A/B antibody titers in results of ABO-incompatible kidney transplantation. Transplantation. 2000;70:1331–1335. doi: 10.1097/00007890-200011150-00011. [DOI] [PubMed] [Google Scholar]
- 100.Gloor JM, Lager DJ, Moore SB, Pineda AA, Fidler ME, Larson TS, Grande JP, Schwab TR, Griffin MD, Prieto M, Nyberg SL, Velosa JA, Textor SC, Platt JL, Stegall MD. ABO-incompatible kidney transplantation using both A2 and non-A2 living donors. Transplantation. 2003;75:971–977. doi: 10.1097/01.TP.0000058226.39732.32. [DOI] [PubMed] [Google Scholar]
- 101.Kayler LK, Farber JL, Colombe B, LaCava D, Friedewald JJ, Ratner LE. Characterization of rejection episodes in patients following positive crossmatch and ABO-incompatible live donor renal transplantation. Transpl Int. 2006;19:128–139. doi: 10.1111/j.1432-2277.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- 102.Ishida H, Kondo T, Shimizu T, Nozaki T, Tanabe K. Postoperative rebound of antiblood type antibodies and antibody-mediated rejection after ABO-incompatible living-related kidney transplantation. Transpl Int. 2015;28:286–296. doi: 10.1111/tri.12482. [DOI] [PubMed] [Google Scholar]
- 103.Chuang JP, Hung CJ, Chang SS, Chou TC, Lee PC. Does immunosuppressive pharmacotherapy affect isoagglutinin titers? Transplant Proc. 2008;40:2685–2687. doi: 10.1016/j.transproceed.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 104.Kato Y, Tojimbara T, Iwadoh K, Koyama I, Nanmoku K, Kai K, Sannomiya A, Nakajima I, Fuchinoue S, Teraoka S. Early steroid withdrawal protocol with basiliximab, cyclosporine and mycophenolate mofetil in renal-transplant recipients. Int Immunopharmacol. 2006;6:1984–1992. doi: 10.1016/j.intimp.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 105.Gloor J, Matas AJ. Steroid-free maintenance immunosuppression and ABO-incompatible transplantation. Transplantation. 2010;89:648–649. doi: 10.1097/TP.0b013e3181c9cc97. [DOI] [PubMed] [Google Scholar]