Abstract
Dialysis vascular access planning, creation, and management is of critical importance to the dialysis patient population. It requires a multidisciplinary approach involving patients and their families, dialysis facility staff, the nephrologist, the surgeon, and the interventionalist. With the emergence of interventional nephrology as a subspecialty of nephrology, the nephrologist is increasingly providing both the nephrology and interventional aspects of care, and in some areas, the surgical functions as well. Most of these interventional nephrologists work in freestanding outpatient dialysis access centers (DACs). Large clinical studies published over the past 10 years demonstrate that the interventional nephrologist can manage the problems associated with dialysis access dysfunction effectively, safely, and economically. A recently published study based upon United States Medicare claims data in which a DAC patient group (n = 27,613) and a hospital outpatient department patient group (HOPD group; n = 27,613) were compared using propensity score matching techniques showed that patients treated in the DACs had significantly better clinical outcomes (P<0.001). This included fewer vascular access-related infections (0.18 vs. 0.29), fewer septicemia-related hospitalizations (0.15 vs. 0.18), and a lower mortality rate (47.9% vs. 53.5%).
Keywords: Dialysis, Dialysis access, Interventional nephrology, Nephrology, Vascular access
Introduction
Hemodialysis is the perfect example of bioengineering being applied to a medical problem to preserve the life of the patient with an otherwise fatal disease. Unfortunately, the interface between the mechanism and the patient, the vascular access, is defective. It is a problem that contributes significantly to the morbidity and mortality associated with the process and the leading cause of hospitalization in the end-stage renal disease (ESRD) patient population [1]. Dialysis vascular access planning, creation, and management are critical in allowing realization of the ESRD patient’s longevity potential. This process is best carried out using a multidisciplinary approach which involves the patient and his/her family, the nephrologist, the dialysis facility personnel, the surgeon, and the interventionalist.
For healthcare professionals, regardless of their specialty, to perform their function in this process in the most optimal fashion, they should possess three critical characteristics [2]. First, they must understand the dialysis process and the dialysis patient. Dialysis patients are unique and have unique problems. Access planning, creation, and management must be individualized. A single algorithm will not serve all cases. Individualization is possible only through understanding of the patient and the process.
Second, the healthcare professional must have a thorough in-depth knowledge of dialysis vascular access. This must include the types of access that are available, the individual characteristics of each, and the appropriate application of each. This must include a knowledge of what not to do and what to do in individual cases.
Third, they must have skill and expertise in the procedures that they are to perform whether this be the cannulation of an arteriovenous access, the surgical creation of the access, or a maintenance procedure performed because of malfunction.
The nephrologist must play a leadership role in the multidisciplinary team overseeing dialysis vascular access. First and foremost, the nephrologist caring for the patient on chronic dialysis therapy has a responsibility to be an advocate for that patient. To perform this function adequately and effectively, it is essential that the physician become knowledgeable in the area of vascular access, develop a vascular access strategy, and oversee its operation. Delegation of this facet of dialysis patient care to other specialists is less than optimum. The physician who knows the most about the patient, is an expert in hemodialysis, and has the deepest understanding of how the vascular access impacts all other facets of the patient׳s care must also be the leader in managing the vascular access.
It is possible for the nephrologist to play an active role in all of the three activities associated with dialysis vascular access—planning, creation, or management—and in some areas, they do. An increasing number of nephrologists have found that the best way to fulfill their obligation to the patient is interventional nephrology (IN). Historically, dialysis vascular access planning, creation, and maintenance have not been done well in many parts of the world [3–7]. Because of this, nephrologists have been becoming increasingly involved over the past 2 decades. This is especially true for dialysis access maintenance procedures. This has given rise to a new subspecialty of nephrology referred to as IN.
Interventional nephrology
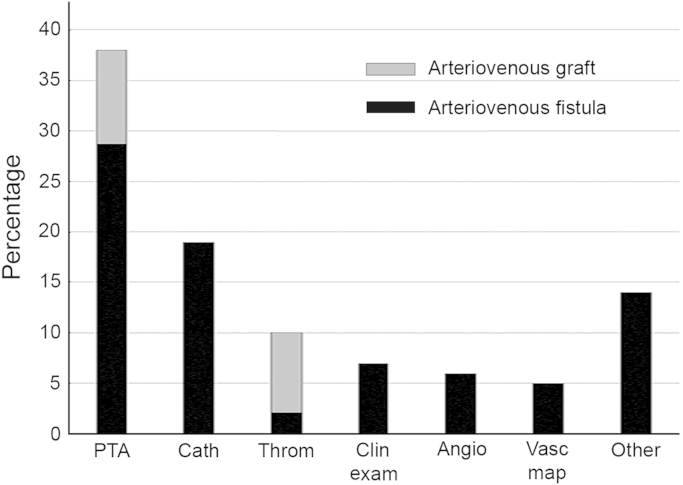
In practice, although some would want to broaden the definition, the term interventional nephrology has come to be defined as that branch of nephrology that deals with the establishment and maintenance of dialysis access, particularly arteriovenous access. A tabulation of the variety of procedures performed in a typical group of facilities is shown in Fig. 1. In the United States, most interventional nephrologists work in freestanding dialysis access centers (DACs), which are designed, equipped, staffed, and supplied specifically for dialysis vascular access management procedures. Their primary goal is to provide an efficient and economical alternative for managing access dysfunction away from the hospital setting. Their efficiency allows for a patient with a thrombosed vascular access to receive therapy and return to the dialysis clinic within a matter of hours, thus avoiding missed treatments.
Figure 1.
Case distribution for IN facility. This is based on 100,000 procedures performed in 75 facilities for the year 2014.
Angio, angiogram; Cath, catheter-based procedures; Clin exam, clinical examination; IN, interventional nephrology; Other, miscellaneous procedures; PTA, percutaneous transluminal angioplasty; Throm, thrombectomy; Vasc map, vascular mapping.
The growth of IN has been almost exponential since its beginning. All of the reasons for this growth are not obvious; however, there are several advantages that relate to improved patient care that have become apparent in dialysis programs in which an interventional nephrologist is involved. The first of these is expeditious management of dialysis access dysfunction. Typically, dialysis access procedures have been given low priority by consultants performing the required procedures. As a result, patients require hospitalization and temporary dialysis catheters frequently [1]. With the advent of IN, these have become outpatient cases associated with a quickly realized, marked decrease in dialysis patient hospitalization and missed dialysis treatments [8].
A second advantage is individualized patient care. The nephrologist trained as an interventionalist can provide the required individualized care, and a prospective approach to the planning for future dialysis vascular access can be provided only by someone who understands the dialysis patient, the dialysis treatment, and vascular access issues.
An additional advantage relates to the opportunity for research and innovation that is offered by involvement in the management of the dialysis patient׳s vascular access. Many dialysis vascular access principles are opinion based. This problem is of obvious paramount importance to the nephrologist providing care to these patients. The nephrologist who is well versed in the basic principles of dialysis vascular access and has the ability to manage these problems independently is in an advantageous position to conduct meaningful research and innovation in this area.
Quality of care provided by interventional nephrologists
Dialysis patients represent a high-risk group for any type of interventional procedure. Published reviews of serious complications encountered in association with procedures performed by interventional radiologists have shown that hemodialysis access management procedures carried a greater risk than other interventional vascular procedures which are primarily arterial [9,10]. When the first nephrologists began to perform as interventionalists, a number of appropriate questions were raised—the quality of medical care provided, the safety of sedation/analgesia (S/A) performed by nonanesthesiologists in an outpatient setting, the safety of radiation-based procedures performed by nonradiologists, and the economic impact of providing the services outside hospital setting. The answers to these questions have been provided by a series of studies reported over the past 10 years.
The first of these was a study conducted to evaluate the efficacy and safety of dialysis access maintenance procedures in the hands of trained nephrologists. In a retrospective cohort study of 14,067 cases performed by 29 interventional nephrologists working in 11 freestanding DACs, a 96.18% clinical success rate was recorded [11]. This study was based on prospectively collected data maintained in an electronic medical record and included six different procedures related to dialysis vascular access management. The clinical success rate and the size for each of these individual procedures were tabulated separately and are shown in Table 1. These data are reflective of the vascular access profile of dialysis patients in the period preceding 2004. Although the number of published studies available for comparison with this report was somewhat limited, the success rate for these cases was equal or superior to the results that were available at that time [11].
Table 1.
Success rates for dialysis access procedures for interventional nephrologists
| Procedure | 2004 |
2014 |
||
|---|---|---|---|---|
| No. | Success rate (%) | No. | Success rate (%) | |
| TDC-Place | 1,765 | 98.24 | 4,038 | 97.89 |
| TDC-Ex | 2,262 | 98.36 | 8,851 | 99.29 |
| AVF-PTA | 1,561 | 96.58 | 32,392 | 99.40 |
| AVG-PTA | 3,560 | 98.06 | 12,418 | 99.56 |
| AVF-T | 228 | 78.10 | 2,613 | 87.94 |
| AVG-T | 4,671 | 93.08 | 8,447 | 94.08 |
| Combined | 14,067 | 96.18 | 68,759 | 98.24 |
AVF, arteriovenous fistula; AVG, arteriovenous graft; Ex, exchange; Place, placement; PTA, percutaneous transluminal angioplasty; T, thrombectomy; TDC, tunneled dialysis catheter.
From “Effectiveness and safety of dialysis vascular access procedures performed by interventional nephrologists,” by G.A. Beathard, T. Litchfield, Physician Operators Forum of RMS Lifeline, Inc., 2004, Kidney Int, 66, p. 1622–1632. Reprinted with permission.
Table 1 also shows data for 2014 from this same DAC group, expanded in number from 11 to 75 facilities. Over this 10-year span, the success rate for all procedures continued to be at a high level. It is obvious that a shift from arteriovenous graft to arteriovenous fistula (AVF) use occurred during this period. In addition, the success of dealing with AVF thrombosis improved by 10%.
This study also addressed procedure-related complications (PRCs) [11]. For the 14,067 cases studied, complications were classified according to the (American) Society of Interventional Radiology complication classification system [12,13]. According to this system, complications are classified as none, minor, or major. In a general sense, minor complications are those that have no permanent sequelae and require no specific therapy. Major complications are those that require a change in medical management or have permanent sequelae. Complication rate thresholds have also been established. In this series of patients, the PRC rates (Table 2) were below the Society of Interventional Radiology thresholds and were less than those previously published from other, but smaller studies. Table 2 also shows data for 2014 from this same DAC group, expanded in number from 11 to 75 facilities. In addition to reflecting the changes brought about by evolving from primarily synthetic grafts to primarily AVFs, the PRC rates are even lower, especially for arteriovenous thrombectomies.
Table 2.
Complication rates for dialysis access procedures for interventional nephrologists
| Procedure | 2004 |
2014 |
||||
|---|---|---|---|---|---|---|
| No. | Minor (%) | Major (%) | No. | Minor (%) | Major (%) | |
| TDC-Place | 1,765 | 1.36 | 0.06 | 4,038 | 0.42 | 0.15 |
| TDC-Ex | 2,262 | 1.37 | 0.04 | 8,851 | 0.19 | 0.15 |
| AVF-PTA | 1,561 | 4.29 | 0.19 | 32,392 | 1.24 | 0.08 |
| AVG-PTA | 3,560 | 1.04 | 0.11 | 12,418 | 0.64 | 0.07 |
| AVF-T | 228 | 6.07 | 0.44 | 2,613 | 4.13 | 0.92 |
| AVG-T | 4,671 | 5.99 | 0.26 | 8,447 | 2.13 | 0.41 |
| Combined | 14,067 | 3.26 | 0.28 | 68,759 | 1.17 | 0.16 |
AVF, arteriovenous fistula; AVG, arteriovenous graft; Ex, exchange; Place, placement; PTA, percutaneous transluminal angioplasty; T, thrombectomy; TDC, tunneled dialysis catheter.
From “Effectiveness and safety of dialysis vascular access procedures performed by interventional nephrologists,” by G.A. Beathard, T. Litchfield, Physician Operators Forum of RMS Lifeline, Inc., 2004, Kidney Int, 66, p. 1622–1632. Reprinted with permission.
In a more recent report, a review of serious complications occurring in a cohort of 84,669 hemodialysis access procedures performed by interventional nephrologists in freestanding DACs (IN group) was presented [14] compared with a concurrent report from an interventional radiology (IR) group [9]. This was from a hospital-based program and included data on 38,927 procedures, 4,132 of which were dialysis access related (IR group). Serious complications were characterized as either a medical emergency (ME) or a cardiopulmonary arrest (CPA). For the purposes of this report, an ME was defined as any change in cardiovascular status or mentation that required the patient to receive a higher-than-planned level of care, including, but not limited to, transfer to the hospital. A CPA was defined as a state of cardiac or pulmonary activity requiring initiation of an advanced cardiac life support protocol or attempted intubation. A combined frequency of 0.61% for ME and CPA for arteriovenous access interventions (angioplasty and thrombectomy) was reported in this IR group (Table 3), whereas IN group data showed a frequency of 0.19%. The IR group had a rate of 0.53% for ME and CPA for dialysis catheter procedures, whereas the IN group showed a rate of 0.09%.
Table 3.
Comparison of dialysis access-related procedure complications
| AV access procedures |
Catheter |
|||||||
|---|---|---|---|---|---|---|---|---|
| Thrombectomy |
Angio/PTA |
Total |
||||||
| IR (N = 393) | IN(N = 11,789) | IR(N = 1,258) | IN(N = 50,300) | IR(N = 1,651) | IN(N = 62,089) | IR(N = 2,088) | IN (N = 22,580) | |
| CPA (%) | 0.51 | 0.10 | 0.08 | 0.02 | 0.18 | 0.03 | 0.29 | 0.01 |
| ME (%) | 0.76 | 0.41 | 0.32 | 0.10 | 0.42 | 0.16 | 0.24 | 0.08 |
| All (%) | 1.27 | 0.51 | 0.40 | 0.11 | 0.61 | 0.19 | 0.53 | 0.09 |
AV, arteriovenous; Angio/PTA, combined angiogram and angioplasty groups; CPA, cardiopulmonary arrest; IN, interventional nephrology; IR, interventional radiology; ME, medical emergency.
From “Medical emergencies and cardiopulmonary arrests in interventional radiology,” by G. Nadolski, A. Praestgaard, R.D. Shlansky-Goldberg, M.C. Soulen, S.W. Stavropoulos, S.O. Trerotola, C. Farrelly, 2013, J Vasc Interv Radiol, 24, p. 1779–1785 and “Dialysis access procedures in the outpatient setting: risky?” by A.Q. Urbanes, 2013, J Vasc Interv Radiol, 24, p. 1787–1789. Reprinted with permission.
Pain management through effective S/A is important in the performance of dialysis access maintenance procedures. However, this aspect of management carries with it a degree of risk, especially considering the ages and comorbidities of the dialysis patient population. To address the question as to whether an interventional nephrologist can administer S/A safely in the outpatient setting, data derived from a cohort of 12,896 hemodialysis patients undergoing dialysis access maintenance procedures performed by interventional nephrologists were analyzed to determine the safety of S/A drug administration in a freestanding DAC [15]. In this study, all medications were administered by the nephrologist performing the procedure who had specific training in S/A. Intravenous midazolam, fentanyl, or a combination of both was used. The S/A goal was moderate sedation as defined by the American Society of Anesthesiology (ASA) [16] in all patients. All patients were evaluated and monitored during the procedure according to ASA guidelines. All drug dosages were individualized and administered in small, incremental doses that were titrated to the patient׳s response. Doses were adjusted to account for patient size, age, and comorbidities at the physician׳s discretion.
All dialysis patients are considered to be at higher risk than normal for these types of procedures; however, within the cohort, an especially high-risk group was identified. The remainder of the cases were grouped together as the lower risk group. The total (all types) complication rate in this cohort of patients was 2.9%, of which 2.4% were minor complications. Complications felt to be directly related to S/A were 0.13% (17 cases). Two-thirds of these occurred in the high-risk group (Table 4). Two deaths occurred, but at a time remote from the procedure and not felt to be related to the procedure (The duration of the complication monitoring period is 30 days). The conclusion of this study was that S/A administered for these types of procedures by interventional nephrologists is a safe procedure even in very high-risk patients.
Table 4.
Complications related to S/A
| Group | No. (%) | Minor | Major | S/A related |
|---|---|---|---|---|
| High risk | 5,415 (42) | 181 (1.40) | 57 (0.44) | 11 (0.085) |
| Lower risk | 7,481 (58) | 123 (0.96) | 10 (0.08) | 6 (0.046) |
| Total | 12,896 | 304 (2.36) | 67 (0.52) | 17 (0.131) |
Data are expressed as n (%).
S/A, sedation/analgesia.
From “The risk of sedation/analgesia in hemodialysis patients undergoing interventional procedures” by G.A. Beathard, A. Urbanes, T. Litchfield, A. Weinstein, 2011, Semin Dial, 24, p. 97–103. Reprinted with permission (Copyright - 2013. Copy right holder - Seminars in Dialysis).
Interventional procedures performed for dialysis vascular access management require the use of fluoroscopy. Any time when radiological equipment is used by a nonradiologist, there are legitimate concerns about radiation exposure to the patient and healthcare team [17,18]. The effects of radiation are cumulative. Most dialysis patients require repetitive procedures that are generally fluoroscopy guided. Most interventional nephrologists and the staff of the DAC spend a major portion of their work time in the procedure room and anticipate doing so for a number of years. Therefore, radiation safety must be of concern.
To analyze this issue as it relates to the interventional nephrologist, a study was conducted to assess the levels of radiation dosage involved with these procedures [19]. Dosimetry information including dose–area product (DAP), reference point air kerma (RPAK), and fluoroscopy time was collected prospectively. Radiation dosage data were collected from 24 centers in various parts of the United States and reflected cases managed by 69 different interventional nephrologists. The data were tabulated separately for eight procedures—fistula angioplasty and thrombectomy, graft angioplasty and thrombectomy, tunneled catheter placement and exchange, as well as vein mapping and cases in which only angiographic evaluation was performed. The number of cases involved for each of these varied. The results are shown in Table 5 as the number of cases reviewed for each procedure and each metric and the geometric mean (the distribution was non-Gaussian) for the group.
Table 5.
Radiation dosages for procedures performed by interventional nephrologists
| Metric | AVF-PTA | AVF-T | AVG-PTA | AVG-T | Angio | Cath-Place | Cath Ex | Map | |
|---|---|---|---|---|---|---|---|---|---|
| FT (s) | Geo. mean | 54.3 | 90 | 47.7 | 88.3 | 30 | 24.6 | 22 | 50 |
| n | 6,126 | 513 | 2,876 | 2,098 | 1,808 | 810 | 2,043 | 978 | |
| RPAK (mGy) | Geo. mean | 2.21 | 4.60 | 2.14 | 5.35 | 1.08 | 0.69 | 1.08 | 2.02 |
| n | 168 | 24 | 126 | 40 | 36 | 44 | 78 | 46 | |
| DAP (Gy cm2) | Geo. mean | 0.741 | 0.873 | 0.798 | 0.903 | 0.590 | 0.832 | 0.892 | 1.142 |
| n | 6,128 | 513 | 2,876 | 2,098 | 1,808 | 810 | 2,043 | 978 | |
Angio, angiogram; AVF, arteriovenous fistula; AVG, arteriovenous graft; Cath, tunneled dialysis catheter; DAP, dose–area product; Ex, exchange; FT, fluoroscopy time; Geo. mean, geometric mean; Map, vein mapping; n, number of cases in group; Place, placement; PTA, percutaneous transluminal angioplasty; RPAK, reference point air kerma; T, thrombectomy.
From “Radiation dose associated with dialysis vascular access interventional procedures in the interventional nephrology facility,” by G.A. Beathard, A. Urbanes, T. Litchfield, 2013, Semin Dial, 26, p. 503–510. Reprinted with permission (Copyright - 2013. Copy right holder - Seminars in Dialysis).
Biologic effects resulting from radiation exposure are traditionally divided into stochastic effects (primarily cancer induction) and deterministic effects (primarily skin injury) [20]. The radiation dose metric used to assess stochastic risk is DAP. Assuming a normal life expectancy, it has been estimated that this risk of experiencing a fatal malignancy is increased 0.5% for each 100 mGy of effective dose of x-ray radiation [21]. The DAP values listed in Table 5 are in Gy × cm2 (radiation dose in gray multiplied by area exposed in square centimeter). It is not possible to convert this information directly into effective dose of radiation; however, the values listed represent a minuscule stochastic risk.
Deterministic effects are characterized by a threshold dose below which they do not occur. In much the same way as sunburn resulting from sun exposure, deterministic effects do not occur until the dose threshold is reached, and then, their severity increases from that point onward. The threshold for the earliest skin changes, erythema, has been established at 2 Gy [22]. The radiation dose metric used to assess deterministic risk is RPAK. The RPAK values listed in Table 5 are in milligray with the highest value for any of the eight procedures being 6.4 mGy for arteriovenous graft thrombectomy. At this level, it would require more than 300 procedures to produce skin erythema provided that the fluoroscopy machine was not moved at any time during the procedures.
Very little has been published in the literature concerning radiation dosage associated with dialysis vascular access maintenance procedures. Comparisons with the studies that are available are published by radiologists working in a hospital setting [23–25]. These show a 3–8 greater radiation dose than in this study. It should be recognized that some of the decrease in dosage is related to the fact that interventional nephrologists working in DAC use a mobile C-arm, whereas those in a hospital setting generally use a fixed C-arm fluoroscopy unit. The dose levels are considerably lower with a mobile C-arm because of the way it operates [26,27].
Clinical value of IN
The vascular access of a dialysis patient is prone to recurrent problems. It is important that these problems be managed effectively, efficiently, and timely. Not infrequently, there is an emergent situation that requires an emergent solution, defined as the absence of a functioning access for hemodialysis in a patient requiring emergent hemodialysis. The goal of dialysis access management should be to avoid missed dialysis treatments and avoid hospitalization. The effectiveness and safety issues discussed previously speak to the clinical value of IN; however, there are also published reports that address the overall value of an IN program working in a freestanding DAC.
Using retrospective data, a study was conducted to compare hospitalization and missed dialysis treatment rates between two patient populations—a test group consisting of a cohort of approximately 6,000 patients receiving dialysis access management at an IN-operated facility and a control group consisting of a national cohort of approximately 290,000 patients [8]. This study looked at a period of 7 years. IN involvement was initiated during the 4th year allowing for a comparison of data within the test group representing a period before and after the initiation of IN involvement. During the first 3 years of the study period, there was no significant difference between the test and control groups. However, with the initiation of IN in the test group, both metrics, hospitalization and missed dialysis treatment rates, declined markedly. By the end of the study period, hospitalization days/patient year decreased 57% in the test group and missed dialysis treatments/patient year decreased 29% in comparison with the control group (P = 0.01).
The clinical value of interventional nephrologist working in a DAC was further demonstrated by a prospective study designed to evaluate the efficiency and outcomes of emergent hemodialysis access procedures over a 3-month period in a high-volume facility [28]. A total of 157 emergent procedures were performed during the period of the study. The procedure was successful in 95% of the cases with 90% being completed within 24 hours of referral. Dialysis treatment was performed within 24 hours in 61% of the cases and within 48 hours in an additional 29%.
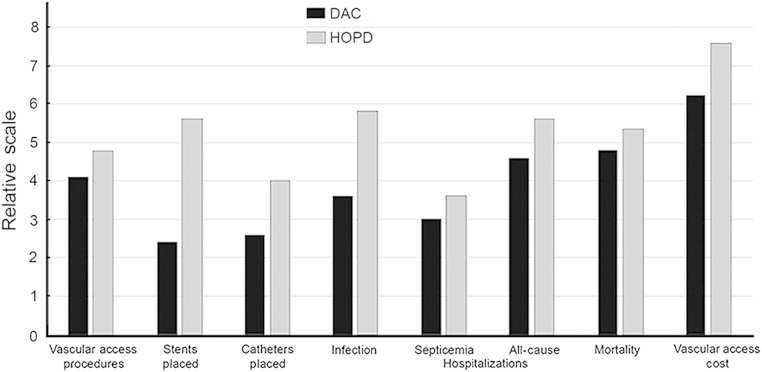
To evaluate the overall clinical effect of a DAC, US Medicare claims data were collected representing incident and prevalent ESRD patients who received at least 80% of their dialysis vascular access management at either a DAC or a hospital outpatient department over a 4-year period [29]. Using propensity score matching techniques [30], cases with a similar clinical and demographic profile from these two sites of service were matched according to 47 different variables. Medicare utilization, payments, and patient outcomes were compared across the matched cohorts. This created a DAC group (n = 27,613) and an hospital outpatient department (HOPD) group (n = 27,613) for comparisons. Patients treated in the DAC (Fig. 2) had significantly better clinical outcomes (P≤0.001). This included fewer vascular access-related infections (0.18 vs. 0.29) and fewer septicemia-related hospitalizations (0.15 vs. 0.18). Mortality rate was lower (47.9% vs. 53.5%).
Figure 2.
Clinical value and economy of DACs. Scale is relative, see the text for actual values; for all values, P≤0.01.
DAC, dialysis access center; HOPD, hospital outpatient department.
From “Clinical and economic value of performing dialysis vascular access procedures in a freestanding office-based center as compared with the hospital outpatient department among Medicare ESRD beneficiaries,” by A. Dobson, A.M. El-Gamil, M.T. Shimer, J.E. DaVanzo, A.Q. Urbanes, G.A. Beathard, T.F. Litchfield, 2013, Semin Dial, 26, p. 624–632. Reprinted with permission (Copyright - 2013. Copy right holder - Seminars in Dialysis).
Although this study was not able to make direct evaluations based on medical specialty, the data did indicate that interventional nephrologists were more likely and interventional radiologists were less likely to be providing most of the patients׳ dialysis vascular access–related care in the DAC than in an HOPD.
The Medicare claims data–based study discussed previously also examined the economic value of a freestanding DAC (Fig. 2). This study showed that matched patients treated in the DAC had fewer dialysis vascular access procedures than those treated in the HOPD (20.5 vs. 23.9, P≤0.01), fewer all-cause hospital admissions (2.3 vs. 2.8, P≤0.001), fewer catheters placed (1.3 vs. 2.0, P≤0.001), and fewer stents placed (0.6 vs. 1.4, P≤0.001) despite having longer episodes and lower mortality rates.
Matched patients who received their care in a freestanding DAC had an average Medicare per member-per month payment for dialysis vascular access services that was $626 lower than those who received care in the HOPD ($3,162 vs. $3,788; confidence interval, –$736 to –$516; P≤0.001). This represented a savings of $7,012 per patient per year or almost $200 million per year for the total cohort of 27,613 patients studied.
Summary
Over the course of the past 20 years, IN has developed as a subspecialty of nephrology specializing in the management of dialysis vascular access problems. Working primarily in freestanding, dedicated dialysis access facilities, interventional nephrologists have demonstrated an ability to provide effective, safe, and economical interventional vascular access management care to the dialysis patient population which they serve. IN has grown to the point that today it is providing most of these essential medical services in many parts of the world.
Conflicts of interest
All authors have no conflicts of interest.
References
- 1.Arora P., Kausz A.T., Obrador G.T., Ruthazer R., Khan S., Jenuleson C.S., Meyer K.B., Pereira B.J. Hospital utilization among chronic dialysis patients. J Am Soc Nephrol. 2000;11:740–746. doi: 10.1681/ASN.V114740. [DOI] [PubMed] [Google Scholar]
- 2.Beathard G.A. Integrated vascular access management. Blood Purif. 2003;21:89–98. doi: 10.1159/000067858. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill C. The new nephrologist. Am J Kidney Dis. 2000;35:978–979. doi: 10.1016/s0272-6386(00)70275-7. [DOI] [PubMed] [Google Scholar]
- 4.Leon F.T., Bermudez C.R., Hernandez V., Silva J., Delpin E.S. Interventional nephrology in Puerto Rico. Semin Dial. 2006;19:176–179. doi: 10.1111/j.1525-139X.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- 5.Nascimento M.M., Chula D., Campos R., Nascimento D., Riella M.C. Interventional nephrology in Brazil: current and future status. Semin Dial. 2006;19:172–175. doi: 10.1111/j.1525-139X.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 6.Rivera M., Quereda C. Diagnostic and interventional nephrology: an opportunity for Spanish nephrologists. Nefrologia. 2011;31:131–133. doi: 10.3265/Nefrologia.pre2011.Feb.10825. [DOI] [PubMed] [Google Scholar]
- 7.Beathard G.A. Interventional nephrology: a part of the solution. Semin Dial. 2006;19:171. doi: 10.1111/j.1525-139X.2006.00145.x. [DOI] [PubMed] [Google Scholar]
- 8.Mishler R., Sands J.J., Ofsthun N.J., Teng M., Schon D., Lazarus J.M. Dedicated outpatient vascular access center decreases hospitalization and missed outpatient dialysis treatments. Kidney Int. 2006;69:393–398. doi: 10.1038/sj.ki.5000066. [DOI] [PubMed] [Google Scholar]
- 9.Nadolski G., Praestgaard A., Shlansky-Goldberg R.D., Soulen M.C., Stavropoulos S.W., Trerotola S.O., Farrelly C. Medical emergencies and cardiopulmonary arrests in interventional radiology. J Vasc Interv Radiol. 2013;24:1779–1785. doi: 10.1016/j.jvir.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Rueb G.R., Brady W.J., Gilliland C.A., Patrie J.T., Saad W.E., Sabri S.S., Park A.W., Stone J.R., Angle J.F. Characterizing cardiopulmonary arrest during interventional radiology procedures. J Vasc Interv Radiol. 2013;24:1774–1778. doi: 10.1016/j.jvir.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Beathard G.A., Litchfield T. Physician Operators Forum of RMS Lifeline, Inc.: Effectiveness and safety of dialysis vascular access procedures performed by interventional nephrologists. Kidney Int. 2004;66:1622–1632. doi: 10.1111/j.1523-1755.2004.00928.x. [DOI] [PubMed] [Google Scholar]
- 12.Sacks D., McClenny T.E., Cardella J.F., Lewis C.A. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 13.Aruny J.E., Lewis C.A., Cardella J.F., Cole P.E., Davis A., Drooz A.T., Grassi C.J., Gray R.J., Husted J.W., Jones M.T., McCowan T.C., Meranze S.G., Van Moore A., Neithamer C.D., Oglevie S.B., Omary R.A., Patel N.H., Rholl K.S., Roberts A.C., Sacks D., Sanchez O., Silverstein M.I., Singh H., Swan T.L., Towbin R.B., Trerotola S.O., Bakal C.W. Society of Interventional Radiology Standards of Practice Committee: Quality improvement guidelines for percutaneous management of the thrombosed or dysfunctional dialysis access. J Vasc Interv Radiol. 2003;14:S247–S253. [PubMed] [Google Scholar]
- 14.Urbanes A.Q. Dialysis access procedures in the outpatient setting: risky? J Vasc Interv Radiol. 2013;24:1787–1789. doi: 10.1016/j.jvir.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Beathard G.A., Urbanes A., Litchfield T., Weinstein A. The risk of sedation/analgesia in hemodialysis patients undergoing interventional procedures. Semin Dial. 2011;24:97–103. doi: 10.1111/j.1525-139X.2011.00844.x. [DOI] [PubMed] [Google Scholar]
- 16.American Society of Anesthesiologists Task Force on Sedation Analgesia by Non-Anesthesiologists: Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1017. [Google Scholar]
- 17.Wagner L.K., Archer B.R., Cohen A.M. Management of patient skin dose in fluoroscopically guided interventional procedures. J Vasc Interv Radiol. 2000;11:25–33. doi: 10.1016/s1051-0443(07)61274-3. [DOI] [PubMed] [Google Scholar]
- 18.Cardella J.F., Miller D.L., Cole P.E., Lewis C.A. Society of Cardiovascular & Interventional Radiology: Society of Cardiovascular & Interventional Radiology position statement on radiation safety. J Vasc Interv Radiol. 2001;12:281. doi: 10.1016/s1051-0443(07)61905-8. [DOI] [PubMed] [Google Scholar]
- 19.Beathard G.A., Urbanes A., Litchfield T. Radiation dose associated with dialysis vascular access interventional procedures in the interventional nephrology facility. Semin Dial. 2013;26:503–510. doi: 10.1111/sdi.12071. [DOI] [PubMed] [Google Scholar]
- 20.Little M.P. Risks associated with ionizing radiation. Br Med Bull. 2003;68:259–275. doi: 10.1093/bmb/ldg031. [DOI] [PubMed] [Google Scholar]
- 21.The 2007 recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Valentin J. Avoidance of radiation injuries from medical interventional procedures. Ann ICRP. 2000;30:7–67. doi: 10.1016/S0146-6453(01)00004-5. [DOI] [PubMed] [Google Scholar]
- 23.Heye S., Maleux G., Oyen R.H., Claes K., Kuypers D.R. Occupational radiation dose: percutaneous interventional procedures on hemodialysis arteriovenous fistulas and grafts. Radiology. 2012;264:278–284. doi: 10.1148/radiol.12110978. [DOI] [PubMed] [Google Scholar]
- 24.Stavas J.M., Smith T.P., DeLong D.M., Miller M.J., Suhocki P.V., Newman G.E. Radiation hand exposure during restoration of flow to the thrombosed dialysis access graft. J Vasc Interv Radiol. 2006;17:1611–1617. doi: 10.1097/01.RVI.0000236842.49430.BD. [DOI] [PubMed] [Google Scholar]
- 25.Vano E., Sanchez R., Fernandez J.M., Gallego J.J., Verdu J.F., de Garay M.G., Azpiazu A., Segarra A., Hernandez M.T., Canis M., Diaz F., Moreno F., Palmero J. Patient dose reference levels for interventional radiology: a national approach. Cardiovasc Intervent Radiol. 2009;32:19–24. doi: 10.1007/s00270-008-9439-9. [DOI] [PubMed] [Google Scholar]
- 26.Fossaceca R., Brambilla M., Guzzardi G., Cerini P., Renghi A., Valzano S., Brustia P., Carriero A. The impact of radiological equipment on patient radiation exposure during endovascular aortic aneurysm repair. Eur Radiol. 2012;22:2424–2431. doi: 10.1007/s00330-012-2492-4. [DOI] [PubMed] [Google Scholar]
- 27.Geijer H., Larzon T., Popek R., Beckman K.W. Radiation exposure in stent-grafting of abdominal aortic aneurysms. Br J Radiol. 2005;78:906–912. doi: 10.1259/bjr/72629938. [DOI] [PubMed] [Google Scholar]
- 28.Kian K., Takesian K., Wyatt C., Vassalotti J., Mishler R., Schon D. Efficiency and outcomes of emergent vascular access procedures performed at a dedicated outpatient vascular access center. Semin Dial. 2007;20:346–350. doi: 10.1111/j.1525-139X.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 29.Dobson A., El-Gamil A.M., Shimer M.T., DaVanzo J.E., Urbanes A.Q., Beathard G.A., Litchfield T.F. Clinical and economic value of performing dialysis vascular access procedures in a freestanding office-based center as compared with the hospital outpatient department among Medicare ESRD beneficiaries. Semin Dial. 2013;26:624–632. doi: 10.1111/sdi.12120. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum P., Rubin D. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]