Abstract
We consider insomnia a disorder of waking rather than a disorder of sleep. This review examines the role of the reticular activating system, especially the pedunculopontine nucleus, in the symptoms of insomnia, mainly representing an overactive waking drive. We determined that high frequency activity during waking and REM sleep is controlled by two different intracellular pathways and channel types in PPN cells. We found three different PPN cell types that have one or both channels and may be active during waking only, REM sleep only, or both. These discoveries point to a specific mechanism and novel therapeutic avenues for insomnia.
Abbreviations: CaMKII, calcium/calmodulin-dependent protein kinase; cAMP, cyclic adenosine monophosphate; EEG, electroencephalogram; KA, kainic acid; NCS-1, neuronal calcium sensor protein 1; NMDA, n methyl d aspartic acid; ω-Aga, ω-agatoxin-IVA; ω-CgTx, ω-conotoxin-GVIA; PGO, ponto-geniculo-occipital; PPN, pedunculopontine nucleus; RAS, reticular activating system; REM, rapid eye movement; SWS, slow wave sleep
Keywords: Calcium channels, Gamma band activity, Neuronal calcium sensor protein, N-type calcium channel, P/Q-type calcium channel
1. Insomnia – symptoms, etiology, and manifestations
We consider that although insomnia is called a “sleep disorder”, it is actually a “waking disorder”, in which the waking system is overactive, intruding excessively into sleep time. Perhaps in treating insomnia, we should not be trying to increase sleep but rather to decrease arousal. Insomnia is termed “primary” if it is not related to some other medical or psychiatric condition [1]. The American Academy of Sleep Medicine defines insomnia as a persistent difficulty with sleep initiation, duration, consolidation, or quality that occurs despite adequate opportunity and circumstances for sleep, and results in some form of daytime impairment [1]. However, insomnia is a hallmark of a number of psychiatric disorders such as schizophrenia, bipolar disorder, and major depression, in which insomnia is termed “secondary”. Insomnia is also present in a number of neurological diseases such as Alzheimer׳s disease and Parkinson׳s disease [1,2]. Insomnia includes difficulty falling asleep (prolonged sleep latency), frequent awakenings (difficulty maintaining sleep), and shortened sleep duration (resulting in daytime sleepiness, irritability and fatigue), all of which leads to impairments in daytime functioning [1]. Almost any condition that affects arousal and vigilance can induce insomnia [2].
Aside from comorbid neurological and psychiatric disorders, risk factors generally include exposure to continued stress. About half of the population experiences insomnia at least half the nights, and about 5% meet clinical criteria. This makes insomnia fairly common in modern society, and, unfortunately, sleeping less than 4 h per night does increase mortality [3]. There may be a genetic component since over one half of patients diagnosed with primary insomnia reported familial insomnia [4]. A number of imaging studies suggest decreased frontal lobe function and/or hypofrontality are present in insomnia [5,6].
The electroencephalographic (EEG) characteristics of patients with insomnia do not show major differences compared to good sleepers, with some studies reporting an increase in low beta and decrease in high beta frequency power [7], as well as decreases in REM sleep [8]. In general, the differences in the EEG are subtle but do suggest intrusion of higher frequency activity during low frequency states, such as the incidence of higher beta activity during slow wave sleep [9–11]. Experts in the field agree that primary insomnia patients not only show hyperarousal at night, but also during the day, manifesting in part as the inability to nap despite sleep deprivation symptoms [4,5]. This particular spectrum suggests that there is high frequency activity during slow wave sleep as well as decreased REM sleep, and the hyperarousal persists during waking. This is why we consider insomnia a “waking disorder”, one in which the mechanisms specifically driving waking are exaggerated [2].
Treatments for insomnia are palliative and include benzodiazepines and non-benzodiazepine hypnotics to increase sleep, but the risk of physical dependence is high when used chronically [1]. However, from the foregoing discussion, it appears that insomnia does tend to manifest specifically with symptoms of excessive waking drive, rather than changes in REM sleep drive.
2. Hyperarousal and the reticular activating system (RAS)
Given the information outlined above, what aspects of RAS function will be involved in insomnia? There are five major factors that apply to disorders involving the RAS. (1) We know that the RAS participates in fight-vs-flight responses; therefore, we would expect that responses to sudden alerting stimuli will be abnormal. For disorders in which the RAS is overactive, this would mean that such stimuli will produce exaggerated responses that would be manifested as exaggerated startle responses or hyperactive reflexes, such as the blink reflex. Few such studies have been performed in patients with insomnia, so we lack critical information about the condition. (2) Another property of the RAS is its rapid habituation to repetitive stimuli. This is reflected in its lack of responsiveness to rapidly repeating stimuli, that is, its rapid habituation. This endows the RAS with its capacity for sensory gating, the property of decreasing responsiveness of repetitive events in favor of novel or different stimuli. For disorders in which this property is affected, we expect a decrease in habituation or a sensory gating deficit. Again, little information on this mechanism is available for insomnia. (3) The RAS controls waking and sleep, so that sleep patterns would be dysregulated. If the RAS is down regulated by a disorder, we expect an inability to remain awake, the presence of excessive daytime sleepiness, and an excess of total sleep time, especially a decrease in slow wave sleep. If, on the other hand, the RAS is up regulated, we expect difficulty in getting to sleep and maintaining sleep. This would be reflected in insomnia or disrupted sleep during the night, and perhaps increased REM sleep drive, which is characterized by vivid nightmares and frequent awakenings. Such a condition would lead to increased REM sleep drive during sleep (resulting in intense dreaming), but perhaps also during waking. That is, resulting in dreaming while awake or hallucinations, along with hypervigilance. Patients with insomnia do not appear to suffer from increased REM sleep drive or hallucinations. Therefore, we assume that the dysregulation in insomnia represents increased waking drive, instead. (4) The RAS also modulates the maintenance of waking, a property ignored by many investigators but one that affects a host of functions. The inability to maintain a steady waking state, in the form of maintained gamma band activity, will interfere with attention, learning, and memory, to name a few processes. The EEG studies mentioned above do suggest excessive high frequency activity in insomnia, further emphasizing that the disorder is one more related to the waking state.
(5) Another factor in all of these disorders is the level of frontal lobe blood flow. Decreased frontal lobe blood flow, or hypofrontality, is present in a number of disorders, e.g. insomnia, schizophrenia, bipolar disorder, to some extent. Such a state during waking would lead to reflexive reactions with lack of consideration of consequences. This condition is probably involved in a lack of habituation to repetitive stimuli, or a sensory gating deficit. Under the condition of decreased cortical modulation, fight-vs-flight responses and reflexes would be exaggerated. Whether hypofrontality is a cause of RAS dysregulation or RAS dysregulation leads to hypofrontality remains to be determined. Decreased frontal lobe function does appear to be present in insomnia, as described above [5,6]. Moreover, successful treatment of the condition should be marked by normalization of frontal lobe blood flow.
3. Pedunculopontine nucleus (PPN) physiology and high frequency activity
The two most important advances on the physiology of the RAS in the last 10 years were, (a) the discovery of electrical coupling in some cells of certain RAS nuclei [12], and (b) the finding that every cell in the same RAS nuclei manifests intrinsic membrane beta/gamma oscillations [13]. These are membrane oscillations mediated by high threshold calcium channels that, when activated by depolarizing inputs, promote firing at the peaks of the oscillations, i.e. at the natural frequency of the oscillations, in this case, at beta/gamma frequencies (20–60 Hz). The first advance helps explain how these brain centers manifest the coherence necessary to maintain neuronal membrane oscillations at both low and high frequencies. The second advance helps explain how these nuclei induce and maintain gamma band activity necessary for the process of awakening and remaining awake. Since insomnia appears to be an increase in waking drive, we will deal with the mechanisms that underlie high frequency, beta/gamma activity in the PPN. Of the three main nuclei in the RAS, the PPN, locus coeruleus, and raphe, it is the PPN that is active during waking and REM sleep [14], the two states of arousal that involve high frequency activity.
The PPN modulates ascending projections through the thalamus (modulating arousal) and descending projections through the pons and medulla (modulating REM sleep and posture and locomotion). The PPN is made up of non-overlapping populations of cholinergic, glutamatergic, and GABAergic neurons [15]. The PPN contains three cell types based on in vitro intrinsic membrane properties [16–18]. Recordings of PPN neurons in vivo identified multiple types of thalamic-projecting PPN cells distinguished by their firing properties relative to ponto-geniculo-occipital (PGO) wave generation [19]. Some neurons exhibited low spontaneous firing frequencies (<10 Hz), but most showed high rates of tonic firing in the beta/gamma range (20–80 Hz). In other in vivo studies, PPN neurons increased firing during REM sleep and were labeled “REM-on” cells, or during both waking and REM sleep and were called “Wake/REM-on” cells, and also during waking only and were called “Wake-on” cells [20–22]. Electrical stimulation of the PPN will potentiate the manifestation of fast (20–40 Hz) oscillations in the cortical EEG, outlasting stimulation by 10–20 s [23]. These results suggest that PPN cells do fire at gamma band frequencies in vivo, and that its outputs can indirectly induce gamma band activity in its targets.
We were the first to report that all PPN cells fired maximally at gamma band frequency when depolarized using current steps applied intracellularly to patch clamped neurons [24]. This is the only property shared by every cell in the PPN, regardless of transmitter type or electrophysiological type. Moreover, cells around the PPN do not share that property [13,24,25]. Further results demonstrated that both voltage-dependent N- and P/Q-type calcium channels mediate the depolarizing phase of gamma band oscillations in the PPN. However, only P/Q-type channels appeared to be essential for gamma oscillation generation. Voltage clamp results suggested that calcium channels are located distally to the cell body, probably in PPN dendritic compartments [25], as has been determined in thalamic neurons [26]. We then confirmed using fast imaging techniques that PPN calcium channel-mediated oscillations are due to P/Q- and N-type channels, and revealed the fact that these channels are distributed along the dendrites of PPN cells [27].
4. Waking vs REM sleep high frequency
The differences between gamma band activities during waking vs REM sleep are unknown. Why is this important? Because it is high frequency, especially beta/gamma band activity that drives our cognitive function during waking but also during REM sleep, two obviously different states. Our studies addressed the differential intracellular mechanisms assumed to subserve high frequency activity during waking vs REM sleep as a prelude to the selective pharmacological modulation of these states. In order to develop an effective treatment for insomnia, we would need to decrease waking drive, partially of course, without affecting REM sleep drive. We know that the two states are differentially regulated in the PPN, and the majority of this work was performed by the Datta lab.
Injections of glutamate into the PPN were shown to increase both waking and REM sleep, but injections of NMDA increased only waking, while injections of kainic acid (KA) increased only REM sleep [28–31]. Thus, the two states are independently activated by NMDA vs KA receptors. Moreover, the intracellular pathways mediating the two states appear to differ. For example, the CaMKII activation inhibitor, KN-93, microinjected into the PPN of freely moving rats resulted in decreased waking but not REM sleep [32]. Increased ERK1/2 signaling in the PPN is associated with maintenance of sleep via suppression of waking [33], and that activation of intracellular protein kinase A (PKA) in the PPN instead contributed to REM sleep recovery following REM sleep deprivation [34]. These authors showed that during REM sleep, pCREB activation in PPN cholinergic neurons was induced by REM sleep, and that PPN intracellular PKA activation and a transcriptional cascade involving pCREB occurred in cholinergic neurons [35]. These results suggest that waking is modulated by the CaMKII pathway while REM sleep is modulated by the cAMP-PKA pathway in the PPN.
As mentioned above, in vivo recording studies have shown that PPN neurons manifest three major types of cellular activities in relation to waking and REM sleep in the form of “Wake/REM on”, “Wake-on”, and “REM on” cells [20–22]. This suggests that some PPN cells fire in relation to waking and REM sleep, only in relation to waking, and others only in relation to REM sleep, presumably through CaMKII and cAMP-PKA pathways vs only the cAMP-PKA pathway, respectively.
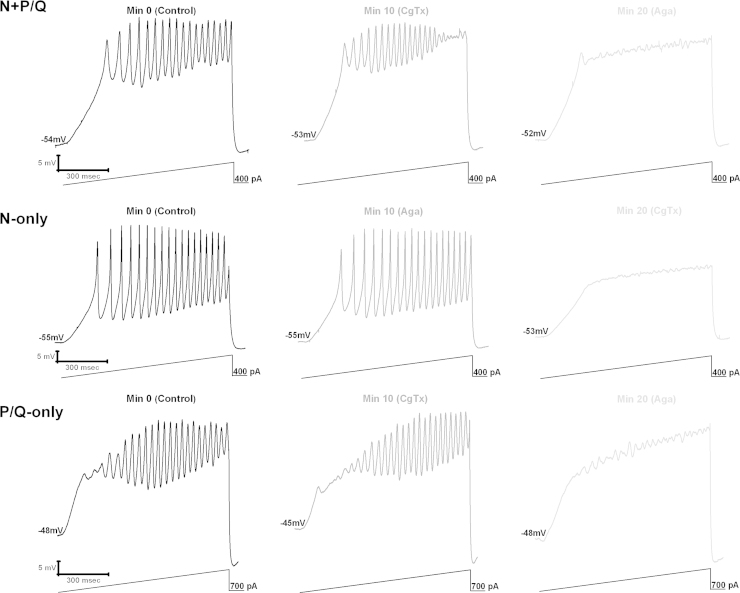
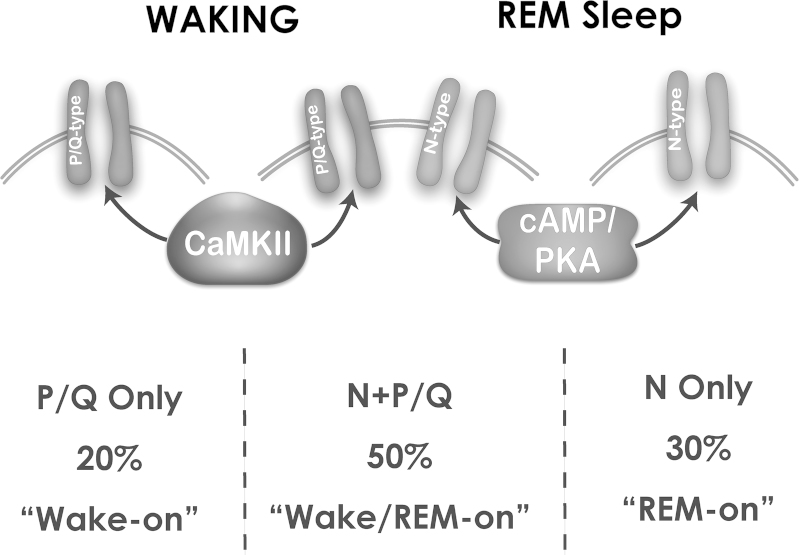
We have breakthrough findings showing that in some PPN cells (50%), the N-type calcium channel blocker ω-conotoxin-GVIA (ω-CgTx) reduced gamma oscillation amplitude, while subsequent addition of the P/Q-type blocker ω-agatoxin-IVA (ω-Aga) blocked the remaining oscillations. Other PPN cells (20%) manifested gamma oscillations that were not significantly affected by the addition of ω-CgTx, however, ω-Aga blocked the remaining oscillations. In the rest of the cells (30%), ω-Aga had no effect on gamma oscillations, while ω-CgTx blocked them. Similar results were found during recordings of voltage-dependent calcium currents. These results confirm the presence of cells in the PPN that manifest gamma band oscillations through only N-type, only P/Q-type, and both N- and P/Q-type calcium channels [36,37]. This new cell type classification suggests that some PPN neurons fire only during REM sleep (“REM-on”, N-type only), only during waking (“Wake-on”, P/Q-type only), or during both waking and REM sleep (“Wake/REM-on”, N-type+P/Q-type) [36,37]. Fig. 1 shows the responses to depolarizing ramps in each of these cell types, with responses being mediated by both N- and P/Q-type calcium channels (partial block by each channel blocker), by N-type only (complete block by ω-CgTx), and by P/Q-type only (complete block by ω-Aga). Fig. 2 shows the distribution and main intracellular control pathways modulating the two channel types, and presumed in vivo firing patterns.
Fig. 1.
PPN cells manifest gamma band activity through N- and P/Q-, N-only, and P/Q-only type Ca2+ channels. Top, N+P/Q). Membrane oscillations recorded during 1 s long ramps in the presence of synaptic blockers and tetrodotoxin (left record, black). Following superfusion with ω-CgTx for 10 min, oscillation amplitude was reduced (middle record, dark gray). Thereafter, ω-Aga was superfused for 10 min blocking the remaining oscillations (right record, light gray). Middle, N-only) Membrane oscillations recorded during 1 s long ramps in the presence of synaptic blockers and tetrodotoxin (left record, black). ω-Aga applied into the bath for 10 min caused no significant effect on the membrane oscillations (middle record, dark gray), that is, the oscillations are NOT reduced by ω-Aga. ω-CgTx was then superfused for 10 min, causing a complete blockade of the membrane oscillations (right record, light gray). Bottom, P/Q-only) Membrane oscillations recorded during 1 s long ramps in the presence of synaptic blockers and tetrodotoxin (left record, black). ω-CgTx was applied for 10 min causing no significant effect on the oscillations (middle record, dark gray), that is, the oscillations are NOT reduced by ω-CgTx. ω-Aga then was superfused for 10 min causing a complete blockade of the membrane oscillations (right record, light gray).
Fig. 2.
PPN cell types manifesting P/Q-type channels only, N-type channels only, or N- and P/Q-type channels. PPN cells with only P/Q-type calcium channels numbered 20%, and are assumed to be “Wake-on” and modulated by CaMKII. PPN cells with both N- and P/Q-type calcium channels numbered 50%, and are assumed to be Wake/REM-on” and modulated by both CaMKII and cAMP/PKA metabolic pathways. PPN cells with only N-type calcium channels numbered 30%, and are assumed to be “REM-on” and modulated by the cAMP/PKA pathway. P/Q-only and N+P/Q cells thus are expected to be active during waking, while N-only and N+P/Q cells are active during REM sleep.
5. Two calcium channels
N- and P/Q-type calcium channel subtypes both are linked to rapid release of synaptic vesicles [38,39], but knockout models manifest markedly different phenotypes [40]. P/Q-type (Cav2.1) knockout animals have deficient gamma band activity in the EEG, abnormal sleep-wake states, ataxia, are prone to seizures (low frequency synchrony), and die by 3 weeks of age [41,42]. On the other hand, N-type (Cav2.2) knockout animals show few sleep-wake abnormalities but exhibit decreased nociceptive responses, and are otherwise normal [40]. While the two types of receptors are modulated by G-protein coupled receptors, they require different G-protein subunits [43]. Intracellularly, protein kinase C (PKC) enhances N-type channel activity but has no effect on P/Q-type channel function [44], but CaMKII was shown to modulate P/Q-type channel function [45]. Again, the two calcium channel subtypes are modulated by different intracellular pathways, N-type by the cAMP/PK pathway, and P/Q-type via the CaMKII pathway. The implication from all of these results is that there is a “waking” pathway mediated by CaMKII and P/Q-type channels, and a “REM sleep” pathway mediated by cAMP/PK and N-type channels.
Interestingly, PPN cells with N-type channels only manifested oscillations in the 50 Hz range, while cells with P/Q-type channels only exhibited oscillations in the 45 Hz range, while cells with both N- and P/Q-type channels showed oscillations in the 37 Hz range. These results suggest that each channel type may have a preferred oscillation frequency, but when channels coexist they may manifest lower frequencies, perhaps due to competition and/or differential location along dendrites [27]. Fig. 3 provides a diagram of the intracellular “waking” pathway and the “REM sleep” pathway, and their modulation of different calcium channel types by separate glutamatergic receptor types. With this information, we can now attempt to selectively modulate waking by affecting P/Q-type calcium channels and/or the CaMKII pathway, or modulate REM sleep by affecting the N-type calcium channels and/or the cAMP/PK pathway.
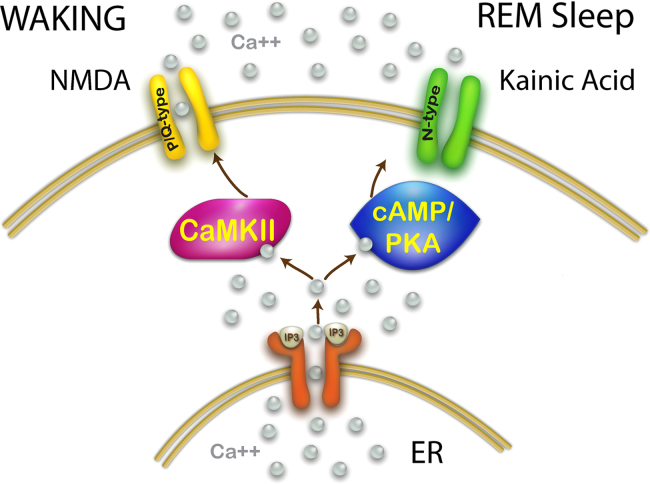
Fig. 3.
Intracellular pathways modulated by different glutamatergic receptors that drive independent calcium channel types. As discussed above, a wealth of information is available to suggest that NMDA promotes waking in the PPN and that this effect is mediated by CaMKII, and that kainic acid promotes REM sleep and that this effect is mediated by cAMP/PKA. Both of these pathways interact with the release of calcium from the endoplasmic reticulum (ER) induced by inositol phosphate 3 (IP3) release from the membrane and binding to the IP3 receptor. In addition, P/Q-type channels are modulated by CaMKII while N-type channels are modulated by cAMP/PKA. This points to a selective participation of P/Q-type channels in generating gamma band activity during waking, and N-type channels generating gamma band activity during REM sleep.
This organization has implications of the differential control of waking vs REM sleep for the developmental decrease in REM sleep seen in between birth and young adulthood. This hypothesis suggests that, early in development, there is increased expression of N-type channels driving exuberant REM sleep that decrease with age. This could be demonstrated by a decrease in N-type calcium channel expression compared to P/Q-type channel expression, but specifically in regions controlling REM sleep such as the PPN, but not in other regions.
6. Novel treatments for insomnia
Given the foregoing discussion, it would appear that the excessive waking drive in insomnia may be mediated by increased activity in the “waking” pathway, either by over expression of P/Q-type calcium channels, over activity in CaMKII modulation of these channels, and/or overdriving of this pathway by another intracellular mechanism.
One such mechanism involves neuronal calcium sensor protein 1 (NCS-1), an intracellular modulator of calcium metabolism, that at low concentrations potentiates beta/gamma oscillations in PPN neurons [46,47]. In insomnia, there may be a dysregulation in this protein that leads to increased high frequency activity. NCS-1 has been shown to be over expressed in schizophrenia and bipolar disorder [48], such that high concentrations of NCS-1 will down regulate gamma band activity [46], and lithium will down regulate the effects of NCS-1 over expression [49]. That is, lithium appears to down regulate excessive NCS-1 to normalize high frequency activity. The use of lithium to alleviate the hypervigilance in schizophrenia and bipolar disorder may also modulate the hyperarousal in insomnia. However, dosage may need to be very low in order to only partially reduce high frequency activity, and to minimize the risk of toxicity. Hopefully, future off label and clinical trials may prove beneficial in this difficult to treat condition, but better animal models of insomnia need to be developed. For example, a genetic mouse model in which over expression of P/Q-type calcium channels can be generated or conditionally induced could be a very valuable approach.
Another potential avenue of treatment for insomnia is the modulation of the CaMKII “waking” pathway. The increased vigilance and decreased REM sleep may indicate that the CaMKII pathway is over activated in relation to the cAMP/PKA pathway. This pathway can be inhibited by KN-93 [47], so that mild down regulation of CaMKII may provide beneficial results in insomnia. This is clinically difficult because of the many functions of CaMKII, so that other approaches may be needed unless more specific agents are forthcoming. A different approach would be partial blockade of P/Q-type calcium channels to reduce gamma oscillations and, therefore, arousal. New agents must be developed to elicit such effects, mimicking the specificity of agatoxin. In summary, this review provides a novel outlook on insomnia as a disorder of waking rather than sleep. This naturally points to a host of information that needs to be gathered (reflex function, startle response, sensory gating), and to novel therapeutic targets (P/Q-type channels, CaMKII, NCS-1) for the treatment of this condition. There may be additional, yet unrecognized, intracellular mechanisms that induce over activity in the function of P/Q-type calcium channels and its pathway, and these can only be identified with further research. These recent findings provide a likely target for such research. In addition, it would be interesting to determine if non-pharmacological therapies such as Cognitive-Behavioral Therapy for Insomnia can modify gamma band activity.
7. Additional implications- cell ensembles
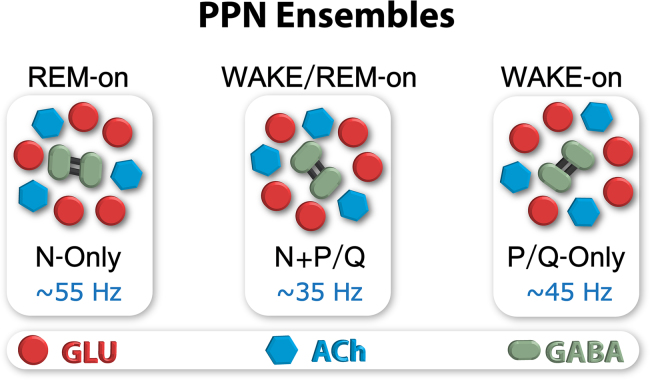
While there are three electrophysiological types of PPN cells in vitro, each of these is represented in the N-only, P/Q-only and N+P/Q cell groups, suggesting that all of the electrophysiological cell types are found in each cell ensemble. Anatomically, neurons in the PPN are scattered such that in the pars compacta there are glutamatergic (GLU), cholinergic (ACh), and GABAergic neurons in the ratio of 5:3:2, respectively [13]. Since all three electrophysiological types are included in the calcium channel segregation, it also means that all of the transmitter types are represented in the calcium channel segregation. This suggests that there are ensembles of N-only cells with glutamatergic, cholinergic, and GABAergic cells, and similarly for P/Q-only and N+P/Q cells. Studies using calcium imaging in the PPN pars compacta reveal an interesting anatomical organization within the nucleus. Pairs of PPN cells are labeled throughout the nucleus even in the control, unstimulated condition [48]. The spatial separation between couplets suggests that there are clusters of cells throughout the nucleus. Since electrically coupled neurons generally represent GABAergic neurons, we speculate that there are 5 GLU and 3 ACh neurons closely associated with each GABAergic pair. That is, there may be clusters of approximately 10 neurons scattered within the pars compacta that may create a functional subgroup. These functional subgroups may each bear a different combination of calcium channels. Much additional evidence is required to support this hypothesis, but it may be possible to dissect such an organization to determine how the nucleus as a whole generates coherent activity at specific frequencies. It is also important to determine how PPN neurons respond to sensory input and how that input generates coherent activity. Fig. 4 provides a proposed organization for these groups, each of which would includes each transmitter type, but all of the cells within the group would express one or both of the channels.
Fig. 4.
Proposed organization of cell ensembles in the PPN containing cells that express only N-type, only P/Q-type, or both calcium channels subtypes. Glutamatergic neurons are shown as red circles, cholinergic neurons as blue hexagons, and GABAergic cells as green ovals, some being electrically coupled (black bars). Groups of cells with a 5:3:2 ratio would all need to express the same channels subtype. Ensembles expressing only N-type channels would fire during REM sleep (REM-on), those expressing only P/Q-type channels would fire during waking (Wake-on), and those expressing both would fire during each state (Wake/REM-on).
Similar functional clustering has been proposed for the hippocampus, especially in relation to epileptic networks [50]. In a study of cell assemblies in the hippocampus, Buzsaki described subsets of about 10 neurons that showed repeated synchronous firing during open field exploration [51]. Interestingly, the timescale of activity between these neurons had a median of 23 ms, and the peak optimal timescale was ~16 ms, that is, most activity occurred in the 40–60 Hz range. We hypothesize that a similar temporal relationship will be evident among cell clusters in the PPN. Moreover, we postulate that there are cell ensembles of 5 GLU, 3 ACh, and 2 GABA cells all of which have N-only calcium channels, representing a cluster firing during REM sleep only (REM-on cluster), and similar ensembles which have only P/Q-type channels that fire only during waking (Wake-on cluster), and ensembles which have both N- and P/Q-type calcium channels that fire during waking and REM sleep (Wake/REM-on clusters). We proposed that each ensemble manifests N-only, P/Q-only, or both N+P/Q calcium channels [2].
8. Conclusion
The implications of the discovery of gamma band activity as a ubiquitous mechanism in RAS nuclei that modulate waking and REM sleep, and activate the cortex as well as postural and locomotor systems, are of critical importance. The fact that these centers can generate gamma band oscillations, however, should not be surprising given the descriptions of gamma activity in other subcortical structures such as the cerebellum, hippocampus, and the basal ganglia. Moreover, gamma band activity between these regions is coherent, so that RAS gamma band activity is probably highly coordinated with cortical and other subcortical gamma band generators, depending on the task. This also should not be surprising, given the need for reverberating circuits and cell assemblies essential for the persistence or maintenance of processes that mediate perception, movement, learning, and memory. These issues are discussed at length in a recent book [2]. The process of maintaining gamma band activity is metabolically demanding. PPN neurons must remain highly active during the waking state. Waking is hard work. When these neurons are over active, the consequences are wide-ranging and exhausting, in keeping with the consequences of increased waking drive in insomnia and sleep-deprivation symptoms. This review strengthens the need to revisit the physiological mechanisms governing the wake/sleep cycle because of the impact it could have on a number of psychiatric and neurological disorders.
Acknowledgments
This work was supported by NIH award R01NS020246, and by core facilities of the Center for Translational Neuroscience supported by NIH award P20 GM103425 and P30 GM110702 to Dr. Garcia-Rill. In addition, this work was supported by Grants from FONCYT-Agencia Nacional de Promoción Científica y Tecnológica; BID 1728 OC.AR. PICT-2012-1769 and UBACYT 2014-2017 #20120130101305BA (to Dr. Urbano) and FONCYT-Agencia Nacional de Promoción Científica y Tecnológica; BID 1728 OC.AR. T 2012-0924 Argentina (to Dr. Bisagno).
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
References
- 1.American Academy of Sleep Medicine. International classification of sleep disorders, 3rd ed.: Diagnostic and coding manual. American Academy of Sleep Medicine: Westchester, Illinois; 2014.
- 2.Garcia-Rill E. Academic Press; New York: 2015. Waking and the reticular activating system in health and disease; p. 330. [Google Scholar]
- 3.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 4.Dauvilliers Y, Morin C, Cervena K, Carlander B, Touchon J, Besset A. Family studies in insomnia. J Psychosom Res. 2005;58:271–278. doi: 10.1016/j.jpsychores.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet M.H., Arand D.L. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Riemann D., Spiegelhalder K., Feige B., Vodeholzer U., Berger M., Perils M. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Cervena K., Espa F., Perogamvros L., Perrig S., Merica H., Ibanez V. Spectral analysis of the sleep onset period in primary insomnia. Clin Neurophysiol. 2014;125:979–987. doi: 10.1016/j.clinph.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Baglioni C., Regen W., Teghen A., Spiegelhalder K., Feige B., Nissen C. Sleep changes in the disorder of insomnia: a meta-analysis of polysomnographic studies. Sleep Med Rev. 2014;18:195–213. doi: 10.1016/j.smrv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Feige B., Baglioni C., Spiegelhalder K., Hirscher V., Nissen C., Riemann D. The microstructure of sleep in primary insomnia: an overview and extension. Int J Psychophysiol. 2013;89:171–180. doi: 10.1016/j.ijpsycho.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Perlis M.L., Smith M.T., Andrews P.J., Orff H., Giles D.E. Beta/gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–117. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 11.Spiegelhalder K., Regen W., Feige B., Holz J., Piosczyk H., Baglioni C. Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol Psychol. 2012;91:329–333. doi: 10.1016/j.biopsycho.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Rill E., Heister D.S., Ye M., Charlesworth A., Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30:1405–1414. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Rill E., Kezunovic N., Hyde J., Beck P., Urbano F.J. Coherence and frequency in the reticular activating system (RAS) Sleep Med Rev. 2013;17:227–238. doi: 10.1016/j.smrv.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steriade M., McCarley R.W. Plenum Press; New York, NY: 1990. Brainstem control of wakefulness and sleep. [Google Scholar]
- 15.Wang H.L., Morales M. Pedunculopontine and laterodorsal tegmental nuclei contan distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonard C.S., Llinas R.R. Electrophysiology of mammalian pedunculopontine and laterodorsal tegmental neurons in vitro: implications for the control of REM sleep. In: Steriade M., Biesold D., editors. Brain cholinergic systems. Oxford Science; Oxford: 1990. pp. 205–223. [Google Scholar]
- 17.Kamondi A., Williams J., Hutcheon B., Reiner P. Membrane properties of mesopontine cholinergic neurons studied with the whole-cell patch-clamp technique: implications for behavioral state control. J Neurophysiol. 1992;68:1359–1372. doi: 10.1152/jn.1992.68.4.1359. [DOI] [PubMed] [Google Scholar]
- 18.Takakusaki K., Kitai S.T. Ionic mechanisms involved in the spontaneous firing of tegmental pedunculopontine nucleus neurons of the rat. Neuroscience. 1997;78:771–794. doi: 10.1016/s0306-4522(96)00540-4. [DOI] [PubMed] [Google Scholar]
- 19.Steriade M., Paré D., Datta S., Oakson G., Dossi R. Curro. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990;10:2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakai K., El Mansari M., Jouvet M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 1990;527:213–223. doi: 10.1016/0006-8993(90)91140-c. [DOI] [PubMed] [Google Scholar]
- 21.Steriade M., Datta S., Paré D., Oakson G., Dossi R. Curro. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datta S., Siwek D.F. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:79–82. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- 23.Steriade M., Curro Dossi R., Paré D., Oakson DG. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Nat Acad Sci. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon C., Kezunovic N., Ye M., Hyde J., Hayar A., Williams D.K. Gamma band unit and population responses in the pedunculopontine nucleus. J Neurophysiol. 2010;104:463–474. doi: 10.1152/jn.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kezunovic N., Urbano F.J., Simon C., Hyde J., Smith K., Garcia-Rill E. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN) Eur J Neurosci. 2011;34:404–415. doi: 10.1111/j.1460-9568.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedroarena C., Llinás R.R. Dendritic calcium conductances generate high-frequency oscillation in thalamocortical neurons. Proc Nat Acad Sci. 1997;94:724–728. doi: 10.1073/pnas.94.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyde J., Kezunovic N., Urbano F.J., Garcia-Rill E. Spatiotemporal properties of high speed calcium oscillations in the pedunculopontine nucleus. J Appl Physiol. 2013;115:1402–1414. doi: 10.1152/japplphysiol.00762.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J Neurophysiol. 2002;87:1790–1798. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- 29.Datta S., Siwek D.F. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induce wakefulness and REM sleep. J Neurophysiol. 1997;77:2975–2988. doi: 10.1152/jn.1997.77.6.2975. [DOI] [PubMed] [Google Scholar]
- 30.Datta S., Spoley E.E., Patterson E.H. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;280:R752–R759. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- 31.Datta S., Patterson E.H., Spoley E.E. Excitation of pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J Neurosci Res. 2001;66:109–116. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- 32.Data S., O’Malley M.W., Patterson E.H. Calcium/calmodulin kinase II in the pedunculopontine tegmental nucleus modulates the initiation and maintenance of wakefulness. J Neurosci. 2010;31:1700–17016. doi: 10.1523/JNEUROSCI.3981-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desarnaud F., Macone B.W., Datta S. Activation of extracellular signal-regulated kinase signaling in the pedunculopontine tegmental cells is involved in the maintenance of sleep in rats. J Neurochem. 2011;116:577–587. doi: 10.1111/j.1471-4159.2010.07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Datta S., Desarnaud F. Protein kinase A in the pedunculopontine tegmental nucleus of rat contributes to regulation of rapid eye movement sleep. J Neurosci. 2010;30:12263–12273. doi: 10.1523/JNEUROSCI.1563-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datta S., Siwek D.F., Stack E.C. Identification of cholinergic and non-cholinergic neurons in the pons expressing phosphorylated cyclic adenosine monophosphate response element-binding protein as a function of rapid eye movement sleep. Neuroscience. 2009;163:397–414. doi: 10.1016/j.neuroscience.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luster B., Hyde J., Donofrio S., Urbano F.J., Garcia-Rill E. Mechanisms behind gamma band activity in the pedunculopontine nucleus (PPN) Neurosci Abstr. 2014;38:257. [Google Scholar]
- 37.Luster B., D’Onofrio S., Urbano F.J., Garcia-Rill E. High-threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN) Physiol Rep. 2015;3:e12431. doi: 10.14814/phy2.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishikawa T., Kaneko M., Shin H.S., Takahashi T. Presynaptic N-type and P/Q-type Ca2+ channels mediating synaptic transmission at the calyx of held of mice. J Physiol. 2005;568:199–209. doi: 10.1113/jphysiol.2005.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reid C.A., Bekker J.M., Clement J.D. Presynaptic Ca2+ channels: a functional patchwork. Trends Neurosci. 2003;26:683–687. doi: 10.1016/j.tins.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Pietrobon D. Function and dysfunction of synaptic Ca2+ channels: insights from mouse models. Curr Opin Neurobiol. 2005;15:257–265. doi: 10.1016/j.conb.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Jun K., Piedras-Rentería E.S., Smith S.M., Wheeler D.B., Lee S.B., Lee T.G. Ablation of P/Q-type Ca2+ channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the a1A-subunit. Proc Natl Acad Sci. 1999;96:15245–15250. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Llinas R.R., Choi S., Urbano F.J., Shin H.S. Gamma band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc Natl Acad Sci. 2007;104:17819–17824. doi: 10.1073/pnas.0707945104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agler H.L., Evans J., Colecraft H.M., Yue D.T. Custom distinctions in the interaction of G-protein b subunits with N-type (Cav2.2) versus P/Q-type (Cav2. 1) Ca2+ channels. J Gen Physiol. 2003;121:495–510. doi: 10.1085/jgp.200208770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stea A., Soomg T.W., Snutch T.P. Determinants of PKC-dependent modulation of a family of neuronal Ca2+ channels. Neuron. 1995;15:929–940. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 45.Jiang X., Lautermilch N.J., Watari H., Westenbroek R.E., Scheuer T., Catterall W.A. Modulation of Cav2.1 channels by Ca+/calmodulin-dependent kinase II bound to the C-terminal domain. Proc Natl Acad Sci. 2008;105:341–346. doi: 10.1073/pnas.0710213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Onofrio S., Kezunovic N., Hyde J., Luster B., Messias E., Urbano F.J. Modulation of gamma oscillations in the pedunculopontine nucleus (PPN) by neuronal calcium sensor protein-1 (NCS-1): relevance to schizophrenia and bipolar disorder. J Neurophysiol. 2015;113:709–719. doi: 10.1152/jn.00828.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Rill E., Kezunovic N., D’Onofrio S., Luster B., Hyde J., Bisagno V. Gamma band activity in the RAS- intracellular mechanisms. Exp Brain Res. 2014;232:1509–1522. doi: 10.1007/s00221-013-3794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koh P.O., Undie A.S., Kabbani N., Levenson R., Goldman-Rakic P., Lidow M.S. Up-regulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients. Proc Natl Acad Sci. 2003;100:313–317. doi: 10.1073/pnas.232693499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Onofrio S., Urbano F.J., Garcia-Rill E. Liithium decreases the effects of neuronal calcium sensor protein 1 in pedunculopontine neurons. Neurosci Abstr. 2015;39:257.19. doi: 10.14814/phy2.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muldoon A.F., Soltesz I., Cossart, R R. Spatially clustered neuronal assemblies comprise the microstructure of synchrony in chronically epileptic networks. Proc Nat Acad Sci. 2013;110:3567–3572. doi: 10.1073/pnas.1216958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buzsaki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]