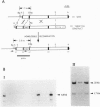
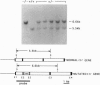
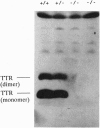
Abstract
Transthyretin (TTR) is thought to play a major role in vitamin A metabolism and thyroid hormone transport in mammals. To investigate the physiological role of the TTR protein in development of the embryo and in the adult, we used gene targeting techniques to generate a null mutation at the mouse ttr locus. The resultant mutant animals are phenotypically normal, viable, and fertile. However, levels of serum retinol, retinol-binding protein, and thyroid hormone are significantly depressed in the mutant animals. These observations demonstrate that the TTR protein maintains normal levels of these metabolites in the circulating plasma.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson M. D. Familial amyloidotic polyneuropathy. Trends Neurosci. 1989 Mar;12(3):88–92. doi: 10.1016/0166-2236(89)90162-8. [DOI] [PubMed] [Google Scholar]
- Berry M. J., Banu L., Larsen P. R. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature. 1991 Jan 31;349(6308):438–440. doi: 10.1038/349438a0. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Geisow M. J., Oatley S. J., Rérat B., Rérat C. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. J Mol Biol. 1978 May 25;121(3):339–356. doi: 10.1016/0022-2836(78)90368-6. [DOI] [PubMed] [Google Scholar]
- Blaner W. S. Radioimmunoassays for retinol-binding protein, cellular retinol-binding protein, and cellular retinoic acid-binding protein. Methods Enzymol. 1990;189:270–281. doi: 10.1016/0076-6879(90)89298-v. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Green M. H., Berg T., Norum K. R. Transport and storage of vitamin A. Science. 1990 Oct 19;250(4979):399–404. doi: 10.1126/science.2218545. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Helgerud P., Rasmussen M., Berg T., Norum K. R. In vivo uptake of chylomicron [3H]retinyl ester by rat liver: evidence for retinol transfer from parenchymal to nonparenchymal cells. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7326–7330. doi: 10.1073/pnas.79.23.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomhoff R., Holte K., Naess L., Berg T. Newly administered [3H]retinol is transferred from hepatocytes to stellate cells in liver for storage. Exp Cell Res. 1984 Jan;150(1):186–193. doi: 10.1016/0014-4827(84)90713-4. [DOI] [PubMed] [Google Scholar]
- Brouwer A., Blaner W. S., Kukler A., Van den Berg K. J. Study on the mechanism of interference of 3,4,3',4'-tetrachlorobiphenyl with the plasma retinol-binding proteins in rodents. Chem Biol Interact. 1988;68(3-4):203–217. doi: 10.1016/0009-2797(88)90017-8. [DOI] [PubMed] [Google Scholar]
- Burr W. A., Ramsden D. B., Hoffenberg R. Hereditary abnormalities of thyroxine-binding globulin concentration. A study of 19 kindreds with inherited increase or decrease of thyroxine-binding globulin. Q J Med. 1980;49(195):295–313. [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- DeChiara T. M., Efstratiadis A., Robertson E. J. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990 May 3;345(6270):78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- Dickson P. W., Howlett G. J., Schreiber G. Rat transthyretin (prealbumin). Molecular cloning, nucleotide sequence, and gene expression in liver and brain. J Biol Chem. 1985 Jul 5;260(13):8214–8219. [PubMed] [Google Scholar]
- Dwulet F. E., Benson M. D. Primary structure of an amyloid prealbumin and its plasma precursor in a heredofamilial polyneuropathy of Swedish origin. Proc Natl Acad Sci U S A. 1984 Feb;81(3):694–698. doi: 10.1073/pnas.81.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felding P., Fex G. Cellular origin of prealbumin in the rat. Biochim Biophys Acta. 1982 Jun 16;716(3):446–449. doi: 10.1016/0304-4165(82)90040-x. [DOI] [PubMed] [Google Scholar]
- Friedman G. D., Blaner W. S., Goodman D. S., Vogelman J. H., Brind J. L., Hoover R., Fireman B. H., Orentreich N. Serum retinol and retinol-binding protein levels do not predict subsequent lung cancer. Am J Epidemiol. 1986 May;123(5):781–789. doi: 10.1093/oxfordjournals.aje.a114307. [DOI] [PubMed] [Google Scholar]
- GOODMAN D. W., HUANG H. S., SHIRATORI T. TISSUE DISTRIBUTION AND METABOLISM OF NEWLY ABSORBED VITAMIN A IN THE RAT. J Lipid Res. 1965 Jul;6:390–396. [PubMed] [Google Scholar]
- Green P. H., Glickman R. M. Intestinal lipoprotein metabolism. J Lipid Res. 1981 Nov;22(8):1153–1173. [PubMed] [Google Scholar]
- Herbert J., Wilcox J. N., Pham K. T., Fremeau R. T., Jr, Zeviani M., Dwork A., Soprano D. R., Makover A., Goodman D. S., Zimmerman E. A. Transthyretin: a choroid plexus-specific transport protein in human brain. The 1986 S. Weir Mitchell award. Neurology. 1986 Jul;36(7):900–911. doi: 10.1212/wnl.36.7.900. [DOI] [PubMed] [Google Scholar]
- Hussain M. M., Mahley R. W., Boyles J. K., Lindquist P. A., Brecht W. J., Innerarity T. L. Chylomicron metabolism. Chylomicron uptake by bone marrow in different animal species. J Biol Chem. 1989 Oct 25;264(30):17931–17938. [PubMed] [Google Scholar]
- Ide M., Mita S., Ikegawa S., Maeda S., Shimada K., Araki S. Identification of carriers of mutant prealbumin gene associated with familial amyloidotic polyneuropathy type I by Southern blot procedures: study of six pedigrees in the Arao district of Japan. Hum Genet. 1986 Aug;73(4):281–285. doi: 10.1007/BF00279086. [DOI] [PubMed] [Google Scholar]
- Kanda Y., Goodman D. S., Canfield R. E., Morgan F. J. The amino acid sequence of human plasma prealbumin. J Biol Chem. 1974 Nov 10;249(21):6796–6805. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen P. R. Thyroid-pituitary interaction: feedback regulation of thyrotropin secretion by thyroid hormones. N Engl J Med. 1982 Jan 7;306(1):23–32. doi: 10.1056/NEJM198201073060107. [DOI] [PubMed] [Google Scholar]
- Makover A., Soprano D. R., Wyatt M. L., Goodman D. S. An in situ-hybridization study of the localization of retinol-binding protein and transthyretin messenger RNAs during fetal development in the rat. Differentiation. 1989 Mar;40(1):17–25. doi: 10.1111/j.1432-0436.1989.tb00809.x. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Martone R. L., Herbert J., Dwork A., Schon E. A. Transthyretin is synthesized in the mammalian eye. Biochem Biophys Res Commun. 1988 Mar 15;151(2):905–912. doi: 10.1016/s0006-291x(88)80367-x. [DOI] [PubMed] [Google Scholar]
- Melhus H., Nilsson T., Peterson P. A., Rask L. Retinol-binding protein and transthyretin expressed in HeLa cells form a complex in the endoplasmic reticulum in both the absence and the presence of retinol. Exp Cell Res. 1991 Nov;197(1):119–124. doi: 10.1016/0014-4827(91)90488-g. [DOI] [PubMed] [Google Scholar]
- Murakami T., Yasuda Y., Mita S., Maeda S., Shimada K., Fujimoto T., Araki S. Prealbumin gene expression during mouse development studied by in situ hybridization. Cell Differ. 1987 Nov;22(1):1–9. doi: 10.1016/0045-6039(87)90408-8. [DOI] [PubMed] [Google Scholar]
- Navab M., Mallia A. K., Kanda Y., Goodman D. S. Rat plasma prealbumin. Isolation and partial characterization. J Biol Chem. 1977 Jul 25;252(14):5100–5106. [PubMed] [Google Scholar]
- Pages R. A., Robbins J., Edelhoch H. Binding of thyroxine and thyroxine analogs to human serum prealbumin. Biochemistry. 1973 Jul 3;12(14):2773–2779. doi: 10.1021/bi00738a034. [DOI] [PubMed] [Google Scholar]
- Robertson E. J. Using embryonic stem cells to introduce mutations into the mouse germ line. Biol Reprod. 1991 Feb;44(2):238–245. doi: 10.1095/biolreprod44.2.238. [DOI] [PubMed] [Google Scholar]
- Robertson E., Bradley A., Kuehn M., Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986 Oct 2;323(6087):445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- Saraiva M. J., Birken S., Costa P. P., Goodman D. S. Amyloid fibril protein in familial amyloidotic polyneuropathy, Portuguese type. Definition of molecular abnormality in transthyretin (prealbumin). J Clin Invest. 1984 Jul;74(1):104–119. doi: 10.1172/JCI111390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Maeda S., Murakami T., Nishiguchi S., Tashiro F., Yi S., Wakasugi S., Takahashi K., Yamamura K. Transgenic mouse model of familial amyloidotic polyneuropathy. Mol Biol Med. 1989 Aug;6(4):333–343. [PubMed] [Google Scholar]
- Soprano D. R., Herbert J., Soprano K. J., Schon E. A., Goodman D. S. Demonstration of transthyretin mRNA in the brain and other extrahepatic tissues in the rat. J Biol Chem. 1985 Sep 25;260(21):11793–11798. [PubMed] [Google Scholar]
- Soprano D. R., Soprano K. J., Goodman D. S. Retinol-binding protein and transthyretin mRNA levels in visceral yolk sac and liver during fetal development in the rat. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7330–7334. doi: 10.1073/pnas.83.19.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelin J., Melhus H., Das S., Eriksson U., Lind P., Trägårdh L., Peterson P. A., Rask L. The primary structure of rabbit and rat prealbumin and a comparison with the tertiary structure of human prealbumin. J Biol Chem. 1985 May 25;260(10):6481–6487. [PubMed] [Google Scholar]
- Tawara S., Nakazato M., Kangawa K., Matsuo H., Araki S. Identification of amyloid prealbumin variant in familial amyloidotic polyneuropathy (Japanese type). Biochem Biophys Res Commun. 1983 Nov 15;116(3):880–888. doi: 10.1016/s0006-291x(83)80224-1. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Vranckx R., Savu L., Maya M., Nunez E. A. Characterization of a major development-regulated serum thyroxine-binding globulin in the euthyroid mouse. Biochem J. 1990 Oct 15;271(2):373–379. doi: 10.1042/bj2710373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasugi S., Maeda S., Shimada K., Nakashima H., Migita S. Structural comparisons between mouse and human prealbumin. J Biochem. 1985 Dec;98(6):1707–1714. doi: 10.1093/oxfordjournals.jbchem.a135442. [DOI] [PubMed] [Google Scholar]
- Wakasugi S., Maeda S., Shimada K. Structure and expression of the mouse prealbumin gene. J Biochem. 1986 Jul;100(1):49–58. doi: 10.1093/oxfordjournals.jbchem.a121705. [DOI] [PubMed] [Google Scholar]
- Wolf G. Multiple functions of vitamin A. Physiol Rev. 1984 Jul;64(3):873–937. doi: 10.1152/physrev.1984.64.3.873. [DOI] [PubMed] [Google Scholar]