Abstract
Schizophrenia is characterized by major sleep/wake disturbances including increased vigilance and arousal, decreased slow wave sleep, and increased REM sleep drive. Other arousal-related symptoms include sensory gating deficits as exemplified by decreased habituation of the blink reflex. There is also dysregulation of gamma band activity, suggestive of disturbances in a host of arousal-related mechanisms. This review examines the role of the reticular activating system, especially the pedunculopontine nucleus, in the symptoms of the disease. Recent discoveries on the physiology of the pedunculopontine nucleus help explain many of these disorders of arousal in, and point to novel therapeutic avenues for, schizophrenia.
Abbreviations: CaMKII, calcium/calmodulin-dependent protein kinase; cAMP, cyclic adenosine monophosphate; EEG, electroencephalogram; EPSC, excitatory postsynaptic potential; GABA, γ aminobutyric acid; InsP, inositol 1,4,5-triphosphate receptor protein; KA, kainic acid; NCS-1, neuronal calcium sensor protein 1; NMDA, n methyl d aspartic acid; ω-Aga, ω-agatoxin-IVA; ω-CgTx, ω-conotoxin-GVIA; Pf, parafascicular nucleus; PGO, ponto-geniculo-occipital; PPN, pedunculopontine nucleus; RAS, reticular activating system; REM, rapid eye movement; SubCD, subcoeruleus dorsalis; SWS, slow wave sleep
Keywords: Calcium channels, Gamma band activity, Neuronal calcium sensor protein, P50 potential
1. Sleep wake dysregulation in schizophrenia
Schizophrenia is a heterogeneous disorder marked by psychotic symptoms such as delusions and hallucinations, as well as attentional impairment, emotional withdrawal, apathy, and cognitive impairment [1]. The symptoms of schizophrenia are typically separated into two categories, positive symptoms and negative symptoms. Positive symptoms include hallucinations, delusions, thought disorder, and agitation, while negative symptoms include lack of affect, anhedonia, and withdrawal. In addition, there are cognitive symptoms. Cognitive symptoms include poor executive function, lack of attention, and disturbed working memory. In addition, abnormal movements have been described. Equally varied causes have been advanced to explain the disease, including cortical atrophy, catecholaminergic abnormalities, and early brain injury [1].
However, schizophrenia is characterized by severe abnormalities in sleep/wake control, including hypervigilance, decreased slow wave sleep (SWS) especially deep sleep stages, increased rapid eye movement (REM) sleep drive, and fragmented sleep [2–6]. These abnormalities basically reflect the presence of increased vigilance and increased REM sleep drive, that is, they reflect an overactive reticular activating system (RAS) output. For this reason, it is important to assess the role in schizophrenia of one of the RAS centers that modulates arousal and REM sleep, the peedunculopontine nucleus (PPN). The increased REM sleep drive in schizophrenia has been proposed to represent REM sleep intrusion during waking, that is, in eliciting hallucinations [7,8]. The sleep/wake disturbances in schizophrenia are correlated better with negative rather than positive symptoms [9]. Other studies emphasized the relationship between negative symptoms in schizophrenia and increased cholinergic output [10,11], including increased output from the cholinergic arm of the RAS, the PPN [12].
There are also postural and motor abnormalities [13,14], as well as eye movement dysregulation [15,16] in schizophrenia. These findings are in keeping with the modulation of motor control by the RAS [17,18]. In addition, these patients suffer from sensory gating deficits determined using habituation of the blink reflex [19]. One of the first studies using the midlatency auditory evoked P50 potential in clinical conditions showed that habituation of the P50 potential using a paired stimulus paradigm was decreased in schizophrenia [20]. The P50 potential is a midlatency auditory evoked response present during waking and REM sleep but absent during slow wave sleep, that is, it is related to arousal states [21]. The P50 potential, as established in human and animal studies, is generated by the PPN and is manifested at the vertex [21,22]. These sensory gating deficits demonstrate a lack of inhibition of responses to repetitive stimuli, which have been found in patients with schizophrenia and in some clinically unaffected first-degree relatives [23].
Gamma oscillations appear to participate in sensory perception, problem solving, and memory [24–29], and coherence at these frequencies may occur at cortical or thalamocortical levels [30,31]. Indeed, synchronous gamma band activation among thalamocortical networks [32], is thought to contribute to the merger, or “binding”, of information originating from separate regions that leads to perception [33]. Conversely, gamma oscillation deficits have been suggested as a pathophysiologic feature of diseases like schizophrenia [34–37]. In addition, aberrant gamma band activity and coherence during cognitive tasks or attentional load have been reported in schizophrenic patients reviewed in Ref. [38]. These results suggest that the generation and maintenance of gamma band activity may be abnormal in schizophrenia. In addition, schizophrenic patients suffer from hypofrontality, or low frontal lobe blood flow [39] which may contribute to the sensory gating deficits, but also to the lack of critical judgment.
The results of electroencephalographic (EEG), reflex, and P50 potential testing all point to increased arousal and increased REM sleep drive. That is, the PPN, as the cholinergic arm of the RAS, is overactive in schizophrenia, but it is overactive in a specific manner. Responses to repetitive stimuli are increased and reflexes are exaggerated suggesting that phasic responses to brief stimuli are dysregulated. For example, exaggerated fight-or-flight responses in response to sudden stimuli are present in schizophrenia with devastating consequences [40]. However, the decreased and interrupted gamma band activity also suggests that gamma oscillations are not properly maintained on a tonic basis. This may have the effect of disturbing processes that depend on continuous gamma oscillations such as sensory perception, problem solving, and memory. This combination of short-term hyperexcitability and long-term diminution of RAS activity is functionally devastating. This is in agreement with findings described above showing that anticholinergic agents appear to alleviate some of the negative symptoms of schizophrenia [10,11], although further work is needed in this area.
2. Role of the reticular activating system (RAS)
The two most important advances on the physiology of the RAS in the last 10 years were, (a) the discovery of electrical coupling in some cells of certain RAS nuclei [41], and (b) the finding that every cell in the same nuclei manifests intrinsic membrane gamma oscillations [17]. The first advance helps explain how these brain centers maintain the coherence necessary to maintain neuronal membrane oscillations at both low and high frequencies. The second advance helps explain how these nuclei induce and maintain gamma band activity necessary for the process of remaining awake and maintaining REM sleep. We will briefly touch on the former, and discuss the latter discovery at length.
Two major elements determining the activity of large assemblies of neurons such as in the EEG are coherence and frequency. Coherence is the term for how groups of neurons, firing in coordination, can create a signal that is mirrored instantaneously and precisely by other groups of neurons across the brain. These transient episodes of coherence across different parts of the brain may be an electrical signature of thought and action. Our recent discovery demonstrated the presence of electrical coupling in three nuclei of the RAS, a mechanism that allows groups of neurons to fire synchronously [41–43]. Briefly, the stimulant modafinil is used for the treatment of narcolepsy. Modafinil increases electrical coupling and, since most coupled neurons in the RAS are GABAergic, the coupling decreases input resistance, decreasing activity in these cells and reducing GABA release, thus disinhibiting other cell types. This disinhibition leads to overall higher frequency in activity, i.e. during sleep and arousal, in the RAS [41,42,44], and thalamocortical systems [45]. In other words, because increased coupling in GABAergic neurons will lead to decreased GABA release, the tendency will be to increase coherence and also disinhibit other transmitter systems, leading to increased excitation, especially during waking. That is, since modafinil increases electrical coupling, it should enable better coherence at all frequencies, during waking and even after its effects are waning, during sleeping. That is why modafinil is also useful in regulating coherence during sleep. Conversely, the most fast-acting anesthetics known, inhaled halothane or injected propofol, both block gap junctions, and both put us to sleep very rapidly [41,42]. That is, the control of gap junctions can determine if we are asleep or awake.
We described the presence of dye and electrical coupling in the RAS, specifically in the intralaminar parafascicular nucleus (Pf), the PPN, and the subcoeruleus dorsalis (SubCD), which modulates REM sleep [42–44]. We also found that the stimulant modafinil decreased the resistance of PPN, Pf, and SubCD neurons [42], in keeping with results in the cortex, reticular thalamus, and inferior olive [45].
3. Pedunculopontine physiology and gamma frequency activity
Gamma oscillations emerge from the dynamic interaction between intrinsic
Neuronal and synaptic properties of thalamocortical networks [34]. Cortical gamma band generation can be influenced by subcortical structures like the hippocampus and cerebellum [46,47]. The neuronal networks behind such activity include inhibitory cortical interneurons with intrinsic membrane potential oscillatory activity in the gamma band range [30,48,49], many of which are electrically coupled [50], as well as rhythmically bursting pyramidal neurons (also electrically coupled) [51]. At the thalamic level, thalamocortical excitatory neurons have intrinsic properties needed to generate subthreshold gamma band membrane potential oscillations [52]. Cortical interneurons can generate membrane potential gamma oscillations through the activation of voltage-dependent, persistent sodium channel-dependent subthreshold oscillations [51], and metabotropic glutamate receptors [53]. In thalamocortical neurons, the mechanism responsible for gamma band activity involves high threshold P/Q-type voltage-gated calcium channels located in the dendrites [52].
In addition to the cortex and thalamus, the hippocampus, cerebellum, and basal ganglia have all been described as manifesting gamma band activity [54–59]. It was reported that gamma band activity in the motor cortex lags behind coherent activity in subcortical structures [60,61]. This led to the suggestion that motor cortex gamma synchronization reflects a momentary arousal-related event for enabling the initiation of movement [62–64]. That is, structures such as the RAS and thalamus may play an early permissive role in the control of movement.
The PPN is most active during waking and REM sleep [65], and modulates ascending projections through the thalamus (modulating arousal), and descending projections through the pons and medulla (modulating REM sleep and posture and locomotion). The PPN is made up of non-overlapping populations of cholinergic, glutamatergic, and GABAergic neurons [66]. The PPN contains three cell types based on in vitro intrinsic membrane properties [67–69]. Recordings of PPN neurons in vivo identified multiple types of thalamic-projecting PPN cells distinguished by their firing properties relative to ponto-geniculo-occipital (PGO) wave generation [70]. Some neurons exhibited low spontaneous firing frequencies (<10 Hz), but most showed high rates of tonic firing in the beta/gamma range (20–80 Hz). In other in vivo studies, PPN neurons increased firing during REM sleep and were labeled “REM-on” cells, or during both waking and REM sleep and were called “Wake/REM-on” cells, and also during waking only and were called “Wake-on” cells [71–73]. Stimulation of the PPN will potentiate the manifestation of fast (20–40 Hz) oscillations in the cortical EEG, outlasting stimulation by 10–20 s [74]. These results suggest that PPN cells do fire at gamma band frequencies in vivo, and that its outputs can indirectly induce gamma band activity in its targets.
We were the first to report that all PPN cells fired maximally at gamma band frequency when depolarized using current steps [75]. This is the only property shared by every cell in the PPN, regardless of transmitter type or electrophysiological type. Further results demonstrated that both voltage-dependent N- and P/Q-type calcium channels mediate the depolarizing phase of gamma band oscillations in the PPN. Voltage clamp results suggested that calcium channels are located distally to the cell body, probably in PPN dendritic compartments [76], as has been determined in thalamic neurons [52]. We then confirmed using fast imaging techniques that PPN calcium channel-mediated oscillations are due to P/Q- and N-type channels, and revealed the fact that these channels are distributed along the dendrites of PPN cells [77].
It has been suggested that consciousness is associated with “continuous” gamma band activity rather than an interrupted pattern of activity [78,79]. The original description of the RAS specifically suggested that it participates in “tonic or continuous” arousal, and that lesions of the RAS eliminated “tonic” arousal [80,81]. RAS structures like the PPN, in which every cell manifests gamma band activity, and in which a subgroup of cells are electrically coupled, then becomes a “gamma-making machine”. We hypothesized that it is the activation of the RAS during waking and REM sleep that induces coherent activity (through electrically coupled cells) and high frequency oscillations (through P/Q-type calcium channel and subthreshold oscillations). This leads to the generation of the background of gamma activity necessary to support a state capable of reliably assessing the world around us on a continuous basis. That is, these mechanisms may underlie the process of preconscious awareness [82,83].
4. Two states, two pathways
Injections of glutamate into the PPN of the rat were found to increase both waking and REM sleep, but injections of NMDA increased only waking, while injections of kainic acid (KA) increased only REM sleep [84–87]. Thus, the two states of waking and REM sleep appear to be independently activated by NMDA vs KA receptors. Moreover, the intracellular pathways mediating the two states are different. For example, the CaMKII activation inhibitor, KN-93, microinjected into the PPN of freely moving rats (in vivo) resulted in decreased waking but not REM sleep [88]. We showed that beta/gamma band oscillations in PPN neurons recorded in vitro were blocked by superfusion of KN-93 [89], suggesting that some cells manifest their oscillations via the CaMKII pathway. Moreover, the effects of the stimulant modafinil, which are mediated by increased electrical coupling, are modulated by the CaMKII pathway since KN-93 inhibits the action of modafinil [41,42,45,90]. These findings suggest that waking in vivo may be modulated by the CaMKII pathway, while REM sleep may be modulated by the cAMP pathway in the PPN [18,83,90]. In addition, it appears that the cAMP-dependent pathway phosphorylates N-type calcium channels [90], while CaMKII regulates P/Q-type calcium channels [91]. Therefore, the presence of P/Q-type calcium channels is related to CaMKII and waking, while the presence of N-type calcium channels is more related to cAMP and REM sleep [89].
We have preliminary findings showing that in some PPN cells (50%), the N-type calcium channel blocker ω-conotoxin-GVIA (ω-CgTx) reduced gamma oscillation amplitude, while subsequent addition of the P/Q-type blocker ω-agatoxin-IVA (ω-Aga) blocked the remaining oscillations. Other PPN cells (20%) manifested gamma oscillations that were not significantly affected by the addition of ω-CgTx, however, ω-Aga blocked the remaining oscillations. In the rest of the cells (30%), ω-Aga had no effect on gamma oscillations, while ω-CgTx blocked them. Similar results were found during recordings of voltage-dependent calcium currents. These results confirm the presence of cells in the PPN that manifest gamma band oscillations through only N-type, only P/Q-type, and both N- and P/Q-type calcium channels. This new cell type classification suggests that some PPN neurons fire only during REM sleep (“REM-on”, N-type only), only during waking (“Wake-on”, P/Q-type only), or during both waking and REM sleep (“Wake/REM-on”, N-type+P/Q-type) [92].
These results point to the presence of an intracellular “waking” pathway and a separate intracellular “REM sleep” pathway, each modulated by different calcium channel types. Armed with this information, we can now attempt to selectively modulate waking by affecting P/Q-type calcium channels and/or the CaMKII pathway, or REM sleep by affecting the N-type calcium channels and/or the cAMP pathway.
5. Neuronal calcium sensor protein 1 (NCS-1) in schizophrenia and bipolar disorder
Human postmortem studies reported increased expression of neuronal calcium sensor protein (NCS-1) in the brains of some bipolar disorder and schizophrenic patients compared to normal controls and major depression patients [93,94]. The distribution of levels of NCS-1 suggest that some patients have a 50% increase in expression, while others fall within the normal range. That is, gamma band activity is reduced or disrupted [95–97] in precisely the same disorders that show brain NCS-1 over expression. However, some studies suggest that other patients show increased gamma band activity [98]. We tested the hypothesis that NCS-1 modulates calcium channels in PPN neurons that generate gamma band oscillations, and that excessive levels of NCS-1, as would be expected with over expression, reduce or block gamma band oscillations in these cells [99].
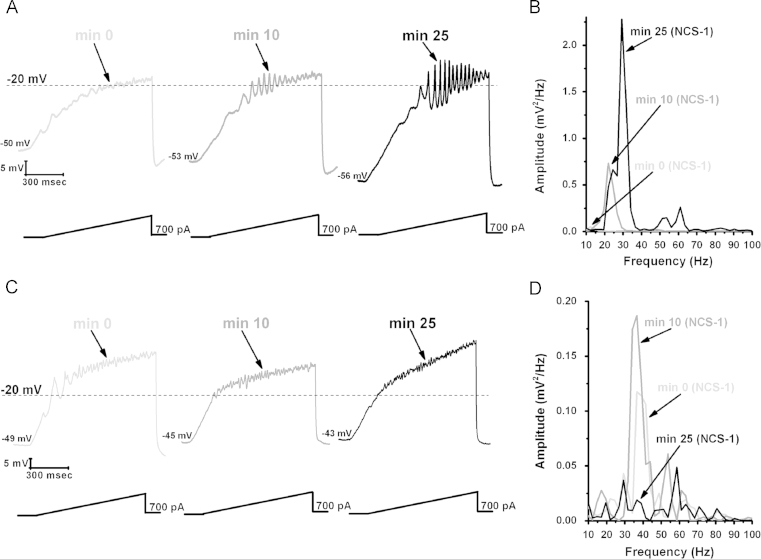
Recordings in PPN neurons using 1 μM NCS-1 were found to increase the amplitude and frequency of ramp-induced oscillations within ~25 min of diffusion into the cell. Fig. 1 is a representative example of ramp-induced membrane potential oscillations in a PPN neuron in the presence of synaptic blockers and tetrodotoxin. Shortly after patching, the ramp typically induced low amplitude oscillations in the beta/gamma range. After 10 min of recording, some increase in the oscillation amplitude and frequency was present. After 25 min of recording, NCS-1 at 1 μM significantly increased the amplitude and frequency of oscillations. Control cells recorded without NCS-1 in the pipette manifested no significant changes in amplitude or frequency throughout the 30 min recording period. These values were not significantly different from each of the 0 min recordings using pipettes with NCS-1, that is, before NCS-1 induced significant effects, so that the 0 min recordings are an accurate representation of control levels.
Fig. 1.
Effects of NCS-1 on gamma oscillations in PPN neurons. (A) Representative 1 s long current ramp-induced oscillations in a PPN neuron in fast synaptic blockers and tetrodotoxin in the extracellular solution and 1 μM NCS-1 in the recording pipette (left record, light gray). After 10 min of NCS-1 diffusing into the cell, the oscillatory activity increased slightly (middle record, dark gray). However, after 25 min of NCS-1 diffusion both oscillation amplitude and frequency were increased (right record, black). (B) Power spectrum of the records shown in (A) showing the increased amplitude and frequency of oscillations after 25 min exposure to 1 μM NCS-1. (C) Representative ramp-induced oscillations recorded during 1 s long current ramps in the presence of fast synaptic blockers and tetrodotoxin and NCS-1 at 10 μM in the recording pipette (left record, light gray). After 10 min of NCS-1 diffusing into the cell, the oscillation amplitude increased slightly (middle record, dark gray). However, testing at 25 min showed a decrease in amplitude compared to both 0 min and 10 min recordings (right record, black). (D) Power spectrum of the records shown in (C) demonstrating the slight increase in amplitude at 10 min, and the subsequent decrease at 25 min.
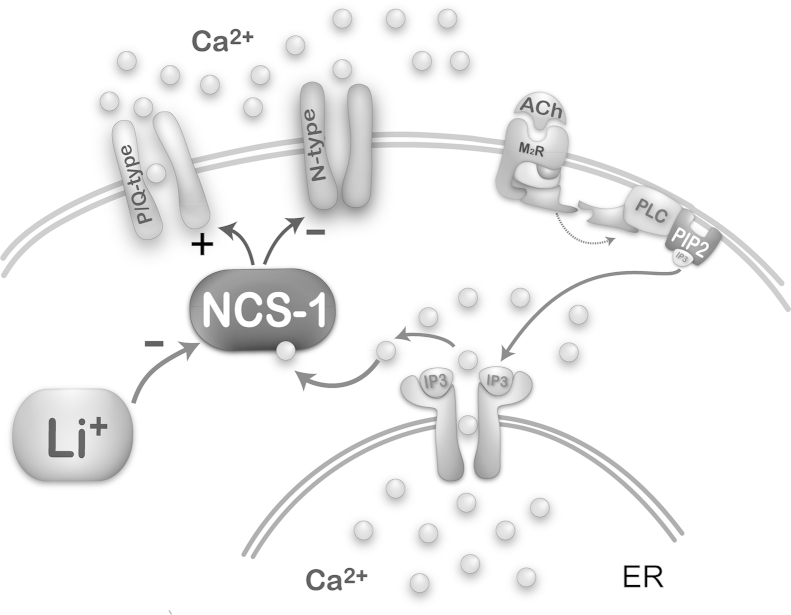
We then carried out a study to determine the effects of NCS-1 concentration on PPN cell ramp-induced oscillations. When using 10 μM NCS-1, the oscillation amplitude immediately increased to four times the levels and but then gradually decreased until it was significantly reduced by 30 min. These effects suggest an immediate increase in amplitude by very high levels of NCS-1 that ultimately led to blockade. Based on these results, 1 μM NCS-1 seems to be the most critical concentration for promoting gamma oscillations, while 10 μM blocked oscillations, in keeping with the effects of NCS-1 over expression [89,99]. Fig. 2 is a diagram of the intracellular pathways at play, showing the NCS-1 may normally stimulate gamma oscillations through P/Q-type calcium channels.
Fig. 2.
Intracellular pathways mediating NCS-1 modulation of intracellular calcium and P/Q-type calcium channels. Representation of effects of acetylcholine (ACh) activation of a muscarinic 2 cholinergic receptor (M2R) acting through G protein coupling to phospholipase C (PLC), that in turn cleaves phospholipid phosphatidylinositol biphosphate (PIP2) into inositol triphosphate (IP3). IP3 is released and binds to IP3 receptors in the endoplasmic reticulum (ER) to release calcium (Ca2+). One of the intracellular pathways activated involves NCS-1, which stimulates (+) P/Q-type calcium channels and somewhat inhibits (−) N-type calcium channels. NCS-1 at low concentrations increases gamma oscillations while NCS-1 at high concentrations blocks them. In addition, NCS-1 over expression is inhibited (−) by lithium (Li+), removing the blockade of gamma oscillations and restoring the maintenance of gamma band activity in these cells.
The postmortem results previously described [79] suggest that only some patients with schizophrenia may suffer from significant over expression of NCS-1, which may be manifested as decreased gamma band activity only in a subpopulation of patients. No human study has measured gamma band activity and correlated it with NCS-1 levels. Unfortunately, serum sampling does not reflect brain levels and, in fact, NCS-1 levels in leukocytes are actually decreased in schizophrenic patients [100]. However, future clinical trials in drug naïve patients with schizophrenia or bipolar disorder may benefit from determination of a significant decrease in gamma band activity prior to pharmacotherapy, which may also help address the heterogeneity of schizophrenia and facilitate the process of identifying more homogeneous groups within the syndrome [101]. It is to those patients that pharmacological targeting to increase gamma band activity may be of benefit. We have preliminary evidence suggesting that the stimulant modafinil may indeed compensate to some extent for excessive amounts of NCS-1. We found a partial return of gamma oscillations after exposure to modafinil that had been suppressed by high levels of NCS-1 [89].
6. One mechanism behind lithium’s action
Serendipitously, the mood disturbances in bipolar disorder (but not schizophrenia) were treated effectively using lithium, an ion that remains one of the best treatment options, although it is limited by side effects [102]. Lithium has also been proposed as a neuroprotective agent. Lithium was proposed to act by inhibiting the interaction between NCS-1 and inositol 1,4,5-triphosphate receptor protein (InsP) [103], and, as we saw above, NCS-1 is over expressed in bipolar disorder and schizophrenia [93,94]. NCS-1 is known to enhance the activity of InsP [104], which is present in the PPN [105]. Our preliminary studies show that lithium at low concentration (1 μM) reduces the effect of NCS-1 on gamma band oscillations, while high levels of lithium have no effect [106]. A diagram of these intracellular pathways appears in Fig. 2. That is, lithium may reduce the effects of over expressed NCS-1 in bipolar disorder, thereby normalizing gamma band oscillations mediated by P/Q-type calcium channels modulated by NCS-1. Therefore, the effects of over expression of NCS-1 in bipolar disorder may be decreased by lithium. We found that excessive NCS-1 decreased gamma oscillations, therefore, lithium may prevent the down regulation of gamma band activity and restore normal levels of gamma band oscillations. These findings taken together resolve the 60-year mystery of the physiology of lithium action in bipolar disorder. An interesting observation is that NCS-1 down regulates N-type calcium channels, at least in some cell lines [107]. This may mean that under some circumstances NCS-1 may inhibit N-type channel function, while promoting P/Q-type channel function. While lithium addresses the mood swings in bipolar disorder, it fails to address the psychotic aspects of schizophrenia. Therefore, a different therapeutic strategy is required for schizophrenia.
7. Dopamine
The dopamine (DA) theory of schizophrenia is a model that draws evidence from the findings that antipsychotics have DA blocking actions. Given the well-known role of DA in schizophrenia, what is the relationship between the substantia nigra and the PPN? Anatomically, the PPN is reciprocally connected to the substantia nigra, with the nigral input being inhibitory to PPN, mostly glutamatergic, neurons, and the PPN input to nigra being excitatory and emanating from both cholinergic and glutamatergic PPN neurons [108–110]. The excitatory input to the nigra from the PPN may be responsible for the activity manifested by nigral neurons, which are active during waking and REM sleep [111,112]. That is, it is likely that normal PPN activity during arousal states helps drive firing in the substantia nigra. However, the hypervigilance and increased REM sleep drive present in schizophrenia suggest that the PPN may overdrive the substantia nigra, thereby potentiating DA release in the striatum. Therefore, it would seem that down regulation of PPN output to the nigra may help alleviate some of the hyperarousal symptoms of the disease.
The first generation antipsychotics included chlorpromazine and haloperidol, which showed DA D2 receptor blockade [113]. Due to limited clinical benefits and long-term side effects such as tardive dyskinesia, new agents had to be developed. A second generation antipsychotic named clozapine was found to act on the same DA receptors in the striatum to help decrease DA tone, but it was also found to affect muscarinic cholinergic and serotonergic receptors. In fact, clozapine was initially developed as an anti-muscarinic cholinergic agent intended to balance the decrease in DA present in Parkinson’s disease, i.e. by decreasing cholinergic tone the idea was to rebalance the striatum [114]. Clozapine thus appears to partially block muscarinic input to the nigra as well as DA input to the striatum. In addition, clozapine acts as a partial serotonin reuptake receptor blocker, thereby increasing inhibition of the PPN, further down regulating the raphe-PPN-nigra-striatum pathway. Despite the better beneficial effects than early antipsychotics, clozapine also shows major side effects, and fails to induce ameliorative effects in many patients. Drug companies have attempted to eliminate the side effects of clozapine, but only olanzapine has retained anti-muscarinic properties, and is, in fact, the most widely used third generation antipsychotic. This suggests that down regulating the muscarinic activation of the nigra by the PPN helps relieve the symptoms of the disease in at least some patients.
8. Conclusion
The PPN simultaneously modulates cortical arousal as well as posture and locomotion. Moreover, in response to sensory inflow, the PPN generates and maintains gamma band activity during waking. These membrane oscillations are mediated by voltage-dependent high threshold N- and P/Q-type calcium channels. It appears that these two types of channels with separate intracellular pathways are involved in selectively controlling high frequency activity. P/Q-type channels are modulated by CaMKII during waking, while N-type channels are modulated by cAMP during REM sleep [89]. In addition to intrinsic membrane oscillations, the maintenance of gamma band activity requires synaptic connectivity within the nucleus and between regions of the brain. PPN circuitry includes cholinergic, glutamatergic, and GABAergic neurons. Some GABAergic cells are electrically coupled to provide coherence [17], and the nucleus may include functional cell clusters [89].
From the moment we awaken, the nucleus ensures that the necessary background of activity is present in order to preconsciously evaluate the world around us [17,18,82,83,89]. Therefore, this process is embedded in the formulation of our perceptions and actions, and modulates higher-level gamma processing through its projections to the intralaminar thalamus, basal ganglia, hypothalamus, and basal forebrain. That is why it affects functions as disparate as waking and REM sleep, mood and perception, and homeostatic regulation. Consequently, dysregulation in PPN processing will be manifested in motor disorders, psychiatric disorders, neurological disease, all of which include sleep disturbances. These issues are discussed at length in a recent book [114].
The implications of PPN dysregulation for schizophrenia suggest that the sleep wake dysfunction in the disease arises from the nucleus. In some patients, over expression of NCS-1 may down regulate high frequency oscillations, especially those mediated by P/Q-type calcium channels. This would lead to interrupted or decreased gamma band activity during waking, disturbing the maintenance of the state of waking and preconscious awareness. It is not clear whether NCS-1 over expression in some way releases N-type channel activity to increase REM sleep drive, but it is worth investigating. In summary, these recent discoveries provide novel therapeutic targets for alleviating some of the arousal and sleep wake disturbances in this devastating disease.
Acknowledgments
This work was supported by NIH award R01 NS020246, and by core facilities of the Center for Translational Neuroscience supported by NIH award P20 GM103425 and P30 GM110702 to Dr. Garcia-Rill. In addition, this work was supported by grants from FONCYT-Agencia Nacional de Promoción Científica y Tecnológica; BID 1728 OC.AR. PICT-2012-1769 and UBACYT 2014-2017 #20120130101305BA (to Dr. Urbano) and FONCYT-Agencia Nacional de Promoción Científica y Tecnológica; BID 1728 OC.AR. T 2012-0924 Argentina (to Dr. Bisagno).
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
References
- 1.Andreasen N.C., Flaum M. Schizophrenia: the characteristic symptoms. Schizophr Bull. 1991;17:27–49. doi: 10.1093/schbul/17.1.27. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell D.F., Domino E.F. Electroencephalographic and eye movement patterns during sleep in chronic schizophrenic patients. Electroencephalogr Clin Neurophysiol. 1997;22:414–420. doi: 10.1016/0013-4694(67)90168-x. [DOI] [PubMed] [Google Scholar]
- 3.Feinberg I., Braun M., Koresko R.L., Gottleib F., Stage F. 4 sleep in schizophrenia. Arch Gen Psychiatry. 1969;21:262–266. doi: 10.1001/archpsyc.1969.01740210006002. [DOI] [PubMed] [Google Scholar]
- 4.Itil T.M., Hsu W., Klingenberg W., Saletu B., Gannon P. Digital computer-analyzed all-night sleep EEG patterns (sleep prints) in schizophrenics. Biol Psychiatry. 1972;4:3–16. [PubMed] [Google Scholar]
- 5.Jus K., Bouchard M., Jus A., Villeneuve A., Lachance R., Sleep R. EEG studies in untreated long-term schizophrenic patients. Arch Gen Psychiatry. 1973;29:286–290. doi: 10.1001/archpsyc.1973.04200030074011. [DOI] [PubMed] [Google Scholar]
- 6.Zarcone V., Azumi K., Dement W.C., Gulevich G., Kraimer H., Pivik R. REM phase deprivation and schizophrenia II. Arch Gen Psychiatry. 1975;32:1431–1436. doi: 10.1001/archpsyc.1975.01760290099012. [DOI] [PubMed] [Google Scholar]
- 7.Dement W.C. Studies on the effects of REM deprovation in humans and animals. Res Publ Assoc Res Nerv Ment Dis. 1967;43:456–467. [PubMed] [Google Scholar]
- 8.Mamelak A.N., Hobson J.A. Dream bizarreness as the cognitive correlate of altered neuronal brain in REM sleep. J Cogn Neurosci. 1989;1 doi: 10.1162/jocn.1989.1.3.201. 2-1-222. [DOI] [PubMed] [Google Scholar]
- 9.Ganguli R., Reynolds D.F., Kupfer D.F. Electroencephalographic sleep in young, never-medicated schizophrenics. Arch Gen Psychiatry. 1987;44:36–44. doi: 10.1001/archpsyc.1987.01800130038006. [DOI] [PubMed] [Google Scholar]
- 10.Janowsky D.S., Davis J.M., Huey L., Judd L.L. Adrenergic and cholinergic drugs as episode and vulnerability markers of affective disorders and schizophrenia. Psychopharmacol Bull. 1979;15:33–34. [PubMed] [Google Scholar]
- 11.Tandon R., Greden J.F., Haskett R.F. Cholinergic hyperactivity and negative symptoms: behavioral effects of physostigmine in normal controls. Schizophr Res. 1993;9:19–23. doi: 10.1016/0920-9964(93)90004-3. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Rill E., Biedermann J.A., Chambers T., Skinner R.D., Mrak R.E., Husain M., Karson C.N. Mesopontine neurons in schizophrenia. Neuroscience. 1995;66:321–335. doi: 10.1016/0306-4522(94)00564-l. [DOI] [PubMed] [Google Scholar]
- 13.King L.J. A sensory-integrative approach to schizophrenia. Am. J. Occup. Ther. 1974;28:529–536. [PubMed] [Google Scholar]
- 14.Manschrek T.C. In: Motor abnormalities in schizophrenia. Nasrallah H.A., Weinberger D.R., editors. Elsevier; Amsterdam: 1986. pp. 65–96. [Google Scholar]
- 15.Holzman P.S., Proctor L.R., Hughes D.W. Eye tracking patterns in schizophrenia. Science. 1973;181:179–181. doi: 10.1126/science.181.4095.179. [DOI] [PubMed] [Google Scholar]
- 16.Karson C.N., Dykman R.A., Paige S.R. Blink rates in schizophrenia. Schizophr Bull. 1990;16:345–354. doi: 10.1093/schbul/16.2.345. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Rill E., Kezunovic N., Hyde J., Beck P., Urbano F.J. Coherence and frequency in the reticular activating system (RAS) Sleep Med Rev. 2013;17:227–238. doi: 10.1016/j.smrv.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urbano F.J., D’Onofrio S.M., Luster B.R., Hyde J.R., Bisagno V., Garcia-Rill E. Pedunculopontine nucleus gamma band activity-preconscious awareness, waking, and REM sleep. Front Sleep Chronobiol. 2014;5:210. doi: 10.3389/fneur.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geyer M.A., Braff D.U. Habituation of the blink reflex in normals and schizophrenic patients. Psychophysiology. 1982;19:1–6. doi: 10.1111/j.1469-8986.1982.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 20.Freedman R., Adler L.E., Waldo M.C., Pachtman E., Franks R.D. Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol Psychiatry. 1983;18:537–551. [PubMed] [Google Scholar]
- 21.Garcia-Rill E., Skinner R.D. The sleep state-dependent P50 midlatency auditory evoked potential. In: Lee-Chiong T.L., Carskadon M.A., Sateia M.J., editors. Vol. 2001. Hanley & Belfus; Philadelphia: 2001. pp. 697–704. (Sleep medicine). [Google Scholar]
- 22.Garcia-Rill E., Skinner R.D., Clothier J., Dornhoffer J., Uc E., Fann A., Mamiya N. The sleep state-dependent midlatency auditory evoked P50 potential in various disorders. Thal Relat Syst. 2002;2:9–19. [Google Scholar]
- 23.Olincy A., Braff D.L., Adler L.E., Cadenhead K.S., Calkins M.E., Dobie D.J., Green M.F., Greenwood T.A., Gur R.E., Gur R.C., Light G.A., Mintz J., Nuechterlein K.H., Radant A.D., Schork N.J., Seidman L.J., Siever L.J., Silverman J.M., Stone W.S., Swerdlow N.R., Tsuang D.W., Tsuang M.T., Turetsky B.i., wagner B.D., Freedman R. Inhibition of the P50 cerebral evoked response to repeated auditory stimuli: results from the consortium on genetics of schizophrenia. Schizophr Res. 2010;119:175–182. doi: 10.1016/j.schres.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckhorn R., Bauer R., Jordan W., Brosch M., Kruse W., Munk M., Reitboeck H.J. Coherent oscillations: a mechanism of feature linking in the visual system? Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- 25.Gray C.M., Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones E.G. Calcium channels in higher-level brain function. Proc Natl Acad Sci USA. 2007;14:17903–17904. doi: 10.1073/pnas.0709509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philips S., Takeda Y. Greater frontal–parietal synchrony at low gamma-band frequencies for inefficient then efficient visual search in human EEG. Int J Psychophysiol. 2009;73:350–354. doi: 10.1016/j.ijpsycho.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Palva S., Monto S., Palva J.M. Graph properties of synchronized cortical networks during visual working memory maintenance. NeuroImage. 2009;49:3257–3268. doi: 10.1016/j.neuroimage.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 29.Voss U., Holzmann R., Tuin I., Hobson J.A. Lucid dreaming: a state of consciousness with features of both waking and non-lucid dreaming. Sleep. 2009;32:1191–1200. doi: 10.1093/sleep/32.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llinas R.R., Grace A.A., Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Natl Acad Sci. 1991;88:897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- 32.Llinas R.R., Leznik E., Urbano F.J. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc Natl Acad Sci USA. 2002;99:449–454. doi: 10.1073/pnas.012604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llinás R.R., Paré D. Of dreaming and wakefulness. Neuroscience. 1991;44:521–535. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- 34.Steriade M., Llinás R.R. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- 35.Ribary U., Ioannides A.A., Singh D., Hasson R., Bolton J.P., Lado F., Mogilner A., Llinás R.R. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci USA. 1991;88:11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stam C.J., van Cappellen van Walsum A.M., Pijnenburg Y.A., Berendse H.W., de Munck J.C., Scheltens P., van Dijk B.W. Generalized synchronization of MEG recordings in Alzheimer’s disease: evidence for involvement of the gamma band. J Clin Neurophysiol. 2002;19:562–574. doi: 10.1097/00004691-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Woo T.U., Spencer K., McCarley R.W. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010;8:173–189. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uhlhaas P.J., Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 39.Kinou M., takizawa R., Marumo K., Kawasaki, Y S., Kawakubo M. Fukuda, Kasai K. Differential spatiotemporal characteristics of the prefrontal hemodynamic response and their association with functional impairment in schizophrenia and major depression. Schizophr Res. 2013;150:459–467. doi: 10.1016/j.schres.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 40.Seeman M.V., Seeman P. Is schizophrenia a dopamine supersensitivity psychotic reaction? Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:155–160. doi: 10.1016/j.pnpbp.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Rill E., Heister D.S., Ye M., Charlesworth A., Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30:1405–1414. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Rill E., Charlesworth A., Heister D.S., Ye M., Hayar A. The developmental decrease in REM sleep: the role of transmitters and electrical coupling. Sleep. 2008;31:673–690. doi: 10.1093/sleep/31.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heister D.S., Hayar A., Garcia-Rill E. Cholinergic modulation of GABAergic and glutamatergic transmission in the dorsal Subcoeruleus: mechanisms for REM sleep control. Sleep. 2009;32:1135–1147. doi: 10.1093/sleep/32.9.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heister D.S., Hayar A., Charlesworth A., Yates C., Zhou Y., Garcia-Rill E. Evidence for electrical coupling in the SubCoeruleus (SubC) nucleus. J Neurophysiol. 2007;97:3142–3147. doi: 10.1152/jn.01316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urbano F.J., Leznik E., Llinas R.R. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc Natl Acad Sci. 2007;104:12554–12559. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soteropoulos D.S., Baker S.N. Cortico-cerebellar coherence during a precision grip task in the monkey. J Neurophysiol. 2006;95:1194–1206. doi: 10.1152/jn.00935.2005. [DOI] [PubMed] [Google Scholar]
- 47.Sirota A., Montgomery S., Isomura S.Y., Buzsáki M.G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Llinas R.R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 49.Steriade M. Cellular substrates of oscillations in corticothalamic systems during states of vigilance. In: Lydic R., Baghdoyan H.A., editors. Handbook of behavioral state control. Cellular and molecular mechanisms. CRC Press; New York, NY: 1999. pp. 327–347. [Google Scholar]
- 50.Gibson J.R., Beierlein M., Connors B.W. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 51.Cunningham M.O., Whittington M.A., Bibbig A., Roopun A., LeBeau F.E., Vogt A., Monyer H., Buhl E.H., Traub R.D. A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proc Natl Acad Sci. 2004;101:7152–7157. doi: 10.1073/pnas.0402060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pedroarena C., Llinás R.R. Dendritic calcium conductances generate high-frequency oscillation in thalamocortical neurons. Proc Natl Acad Sci. 1997;94:724–728. doi: 10.1073/pnas.94.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whittington M.A., Traub R.D., Jefferys J.G. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 54.Bussey T.J., Muir J.L., Aggleton J.P. Functionally dissociating aspects of event memory: the effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J Neurosci. 1999;19:495–502. doi: 10.1523/JNEUROSCI.19-01-00495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Charpak S., Paré D., Llinás R.R. The entorhinal cortex entrains fast CA1 hippocampal oscillations in the anaesthetized guinea-pig: role of the monosynaptic component of the perforant path. Eur J Neurosci. 1995;7:1548–1557. doi: 10.1111/j.1460-9568.1995.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 56.Colgin L.L., Denninger T., Fyhn M., Hafting T., Bonnevie T., Jensen O., Moser M.B., Moser E.I. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 57.Lang E.J., Sugihara I., Llinás R.R. Olivocerebellar modulation of motor cortex ability to generate vibrissal movements in rat. J Physiol. 2006;571:101–120. doi: 10.1113/jphysiol.2005.102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Middleton S.J., Racca C., Cunningham M.O., Traub R.D., Monyer H., Knöpfel T., Schofield I.S., Jenkins A., Whittington M.A. High-frequency network oscillations in cerebellar cortex. Neuron. 2008;58:763–774. doi: 10.1016/j.neuron.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trottenberg T., Fogelson N., Kuhn A.A., Kivi A., Kupsch A., Schneider G.H., Brown P. Subthalamic gamma activity in patients with Parkinson’s disease. Exp Neurol. 2006;200:56–65. doi: 10.1016/j.expneurol.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Lalo E., Thobois S., Sharott A., Polo G., Mertens P., Pogosyan A. Patterns of bidirectional communication between cortex and basal ganglia during movement in patients with Parkinson disease. J Neurosci. 2008;28:3008–3016. doi: 10.1523/JNEUROSCI.5295-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Litvak V., Eusebio A., Jha A., Oosterveld R., Barnes G., Foltynie T. Movement-related changes in local and long-range synchronization in Parkinson’s disease revealed by simultaneous magnetoencephalography and intracranial recordings. J Neurosci. 2012;32:10541–10553. doi: 10.1523/JNEUROSCI.0767-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brucke C., Huebl J., Schonecker T., Wolf-Julian N., Yarrow K., Kupsch A., Blahak C., Lutjens G., Brown P., Krauss J., Schneider G.H., Kuhn A. Scaling of movement is related to pallidal g oscillations in patients with dystonia. J Neurosci. 2012;32:1008–1019. doi: 10.1523/JNEUROSCI.3860-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheyne G., Ferrari P. MEG studies of motor cortex gamma oscillations: evidence for a gamma fingerprint in the brain? Front Hum Neurosci. 2013;7:#575. doi: 10.3389/fnhum.2013.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jenkinson N., Kuhn A.A., Brown P. Gamma oscillations in the human basal ganglia. Exp Neurol. 2013;245:72–76. doi: 10.1016/j.expneurol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Steriade M., McCarley R.W. Plenum Press; New York, NY: 1990. Brainstem control of wakefulness and sleep. [Google Scholar]
- 66.Wang H.L., Morales M. Pedunculopontine and laterodorsal tegmental nuclei contan distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leonard C.S., Llinas R.R. Electrophysiology of mammalian pedunculopontine and laterodorsal tegmental neurons in vitro: implications for the contol of REM sleep. In: Steriade M., Biesold D., editors. Brain cholinergic systems. Oxford Science; Oxford: 1990. pp. 205–223. [Google Scholar]
- 68.Kamondi A., Williams J., Hutcheon B., Reiner P. Membrane properties of mesopontine cholinergic neurons studied with the whole-cell patch-clamp technique: implications for behavioral state control. J Neurophysiol. 1992;68:1359–1372. doi: 10.1152/jn.1992.68.4.1359. [DOI] [PubMed] [Google Scholar]
- 69.Takakusaki K., Kitai S.T. Ionic mechanisms involved in the spontaneous firing of tegmental pedunculopontine nucleus neurons of the rat. Neuroscience. 1997;78:771–794. doi: 10.1016/s0306-4522(96)00540-4. [DOI] [PubMed] [Google Scholar]
- 70.Steriade M., Paré D., Datta S., Oakson G., Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990;10:2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sakai K., El Mansari M., Jouvet M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 1990;527:213–223. doi: 10.1016/0006-8993(90)91140-c. [DOI] [PubMed] [Google Scholar]
- 72.Steriade M., Datta S., Paré D., Oakson G., Curro Dossi R. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Datta S., Siwek D.F. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:79–82. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- 74.Steriade M., Curro Dossi R., Paré D., Oakson DG. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Natl Acad Sci. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simon C., Kezunovic N., Ye M., Hyde J., Hayar A., Williams D.K., Garcia-Rill E. Gamma band unit and population responses in the pedunculopontine nucleus. J Neurophysiol. 2010;104:463–474. doi: 10.1152/jn.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kezunovic N., Urbano F.J., Simon C., Hyde J., Smith K., Garcia-Rill E. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN) Eur J Neurosci. 2011;34:404–415. doi: 10.1111/j.1460-9568.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hyde J., Kezunovic N., Urbano F.J., Garcia-Rill E. Spatiotemporal properties of high speed calcium oscillations in the pedunculopontine nucleus. J Appl Physiol. 2013;115:1402–1414. doi: 10.1152/japplphysiol.00762.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vanderwolf C.H. What is the significance of gamma wave activity in the pyriform cortex? Brain Res. 2000;877:125–133. doi: 10.1016/s0006-8993(00)02568-3. [DOI] [PubMed] [Google Scholar]
- 79.Vanderwolf C.H. Are neocortical gamma waves related to consciousness? Brain Res. 2000;855:217–224. doi: 10.1016/s0006-8993(99)02351-3. [DOI] [PubMed] [Google Scholar]
- 80.Moruzzi G., Magoun H.W. Brainstem reticular formation and activation. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 81.Watson R.T., Heilman K.M., Miller B.D. Neglect after mesencephalic reticular formation lesions. Neurology. 1974;24:294–298. doi: 10.1212/wnl.24.3.294. [DOI] [PubMed] [Google Scholar]
- 82.Garcia-Rill E., Kezunovic N., Hyde J., Beck P., Urbano F.J. Coherence and frequency in the reticular activating system (RAS) Sleep Med Rev. 2013;17:227–238. doi: 10.1016/j.smrv.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Urbano F.J., Kezunovic N., Hyde J., Simon C., Beck P., Garcia-Rill E. Gamma band activity in the reticular activating system (RAS) Front Neurol Sleep Chronobiol. 2012;3(6):1–16. doi: 10.3389/fneur.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J Neurophysiol. 2002;87:1790–1798. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- 85.Datta S., Siwek D.F. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induce wakefulness and REM sleep. J Neurophysiol. 1997;77:2975–2988. doi: 10.1152/jn.1997.77.6.2975. [DOI] [PubMed] [Google Scholar]
- 86.Datta S., Spoley E.E., Patterson E.H. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;280:R752–R759. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- 87.Datta S., Patterson E.H., Spoley E.E. Excitation of pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J Neurosci Res. 2001;66:109–116. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- 88.Datta S., O’Malley M.O., Patterson E.H. Calcium/calmodulin kinase II in the pedunculopontine tegmental nucleus modulates the initiation and maintenance of wakefulness. J Neurosci. 2011;23:17007–17016. doi: 10.1523/JNEUROSCI.3981-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garcia-Rill E., Kezunovic N., D’Onofrio S., Luster B., Hyde J., Bisagno V., Urbano F.J. Gamma band activity in the RAS-intracellular mechanisms. Exp Brain Res. 2014;232:1509–1522. doi: 10.1007/s00221-013-3794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hell J.W., Yokoyama C.T., Breeze L.J., Chavkin C., Catterall W.A. Phosphorylation of presynaptic and postsynaptic Ca2+ channels by cAMP-dependent protein kinase in hippocampal neurons. Eur Mol Biol Org J. 1995;13:3036–3044. doi: 10.1002/j.1460-2075.1995.tb07306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jenkins M.A., Christel C.J., Jiao X., Abiria S., Kim K.Y., Usachev Y.M., Obermair G.J., Colbran R.J., Lee A. Ca2+-dependent facilitation of Cav1.3 Ca2+ channels by densin and Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30:5125–5135. doi: 10.1523/JNEUROSCI.4367-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luster B., Hyde J., Donofrio S., Urbano F.J., Garcia-Rill E. Mechanisms behind gamma band activity in the pedunculopontine nucleus (PPN) Neurosci Abstr. 2014;38:257.20. [Google Scholar]
- 93.Bergson C., Levenson R., Goldman-Rakic P., Lidow M.S. Dopamine receptor-interacting proteins: the Ca2+ connection in dopamine signaling. Trends Pharmacol Sci. 2003;24:486–492. doi: 10.1016/S0165-6147(03)00232-3. [DOI] [PubMed] [Google Scholar]
- 94.Koh P.O., Undie A.S., Kabbani N., Levenson R., Goldman-Rakic P., Lidow M.S. Up-regulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients. Proc Natl Acad Sci. 2003;100:313–317. doi: 10.1073/pnas.232693499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leicht G., Andreou C., Polomac N., lanig C., Schottle D., lambert M., Mulert C. Reduced auditory evoked gamma band response and cognitive processing deficits in first episode schizophrenia. World J Biol Psychiatry. 2015;16:1–11. doi: 10.3109/15622975.2015.1017605. [DOI] [PubMed] [Google Scholar]
- 96.Senkowsky D., Gallinat J. Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biol Psychiatry Epub. 2015 doi: 10.1016/j.biopsych.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 97.Wynn J.K., Roach B.J., Lee J., Horan W.p., Ford J.M., Jimenez A.M., Green M.F. EEG findings of reduced neural synchronization during visual integration in schizophrenia. PLoS One. 2015;10:e0119849. doi: 10.1371/journal.pone.0119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hirano Y., Oribe N., kanba S., Onitsuka T., Nestor P.G., Spencer K.M. Spontaneous gamma activity in schizophrenia. JAMA Psychiatry Epub. 2015 doi: 10.1001/jamapsychiatry.2014.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.D’Onofrio S., Kezunovic N., Hyde J., Luster B., Messias E., Urbano F.J., Garcia-Rill E. Modulation of gamma oscillations in the pedunculopontine nucleus (PPN) by neuronal calcium sensor protein-1 (NCS-1): relevance to schizophrenia and bipolar disorder. J Neurophysiol. 2015;113:709–719. doi: 10.1152/jn.00828.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Torres K.C., Souza B.R., Miranda D.M., Sampiao A.M., Nicolato R., Neves F.S., Barros A.G., Dutra W.O., Gollob K.J., Correa H., Romano-Silva M.A. Expression of neuronal calcium sensor-1 (NCS-1) is decreased in leukocytes of schizophrenia and bipolar disorder patients. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:229–234. doi: 10.1016/j.pnpbp.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 101.Picardi A., Viroli C., Tarsitani L., Miglio R., de Girolamo G., Dell’Acqua G., Biondi M. Heterogeneity and symptom structure of schizophrenia. Psychiatry Res. 2012;198:386–394. doi: 10.1016/j.psychres.2011.12.051. [DOI] [PubMed] [Google Scholar]
- 102.Brown K.M., Tracy D.K. Lithium: the pharmacodynamics actions of the amazing ion. Ther Adv Psychopharmacol. 2012;3:163–176. doi: 10.1177/2045125312471963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schleker C., Boehmerie W., Jeromin A., DeGray B., Varshey A., Sharma Y., Szigeti-Buck K., Ehrlich B.E. Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium. J Clin Invest. 2006;116:1668–1674. doi: 10.1172/JCI22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kasri N.N., Holmes A.M., Bultynk G., Parys J.B., Bootman M.D., Rietdorf K., Missiaen L., McDonald F., Smedt H., Conway S.J., Holmes A.B., Berridge M.J., Roderick H.I. Regulation of InsP3 receptor activity by neuronal Ca2+ -binding proteins. Eur Mol Biol Org J. 2004;23:312–321. doi: 10.1038/sj.emboj.7600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rodrigo J., Suburu A.M., Bentura M.L., Fernandez T., Nakada S., Mikoshiba K., Martinez-Murillo R., Polak J.M. Distribution of the inositol 1,4,5-triphosphate receptor, P400, in adult rat brain. J Comp Neurol. 1993;337:493–517. doi: 10.1002/cne.903370311. [DOI] [PubMed] [Google Scholar]
- 106.D’Onofrio S., Urbano F.J., Garcia-Rill E. Liithium decreases the effects of neuronal calcium sensor protein 1 in pedunculopontine neurons. Neurosci Abstr. 2015;39 doi: 10.14814/phy2.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gambino F., Pavlowsky A., Begle A., Dupont J.L., Bahi N., Courjaret R., Gardette R., Hdjkacem H., Skala H., Poulain B., Vitale N., Humeau Y. IL1-receptor accessory protein-like 1 (IL1RAPL1), a protein involved in cognitive functions, regulates N-type Ca2+-channel and neurite elongation. Proc Natl Acad Sci. 2007;104:9063–9068. doi: 10.1073/pnas.0701133104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Futami T., Takakusaki K., Kitai S.T. Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci Res. 1995;21:331–342. doi: 10.1016/0168-0102(94)00869-h. [DOI] [PubMed] [Google Scholar]
- 109.Granata A.R., Kitai S.T. Inhibitory substantia nigra inputs to the pedunculopontine neurons. Exp Brain Res. 1991;86:459–466. doi: 10.1007/BF00230520. [DOI] [PubMed] [Google Scholar]
- 110.Grofova I., Zhou M. Nigral innervation of cholinergic and glutamatergic cells in the rat mesopontine tegmentum: light and electron microscopic anterograde tracing and immunohistochemical studies. J Comp Neurol. 1998;395:359–379. [PubMed] [Google Scholar]
- 111.Datta S., Curro Dossi R., pare D., Oakson G., Steriade M. Substantia nigra reticulate neurons during sleep-waking states: relation with ponto-geniculo-occipital waves. Brain Res. 1991;566:344–347. doi: 10.1016/0006-8993(91)91723-e. [DOI] [PubMed] [Google Scholar]
- 112.Leger L., Sapin E., Goutany R., Petron C., Salvert D., Fort P., Luppi P.H. Dopaminergic neurons expressing Fos during waking and paradoxical sleep in the rat. J Chem Neuroanat. 2010;39:262–271. doi: 10.1016/j.jchemneu.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 113.Sawa A., Snyder S.H. Schizophrenia: neural mechanisms for novel therapies. Mol Med. 2003;9:3–9. [PMC free article] [PubMed] [Google Scholar]
- 114.Garcia-Rill E. Academic Press; New York, NY: 2015. Waking and the reticular activating system in health and disease; p. 360. ISBN 9780128013854. [Google Scholar]