Abstract
Background and aims
Aging is a multifactorial process that elicits changes in the duration and quality of sleep. Polysomnography is considered to be the standard examination for the analysis of sleep and consists of the simultaneous recording of selected physiological variables during sleep.
Objective
The objective of this study was to use polysomnography to compare sleep reported by senior citizens.
Methods
We selected 40 patients, both male and female, with ages ranging from 64 to 89 years from the Center for the Study of Aging at the Federal University of São Paulo. Patients answered questions about sleep on the Comprehensive Geriatric Assessment and underwent polysomnography.
Results
The results were compared, and agreement between perceived sleep and polysomnography was found in several areas. There was an association between difficulty sleeping and sleep onset latency (p=0.015), waking up at night with sleep onset latency (p=0.005), total sleep time with daytime sleepiness (0.005) and snoring (0.027), sleep efficiency with sleepiness (0.004), snoring (0.033) and pause in breathing (p=0.024), awakenings with snoring (p=0.012) and sleep apnea with pauses in breathing (p=0.001).
Conclusion
These results suggest that the older adult population have a good perception of their sleep. The questionnaires aimed at this population should be used as an alternative to polysomnography.
Keywords: Aging, Sleep, Polysomnography
1. Introduction
Aging is associated with a set of morphological and physiological changes resulting from the action of time on living beings, can pass on the various body systems, including on the duration and quality of sleep, with decreased slow-wave sleep, fragmented sleep, increased sleep latency and periodic leg movements, with effects on metabolic function and immune responses [1,2]. Moreover, older people are more alert in the early morning, but sleepier in the early afternoon and also experience daytime sleepiness [3,4]. In a study from the National Institute on Aging involving more than 9000 individuals aged 65 and older, it was reported that more than 50% of participants had at least one chronic sleep complaint [5].
Typical sleep problems in the older adult include difficulty falling asleep and maintaining sleep, early-morning awakening and excessive daytime sleepiness [6]. A variety of processes may interfere with sleep and wakefulness in the older adult. Among these processes, we can list acute and chronic medical illnesses, medication effects, psychiatric disorders, primary sleep disorders, social changes, poor sleep habits and circadian rhythm shifts [7,8].
Several generalizations can be made regarding aging and sleep characteristics, however, there are controversies regarding the sleep time required for the older adult. According to Guimaraes et al. [9], there is definitive evidence that the required amount of sleep decreases with the aging process. However, it seems that the older adult generally has a shorter duration of sleep, increased nocturnal awakenings, and consequently more naps. Unruh et al. stated that sleep efficiency, which is the ratio between the time that the person can actually fall asleep and time spent in bed with the intention of sleeping, appears to be reduced in older adult and the difficulty of maintaining nighttime sleep contributes to the decrease in the quality of sleep [10]. Overall, the sleep–wake cycle in the older adult may be fragmented, with interrupted nighttime sleep and daytime sleepiness interrupted by naps. The deepest stages of non-REM sleep are frequently reduced or nonexistent in older adult; however, the REM sleep tends to be preserved [11]. Authors believe these changes in the sleep of older adult may be due to alterations in the quality of transmission of afferent information from the retina to the optic central markers (Suprachiasmatic nucleus), which lose their ability to respond to information, changing the sleep–wake cycle, as well as environmental, behavioral, social and physical changes [12,13,6].
Among sleep disorders, obstructive sleep apnea syndrome (SAOS) is very common among the older adult [14,15]. SAOS occurs when there is a repeated obstruction of the upper airway during sleep for 10 s or more, accompanied by oxyhemoglobin desaturation, causing micro-arousals and awakenings. SAOS may be followed by daytime sleepiness and fatigue, an increase in naps and increased cardiovascular morbidity and mortality [16,17]. In the older adult, there is still no consensus on the diagnostic criteria for sleep apnea. Some authors consider the values similar to adults, i.e., an index of apnea/hypopnea episodes per hour up to 5 is considered to be normal, from 21 to 50: moderate, over 50 are considered to be high [18].
However, it is estimated that the diagnosis of SAOS is not made in 82% of men and 93% of women with moderate to severe SAOS. The low frequency of diagnosis may reflect the reduced perception of symptoms of sleep as a problem by the patient and their relatives, the difficult access to associated diagnostics and the possibility of insufficient training in sleep medicine [19].
The gold standard method for the diagnosis of sleep disorders is polysomnography, which is a study that is performed throughout the night in a laboratory [20]. However, other diagnostic methods can be used for the investigation of sleep disorders and, subjective and objective assessments of sleep parameters may differ due to sleep misperception and measurement effects. While subjective estimates may be biased by a person’s own sleep perception, objective assessment methods (such as PSGs), may be considered distressing, thus changing the quality and quantity of a person’s usual sleep. Exposure to polysomnographic equipment, e.g., head and chest sensors, or sleeping in an unfamiliar setting such as a laboratory, may interfere with the person’s habitual sleep pattern [21,22].
Subjective measures can also be used in clinical practice to assess sleep. Most sleep questionnaires were developed in countries other than Brazil, and a few were written in Portuguese, which lead us to consider cultural misunderstandings may influence the specificity and accuracy of these methods.
Some variables hamper the achievement of polysomnography in the older adult, in addition to the inconveniences above, as the difficulty in taking older adult to sleep at hospitals or medical clinics besides the cost of the exam itself. Due to these difficulties as well as to the lack of agreement between subjective sleep and objective method, we became interested in investigating the relationship of sleep reported and polysomnography, making it possible to analyze how the older adult population understands his/her own sleep.
Therefore, this study aimed to compare perceived sleep with the polysomnography of older adult evaluated at the Center for the Study of Aging UNIFESP, given the subjective and objective assessments of sleep may be discrepant due to sleep misperception and measurement effects.
2. Methodology
The protocol was approved by the Ethics Committee and Research of the Federal University of São Paulo on April 1, 2011 (0330/11), and all of the individuals involved provided written consent document.
The older adult people included for this study belong to a cohort of those who are 60 or older living in the neighborhood of Vila Clementino, district of São Paulo, known as EPIDOSO. These older adults underwent a series of medical evaluations with several health professionals (physician, nutritionist, physical educator and psychologist) to complete the Comprehensive Geriatric Assessment (CGA).
Among the numerous medical evaluations in the Comprehensive Geriatric Assessment (CGA), there is a “Sleep Habits Questionnaire” with questions about perceived sleep (difficulty sleeping, waking up during the night, difficulty to get back to sleep and waking up too early in the morning). Daytime characteristics described by the patients were tiredness, sleepiness and lack of enough sleep. The participants included also asking about some unusual behavior during sleep, such as leg movements, excessive snoring and pauses in breathing. All questions about sleep are marked with yes or no, which shall be completed only with questions about unusual behavior during sleep and daytime evidences. This questionnaire was precisely applied on this search.
The inclusion criteria were older adults participating in the study must be over 60, have responded to the sleep habits questionnaire and acceptance of the procedures (polysomnography). The exclusion criteria were refusal to perform polysomnography and/or problems that interfere with the examination, such as flu or fever.
Thus, a group of 40 individuals were chosen. The mean age was 73.68 years (range: 64–89), with female predominance (55%) and high BMI (27.36). The predominance of females and BMI did not cause statistical differences in the variables studied.
Polysomnography was requested for this group of individuals in order to compare these results with the measured sleep in the sleep habits questionnaire of Comprehensive Geriatric Assessment (CGA).
The polysomnography was conducted at the Sleep Institute (Department of Psychobiology) and the whole night of sleep was registered using the polygraph Sleep Analyzing Computer SAC Version 8.1 (Oxford Instruments Inc.). The examination included electroencephalogram record, electrooculogram record, electromyogram of the submentonian muscles and tibialis record, electrocardiogram record, record of oronasal flow, thoracic and abdominal movement, record of snore, body position and oximetry records. The sleep efficiency was considered decreased when it was below 85% and awakenings index normal until 10 events per hour. It was considered as an objective polysomnographic diagnosis of apnea when the apnea–hypopnea index (AHI) was higher than or equal to 15 per hour and the occurrence of periodic leg movements (PLM) when registering more than 15 events per hour.
3. Statistical analysis
The sample size was based on the population of older adult at the Center for the Study of Aging who answered the sleep habits questionnaire, with Hypothesis test average, which met the needed amount of participants to produce a reliable statistical analysis. The independent t test was used to compare the presence or absence of symptoms depending on the variables of polysomnography. The older adults were divided in two groups: have “symptoms” and the ones who do not have symptoms. In order to verify the association between the normative values of polysomnography with the presence of symptoms, we used the chi-square test. For meaningful comparisons, the data are represented by bar charts with confidence intervals (95%). A p-value less than 0.05 was considered statistically significant.
4. Results
We compared all of the variables on the polysomnography with the questions on the CGA (sleep habits questionnaire). The results are shown in Tables 1 and 2.
Table 1.
Relationship between polysomnography and comprehensive geriatric assessment.
| Polysomnography variables |
Difficulty sleeping |
Waking up at night |
Difficulty sleeping and waking up at night |
Sleepiness |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P | Yes | No | P | Yes | No | P | Yes | No | P | |
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | |||||
| Sleep onset latency | 74.0±75.6 | 27.8±29.3 | 0.015⁎ | 51.30±68.31 | 31.94±34.76 | 0.005⁎ | 29.96±23.52 | 31.94±34.76 | 0.278 | 30.32±38.95 | 32.82±37.61 | 0.976 |
| Total sleep time | 304.43±89.14 | 297.40±74.44 | 0.409 | 314.66±106.49 | 297.40±74.44 | 0.162 | 304.83±80.08 | 297.40± | 0.931 | 287.73±205.05 | 311.17±63.56 | 0.005⁎ |
| Sleep efficiency | 71.12±21.46 | 69.44±17.34 | 0.399 | 69.40±25.16 | 69.44±17.34 | 0.158 | 67.92±18.04 | 69.44±17.34 | 0.933 | 59.8±24.1 | 76.41±10.5 | 0.004⁎ |
| Stage 1 | 17.31±11.77 | 17.17±11.59 | 0.962 | 14.61±7.22 | 17.17±11.59 | 0.249 | 19.94±12.13 | 17.17±11.59 | 0.889 | 19.52±11.27 | 16.26±10.83 | 0.572 |
| Stage 2 | 48.05±12.82 | 42.51±12.30 | 0.868 | 42.61±14.63 | 42.51±12.30 | 0.885 | 43.32±7.46 | 42.51±12.30 | 0.075 | 47.67±11.86 | 41.52±11.18 | 0.98 |
| Stage 3 | 21.21±14.52 | 22.87±8.96 | 0.249 | 28.77±19.17 | 2287±8.96 | 0.095 | 20.79±12.98 | 22.87±8.96 | 0.141 | 17.84±11.56 | 25.77±12.28 | 0.895 |
| REM | 18.04±7.0 | 18.68±6.55 | 0.629 | 16.86±5.23 | 18.68±6.55 | 0.78 | 15.93±5.10 | 18.68±6.55 | 0.599 | 16.17±5.92 | 18.65±6.08 | 0.734 |
| Awakenings | 117.88±62.28 | 83.16±55.38 | 0.453 | 59.67±20.93 | 83.16±55.38 | 0.139 | 131.11±64.40 | 83.16±55.38 | 0.61 | 92.47±50.61 | 99.04±64.27 | 0.372 |
| Leg movements | 34.97±48.08 | 17.75±29.79 | 0.261 | 5.46±9.96 | 17.75±29.74 | 0.192 | 23.63±20.28 | 17.52±29.74 | 0.732 | 31.12±31.46 | 14.65±29.54 | 0.537 |
| Apnea/hipopnea index | 16.46±14.68 | 19.62±21.35 | 0.226 | 11.53±13.44 | 19.62±21.335 | 0.192 | 27.54±21.32 | 19.62±21.35 | 0.452 | 19.53±18.36 | 19.57±20.14 | 0.931 |
Data represented as mean± standard deviation. P: 0.005.
Table 2.
Relationship between polysomnography and comprehensive geriatric assessment.
| Polysomnography variables |
Snoring |
Pause in breathing |
Leg movements |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P | Yes | No | P | Yes | No | P | |
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| Sleep onset latency | 32.33±35.54 | 31.21±42.45 | 0.94 | 25.26±35.90 | 32.44±38.17 | 0.906 | 48.50±51.52 | 30.65±36.95 | 0.415 |
| Total sleep time | 292.48±91.31 | 321.38±53.45 | 0.027⁎ | 319.83±13.69 | 301.49±83.27 | 0.058 | 352.16±58.77 | 299.0±81.12 | 0.528 |
| Sleep efficiency | 66.69±21.14 | 74.36±12.70 | 0.033⁎ | 69.36±1.72 | 69.43±19.49 | 0.024⁎ | 77.80±13.16 | 68.78±19.08 | 0.469 |
| Stage 1 | 18.13±11.88 | 16.15±9.35 | 0.266 | 28.33±8.73 | 16.58±10.75 | 0.654 | 6.80±3.78 | 18.24±10.93 | 0.153 |
| Stage 2 | 45.5±12.63 | 40.51±9.19 | 0.075⁎ | 44.13±10.12 | 43.72±11.89 | 0.478 | 47.16±11.69 | 43.49±11.77 | 0.73 |
| Stage 3 | 21.16±12.11 | 26.06±12.91 | 0.754 | 25.10±3.39 | 22.88±12.80 | 0.202 | 26.93±4.21 | 22.68±12.90 | 0.164 |
| REM | 17.28±6.82 | 18.54±4.47 | 0.083 | 10.77±3.42 | 18.29±5.910 | 0.323 | 19.07±4.50 | 17.61±6.21 | 0.447 |
| Awakenings | 104.89±67.03 | 81.93±39.46 | 0.012⁎ | 221.67±39.31 | 87.08±48.70 | 0.709 | 64.0±4.42 | 99.21±59.68 | 0.71 |
| Leg movements | 26.32±35.85 | 10.12±15.11 | 0.116 | 12.23±21.18 | 21.17±31.63 | 0.782 | 20.70±16.02 | 20.52±31.89 | 0.55 |
| Apnea/hipopnea index | 20.98±19.89 | 16.99±18.58 | 0.626 | 55.6±29.5 | 16.8±15.4 | 0.001⁎ | 11.26±14.57 | 20.20±19.62 | 0.591 |
Data represented as mean± standard deviation. P: 0.005.
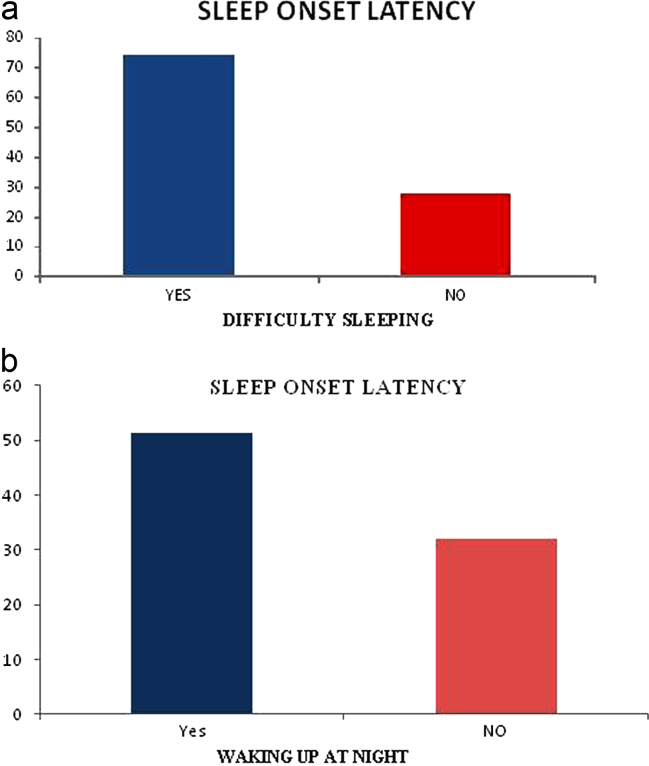
The sleep onset latency on the polysomnography showed relation with the sleep habits questionnaire, when the older adults answered difficulty sleeping on the CGA (p=0.015) and, when they reported waking up at night on the CGA (p=0.005) – graphic 1
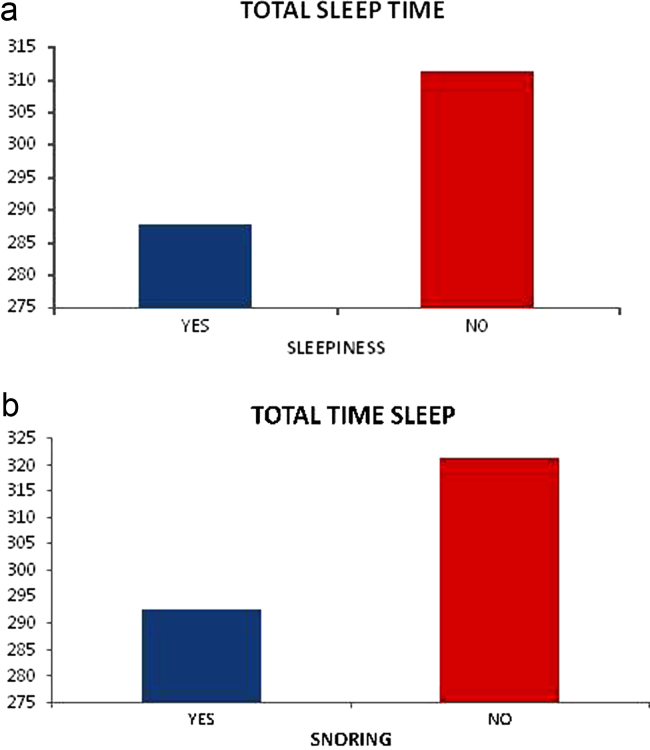
We noted associations between total sleep time reduction on the polysomnography with daytime sleepiness on the CGA (0.005) and snoring on the CGA (0.027) – graphic 2
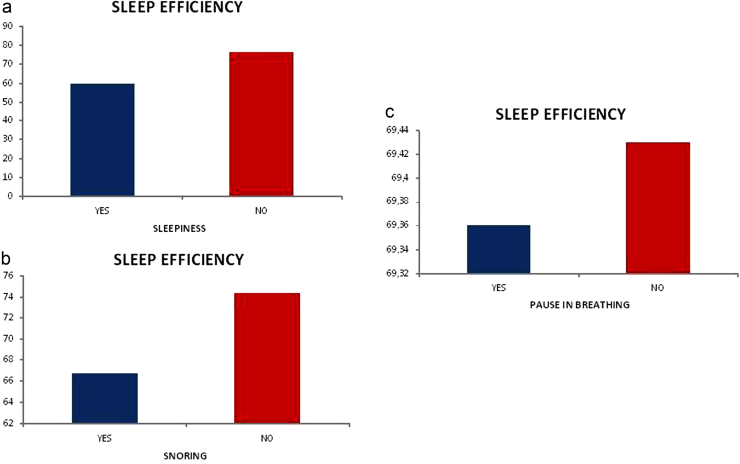
Sleep efficiency reduction on the polysomnography has an association with sleepiness on the CGA (0.004), snoring on the CGA (0.033) and pause in breathing on the CGA (p=0.024) – graphic 3;
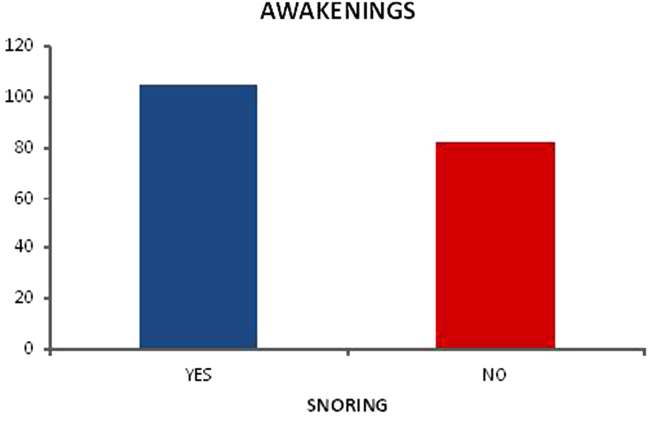
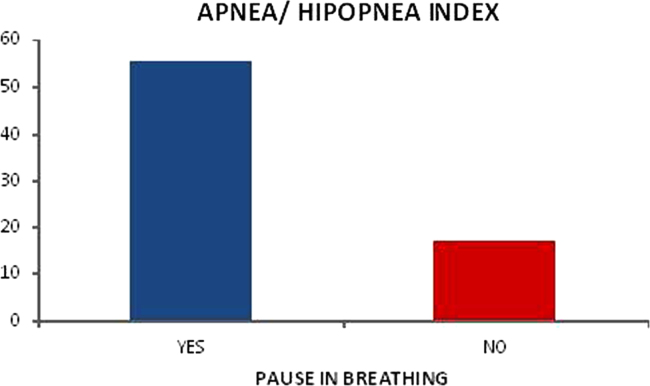
The awakenings index on the polysomnography indicated association with snoring on the CGA (p=0.012) – graphic 4 and when older adults noticed there was apnea on the polysomnography and reported the feeling of a pause in breathing on the questionnaire (p=0.001) – graphic 5.
A new analysis of polysomnography compared with data from CGA, rated Normal/Changed was performed. We used the Chi-Square for Independence test for this analysis (Table 3).
Table 3.
Relationship and/or sleep association referred to polysomnography.
| Polysomnography |
Comprehensive geriatric assessment |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| No |
Yes |
Total |
||||||
| N | % | N | % | N | % | |||
| Awakenings | Altered | 35 | 100 | 9 | 100 | 44 | 100 | -x- |
| Normal | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Sleep onset latency | Altered | 12 | 30 | 2 | 50 | 14 | 32 | 0.413 |
| Normal | 28 | 70 | 2 | 50 | 30 | 68 | ||
| Total sleep time | Altered | 5 | 22 | 11 | 52 | 16 | 36 | 0.035 |
| Normal | 18 | 78 | 10 | 48 | 28 | 64 | ||
| Sleep efficiency | Altered | 7 | 30 | 13 | 62 | 20 | 45 | 0.036 |
| Normal | 16 | 70 | 8 | 38 | 24 | 55 | ||
| Apnea/hipopnea index | Altered | 17 | 43 | 3 | 75 | 20 | 45 | 0.213 |
| Normal | 23 | 58 | 1 | 25 | 24 | 55 | ||
| Leg movements | Altered | 19 | 46 | 2 | 67 | 21 | 48 | 0.496 |
| Normal | 22 | 54 | 1 | 33 | 23 | 52 | ||
P: 0.005.
5. Discussion
It is often difficult to create a sleep questionnaire which is easy to understand and following the time needed for individual clinical care with the older adult. Based on these needs, the simple sleep questions on the Comprehensive Geriatric Assessment (CGA) have been written. However, there is still the need to compare these questionnaires with the polysomnography.
The major finding of this research was that the older adults have an accurate perception of the time they spend to fall asleep, which is consistent with the findings of O’Donnell et al. [22]. Those authors assessed 24 healthy adults through questionnaires and polysomnography and found correlations in the total sleep time associated with an increase in slow-wave sleep and sleep onset latency. Our findings of the association between increased in the sleep onset latency with difficulty sleeping and waking up at night is similar to other studies [23,24], and so are our results about total sleep time reduced with daytime sleepiness and snoring (TST) [23–25].
The older adult, who demonstrated a lower total sleep time and lower efficiency on the polysomnography, reported daytime sleepiness on the CGA. Studies have shown changes in the structure of sleep in older adults with decreased total sleep time, as well as decreased in sleep efficiency, that may lead to daytime sleepiness in the older adult [26].
A relationship has also been reported regarding apnea on the polysomnography and the question on the CGA when the patient reported waking up with a feeling of suffocation and the individual’s partner informed that the person has “stopped breathing” during sleep. Studies have shown that apnea is a common problem of aging and a risk factor for mortality in older adults [26].
We noted an association between reported snoring in patients with decreased total sleep time, efficiency and awakenings on the polysomnography. These data are consistent with the literature; snoring is a major symptom of sleep apnea, leading to decreased total sleep time, sleep efficiency and increased nocturnal awakenings [27]. The pause in breathing was also associated with decreased sleep efficiency. We know that the pause in breathing is an indication of sleep apnea, which decreases the efficiency of sleep due to an increase in the number of awakenings.
Although the literature states that women complain more about sleep difficulties [28], there was no difference in the perception of subjective and objective sleep between females and males.
Finally, through this study, we intend to stimulate he need for further studies on the relationship between perceived sleep and polysomnography in older adults. However, our findings suggest that studies objectively assessing sleep times may be comparable to those using objective determinations, often facilitating clinical practice in the sleep disorders investigations in older adults (Fig. 1–5).
Fig. 1.
(a) Relationship between sleep onset latency and difficulty sleeping. (b) Relationship between sleep onset latency and waking up at night.
Fig. 2.
(a) Relationship between total sleep time and sleepiness. (b) Relationship between total sleep time and snoring.
Fig. 3.
(a) Relationship between sleep efficiency and sleepiness. (b) Relationship between sleep efficiency and snoring. (c) Relationship between sleep efficiency and pause in breathing.
Fig. 4.
Relationship between awakenings and snoring.
Fig. 5.
Relationship between apnea/hipopnea and pause in breathing.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Acknowledgments
We would like to offer our special thanks to the Agency for its support and evaluation of the Graduate Education (CAPES) for its valuable contribution to this research.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
References
- 1.Ladislas R. Cellular and molecular mechanisms of aging and age related diseases. Pathol Oncol Res. 2000;6(1):3–9. doi: 10.1007/BF03032651. [DOI] [PubMed] [Google Scholar]
- 2.Avidan AY. Sleep disorders in the older patient. Prim Care. 2005;32(2):563–586. doi: 10.1016/j.pop.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Harrigton JJ., Lee-Chiong T., Jr. Sleep and older. Clin Chest Med. 2007;28(4):673–684. doi: 10.1016/j.ccm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Wolkove N., Elkholy O., Baltzan M., Palayew M. Sleep and aging: 1. Sleep disorders commonly found in older people. Can Med Assoc J. 2007;176(9):1299–1304. doi: 10.1503/cmaj.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley DJ., Monjan AA., Brown SL., Simonsick EM., Wallace RB., Blazer DG. Sleep complaints among elderly people: an epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 6.Duffy J.F., Czeisler C.A. Age-related change in the relationship between circadian period. circadian phase and diurnal preference in humans. Neurosci Lett. 2002;318:117–120. doi: 10.1016/s0304-3940(01)02427-2. [DOI] [PubMed] [Google Scholar]
- 7.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 8.Neubauer DN., Smith PL., Earley CJ. Sleep disorders. In: Barker LR., Burton JR., Zieve PD., editors. Principles of ambulatory medicine. 5th ed. Williams & Wilkins; Baltimore: 1999. pp. 1314–1328. [Google Scholar]
- 9.Guimarães LHCT., Lima MD., Souza JÁ. Physical activity in sedentary group improves sleep in sedentary elder women. Mag Neurosci. 2007;15(3):203–206. [Google Scholar]
- 10.Unruh ML., Redline S., An MW., Buysse DJ., Nieto FJ., Yeh JL., Newman AB. Subjective and objective sleep quality and aging in the sleep heart health study. J Am Geriatr Soc. 2008;56(7):1218–1227. doi: 10.1111/j.1532-5415.2008.01755.x. [DOI] [PubMed] [Google Scholar]
- 11.Avidan A.Y. Sleep changes and disorders in the elderly patient. Curr Neurol Neurosci Rep. 2002;2(2):178–185. doi: 10.1007/s11910-002-0028-z. [DOI] [PubMed] [Google Scholar]
- 12.Weinert D. Age-dependent changes of the circadian system. Chronobiol Int. 2000;17:261–283. doi: 10.1081/cbi-100101048. [DOI] [PubMed] [Google Scholar]
- 13.Koller A.., Turek F.W. Circadian rhythms and sleep in aging rodends. In: Hof PR., Mobbs CV., editors. Functional neurobiology of aging. Academic Press; San Diego: 2001. pp. 855–868. [Google Scholar]
- 14.Dealberto MJ., Ferber C., Garma L., Lemoine P., Alpérovitch A. Factors related to sleep apnea syndrome in sleep clinic patients. Chest. 1994;105(6):1753–1758. doi: 10.1378/chest.105.6.1753. [DOI] [PubMed] [Google Scholar]
- 15.Geib LTC. Sono e envelhecimento. Ver. Psiquiatr. Rio Grande do Sul; 2003.
- 16.American Academy of Sleep Medicine . 2nd ed. American Academy of sleep Medicine; Westchester JL: 2005. International classification of sleep disorders: diagnostic and coding manual. [Google Scholar]
- 17.Floyd JA. Sleep and aging. Nurs Clin N Am. 2002;37(4):719–731. doi: 10.1016/s0029-6465(02)00034-8. [DOI] [PubMed] [Google Scholar]
- 18.Martinez D. Syndrome of obstructive sleep apnea. In: Silva LCC, editor. Conduct in pulmonology. Revinter; Rio de Janeiro (RJ): 2001. pp. 757–769. [Google Scholar]
- 19.Noal RB., Menezes AMB, Canani SF., Siqueira FV. Usual snoring and obstructive sleep apnea in adults: population-based study. Pelotas. Brazil. Rev Saude Publica. 2008;42(2):224–233. doi: 10.1590/s0034-89102008000200006. [DOI] [PubMed] [Google Scholar]
- 20.Strollo PJ, Sanders MH. Constantino Jp et al. Split-Night studies for the diagnosis and treatment of sleep-disordered breathing. Sleep. 1996;19(10):S255–S259. doi: 10.1093/sleep/19.suppl_10.s255. [DOI] [PubMed] [Google Scholar]
- 21.Rechtschafen A., Kales A. UCLA Brain Information Service; Los Angeles: 1968. A manual of standardized terminology, techniques and scoring system and sleep stages of human individuals. [Google Scholar]
- 22.O’Donnell D., Silva EJ, Munch M., Ronda JM., Wang W., Duffy JF. Comparison of subjective and objective assessments of sleep in healthy older individuals without sleep complaints. J Sleep Res. 2009;18(2):254–263. doi: 10.1111/j.1365-2869.2008.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akerstedt T., Hume K., Minors D., Waterhouse J. Good sleep—its timing and physiological sleep characteristics. J Sleep Res. 1997;6(4):221–229. doi: 10.1111/j.1365-2869.1997.00221.x. [DOI] [PubMed] [Google Scholar]
- 24.Hoch CC., Reynolds CF, 3rd, Kupfer DJ., Berman SR., Houck PR., Stack JA. Empirical note: self-report versus recorded sleep in healthy seniors. Psychophysiology. 1987;24(3):293–299. doi: 10.1111/j.1469-8986.1987.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 25.Keklund G., Akerstedt T. Objective components of individual differences in subjective sleep quality. J Sleep Res. 1997;6(4):217–220. doi: 10.1111/j.1365-2869.1997.00217.x. [DOI] [PubMed] [Google Scholar]
- 26.Gooneratne NS., Richards KC., Joffe M., Lam RW., Pack F., Staley B. Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults. Sleep. 2011;34(4):435–442. doi: 10.1093/sleep/34.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan AM, Appel DW. Anemia of aging, inflammation and obstructive sleep apnea. Med Hypotheses. 2008;71(4):606. doi: 10.1016/j.mehy.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 28.Pallesen S., Sivertsen B., Nordhus IH., Bjorvatn B. A 10-year trend of insomnia prevalence in the adult Norwegian population. Sleep Med. 2013;15(2):173–179. doi: 10.1016/j.sleep.2013.10.009. [DOI] [PubMed] [Google Scholar]