Abstract
Age-related macular degeneration (AMD) is a neurodegenerative disease that causes adult-onset blindness. There are 2 forms of this progressive disease: wet and dry. Currently there is no cure for AMD, but several treatment options have started to emerge making early detection critical for therapeutic success. Analysis of the eyes of Abca4−/−Rdh8−/− mice that display light-induced retinal degeneration indicates that 11-cis-retinal and docosahexaenoic acid (DHA) levels were significantly decreased as compared with the eyes of control dark-adapted C57BL/6J mice. In addition, exposure to intense light correlated with higher levels of prostaglandin G2 in the eyes of Abca4−/−Rdh8−/− mice. Intense light exposure also lowered DHA levels in the eyes of wild-type C57BL/6J mice without discernible retinal degeneration. Analysis of human serum from patients with AMD recapitulated these dysregulated DHA levels and revealed dysregulation of arachidonic acid (AA) levels as well (∼32% increase in patients with AMD compared with average levels in healthy individuals). From these observations, we then built a statistical model that included levels of DHA and AA from human serum. This model had a 74% probability of correctly identifying patients with AMD from controls. Addition of a genetic analysis for one of the most prevalent amino acid substitutions in the age-related maculopathy susceptibility 2 gene linked to AMD, Ala69→Ser, did not improve the statistical model. Thus, we have characterized a reliable method with the potential to detect AMD without a genetic component, paving the way for a larger-scale clinical evaluation. Our studies on mouse models along with the analysis of human serum suggest that our small molecule-based model may serve as an effective tool to estimate the risk of developing AMD.—Orban, T., Johnson, W. M., Dong, Z., Maeda, T., Maeda, A., Sakai, T., Tsuneoka, H., Mieyal, J. J., Palczewski, K. Serum levels of lipid metabolites in age-related macular degeneration.
Keywords: docosahexaenoic acid, arachidonic acid, risk evaluation, serum, mouse model
Age-related degenerative processes often contribute to various diseases (1–6). Depending on factors such as genetic background, environmental exposure, and diet, the rate of pathologic changes can differ among individuals, but the major contributor to the progression of disease is age itself (7–9). Alleviation of the ensuing diseases requires early detection and prophylactic treatment of the affected tissue(s), effective therapy of the most adverse pathology in fully developed disease, and radical tissue/cell replacement of degenerated cells at the final stage of disease. Confounding effects of many age-related changes affect straightforward elucidation of their underlying etiology; the processes can complicate the development of mechanism-based treatment, as many biochemical pathways can be adversely affected. Moreover, preclinical and early clinical tests of experimental approaches are both lengthy and costly. These examinations can involve either a combination of surrogate animal models, a large number of randomly selected individuals for long periods with disease that may or may not progress, or a group of patients with end-stage disease wherein only a few treatment options exist. Thus, reliable early detection is essential for developing treatments for these diseases, as this will allow many more therapeutic approaches to be tested in a shorter time at much lower cost. Ultimately, only susceptible individuals will require treatment before irreversible pathology sets in.
Age-related macular degeneration (AMD) is a debilitating multifaceted blinding disease. The diversity of risk factors identified to date indicates that AMD can result from a multitude of dysregulated physiologic pathways within the eye (10). At its final stage, there are a reduced number of photoreceptor cells and cells in the retinal pigmented epithelium that are essential for photoreceptor function and survival. Imaging techniques such as scanning laser ophthalmoscopy and optical coherent tomography have revolutionized current ophthalmology, improving the diagnosis of retinal diseases, monitoring their progression, evaluating treatment options, and dramatically advancing in vivo retinal research (11–17). However, by the time structural changes are observed in the retina with current methodology, the pathology is already well established. And once the retina is committed to degeneration, merely slowing the rate of deterioration only prolongs the onset of symptoms. New 2-photon imaging will enable functional imaging with improved resolution, but this method is still in early development (18–21). Because an optimal therapeutic intervention would target early stages of disease, there is a great need for safe and effective ways to detect AMD early and safely monitor its progression. Decline in visual functions, particularly dark adaptation, becomes yet another potential biomarker in monitoring/predicting pathologies leading to AMD (22–24).
Although development of a large number of animal models has helped to advance our understanding of retinal pathobiology and identify specific genetic factors that contribute to photoreceptor degeneration, inflammation, and the biochemistry of many retinal processes, these models all have limitations (25–28). No one animal model fully recapitulates all the phenotypes of human retinal pathology, and patients with AMD also do not fully resemble each other. Though genetically manipulated rodents and other small rapidly reproducing animals do not have maculae, they can serve as limited surrogate models of photoreceptor/retinal pigmented epithelium degeneration with drusen and lipofuscin accumulation similar to that observed with humans with AMD. One of these models is a mouse with a double knockout of both the ATP-binding cassette transporter 4 and retinol dehydrogenase 8, a transporter and enzyme involved in all-trans-retinal clearance (29). In this model, the pathology and death of photoreceptors can be induced in a cohort of animals by simple exposure to light in a synchronized manner. Retinal bleaching requires shorter and lower-intensity light than previously used for albino mouse or rat models (30, 31).
Here, in proof of concept studies, we set out to identify early molecular markers for retinal degeneration/stress by using mass spectrometric analyses in both Abca4−/−Rdh8−/− mice and wild-type mice exposed to bright light stress. The mouse model studies then were used to inform the analyses of sera from a group of human volunteers and patients with AMD (with and without the Ala69→Ser change in age-related maculopathy susceptibility 2 (ARMS2) gene that was previously associated with AMD) (32) to determine if the molecular markers identified in the mouse models would be indicative of retinal degeneration (a hallmark of AMD). This approach of measuring levels of small molecules in human serum constitutes an unbiased strategy to generate a risk assessment model that may be further verified in longitudinal clinical studies to predict populations at risk of developing AMD.
MATERIALS AND METHODS
Materials
3-(N-morpholino)propanesulfonic acid, formic acid (FA), and trichloroacetic acid were from Sigma (St. Louis, MO, USA). Docosahexaenoic acid (DHA) and DHA with 5 deuterium atoms at the 21, 21, 22, 22, and 22 positions (DHA-d5); arachidonic acid (AA) and AA with 8 deuterium atoms at the 5, 6, 8, 9, 11, 12, 14, and 15 positions (AA-d8); eicosapentaenoic acid (EPA) and EPA with 5 deuterium atoms at the 19, 19′, 20, 20, and 20 positions (EPA-d5) were from Cayman Chemicals (Ann Arbor, MI, USA).
Methods
Human samples
All patients underwent a complete ophthalmic examination and visual function tests by a physician, and informed consent was obtained from each person for the present study. Procedures followed the Declaration of Helsinki guidelines and were approved by the Institutional Review Boards of Jikei University School of Medicine and Case Western Reserve University School of Medicine. To minimize ambiguity from different racial backgrounds, blood samples were collected only from Japanese patients (average age 68.9 ± 13.1 yr). Approximately equal numbers of males and females were selected and patients diagnosed with the wet form of AMD (n = 22), which is prevalent in the Japanese population (33), were compared with controls (n = 22) who had no retinal degeneration as determined by ophthalmologic examination. After standard venipuncture, human blood samples were collected in tubes containing disodium ethylenediaminetetraacetate.
Animals
Abca4−/−Rdh8−/− mice were generated as described previously (34, 35). Such mice used in this study were homozygous for the Leu450 allele of Rpe65 as determined by a published genotyping protocol (36) and free of the Crb1/rd8 (37) and rd/rd (38) mutations. All mice were genotyped by PCR with the following primers: ABCR1 (5′-GCCCAGTGGTCGATCTGTCTAGC-3′) and ABCR2 (5′-CGGACACAAAGGCCGCTAGGACCACG-3′) for wild-type (619 bp) and A0 (5′-CCACAGCACACATCAGCATTTCTCC-3′) and N1 (5′-TGCGAGGCCAGAGGCCACTTGTGTAGC-3′) for the targeted deletion (455 bp) as published previously (29). Animals were provided with standard chow (LabDiet 5053; Purina Mills St. Louis, MO, USA) and maintained under a 12/12-h light–dark cycle. Wild-type mice (C57BL/6J) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Equal numbers of male and female mice were employed. All animals were housed in the Animal Resource Center at the Case Western Reserve University School of Medicine (Cleveland, OH, USA), where they were maintained in complete darkness. Experimental manipulations in the dark were done under dim red light transmitted through a Kodak No. 1 safelight filter (transmittance > 560 nm; Eastman Kodak, Rochester, NY, USA). All animal procedures and experiments were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University and conformed to recommendations of both the American Veterinary Medical Association Panel on Euthanasia and the Association of Research for Vision and Ophthalmology.
Light-induced photoreceptor damage
Mice were dark adapted for 3 d before exposure to light. Pupils were dilated with a mixture of 0.5% tropicamide and 0.5% phenylephrine hydrochloride (Midorin-P; Santen Pharmaceutical Co., Ltd., Osaka, Japan). Light-induced photoreceptor damage was accomplished by exposing mice to 10,000 lux of diffuse white fluorescent light (150 W spiral lamp; Commercial Electric, Cleveland, OH, USA) for 1 h. After exposure, animals were kept in the dark for 3 d until evaluation.
Spectral domain–optical coherence tomography
In vivo spectral domain–optical coherence tomography (SD-OCT; Bioptigen, Durham, NC, USA) was used to image whole layers of mouse retina. Mice were anesthetized with an intraperitoneal injection of an anesthetic cocktail consisting of ketamine (16.5 mg/ml) and xylazine (1.65 mg/ml) diluted in 10 mM sodium phosphate, pH 7.2, and 100 mM NaCl. The dose was set to 8–12 µl/g of body weight. Before imaging, pupils were dilated with a topical solution of 1% tropicamide. Five frames of SD-OCT images were acquired in the B-scan mode and averaged for image presentation and analysis. The thickness of the retinal outer nuclear layer (ONL) was measured at 500 μm from the optic nerve head in 4 directions (superior, inferior, temporal, and nasal). For light-exposed mice, SD-OCT images were acquired 3 d after light exposure. Three-dimensional images of the SD-OCT slices were generated with ImageJ software (version 1.40g; National Institutes of Health, Bethesda, MD, USA) (39).
Untargeted analysis of hydrolyzed mouse samples
Abca4−/−Rdh8−/− mouse eye samples (n = 22) were divided into dark-adapted and light-exposed samples. Samples (2 eyes per sample) were hydrolyzed by addition of 200 µl of 10 M NaOH and incubation at 56°C for 24 h. Next, samples were acidified by adding 200 µl of 12 M HCl and subjected to extraction with a chloroform:methanol:water mixture (6:3:0.5; v:v:v), after which the organic layer was dried by evaporation and resolubilized in acetonitrile containing 0.1% FA. Samples were analyzed by HPLC with a variety of C18 columns selected to optimize the analytical protocol. An Aeris Peptide 100 × 2.10 mm 3.6µ XB-C18 column (Phenomenex, Torrance, CA, USA) produced the desired group separation (Supplemental Fig. 1) for principal component analyses. The elution program consisted of a gradient from 0 to 100% acetonitrile over 50 min at 37°C. The eluant was analyzed with an LTQ Velos mass spectrometer (Thermo Scientific, Waltham, MA, USA) equipped with an electrospray source operated at 350°C. For mass spectrometry, both positive and negative ionization modes were considered. Untargeted evaluation of the chromatograms was further subjected to principal component analysis with XCMS online (40), and loadings that generated separations between groups of metabolites were identified, evaluated, and quantified (vide infra). The main leads (high score on the tandem mass spectrometry (MS/MS) compared with those obtained from the database) were finally identified with authentic standards.
Targeted analyses of DHA, EPA, AA, linoleic acid, palmitic acid, and oleate
Samples (200 µl of serum or 2 eyes) were first added to 500 µl of deionized H2O. The hydrolysis reaction then was initiated by adding 200 µl of 10 M NaOH solution containing authentic deuterated standards: DHA-d5 (6 pg/μl), AA-d8 (2 pg/μl), and EPA-d5 (2 pg/μl) (Cayman Chemicals). Reaction tubes were capped and incubated at 56°C for 24 h. Samples were acidified by adding 200 µl of 12 M HCl to reform the free fatty acids from their Na+ salts produced after the base-catalyzed hydrolysis. Free fatty acids were extracted with 3 ml of a chloroform:methanol:water mixture (6:3:0.5; v:v:v) followed by vigorous shaking. Samples were centrifuged and the bottom organic layer collected; this extraction was repeated twice. Organic phases then were combined and dried down in a Speedvac (Thermo Electron, Waltham, MA, USA) for 2 h. Dried samples were suspended in 500 µl of an HPLC mobile phase comprised of 85% methanol and 15% of 2 mM ammonium acetate with 0.1% FA and 1 mM acetic acid. Samples were numbered and scrambled to introduce a random running pattern. Washes (mock injections of 10 μl of water) were introduced after each run to test for carryover from one run to another. Replicate runs from the same samples were analyzed over 5 consecutive days to assess the day-to-day coefficient of variation (CV). Each sample (100 µl) was injected onto an Aeris Peptide 100 × 2.10 mm 3.6µ XB-C18 column with an autosampler operated at 4°C. The eluant was directed to an LXQ mass spectrometer (Thermo Scientific) operated in negative ionization mode with a temperature set to 350°C. Selected ion monitoring (SIM) experiments were set up with the following parent and reporter ions: EPA: mass to charge ratio (m/z) 301→257; AA: m/z 303→259; DHA: m/z 327→283; AA-d8: m/z 311→267; EPA-d5: m/z 306→213; DHA-d5: m/z 332→288; linoleic acid (LA): m/z 279→211; OA: m/z 281→212; and palmitic acit (PA): m/z 255→237.
Mass spectrometry analyses indicated that in the 0.01–3 µmol concentration range, the area under the curve of the intensity peak for DHA-d5 was linear with its concentration (R2 = 0.9974). The sample (2 eyes of a C57BL/6J mouse) was spiked with authentic deuterated standard before the hydrolysis reaction step. The run-to-run coefficient of variation based on 20 runs with the same sample was between 0.8 and 8.2%. Sample to sample carryover was below the limit of detection. A similar coefficient of variation and serum concentration range was obtained with deuterated forms of AA and EPA. Both AA and EPA were added to the samples (eye homogenate of a C57BL/6J mouse) before the hydrolysis reaction step. To quantify LA, OA, and PA, we used AA-d8 and found no statistically significant differences (P > 0.05).
Analyses of AA nonenzymatic oxidation and peroxidation products
These products were evaluated by SIM with the following precursor and reporter ions: 5-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid (5-HETE): m/z 319→115; 8-hydroxy-5Z,9E,11Z,14Z-eicosatetraenoic acid (8-HETE): m/z 319→155; 9-hydroxy-5Z,7E,11Z,14Z-eicosatetraenoic (9-HETE): m/z 319→151; 11-hydroxy-5Z,8Z,12E,14Z-eicosatetraenoic acid (11-HETE): m/z 319→167; 12-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12-HETE): m/z 319→179; 15-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid (15-HETE): m/z 319→175; and 9α,11α,15R-trihydroxy-(8β)-prosta-5Z,13E-dien-1-oic acid (F2 isoprostane): m/z 353→309.
Analyses of LA nonenzymatic oxidation and peroxidation by-products
By-products of LA were evaluated by using SIM of the following parent and reporter ions: 9-hydroxy-10E,12Z-octadecadienoic acid (9-HODE): m/z 295→171; 13-hydroxy-9Z,11E-octadecadienoic acid (13-HODE): m/z 295→195.
Mass spectrometry analysis indicated no statistically significant differences between light- and dark-adapted samples of both mouse models for the following by-products tested: 5-HETE, 8-HETE, 9-HETE, 11-HETE, 12-HETE, 15-HETE, F2 isoprostane, 9-HODE, and 13-HODE (P > 0.05).
Analyses of 11-cis-retinal levels in mouse eyes
Two frozen mouse eyes per sample were homogenized with a glass-on-glass homogenizer in 2 ml of 50 mM MOPS, pH 6.5, and 50 mM NH2OH in 50% ethanol. Homogenization was achieved with 10 passes of the pestle. Homogenates then were incubated at 27°C for 30 min to allow formation of retinal oximes. Retinyl acetate (2 nmol/sample in ethanol) was added as an internal standard for quantitative analysis. Next, 4 ml of hexanes solvent were added and the sample was mixed with a Pasteur pipette. The sample then was centrifuged at 900 g for 5 min to create a clear phase separation. The upper phase was removed and the extraction was repeated twice after each addition of 4 ml of hexanes to the sample. The combined upper phases from 3 consecutive extractions were dried down in a Speedvac. The dried sample was redissolved in 200 µl of hexanes and loaded onto a silica HPLC column Supelcosil LC-SI, 150 × 4.5 mm, 3 µm particle size (Supelco, Bellefonte, PA, USA). The column had been equilibrated with hexanes for 10 min prior to sample injection. Retinoids were eluted according to the following HPLC program: 0–10 min, 100:0 (hexanes:ethyl acetate, v/v); 10–20 min, 99.5:0.5; 20–35 min, 90:10; 35–45 min, 100:0. The flow rate was set to 1 ml/min.
Analysis of the Ala69→Ser change in ARMS2
The amino acid change from Ala69→Ser was evaluated by Sanger sequencing that identified the rs10490924 G→T mutation. DNA was isolated from whole blood with the Qiamp DNA Blood Mini Kit (Qiagen Sciences, Germantown, MD, USA) following the manufacturer’s instructions. PCR amplifications were performed in 50 μl final volumes. Each PCR mixture contained 10 μl of purified DNA (∼100 ng), 10 μl 5× GoTaq buffer, 1 μl (1 mM final concentration) primers, forward 5′-CCTTATTTGGAAAATGGATATAATCAAAATC-3′; reverse 5′ GTGTACTTACTGACACGGATGC-3′ (Operon, Huntsville, AL, USA), 1 μl deoxynucleotide mix (200 mM final concentration), 0.25 μl GoTaq2 (Promega, Madison, WI, USA), and 26.75 μl H2O. After an initial 2 min at 95°C, samples were processed through 34 cycles of 30 s at 95°C, 30 s at 54°C, 1 min at 72°C, and a final 5 min at 72°C. Then, 10 μl of each reaction was run on a 1% agarose gel and stained with ethidium bromide to visualize products of the reaction. Sanger sequencing was performed (Genomics Core, Cleveland Clinic, Cleveland, OH, USA) with the primer: 5′ CTTTAGTTCGTCTTCAGTTATACATT. Sequencing data were checked for the G/T mutation with FinchTV software (version 1.4.0; Geospiza/PerkinElmer, Seattle, WA, USA).
Receiver operator characteristic curves
Receiver operator characteristic (ROC) curves were constructed to assess the biomarker value of 1 or more compounds (41). Data analyses and visualization were performed with the Web version of ROCCET software (The Metabolomics Innovation Centre, Edmonton, AB, Canada) (42, 43). Model construction was achieved with 2 sets of latent variables grouped as AMD and controls.
Statistical analyses
Statistical analyses [evaluation of odds ratios (ORs) and P values] were performed using custom code in the R package software (version 3.1.1; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Changes observed in Abca4−/−Rdh8−/− mice
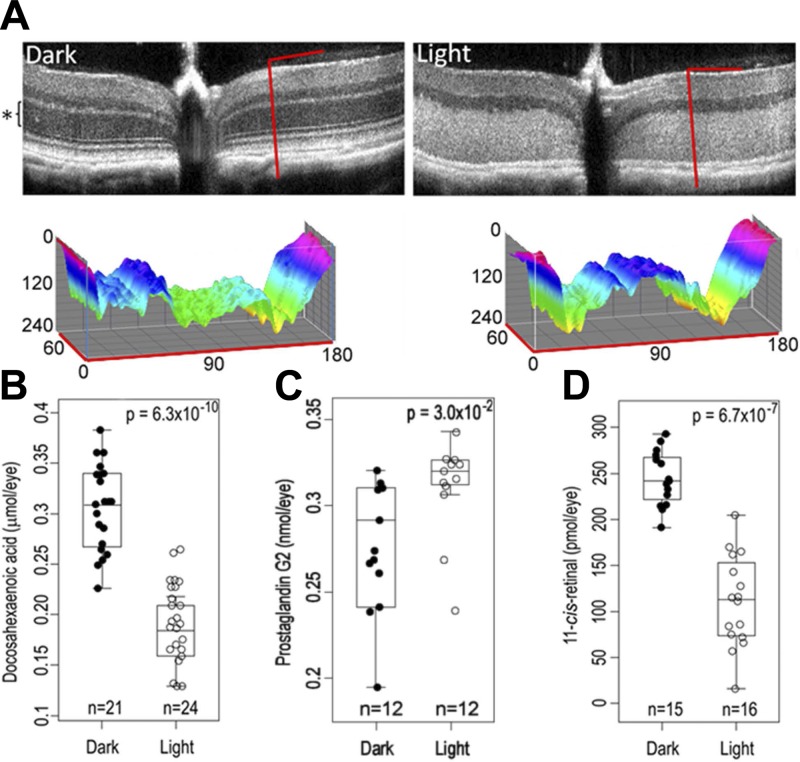
Because Abca4−/−Rdh8−/− mice develop severe massive synchronized photoreceptor degeneration following bright light exposure (44, 45), this model can reveal what is released when photoreceptors disintegrate. SD-OCT images collected from dark-adapted (Fig. 1A, left) and light-exposed (Fig. 1A, right) retinal samples demonstrated retinal degeneration in the light-exposed sample evidenced by a thinner ONL (compare the ONL thickness in Fig. 1A, black asterisk, with that in Fig. 1B). Mass spectrometry analyses of the chloroform/methanol/water organic layer that extracted lipids from the hydrolyzed Abca4−/−Rdh8−/− whole-eye samples indicated several statistically different features between dark-adapted and light-exposed preparations following our principal component analysis (Supplemental Fig. 1). Two major components, DHA and prostaglandin G2, were found to be dysregulated between light-exposed and dark-adapted samples. Initially, these 2 molecules were found in the list of identified loadings (based on MS/MS data from the XCMS database). Then both molecules were verified by comparing their MS/MS profiles to those of authentic standards (Supplemental Fig. 2).
Figure 1.
Characterization of Abca4−/−Rdh8−/− mouse retinas by SD-OCT and of Abca4−/−Rdh8−/− whole eye homogenates by mass spectrometry. A) Representative SD-OCT images of Abca4−/−Rdh8−/− mouse eyes recorded from either dark-adapted (left) or light-exposed (right) retinas. The ONL seen in the image recorded for the dark-adapted eye is labeled with a black asterisk. Slices (denoted by the inverted L-shaped red lines in the optical coherent tomography images) are presented in 3 dimensions based on the following information: the OZ axis presents a normalized intensity of pixels (0–240, black to white), the OX axis (0–180) shows the vertical slice of the SD-OCT image, whereas the OY axis (0–60) reveals the horizontal slice of the SD-OCT image. The surface is colored using the color wheel with both the brightest (OZ = 0, black) and dimmest (OZ = 240, white) points mapped to red. B–D) Ocular levels of DHA (B), prostaglandin G2 (C), and 11-cis-retinal (D).
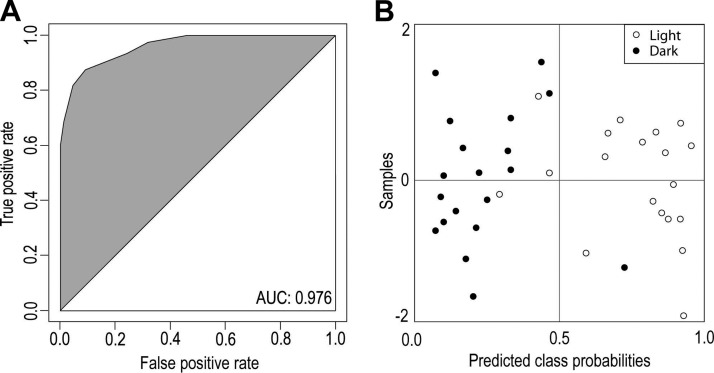
DHA levels were decreased in the light-exposed samples (Fig. 1B). The ROC curve constructed for DHA, similar to that for 11-cis-retinal, indicated that ocular levels of DHA were sufficiently different in light-exposed vs. dark-adapted samples to distinguish them from each other [area under the curve (AUC) = 0.999]. The feature identified as prostaglandin G2 (with an authentic standard; Supplemental Fig. 2) was also found to differ between light-exposed versus dark-adapted samples with levels (Fig. 1C) increased in light-exposed samples (P = 0.03). Following light exposure, retinas of these mice also contained reduced levels of 11-cis-retinal (Fig. 1D). A statistical model solely employing 11-cis-retinal sufficed to delineate between light- and dark-adapted samples as evidenced by its ROC curve (42) (AUC = 0.998). The ROC curve constructed for prostaglandin G2 indicated a lower predictive value for retinal degeneration (AUC = 0.790) as compared with either 11-cis-retinal or DHA. We found no statistically significant differences in levels of AA, EPA, LA, OA, or PA (P > 0.05). Thus a model that included DHA, 11-cis-retinal, and prostaglandin G2 resulted in a ROC curve that had an AUC = 0.976 (Fig. 2A). The model that included all 3 parameters (i.e., DHA, 11-cis-retinal, and prostaglandin G2; Fig. 2B) placed 94% of the dark-adapted samples into the correct group.
Figure 2.
Predicted class probabilities based on AUC values. In a model developed with the ROCCET online server, the classification boundary was set at the center (x = 0.5). A) ROC curve for the 3 combined biomarkers. B) Predicted class probabilities for the Abca4−/−Rdh8−/− mouse model samples (dark-adapted vs. light-exposed) constructed from 3 variables: DHA, 11-cis-retinal, and prostaglandin G2. This model places 85% of the samples in the correct group.
Changes observed in C57BL/6J mice
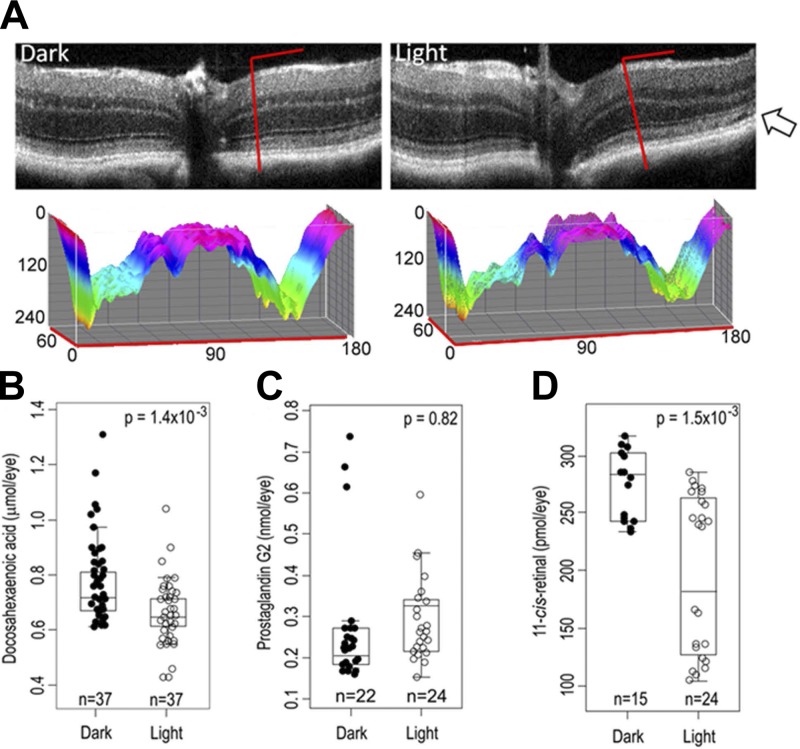
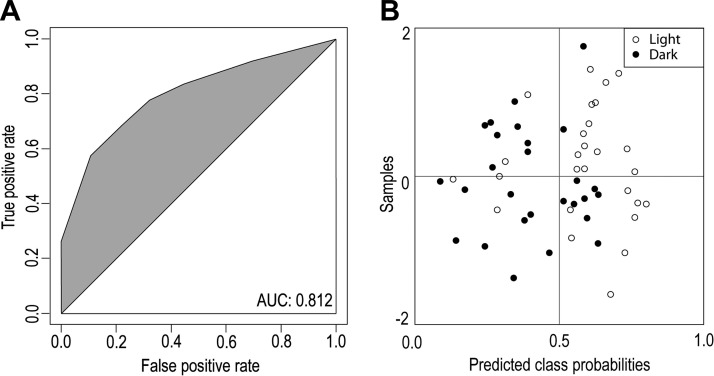
We then tested whether the same biomarkers (i.e., DHA, 11-cis-retinal, and prostaglandin G2) would be dysregulated in C57BL/6J mice, a model that displays a more gradual death of photoreceptors after bright light exposure (Fig. 2). Representative optical coherent tomography images collected for C57BL/6J mouse model samples exposed to intense light did not reproduce the increased retinal degeneration of the ONL seen in Abca4−/−Rdh8−/− mice (Fig. 1A). This was evidenced mainly by the intact retinal layers in samples from C57BL/6J mice (Fig. 3A). However, some light-exposed samples did show a thinning of the ONL despite having all their retinal layers intact (Fig. 3A, arrow). DHA levels were decreased in light-exposed samples (Fig. 3B), whereas prostaglandin G2 levels were unaffected (Fig. 3C). For 11-cis-retinal, a statistically significant difference was observed for the mean values of samples from light-exposed versus dark-adapted animals. However, values for the light-exposed samples displayed a bimodal distribution. About half (54%) of light-exposed C57BL/6J samples showed a full recovery of 11-cis-retinal as evidenced by levels similar to those in the dark-adapted samples (average of 276 ± 31 pmol/eye; Fig. 3D). The remaining light-exposed samples had an average value of ∼191 ± 71 pmol/eye. The likely reason for this bimodal distribution is that some C57BL/6J samples did not fully regenerate 11-cis-retinal levels to their original dark-adapted concentrations. We found no statistically significant differences in ocular levels of AA, EPA, LA, OA, or PA (P > 0.05). A model that included DHA and 11-cis-retinal resulted in a ROC curve that had an AUC = 0.812 (Fig. 4A) and, placed 62% of the dark-adapted samples in the correct group (Fig. 4B).
Figure 3.
Characterization of C57BL/6J mouse retinas by SD-OCT and of C57BL/6J whole eye homogenates by mass spectrometry. A) Representative SD-OCT images recorded from dark-adapted (left) and light-exposed (right) retinas from C57BL/6J mice. The ONL is visible in both dark-adapted and light-exposed retinas. Slices (denoted by the inverted L-shaped red lines in the optical coherent tomography images) are represented in 3 dimensions based on the following information: the OZ axis presents a normalized intensity of pixels (0–240, black to white), the OX axis (0–180) shows the vertical slice of the SD-OCT image whereas the OY (0–60) axis reveals the horizontal slice of the SD-OCT image. The surface is colored using a color wheel with both the brightest (OZ = 0, black) and dimmest (OZ = 240, white) points mapped to red. The rare occurrence of thinning of the ONL is shown with an arrow. B–D) Ocular levels of DHA (B), prostaglandin G2 (C), and 11-cis-retinal (D).
Figure 4.
Predicted class probabilities based on AUC values. The ROCCET online server (43) was used to develop this model with the classification boundary set at the center (x = 0.5). A) ROC curve for the 3 combined biomarkers. B) Predicted class probabilities for the C57BL/6J mouse samples (dark-adapted and light-exposed) constructed from 2 variables, namely DHA and 11-cis-retinal. This model placed 81% of the samples in the correct group.
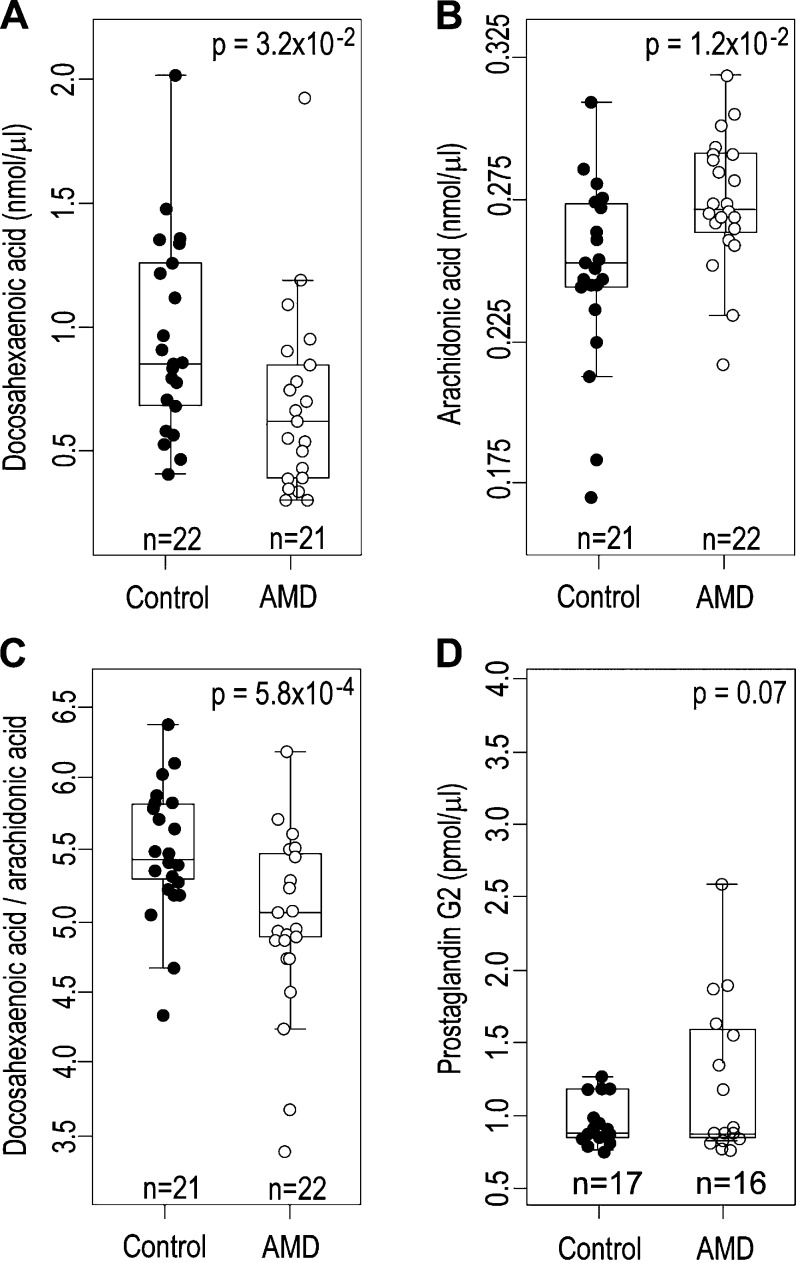
General characterization of human blood samples
We then obtained 21 blood samples from individuals with AMD, and 24 individuals without discernible signs of this disease. First, we performed a genetic screen for the Ala69→Ser change (G→T mutation) in the ARMS2 gene, which is implicated in development of AMD (32). Mass spectrometry analysis of serum lipids indicated that DHA levels in the AMD samples were decreased as compared with those in the non-AMD control group (Fig. 5A). In addition, we also found a statistically significant difference in AA serum levels and the ratios of DHA/AA serum levels between AMD and control samples (Fig. 5B, C). Prostaglandin G2 levels were not statistically different between the 2 groups (Fig. 5D, P = 0.07), but there was a correlation between AMD and higher levels of prostaglandin G2 (OR = 4.5, 95% confidence interval 0.75–26.9). Statistical modeling indicated only a small correlation (OR = 2.6, 95% confidence interval 0.79–8.9; Table 1) between the G→T mutation previously associated with AMD. As a consequence, the genetic screen for the Ala69→Ser change (G→T mutation) in the ARMS2 gene alone was not a good predictor of AMD development in the samples we studied.
Figure 5.
Representative compounds quantified from human serum samples. A–C) Serum concentrations of DHA (A), AA (B), and the ratios between serum DHA and AA (C). D) Serum concentrations of prostaglandin G2.
TABLE 1.
Frequency of the G→T mutation (Ala69→Ser change, rs10490924) in the ARMS2 gene in individuals with AMD and controls
OR = 2.6; 95% confidence interval 0.79–8.9; P = 0.1926.
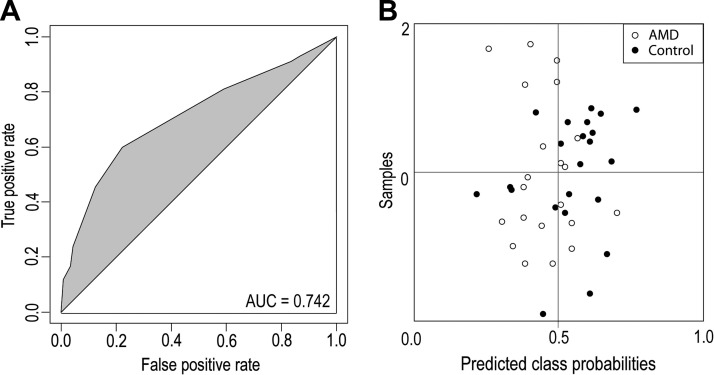
ORs of deidentified human serum samples for tobacco usage and sex were completed. The OR calculated for tobacco usage was 1.6 (95% confidence interval 0.7–3.6, P = 0.1); the OR for gender was 0.3 (95% confidence interval 0.15–0.8, P = 0.015). These statistical data indicate that tobacco use did not correlate significantly with an increased incidence of AMD in our sample cohort whereas females were more susceptible to AMD than males. Generation of a ROC curve constructed using DHA:AA ratios resulted in an AUC = 0.742 (Fig. 6A), placing 74% of the samples in the correct group (Fig. 6B). Inclusion of the genetic screen for the ARMS2 G→T mutation derived from human serum samples (Table 1) in the risk evaluation of DHA:AA ratio failed to improve this model. Moreover, prostaglandin G2 levels did not statistically differ in the AMD group as compared with controls.
Figure 6.
Predicted class probabilities based on AUC values. This model also was developed with the ROCCET online server (43) with the classification boundary set at the center (x = 0.5). A) ROC curve for the DHA:AA ratios. B) Predicted class probabilities for the human serum samples (AMD-diagnosed and controls without AMD) constructed from 2 variables, namely DHA and AA. This model placed 74% of the samples in the correct group.
DISCUSSION
Complex diseases progress through complex pathways. Each individual may display a different set of early changes that could contribute to the final outcome. AMD is a multifactorial disease with a complex and not yet fully explored etiology. The final stages of this disease can be followed by optical methods that currently include fundus pictures, electroretinography, optical coherent tomography, and scanning laser ophthalmoscopy imaging (46–48). These imaging techniques are applicable to the final stages of AMD because of the major structural changes that occur such as geographic atrophy or demise of photoreceptor cells. Some of the other structural changes could possibly be reversible. Functional assays of dark adaptation are also important for monitoring progression of the disease (22–24, 49, 50). In summary, current diagnostic tools for AMD are effective only after the disease is well advanced. Thus, there is an urgent medical need for development of biomarkers and early risk assessment models that could facilitate detection of AMD onset and increase the potential efficacy of therapeutic interventions.
Our aim in this study of AMD mouse models and patient samples was to create a new risk evaluation model with the potential to predict generation of AMD, based on dysregulated levels of small molecules. Photoreceptor cell death is a fundamental trademark of AMD. Thus, the initial hypothesis was that photoreceptor degeneration is accompanied by a cohort of molecules that are either released into or depleted in the serum of patients with AMD. Moreover, dysregulation of retinal layers observed in SD-OCT images (photoreceptor layers) correlates with retinal degeneration due to increased photoreceptor cell death.
This study was divided into 3 sets of experiments. We first started with genetically altered Abca4−/−Rdh8−/− mice that exhibit light-induced (10,000 lux for 1 h) photoreceptor degeneration. Second, we followed with light-exposed wild-type mice that display only mild, if any, retinal degeneration. Using mass spectrometry, we identified changes in prostaglandin G2 levels as one feature of dysregulated metabolism in the eyes of Abca4−/−Rdh8−/− mice following light exposure. Because prostaglandin G2 is a product of AA metabolism by cyclooxygenase-1, an enzyme involved in inflammation (51), we conclude that its altered levels in light-exposed eyes are likely due to an inflammatory process. Differences in DHA between light-exposed and dark-adapted samples of C57BL/6J mouse eyes were statistically significant but not as prominent as those in Abca4−/−Rdh8−/− mouse eyes (P = 1.4 × 10−2 vs. P = 6.4 × 10−10, respectively). We also found that dark-adapted baseline levels of DHA were more than twice as high in C57BL/6J mice as compared with Abca4−/−Rdh8−/− mice (0.7 μmol/eye vs. 0.3 μmol/eye). These higher baseline levels in C57BL/6J mice could explain the increased resistance to photoreceptor damage following exposure to intense light. Prostaglandin G2 levels were not significantly different between light-exposed and dark-adapted groups of C57BL/6J mice, but we did note a potential correlation between higher levels of prostaglandin G2 and light exposure (P = 0.82, OR = 4.2, 95% confidence interval 0.97–18.1). This result also could be explained by the higher resistance of these mice to light-induced photoreceptor degeneration together with reduced retinal inflammation. Proinflammatory levels of chemokine (C-C motif) ligand 2 and chemokine (C-C motif) ligand 3 cytokines were reportedly elevated in Abca4−/−Rdh8−/− mice after intense light exposure (52, 53). DHA and 11-cis-retinal levels are positively correlated with photoreceptor numbers and health. DHA is the major fatty acid component of the retina in humans and other vertebrate animals (54), whereas 11-cis-retinal is the chromophore for rhodopsin, the most abundant protein in photoreceptor cells. That AMD results in photoreceptor death is clearly reflected in our measurements of DHA and 11-cis-retinal (Figs. 5, 6).
Finally, in our third set of experiments, we analyzed serum samples from both patients with AMD and controls. We found that AA levels were dysregulated between AMD and control serum samples (AA levels within the eye did not statistically differ in either mouse model under study). Further studies are needed to establish the full mechanism by which the DHA:AA ratio relates to AMD. Our human serum profiles for prostaglandin G2 levels did not reproduce the differences observed in Abca4−/−Rdh8−/− mouse eyes (Fig. 5D). Instead, the profile reproduced observations drawn from the C57BL/6J mouse model wherein there was a correlation between the higher G2 levels and the presence of AMD (OR = 5.8, 95% confidence interval 0.98–34.4) even though prostaglandin G2 levels did not differ between the AMD and control groups. The importance of the Ala69→Ser change in ARMS2 mutation in predicting the incidence of AMD was previously described (32). Our finding that addition of this second parameter did not improve our model is not surprising. In a similar manner, when C-reactive protein levels, known to be associated with an increased incidence of coronary heart disease, were added to the modified Framingham risk scores, only a limited improvement in the prediction of coronary heart disease was detected (55). In an ROC curve, the importance of both the sensitivity and the specificity of the biomarker are equally weighed. However, clinically, specificity might be favored over sensitivity. For example, when screening the general population for a disease with a low incidence requiring expensive or invasive follow-ups, high specificity is preferred. In contrast, cancer screening in a high-risk population would benefit from a biomarker that has higher sensitivity (56). Thus, instead of using 1 model that bundles together all biomarkers, it seems more advisable to use a cumulative evaluation with different biomarkers. This strategy could embrace all known biomarkers and also balance the need for both specificity and sensitivity when applied to a general population.
DHA was the only statistically significant dysregulated feature identified from our mouse model analyses that was translatable to human AMD serum samples. Recently, several cytokines were proposed to be involved in AMD, further implicating inflammation in this disease (57). Exposure to high-intensity light for a prolonged period was previously shown to induce an inflammatory response manifested by anti-inflammatory molecules such as complement factor H, hepatic arginase, and TGF-β1 (58). We measured the expression of the ELOVL-4 gene, which codes for an enzyme that has a role in fatty acid elongation and that was previously associated with Stargardt-like macular degeneration phenotype (59, 60). At the RNA level, no statistically significant differences were observed between dark- and light-exposed samples (data not shown). Our studies recapitulated the results obtained in patients with AMD (61, 62), further denoting the complexity of the macular degeneration phenotypes. However, very-long-chain polyunsaturated fatty acids (whose production is catalyzed by ELOVL-4) are similar to DHA because of their role in photoreceptor function and integrity (63). As a consequence, it is reasonable to expect that, analogous to DHA, changes in levels of the very long polyunsaturated fatty acids could be early indicators of declining photoreceptor health. Our study is in line with recent studies that show promising value in evaluation of AMD risk factors or monitor progression of the disease in patients diagnosed with AMD, by measuring serum levels of polyunsaturated fatty acids from serum (64). Our study was performed on Japanese patients who have high dietary intake of polyunsaturated fatty acids resulting from a diet rich in fish. Further dissection of the specific relevance of our findings in a heterogeneous population would be a natural progression to test the applicability of our findings for non-Japanese patients.
Here, we describe a simple and rapid diagnostic tool that employs the ratio between DHA and AA to assess the potential risk for AMD, using mass spectrometric analysis of serum samples. Our final analysis of just these 2 lipids allows a 74% probability of correctly distinguishing AMD samples from control samples. This model is on par with other medical assays. For example, photostress recovery evaluation was used to predict AMD with a 71% success rate (24), and optical coherence tomography was shown to detect the presence of AMD based on the presence of a prominent hyper-reflective haze present in the photoreceptor nuclear layer over drusen in 67% of patients with AMD (65). Small molecule biomarkers from the protein adduct class of compounds such as carboxyethylpyrrole were shown to discriminate between AMD and control plasma donors with ∼76% accuracy (66). When carboxyethylpyrrole–protein adduct markers were used in combination with genomic markers, they were shown to provide up to ∼80% discrimination accuracy. A follow-up study also showed that plasma protein Nε-carboxymethyllysine, carboxyethylpyrrole protein adducts, and pentosidine alone discriminated between AMD and control subjects with accuracies between 78 and 88%. When these parameters were used in combination, the accuracy levels of correctly identifying patients with AMD was found to be between 80 and 90% (67). A proteomics study found that vinculin had an 80% accuracy for AMD detection with an added 10% improvement upon adding a test for the ARMS2 gene (68). Oxidative stress by-products such as malonaldehyde and 4-hydroxy-2-nonenal were also found to be elevated in patients with AMD (69). In addition, biomarkers that are relevant in other diseases, such as cardiovascular disease, were also evaluated. Among these, homocysteine and C-reactive protein seem to be elevated in patients with AMD (70, 71). Improvement in the sample size and similar analysis of a larger number of lipids could improve the predictability of our approach.
In conclusion, in proof-of-concept studies we identified early molecular markers, DHA and AA, for retinal degeneration by using mass spectrometric analyses of mouse models. These small molecules were also found to be dysregulated in human serum samples from individuals suffering from AMD. Thus, using our unbiased strategy we have generated a risk assessment model that may be predictive for identifying humans at risk of developing this blinding disease.
Acknowledgments
The authors thank Drs. Leslie T. Webster, Jr., Daniel Figeys, and members of the K.P. laboratory for helpful comments on this manuscript; and Drs. Philip D. Kiser, Marcin Golczak, and Yaroslav Tsybovsky (all from Case Western Reserve University) for support and advice during this study. This work was supported by funding from the U.S. National Institutes of Health, National Eye Institute Grants EY009339 and EY021126 (to K.P.), the Foundation Fighting Blindness, and the Arnold and Mabel Beckman Foundation. K.P. is a John H. Hord Professor of Pharmacology. The authors declare no conflicts of interest.
Glossary
- 5-HETE
5-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid
- 8-HETE
8-hydroxy-5Z,9E,11Z,14Z-eicosatetraenoic acid
- 9-HETE
9-hydroxy-5Z,7E,11Z,14Z-eicosatetraenoic
- 9-HODE
9-hydroxy-10E,12Z-octadecadienoic acid
- 11-HETE
11-hydroxy-5Z,8Z,12E,14Z-eicosatetraenoic acid
- 12-HETE
12-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid
- 13-HODE
13-hydroxy-9Z,11E-octadecadienoic acid
- 15-HETE
15-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid
- AA
arachidonic acid
- AA-d8
arachidonic acid with 8 deuterium atoms at the 5, 6, 8, 9, 11, 12, 14, and 15 positions
- AMD
age-related macular degeneration
- ARMS2
age-related maculopathy susceptibility 2
- DHA
docosahexaenoic acid
- DHA-d5
docosahexaenoic acid with 5 deuterium atoms at the 21, 21, 22, 22, and 22 positions
- EPA
eicosapentaenoic acid
- EPA-d5
eicosapentaenoic acid with 5 deuterium atoms at the 19, 19′, 20, 20, and 20 positions
- F2 isoprostane
9α,11α,15R-trihydroxy-(8β)-prosta-5Z,13E-dien-1-oic acid
- FA
formic acid
- LA
linoleic acid
- MS/MS
tandem mass spectrometry
- m/z
mass to charge ratio
- ONL
outer nuclear layer
- OR
odds ratio
- PA
palmitic acid
- ROC
receiver operator characteristic
- SIM
selected ion monitoring
- SD-OCT
spectral domain–optical coherence tomography
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Gelfant S., Smith J. G. Jr (1972) Aging: noncycling cells an explanation. Science 178, 357–361 [DOI] [PubMed] [Google Scholar]
- 2.Shafto M. A., Tyler L. K. (2014) Language in the aging brain: the network dynamics of cognitive decline and preservation. Science 346, 583–587 [DOI] [PubMed] [Google Scholar]
- 3.Lindenberger U. (2014) Human cognitive aging: corriger la fortune? Science 346, 572–578 [DOI] [PubMed] [Google Scholar]
- 4.Gutchess A. (2014) Plasticity of the aging brain: new directions in cognitive neuroscience. Science 346, 579–582 [DOI] [PubMed] [Google Scholar]
- 5.Guarente L. (1997) Aging. What makes us tick? Science 275, 943–944 [DOI] [PubMed] [Google Scholar]
- 6.Finch C. E., Tanzi R. E. (1997) Genetics of aging. Science 278, 407–411 [DOI] [PubMed] [Google Scholar]
- 7.Van Deursen J. M. (2014) The role of senescent cells in ageing. Nature 509, 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop N. A., Lu T., Yankner B. A. (2010) Neural mechanisms of ageing and cognitive decline. Nature 464, 529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vijg J., Campisi J. (2008) Puzzles, promises and a cure for ageing. Nature 454, 1065–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nussenblatt R. B., Lee R. W., Chew E., Wei L., Liu B., Sen H. N., Dick A. D., Ferris F. L. (2014) Immune responses in age-related macular degeneration and a possible long-term therapeutic strategy for prevention. Am. J. Ophthalmol. 158, 5–11.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan J., Huckfeldt R., Wong R. O. (2005) Imaging techniques in retinal research. Exp. Eye Res. 80, 297–306 [DOI] [PubMed] [Google Scholar]
- 12.Costa R. A., Skaf M., Melo L. A. Jr., Calucci D., Cardillo J. A., Castro J. C., Huang D., Wojtkowski M. (2006) Retinal assessment using optical coherence tomography. Prog. Retin. Eye Res. 25, 325–353 [DOI] [PubMed] [Google Scholar]
- 13.Van Velthoven M. E., Faber D. J., Verbraak F. D., van Leeuwen T. G., de Smet M. D. (2007) Recent developments in optical coherence tomography for imaging the retina. Prog. Retin. Eye Res. 26, 57–77 [DOI] [PubMed] [Google Scholar]
- 14.Drexler W., Fujimoto J. G. (2008) State-of-the-art retinal optical coherence tomography. Prog. Retin. Eye Res. 27, 45–88 [DOI] [PubMed] [Google Scholar]
- 15.Gabriele M. L., Wollstein G., Ishikawa H., Xu J., Kim J., Kagemann L., Folio L. S., Schuman J. S. (2010) Three dimensional optical coherence tomography imaging: advantages and advances. Prog. Retin. Eye Res. 29, 556–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll J., Kay D. B., Scoles D., Dubra A., Lombardo M. (2013) Adaptive optics retinal imaging—clinical opportunities and challenges. Curr. Eye Res. 38, 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heimann H., Jmor F., Damato B. (2013) Imaging of retinal and choroidal vascular tumours. Eye (Lond.) 27, 208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palczewska G., Dong Z., Golczak M., Hunter J. J., Williams D. R., Alexander N. S., Palczewski K. (2014) Noninvasive two-photon microscopy imaging of mouse retina and retinal pigment epithelium through the pupil of the eye. Nat. Med. 20, 785–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter J. J., Masella B., Dubra A., Sharma R., Yin L., Merigan W. H., Palczewska G., Palczewski K., Williams D. R. (2010) Images of photoreceptors in living primate eyes using adaptive optics two-photon ophthalmoscopy. Biomed. Opt. Express 2, 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palczewska G., Vinberg F., Stremplewski P., Bircher M. P., Salom D., Komar K., Zhang J., Cascella M., Wojtkowski M., Kefalov V. J., Palczewski K. (2014) Human infrared vision is triggered by two-photon chromophore isomerization. Proc. Natl. Acad. Sci. USA 111, E5445–E5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palczewska G., Golczak M., Williams D. R., Hunter J. J., Palczewski K. (2014) Endogenous fluorophores enable two-photon imaging of the primate eye. Invest. Ophthalmol. Vis. Sci. 55, 4438–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owsley C., Jackson G. R., Cideciyan A. V., Huang Y., Fine S. L., Ho A. C., Maguire M. G., Lolley V., Jacobson S. G. (2000) Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 41, 267–273 [PubMed] [Google Scholar]
- 23.Dimitrov P. N., Robman L. D., Varsamidis M., Aung K. Z., Makeyeva G., Busija L., Vingrys A. J., Guymer R. H. (2012) Relationship between clinical macular changes and retinal function in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 53, 5213–5220 [DOI] [PubMed] [Google Scholar]
- 24.Dimitrov P. N., Robman L. D., Varsamidis M., Aung K. Z., Makeyeva G. A., Guymer R. H., Vingrys A. J. (2011) Visual function tests as potential biomarkers in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 52, 9457–9469 [DOI] [PubMed] [Google Scholar]
- 25.Chader G. J. (2002) Animal models in research on retinal degenerations: past progress and future hope. Vision Res. 42, 393–399 [DOI] [PubMed] [Google Scholar]
- 26.Pennesi M. E., Neuringer M., Courtney R. J. (2012) Animal models of age related macular degeneration. Mol. Aspects Med. 33, 487–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fletcher E. L., Jobling A. I., Vessey K. A., Luu C., Guymer R. H., Baird P. N. (2011) Animal models of retinal disease. Prog. Mol. Biol. Transl. Sci. 100, 211–286 [DOI] [PubMed] [Google Scholar]
- 28.Ding X., Patel M., Chan C. C. (2009) Molecular pathology of age-related macular degeneration. Prog. Retin. Eye Res. 28, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda A., Maeda T., Golczak M., Palczewski K. (2008) Retinopathy in mice induced by disrupted all-trans-retinal clearance. J. Biol. Chem. 283, 26684–26693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y., Palczewska G., Mustafi D., Golczak M., Dong Z., Sawada O., Maeda T., Maeda A., Palczewski K. (2013) Systems pharmacology identifies drug targets for Stargardt disease-associated retinal degeneration. J. Clin. Invest. 123, 5119–5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeda A., Golczak M., Chen Y., Okano K., Kohno H., Shiose S., Ishikawa K., Harte W., Palczewska G., Maeda T., Palczewski K. (2012) Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat. Chem. Biol. 8, 170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanda A., Chen W., Othman M., Branham K. E., Brooks M., Khanna R., He S., Lyons R., Abecasis G. R., Swaroop A. (2007) A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc. Natl. Acad. Sci. USA 104, 16227–16232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida T., DeWan A., Zhang H., Sakamoto R., Okamoto H., Minami M., Obazawa M., Mizota A., Tanaka M., Saito Y., Takagi I., Hoh J., Iwata T. (2007) HTRA1 promoter polymorphism predisposes Japanese to age-related macular degeneration. Mol. Vis. 13, 545–548 [PMC free article] [PubMed] [Google Scholar]
- 34.Maeda A., Maeda T., Sun W., Zhang H., Baehr W., Palczewski K. (2007) Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc. Natl. Acad. Sci. USA 104, 19565–19570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeda A., Maeda T., Golczak M., Chou S., Desai A., Hoppel C. L., Matsuyama S., Palczewski K. (2009) Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J. Biol. Chem. 284, 15173–15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimm C., Wenzel A., Stanescu D., Samardzija M., Hotop S., Groszer M., Naash M., Gassmann M., Remé C. (2004) Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. J. Neurosci. 24, 5651–5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattapallil M. J., Wawrousek E. F., Chan C. C., Zhao H., Roychoudhury J., Ferguson T. A., Caspi R. R. (2012) The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest. Ophthalmol. Vis. Sci. 53, 2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pittler S. J., Baehr W. (1991) Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc. Natl. Acad. Sci. USA 88, 8322–8326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider C. A., Rasband W. S., Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patti G. J., Tautenhahn R., Rinehart D., Cho K., Shriver L. P., Manchester M., Nikolskiy I., Johnson C. H., Mahieu N. G., Siuzdak G. (2013) A view from above: cloud plots to visualize global metabolomic data. Anal. Chem. 85, 798–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zweig M. H., Campbell G. (1993) Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39, 561–577 [PubMed] [Google Scholar]
- 42.Lasko T. A., Bhagwat J. G., Zou K. H., Ohno-Machado L. (2005) The use of receiver operating characteristic curves in biomedical informatics. J. Biomed. Inform. 38, 404–415 [DOI] [PubMed] [Google Scholar]
- 43.Xia J., Broadhurst D. I., Wilson M., Wishart D. S. (2013) Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics 9, 280–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maeda A., Palczewski K. (2013) Retinal degeneration in animal models with a defective visual cycle. Drug Discov. Today Dis. Models 10, e163–e172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maeda A., Palczewska G., Golczak M., Kohno H., Dong Z., Maeda T., Palczewski K. (2014) Two-photon microscopy reveals early rod photoreceptor cell damage in light-exposed mutant mice. Proc. Natl. Acad. Sci. USA 111, E1428–E1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitz-Valckenberg S., Fleckenstein M., Helb H. M., Charbel Issa P., Scholl H. P., Holz F. G. (2009) In vivo imaging of foveal sparing in geographic atrophy secondary to age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 50, 3915–3921 [DOI] [PubMed] [Google Scholar]
- 47.Holz F. G., Strauss E. C., Schmitz-Valckenberg S., van Lookeren Campagne M. (2014) Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology 121, 1079–1091 [DOI] [PubMed] [Google Scholar]
- 48.Holz F. G., Bindewald-Wittich A., Fleckenstein M., Dreyhaupt J., Scholl H. P., Schmitz-Valckenberg S.; FAM-Study Group (2007) Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am. J. Ophthalmol. 143, 463–472 [DOI] [PubMed] [Google Scholar]
- 49.Gaffney A. J., Binns A. M., Margrain T. H. (2013) The effect of pre-adapting light intensity on dark adaptation in early age-related macular degeneration. Doc. Ophthalmol. 127, 191–199 [DOI] [PubMed] [Google Scholar]
- 50.Owsley C., Huisingh C., Jackson G. R., Curcio C. A., Szalai A. J., Dashti N., Clark M., Rookard K., McCrory M. A., Wright T. T., Callahan M. A., Kline L. B., Witherspoon C. D., McGwin G. Jr (2014) Associations between abnormal rod-mediated dark adaptation and health and functioning in older adults with normal macular health. Invest. Ophthalmol. Vis. Sci. 55, 4776–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marnett L. J., Rowlinson S. W., Goodwin D. C., Kalgutkar A. S., Lanzo C. A. (1999) Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J. Biol. Chem. 274, 22903–22906 [DOI] [PubMed] [Google Scholar]
- 52.Kohno H., Chen Y., Kevany B. M., Pearlman E., Miyagi M., Maeda T., Palczewski K., Maeda A. (2013) Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal. J. Biol. Chem. 288, 15326–15341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kohno H., Maeda T., Perusek L., Pearlman E., Maeda A. (2014) CCL3 production by microglial cells modulates disease severity in murine models of retinal degeneration. J. Immunol. 192, 3816–3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fliesler S. J., Anderson R. E. (1983) Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 22, 79–131 [DOI] [PubMed] [Google Scholar]
- 55.De Ruijter W., Westendorp R. G., Assendelft W. J., den Elzen W. P., de Craen A. J., le Cessie S., Gussekloo J. (2009) Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ 338, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grund B., Sabin C. (2010) Analysis of biomarker data: logs, odds ratios, and receiver operating characteristic curves. Curr. Opin. HIV AIDS 5, 473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nassar K., Grisanti S., Elfar E., Lüke J., Lüke M., Grisanti S. (2015) Serum cytokines as biomarkers for age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 253, 699–704 [DOI] [PubMed] [Google Scholar]
- 58.Guymer R. H., Tao L. W., Goh J. K., Liew D., Ischenko O., Robman L. D., Aung K., Cipriani T., Cain M., Richardson A. J., Baird P. N., Langham R. (2011) Identification of urinary biomarkers for age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 52, 4639–4644 [DOI] [PubMed] [Google Scholar]
- 59.McMahon A., Butovich I. A., Mata N. L., Klein M., Ritter R. III, Richardson J., Birch D. G., Edwards A. O., Kedzierski W. (2007) Retinal pathology and skin barrier defect in mice carrying a Stargardt disease-3 mutation in elongase of very long chain fatty acids-4. Mol. Vis. 13, 258–272 [PMC free article] [PubMed] [Google Scholar]
- 60.McMahon A., Jackson S. N., Woods A. S., Kedzierski W. (2007) A Stargardt disease-3 mutation in the mouse Elovl4 gene causes retinal deficiency of C32-C36 acyl phosphatidylcholines. FEBS Lett. 581, 5459–5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeAngelis M. M., Ji F., Kim I. K., Adams S., Capone A. Jr., Ott J., Miller J. W., Dryja T. P. (2007) Cigarette smoking, CFH, APOE, ELOVL4, and risk of neovascular age-related macular degeneration. Arch. Ophthalmol. 125, 49–54 [DOI] [PubMed] [Google Scholar]
- 62.Ayyagari R., Zhang K., Hutchinson A., Yu Z., Swaroop A., Kakuk L. E., Seddon J. M., Bernstein P. S., Lewis R. A., Tammur J., Yang Z., Li Y., Zhang H., Yashar B. M., Liu J., Petrukhin K., Sieving P. A., Allikmets R. (2001) Evaluation of the ELOVL4 gene in patients with age-related macular degeneration. Ophthalmic Genet. 22, 233–239 [DOI] [PubMed] [Google Scholar]
- 63.Agbaga M. P., Mandal M. N., Anderson R. E. (2010) Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. J. Lipid Res. 51, 1624–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Merle B. M. J., Benlian P., Puche N., Bassols A., Delcourt C., Souied E. H.; Nutritional AMD Treatment 2 Study Group (2014) Circulating omega-3 fatty acids and neovascular age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 55, 2010–2019 [DOI] [PubMed] [Google Scholar]
- 65.Schuman S. G., Koreishi A. F., Farsiu S., Jung S. H., Izatt J. A., Toth C. A. (2009) Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography. Ophthalmology 116, 488–496.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu J., Pauer G. J., Yue X., Narendra U., Sturgill G. M., Bena J., Gu X., Peachey N. S., Salomon R. G., Hagstrom S. A., Crabb J. W.; Clinical Genomic and Proteomic AMD Study Group (2009) Assessing susceptibility to age-related macular degeneration with proteomic and genomic biomarkers. Mol. Cell. Proteomics 8, 1338–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ni J., Yuan X., Gu J., Yue X., Gu X., Nagaraj R. H., Crabb J. W.; Clinical Genomic and Proteomic AMD Study Group (2009) Plasma protein pentosidine and carboxymethyllysine, biomarkers for age-related macular degeneration. Mol. Cell. Proteomics 8, 1921–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim H. J., Woo S. J., Suh E. J., Ahn J., Park J. H., Hong H. K., Lee J. E., Ahn S. J., Hwang D. J., Kim K. W., Park K. H., Lee C. (2014) Identification of vinculin as a potential plasma marker for age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 55, 7166–7176 [DOI] [PubMed] [Google Scholar]
- 69.Weismann D., Hartvigsen K., Lauer N., Bennett K. L., Scholl H. P., Charbel Issa P., Cano M., Brandstätter H., Tsimikas S., Skerka C., Superti-Furga G., Handa J. T., Zipfel P. F., Witztum J. L., Binder C. J. (2011) Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature 478, 76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vine A. K., Stader J., Branham K., Musch D. C., Swaroop A. (2005) Biomarkers of cardiovascular disease as risk factors for age-related macular degeneration. Ophthalmology 112, 2076–2080 [DOI] [PubMed] [Google Scholar]
- 71.Keles S., Ates O., Kartal B., Alp H. H., Ekinci M., Ceylan E., Ondas O., Arpali E., Dogan S., Yildirim K., Keles M. S. (2014) Evaluation of cardiovascular biomarkers in patients with age-related wet macular degeneration. Clin. Ophthalmol. 8, 1573–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]