Abstract
Coxsackievirus B3 (CVB3), an important human causative pathogen for viral myocarditis, pancreatitis, and meningitis, has evolved different strategies to manipulate the host signaling machinery to ensure successful viral infection. We previously revealed a crucial role for the ERK1/2 signaling pathway in regulating viral infectivity. However, the detail mechanism remains largely unknown. Grb2-associated binder 1 (GAB1) is an important docking protein responsible for intracellular signaling assembly and transduction. In this study, we demonstrated that GAB1 was proteolytically cleaved after CVB3 infection at G175 and G436 by virus-encoded protease 2Apro, independent of caspase activation. Knockdown of GAB1 resulted in a significant reduction of viral protein expression and virus titers. Moreover, we showed that virus-induced cleavage of GAB1 is beneficial to viral growth as the N-terminal proteolytic product of GAB1 (GAB1-N1–174) further enhances ERK1/2 activation and promotes viral replication. Our results collectively suggest that CVB3 targets host GAB1 to generate a GAB1-N1–174 fragment that enhances viral infectivity, at least in part, via activation of the ERK pathway. The findings in this study suggest a novel mechanism that CVB3 employs to subvert the host signaling and facilitate consequent viral replication.—Deng, H., Fung, G., Shi, J., Xu, S., Wang, C., Yin, M., Hou, J., Zhang, J., Jin, Z.-G., Luo, H. Enhanced enteroviral infectivity via viral protease-mediated cleavage of Grb2-associated binder 1.
Keywords: coxsackievirus B3, viral protease 2Apro, ERK1/2, signal transduction
Coxsackievirus B3 (CVB3), an enterovirus in the Picornaviridae family, is a common human pathogen associated with several fatal diseases, including viral myocarditis, pancreatitis, and meningitis, in infants and young children (1). Similar to many other viruses, CVB3 has evolved diverse mechanisms to modulate the host signaling machinery to ensure successful viral infection (2–4). We previously revealed an important role for the ERK1/2 signaling pathway in regulating viral replication (5). We demonstrated that CVB3 induces biphasic ERK1/2 activation: the early transient activation triggered by virus-receptor interaction and the late sustained activation mediated by intracellular signaling events depending on the production of virus-encoded proteins (5). Inhibition of the ERK1/2 pathway greatly blocks the virulence of CVB3 and attenuates virus-mediated pathogenesis (5). Despite the profound effects of the ERK1/2 signaling pathway on virus propagation, the upstream factors that regulate its activation remain poorly understood.
Grb2-associated binder 1 (GAB1) is a docking/scaffolding adaptor protein belonging to the family of insulin receptor substrate 1–like multisubstrate proteins (6). Emerging evidence has suggested that signaling mediated through GAB1 plays a critical role in the regulation of a variety of cellular processes, including cell proliferation, cell differentiation, apoptosis, and stress responses (7). GAB1 deficiency results in embryonic lethality due to severe defects in heart, placenta, liver, and spleen development (8). Disruption of GAB1-mediated signaling has been associated with multiple human diseases, including tumor, cardiovascular disease, and inflammation (9). GAB1 functions as a platform for assembling multiple intracellular signaling pathways evoked by various extracellular stimuli via its multiple functional domains, including a highly conserved pleckstrin homology (PH) domain at its N-terminal, a specific c-Met binding domain, proline-rich regions, and multiple tyrosine and serine/threonine phosphorylation residues (7, 10–12). Upon activation, GAB1 translocates from cytoplasm to the cellular membrane, where it promotes signaling amplification and transduction by tyrosine phosphorylation and recruitment of downstream proteins such as Src-homology-2-containing protein tyrosine phosphatase 2 (SHP2), p85, Crk, and phospholipase C γ, which further contributes to the activation of ERK1/2, PI3K, JNK, and STAT5 signaling pathways, respectively (10, 13, 14).
Given the important function of GAB1 in transducing signals from extracellular cues, we questioned whether CVB3 could manipulate GAB1 to gain advantage on viral replication. In this study, we demonstrated that GAB1 is cleaved during CVB3 infection by virus-encoded protease 2Apro, leading to the generation of an N-terminal cleaved fragment that facilitates viral replication, at least in part, through the activation of ERK1/2 signaling pathway.
MATERIALS AND METHODS
Cell culture and virus infection
HeLa cells were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM supplemented with 10% fetal bovine serum at 37°C in a humidified incubator with 5% CO2. CVB3 (Nancy strain) was propagated in HeLa cells and stored at −80°C. For viral infection, HeLa cells were incubated with serum-free medium containing either CVB3 at different multiplicity of infections or PBS (sham infection) for different period of time.
Plasmids and small interfering RNAs
The 3×Flag-GAB1G175E and 3×Flag-GAB1G436E mutants were established by replacing the glycine (G) of wild-type (WT) GAB1 at amino acids 175 and 436 with glutamic acid (E), respectively. The 3×Flag-GAB1WT was used as a template to generate 2 truncated fragments of GAB1 (3×Flag-GAB1-N1–174 and 3×Flag-GAB1-C175–694). The small interfering RNA (siRNA) against human GAB1 was purchased from Dharmacon (#L-131213; Dharmacon, Ottawa, ON, Canada).
Transient transfection and drug treatments
For plasmid or siRNA transfection, HeLa cells were transiently transfected with plasmid or siRNA using Lipofectamine 2000 (#11668019; Invitrogen, Burlington, ON, Canada) or Oligofectamine (#12252-011; Invitrogen), respectively, according to manufacturer’s instructions. For caspase-inhibition experiments, z-VAD-fmk (50 µM, #550377; BD Biosciences, San Jose, CA, USA), a pan-caspase inhibitor, was added to the medium of CVB3-infected HeLa cells at 1 h postinfection. For experiments involving ERK inhibition, HeLa cells cultured in serum-free medium were incubated with MEK inhibitor, U0126 (20 µM, #9903; Cell Signaling, Beverly, MA, USA), starting at 30 min prior to infection until the end of experiments.
Western blot analysis
Cells were harvested using modified oncogene science lysis buffer (250 mM NaCl, pH 7.2, 50 mM Tris-HCl, 0.1% NP-40, 2 mM EDTA, and 10% glycerol) supplemented with protease inhibitors. Western blotting was performed as previously described (15). Briefly, equal amounts of proteins were subjected to SDS-PAGE and then transferred to nitrocellulose membranes. After blocking with 5% nonfat dry milk solution containing 0.1% Tween-20 for 1 h, the membranes were incubated with primary antibodies at 4°C for overnight, followed by the incubation with horseradish peroxidase–conjugated secondary antibodies at room temperature for 1 h. The immunoreactive bands were visualized by enhanced chemiluminescence. Primary antibodies used in this study were 1) anti-human GAB1 (#3232; Cell Signaling); 2) anti-Flag (sc-807; Santa Cruz, Dallas, TX, USA); 3) anti-viral capsid protein VP1 (NCL-ENTERO; Leica Biosystems, Concord, ON, Canada); 4) anti-p-ERK (#4370; Cell Signaling); 5) anti-cleaved caspase-3 (#9661; Cell Signaling); 6) anti-β-actin (#2228; Sigma-Aldrich, St. Louis, MO, USA); and 7) anti-low-density lipoprotein receptor-related protein 6 (LRP6) (#2560; Cell Signaling).
Cell fractionation
Cell fractionation was performed as previously described (16, 17). Briefly, HeLa cells were incubated with hypotonic lysis buffer (20 mM HEPES, pH 7.4, 10 mM KCl with phosphatase and protease inhibitors) for 20 min on ice. Cell lysates were collected and homogenized with 30 strokes in a Dounce homogenizer, followed by centrifugation at 3,000 rpm for 5 min to remove nuclei and unbroken cells. The supernatant was further centrifuged at 55,000 rpm for 1 h to separate the cytoplasm from the membrane fraction. The purity of the membrane fraction was verified by the absence of the intracellular protein β-actin and the presence of the membrane protein LRP6 using Western blotting.
Viral plaque assay
The viral titer in the supernatant was examined by plaque assay as previously described (18). In brief, cell supernatant was serially diluted and overlaid on a monolayer of HeLa cells. After 1 h incubation, the medium was replaced by complete DMEM containing 0.75% agar. Seventy-two hours later, the cells were fixed with Carnoy’s fixative (75% ethanol and 25% acetic acid) for 30 min and then stained with 1% crystal violet. The plaques were counted and the viral titers were calculated and represented as plaque forming units per milliliter.
In vitro cleavage assay
Purified viral protease 2Apro and catalytically inactive 2Apro were generous gifts from Dr. Eric Jan (University of British Columbia). In vitro cleavage assay was conducted as previously described (19, 20). In brief, HeLa cell lysates (50 µg) were incubated with either viral protease 2Apro or catalytically inactive 2Apro in cleavage reaction buffers (20 mM HEPES-pH 7.4, 150 mM KOAc, and 1 mM DTT) at 37°C for overnight. The reaction was stopped by adding 6× sample buffers. Then the cell lysates were processed for the determination of GAB1 cleavage by Western blotting.
Immunocytochemical staining and confocal laser scanning microscopy
The immunocytochemical staining was performed as previously described (21). Briefly, cells were fixed with 4% paraformaldehyde, followed by permeabilization with 0.1% Triton X-100 and blocked with 5% bovine serum album plus Tween 20. Coverslips were then incubated with primary antibodies at 4°C overnight, followed by the incubation with secondary antibodies at room temperature for 1 h. After washing with PBS, the coverslips were counterstained with DAPI (#H1200; Vector Laboratories, Burlington, ON, Canada). Images were visualized by using a Leica SP2 AOBS inverted confocal laser scanning microscope (Leica, Wetzlar, Germany).
Statistical analysis
All results presented are representative of at least 3 independent experiments. Results are expressed as means ± sds. Statistical analysis was performed with unpaired Student’s t test. Values of P < 0.05 were considered to be statistically significant.
RESULTS
GAB1 is cleaved during CVB3 infection
Given the significance of GAB1 in the activation of the mitogen-activated protein kinase (MAPK)/ERK pathway, we first determined the effect of CVB3 infection on the protein expression of GAB1. We demonstrated that the protein level of GAB1 decreased following CVB3 infection, accompanied by the manifestation of 2 additional bands (∼75 and ∼40 kDa, respectively) using an anti-GAB1 antibody that targets residues surrounding Tyr472 of human GAB1 (Fig. 1A). To verify whether the production of these extra bands is a result of GAB1 cleavage, we transiently transfected a plasmid expressing N-terminal Flag-tagged GAB1 into HeLa cells, then infected cells with CVB3. Western blotting using an anti-Flag antibody that targets the N-terminal region of GAB1 detected 2 cleavage fragments of GAB1 (∼70 and ∼35 kDa, respectively), corresponding well with the findings of endogenous GAB1 (Fig. 1B). The structure of full-length GAB1 with various functional domains, the resulting cleavage fragments, and the regions that individual antibodies detected are illustrated in Fig. 1C. Together, our results suggest that GAB1 is cleaved during CVB3 infection.
Figure 1.
GAB1 is cleaved during CVB3 infection. A) Cleavage of endogenous GAB1 following CVB3 infection. HeLa cells were sham- or CVB3-infected at multiplicity of infections 10 for various time points as indicated. Cell lysates were collected and processed for Western blotting for detection of viral capsid protein VP1 and GAB1 protein expression (using an anti-GAB1 antibody targeting residues surrounding Tyr472 of human GAB1). The protein level of β-actin was examined as a loading control. B) Cleavage of exogenous GAB1 following CVB3 infection. HeLa cells were transiently transfected with a plasmid expressing N-terminal Flag-tagged GAB1 (3×Flag-GAB1) for 24 h, followed by CVB3 infection for different time points as indicated. Western blotting was performed to assess the protein levels of exogenous GAB1 (using anti-Flag antibody), VP1, and β-actin. Arrowheads indicate CVB3-induced GAB1 cleavage fragments. C) Schematic diagram of full-length GAB1 with various functional domains, the resulting cleavage fragments, and the regions that individual antibodies detected. Red arrows indicate 2 potential cleavage sites. pi, post infection.
GAB1 is cleaved by viral protease 2Apro
To further determine the potential mechanisms by which CVB3 infection results in the cleavage of GAB1, we utilized a UV-irradiated CVB3 (UV-CVB3) that is unable to self-replicate but maintains virus–host receptor binding. Similar to the result shown in Fig. 1, infection with WT CVB3 led to the formation of the 75 and 40 kDa cleavage fragments, although these proteolytic products were not detected in UV-CVB3–infected cells (Fig. 2A), indicating that GAB1 cleavage is associated with CVB3 replication. We then questioned whether this cleavage is mediated through the function of virus-encoded proteases. In vitro cleavage assay showed that incubation with WT viral protease 2Apro, but not catalytic inactive 2Apro (Fig. 2B) nor viral protease 3Cpro (data not shown), induced the production of cleaved GAB1 fragments, suggesting that cleavage of GAB1 is triggered by viral protease 2Apro. It was previously reported that GAB1 can be cleaved by caspases in cells undergoing apoptosis. Caspase activation is a late cellular event compared with accumulation of viral proteases during CVB3 infection. However, to further eliminate the possibility of caspase-induced cleavage of GAB1, HeLa cells were treated with z-VAD-fmk, a pan-caspase inhibitor. As shown in Fig. 2C, caspase inhibition did not block the cleavage of GAB1. Collectively, our results suggest that CVB3-induced GAB1 cleavage is an outcome of viral replication, relying on the function of viral protease 2Apro, but independent of caspase activities.
Figure 2.
GAB1 is cleaved by viral protease 2Apro. A) CVB3-induced cleavage of GAB1 is dependent on viral protein production. HeLa cells infected with either WT CVB3 or UV-CVB3 were harvested at 7 h postinfection (pi). Cell lysates were processed for Western blotting to determine the protein expression levels of GAB1 (using anti-GAB1 antibody), VP1, and β-actin. B) Cleavage of GAB1 is mediated by viral protease 2Apro. Cell lysates (50 μg) from HeLa cells transfected with Flag-GAB1 were incubated with either purified viral protease 2Apro (0.1 or 0.4 µg) or catalytically inactive mutant 2Apro (0.4 µg) overnight and in vitro cleavage assay was conducted as described in the Materials and Methods. Protein levels of GAB1 (using anti-GAB1 antibody), VP1, and β-actin were examined by Western blotting. CVB3-infected HeLa cell lysates (7 h pi) were loaded (right lane) as a positive control for GAB1 cleavage. C) Cleavage of GAB1 following CVB3 infection is independent of caspase activation. HeLa cells were infected with CVB3 in the presence or absence of pan-caspase inhibitor, z-VAD-fmk (50 µM), for 7 h. Protein levels of GAB1 (using anti-GAB1 antibody), VP1, cleaved caspase-3, and β-actin were examined by Western blotting. Arrowheads indicate CVB3-induced GAB1 cleavage fragments.
Viral protease 2Apro cleaves GAB1 at G436 and G175
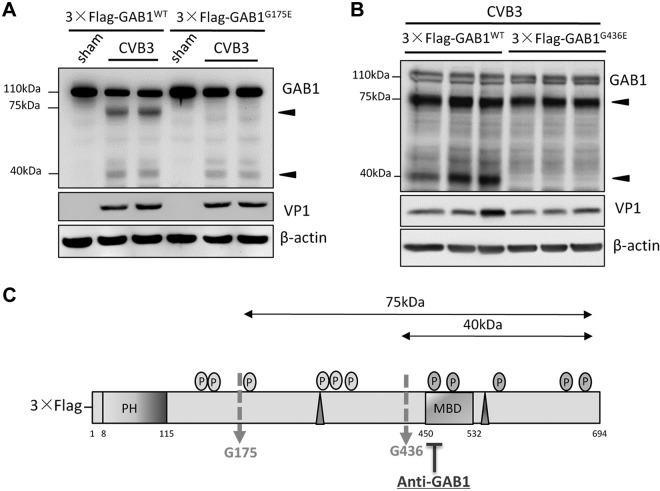
To identify the potential cleavage sites on GAB1 by viral protease 2Apro, amino acid sequence of human GAB1 was analyzed and 2 potential cleavage sites (glycine175 and glycine436) were identified based on the consensus cleavage motif by 2Apro and the size of the cleavage products. [The cleavage recognition site by 2Apro protease usually contains a T (threonine), S (serine), or N (asparagine) at position P2 and an L (leucine), I (isoleucine), or M (methionine) at position P4. A G (glycine) residue at the P1′ C-terminal side of the cleavage site takes place in all known substrates of 2Apro (18).] Two GAB1 mutants were then established by site-directed mutagenesis through replacing the glycine (G) at amino acids 175 and 436 with glutamic acid (E), respectively. Using anti-GAB1 antibody, we demonstrated that CVB3 infection failed to induce the generation of the 75 kDa band in cells expressing 3×Flag-GAB1G175E (Fig. 3A) and the formation of the 40 kDa products in cells expressing 3×Flag-GAB1G436E (Fig. 3B) as compared with WT GAB1 control. These results indicate that G175 and G436 are targeted during CVB3 infection by 2Apro, generating the 75 and 40 kDa products, respectively (Fig. 3C).
Figure 3.
GAB1 is cleaved at G175 and G436 during CVB3 infection. A) GAB1G175E mutant blocks the generation of the 75 kDa cleavage product triggered by CVB3 infection. HeLa cells were transiently transfected with either 3×Flag-GAB1WT or 3×Flag- GAB1G175E for 24 h, followed by sham or CVB3-infection for 7 h. Cell lysates were harvested for Western blot analysis to detect the cleaved fragments of GAB1 using anti-GAB1 antibody. VP1 and β-actin were determined as an infection and a loading control, respectively. B) GAB1G436E mutant inhibits the production of the 40 kDa product. The same protocol was performed as described above using either plasmid of 3×Flag-GAB1WT or 3×Flag-GAB1G436E. Arrowheads indicate CVB3-induced GAB1 cleavage fragments detected by anti-GAB1 antibody. C) Schematic diagram of full-length GAB1 with 2 cleavage sites at amino acid G175 and G436, respectively.
Knockdown of GAB1 inhibits viral replication
To determine the functional significance of GAB1 in the course of CVB3 infection, GAB1 was knocked down by siRNA in HeLa cells. We demonstrated that gene silencing of GAB1 led to a marked reduction of viral protein expression and ERK1/2 phosphorylation (Fig. 4A), as well as a significant decrease of virus titers (∼2.9-fold; Fig. 4C). Our results indicate a proviral function of GAB1, probably through regulating the activation of the MAPK/ERK signaling pathway.
Figure 4.
Knockdown of GAB1 inhibits CVB3-induced ERK phosphorylation and viral replication. A) HeLa cells were treated with either control siRNA (siCon) or GAB1-targeting siRNA (siGAB1) for 48 h, followed by CVB3 infection for 7 h. Cell lysates were harvested to examine the protein levels of GAB1 (using anti-GAB1 antibody), VP1, p-ERK1/2, and β-actin by Western blotting. Protein levels of VP1 were quantitated by densitometric analysis using ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA), normalized to β-actin, and presented underneath as fold changes compared with control group, which was arbitrarily set a value of 1. B) HeLa cells were transiently transfected with 3×Flag-GAB1WT, or corresponding vector control for 24 h, followed by CVB3 infection for 7 h. Western blotting was performed and analyzed as described above. C, D) Supernatants were collected from (A) and (B) for plaque assay and the results are presented as means ± sd (n = 4). **P < 0.001.
We also examined the effects of overexpression of GAB1 on viral replication. Interestingly, we found that forced expression of GAB1 did not further increase viral protein expression (Fig. 4B) and virus titers (Fig. 4C), suggesting that the level of endogenous GAB1 may be already high or saturated and exogenous addition of GAB1 fails to trigger enhanced viral replication.
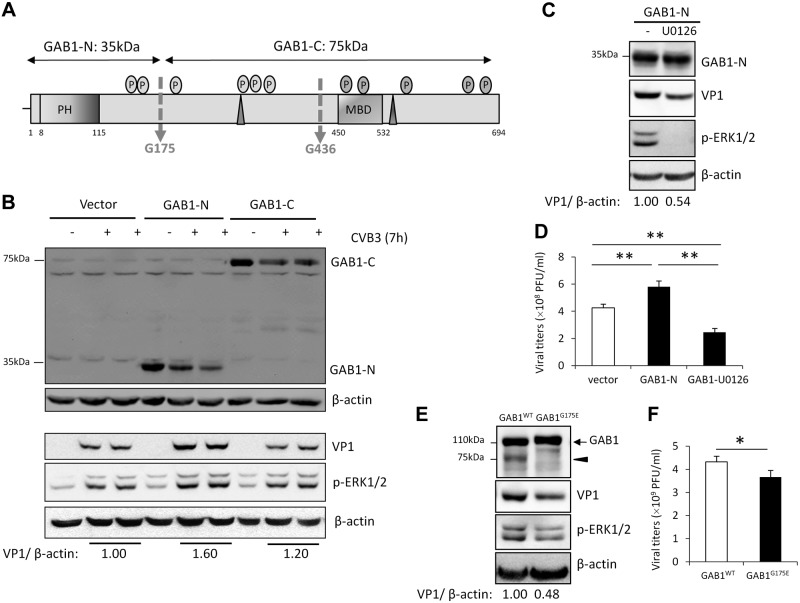
The N-terminal cleavage fragment of GAB1 (GAB1-N) promotes CVB3 replication via further enhancing ERK1/2 phosphorylation
Next, we asked whether CVB3-induced GAB1 cleavage results in a loss of function or a gain of function of GAB1 in the context of viral replication. The N-terminal (N1–174) and C-terminal (C175–694) fragments of GAB1 (Fig. 5A), the predominant cleavage products observed during CVB3 infection, were subcloned into a Flag-tagged vector. Figure 5A showed that expression of GAB1-N1–174, but not GAB1-C175–694, further enhances viral protein expression and ERK phosphorylation (Fig. 5B). Viral plaque assay results also demonstrated a significantly increase in virus titers in the supernatants of cells expressing GAB1-N1–174 (Fig. 5D). To further explore the role of ERK activation in GAB1-N–induced viral replication, HeLa cells were treated with MEK inhibitor U0126. We demonstrated that inhibition of ERK phosphorylation attenuated GAB1-N–induced augmentation of viral protein expression (Fig. 5C) and virus titers (Fig. 5D), indicating that GAB1-N1–174 promotes CVB3 replication, at least in part, via enhancement of ERK1/2 phosphorylation.
Figure 5.
The N-terminal cleavage fragment of GAB1 promotes CVB3 replication by further enhancing ERK1/2 phosphorylation. A) Schematic diagram of the N- and C-terminal cleaved fragments of GAB1 used in this figure and Fig. 6 hereafter. B) HeLa cells were transiently transfected with 3×Flag-GAB1-N1–174, 3×Flag-GAB1-C175–694, or corresponding empty vector (3×Flag) for 24 h, followed by CVB3 infection for 7 h. Cell lysates were harvested to determine protein levels of VP1, GAB1-N1–174, or GAB1-C175–694 (using anti-Flag antibody), p-ERK1/2, and β-actin. Densitometric analysis was conducted as in Fig. 4. C, D) Inhibition of ERK1/2 activation attenuates CVB3 replication induced by GAB1-N. HeLa cells were transiently transfected with 3×Flag-GAB1-N1–174 for 24 h, followed by CVB3 infection in the presence or absence of MEK inhibitor U0126 (20 µM). C) Protein levels of GAB1-N1–174 (using anti-Flag antibody), VP1, and β-actin were examined by Western blotting. D) Supernatants were collected for plaque assay (mean ± sd, n = 4). **P < 0.001. E, F) HeLa cells were transiently transfected with 3×Flag-GAB1WT or 3×Flag-GAB1G175E, followed by viral infection as described above. Protein expression of GAB1 (using anti-GAB1 antibody) and VP1 was assessed by Western blotting (E) and plaque assay was conducted to determine virus titer in supernatants (F) (mean ± sd; n = 4). *P < 0.05.
The proviral role of the cleavage products of GAB1 in viral replication was further supported by the findings that expression of a noncleavable GAB1 mutant (GAB1G175E), which fails to produce GAB1-N1–174, resulted in decreased viral protein expression (Fig. 5E) and reduced virus titers compared with GAB1WT control (Fig. 5F).
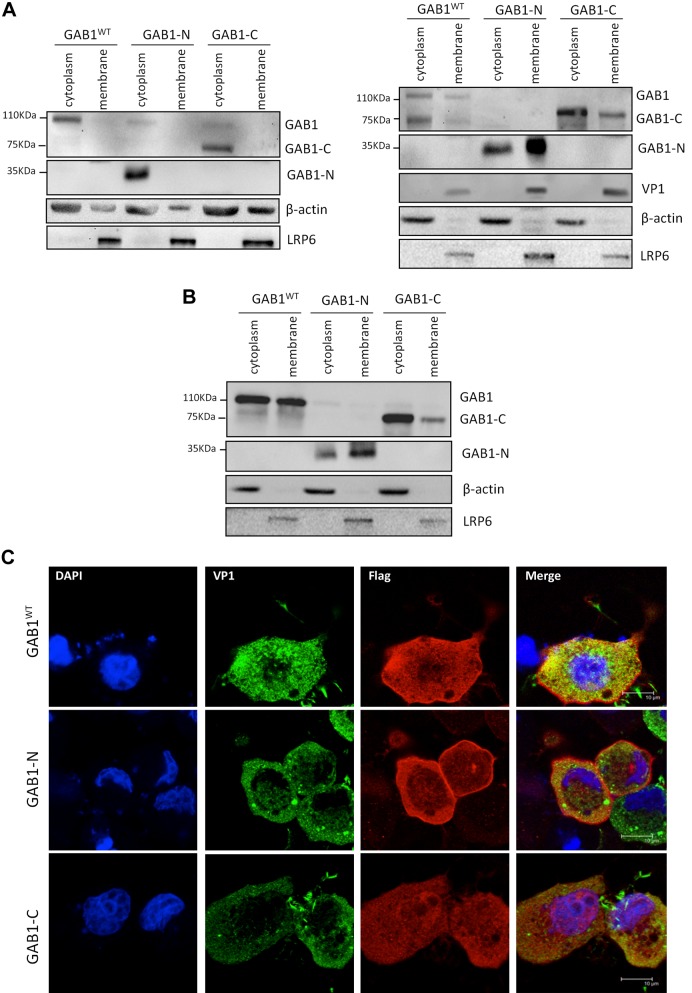
The N-terminal cleavage fragment of GAB1 is constitutively localized to the cellular membrane
Recruitment of GAB1 from the cytoplasm to the cellular membrane is a crucial step in the activation of the MAPK-ERK pathway. We then questioned whether the cleavage fragments of GAB1 could be recruited to the plasma membrane upon viral infection, contributing to enhanced ERK1/2 activation and increased viral replication. Cell fractionation was conducted, and the results showed that majority of GAB1WT and GAB1-C were present in the cytoplasmic fractions either under baseline condition (Fig. 6A, left panel) or upon viral infection (Fig. 6A, right panel), although GAB1-N was detected mainly in cytoplamic fractions in sham-infected cells (Fig. 6A, left panel) and translocation of GAB1-N from cytoplasm to membrane was increased following CVB3 infection (Fig. 6A, right panel). Consistent with previous studies (22), GAB1WT was found to translocate to the membrane fraction in cells treated with growth factors (Fig. 6B). Confocal microscopy analysis further confirmed that GAB1-N is predominantly localized to the cellular membranes, and GAB1WT and GAB1-C largely retained in the cytoplasm of CVB3-infected cells (Fig. 6C). Taken together, our finding suggests that the proviral activity of GAB1-N may be related to its constitutive presence in cellular membrane fraction and preference to translocate to cellular membrane upon stimulation, and subsequently activation of the MAPK/ERK pathway that favors viral replication.
Figure 6.
The N-terminal cleavage fragment of GAB1 is constitutively localized in the cell plasma membrane. A, B) HeLa cells were transiently transfected with 3×Flag-GAB1WT, 3×Flag-GAB1-N1–174, or 3×Flag-GAB1-C175–694 for 24 h, followed by sham (A, left panel), or CVB3 infection (A, right panel), or treatment with 10% fetal bovine serum (B). Cells were collected for cell fractionation. Plasma membrane and cytoplasm fractions were subjected to Western blot analysis of protein levels of WT GAB1 and GAB1-C175–694 (using anti-GAB1 antibody), GAB1-N1–174 (using anti-Flag antibody), VP1, LRP6, and β-actin. C) Cells were transfected and infected as above and then immunocytochemical staining was conducted using anti-Flag (red) and anti-VP1 antibody (green). Nuclei were counterstained by DAPI (blue). Scale bars, 10 μM.
DISCUSSION
Our findings in this study reveal a novel mechanism by which CVB3 employs to trigger ERK1/2 activation and promote consequent viral replication. Enteroviral protease 2Apro plays an essential role in ensuring successful completion of viral life cycle through direct processing viral polyprotein and by targeting host proteins for proteolytic degradation to create a favorable microenvironment for viral growth (3, 23, 24). Several mechanisms have been proposed with regard to the latter proviral strategy. For example, it was well documented that 2Apro cleaves host eukaryotic initiation factor 4-γ and poly (A)-binding protein, resulting in the shutoff of host protein synthesis to benefit viral mRNA translation (25, 26). In addition, it was reported that enteroviral protease 2Apro mediates the cleavage of MDA5 and MAVS, 2 critical regulators in type I interferon responses, to escape host antiviral immune surveillance (27, 28). Our results in this study suggest, for the first time, that 2Apro can also support enteroviral infection by manipulating and usurping the host signaling machinery. We demonstrated that adaptor protein GAB1 is proteolytically cleaved by enteroviral protease 2Apro during CVB3 infection, which leads to the release of the N-terminal PH domain-containing fragment that facilitates viral infectivity.
We previously demonstrated that CVB3 infection mediates a late, persistent ERK1/2 activation that depends on viral protein production (5). The present study identified the cleavage of GAB1 as a mechanism triggering the late phase activation of ERK1/2 during CVB3 infection. We reported that expression of the N-terminal cleavage truncation of GAB1 induces ERK1/2 activation, which appears to be related to its constitutive membrane association. Unlike stimulation by growth factors, such as hepatocyte growth factor, we found that upon CVB3 infection, GAB1WT failed to translocate from the cytoplasm to the cellular membrane, whereas the GAB1-N1–174 is constantly detected in the membrane fractions. GAB1-N1–174 contains several potential phosphorylation sites and the PH domain that is known to bind with the plasma membrane enriched in phosphatidylinositol lipids (Fig. 1C). The exact mechanism by which GAB1-N1–174 promotes ERK1/2 activation is currently unclear. We hypothesize that GAB1-N1–174 preoccupies the PH-domain binding sites that are necessary for recruiting the upstream inhibitory modulators of the MAPK/ERK signaling pathway, which results in sustained activation of ERK1/2. For instance, SAPK-interacting protein 1 and Dok (for downstream of tyrosine kinases) were reported to contain PH domain, and membrane binding is required for their function in inhibiting the activities of small GTPase Ras and protein kinases upstream of ERK1/2 (29, 30). Thus, the incapability of being recruited to lipid-enriched membrane due to preoccupation of these regions by GAB1-N1–174 may result in the relief of their inhibitory effects on ERK1/2 activation. Further studies are needed to define more specifically the mechanism of enhanced ERK1/2 activation and viral replication by cleaved GAB1 fragments.
The best characterized pathway for GAB1 activation of MAPK/ERK pathway is through interaction with SHP2 (31). Although the C-terminal cleavage product of GAB1 contains the SHP2 binding site (Y627 and Y659), it lacks the PH domain and fails to be recruited to the plasma membrane. Previous studies have suggested an important role for PH domain in GAB1 function. It was reported that GAB1 mutant with PH domain deletion fails to be tyrosine phosphorylated upon growth hormone receptor stimulation, resulting in impaired ERK1/2 activation (32). Thus we speculate that the inability of GAB1-C175–694 to function in activating ERK signaling may correlate with its failure to translocate to the plasma membrane.
In conclusion, our study demonstrated the cleavage of GAB1 by CVB3-encoded viral protease 2Apro at G175 and G436, producing the predominant functional cleavage fragment GAB1-N1–174. GAB1-N1–174 further enhances viral replication by up-regulating host ERK1/2 signaling. These findings present a new mechanism by which CVB3 contributes to the pathogenesis of enterovirus infection.
Acknowledgments
This work was supported by Canadian Institutes of Health Research Grants MOP-119274 and CCI-125685 to H.L., U.S. National Institutes of Health National Heart, Lung, and Blood Institute Grants R01HL109502 and R01HL114570 to Z.G.J., and American Diabetes Association Grant 1-12-BS-92-R1 to Z.G.J. The authors declare no conflicts of interest.
Glossary
- CVB3
coxsackievirus B3
- GAB1
Grb2-associated binder 1
- LRP6
low-density lipoprotein receptor-related protein 6
- PH
pleckstrin homology
- SHP2
Src-homology-2-containing protein tyrosine phosphatase 2
- siRNA
small interfering RNA
- UV-CVB3
UV-irradiated coxsackievirus B3
- WT
wild-type
REFERENCES
- 1.Esfandiarei M., McManus B. M. (2008) Molecular biology and pathogenesis of viral myocarditis. Annu. Rev. Pathol. 3, 127–155 [DOI] [PubMed] [Google Scholar]
- 2.Jensen K. J., Garmaroudi F. S., Zhang J., Lin J., Boroomand S., Zhang M., Luo Z., Yang D., Luo H., McManus B. M., Janes K. A. (2013) An ERK-p38 subnetwork coordinates host cell apoptosis and necrosis during coxsackievirus B3 infection. Cell Host Microbe 13, 67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo H., Wong J., Wong B. (2010) Protein degradation systems in viral myocarditis leading to dilated cardiomyopathy. Cardiovasc. Res. 85, 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchant D., Si X., Luo H., McManus B., Yang D. (2008) The impact of CVB3 infection on host cell biology. Curr. Top. Microbiol. Immunol. 323, 177–198 [DOI] [PubMed] [Google Scholar]
- 5.Luo H., Yanagawa B., Zhang J., Luo Z., Zhang M., Esfandiarei M., Carthy C., Wilson J. E., Yang D., McManus B. M. (2002) Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J. Virol. 76, 3365–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W., Xu S., Yin M., Jin Z. G. (2015) Essential roles of Gab1 tyrosine phosphorylation in growth factor-mediated signaling and angiogenesis. Int. J. Cardiol. 181, 180–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wöhrle F. U., Daly R. J., Brummer T. (2009) Function, regulation and pathological roles of the Gab/DOS docking proteins. Cell Commun. Signal. 7, 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itoh M., Yoshida Y., Nishida K., Narimatsu M., Hibi M., Hirano T. (2000) Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol. Cell. Biol. 20, 3695–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakaoka Y., Komuro I. (2013) Gab docking proteins in cardiovascular disease, cancer, and inflammation. Int. J. Inflamm. 2013, 141068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishida K., Hirano T. (2003) The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Sci. 94, 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maroun C. R., Holgado-Madruga M., Royal I., Naujokas M. A., Fournier T. M., Wong A. J., Park M. (1999) The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol. 19, 1784–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weidner K. M., Di Cesare S., Sachs M., Brinkmann V., Behrens J., Birchmeier W. (1996) Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 384, 173–176 [DOI] [PubMed] [Google Scholar]
- 13.Gu H., Neel B. G. (2003) The “Gab” in signal transduction. Trends Cell Biol. 13, 122–130 [DOI] [PubMed] [Google Scholar]
- 14.Liu Y., Rohrschneider L. R. (2002) The gift of Gab. FEBS Lett. 515, 1–7 [DOI] [PubMed] [Google Scholar]
- 15.Wong J., Si X., Angeles A., Zhang J., Shi J., Fung G., Jagdeo J., Wang T., Zhong Z., Jan E., Luo H. (2013) Cytoplasmic redistribution and cleavage of AUF1 during coxsackievirus infection enhance the stability of its viral genome. FASEB J. 27, 2777–2787 [DOI] [PubMed] [Google Scholar]
- 16.Yuan J., Zhang J., Wong B. W., Si X., Wong J., Yang D., Luo H. (2005) Inhibition of glycogen synthase kinase 3beta suppresses coxsackievirus-induced cytopathic effect and apoptosis via stabilization of beta-catenin. Cell Death Differ. 12, 1097–1106 [DOI] [PubMed] [Google Scholar]
- 17.Krebs J. F., Armstrong R. C., Srinivasan A., Aja T., Wong A. M., Aboy A., Sayers R., Pham B., Vu T., Hoang K., Karanewsky D. S., Leist C., Schmitz A., Wu J. C., Tomaselli K. J., Fritz L. C. (1999) Activation of membrane-associated procaspase-3 is regulated by Bcl-2. J. Cell Biol. 144, 915–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong J., Zhang J., Yanagawa B., Luo Z., Yang X., Chang J., McManus B., Luo H. (2012) Cleavage of serum response factor mediated by enteroviral protease 2A contributes to impaired cardiac function. Cell Res. 22, 360–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi J., Fung G., Piesik P., Zhang J., Luo H. (2014) Dominant-negative function of the C-terminal fragments of NBR1 and SQSTM1 generated during enteroviral infection. Cell Death Differ. 21, 1432–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi J., Wong J., Piesik P., Fung G., Zhang J., Jagdeo J., Li X., Jan E., Luo H. (2013) Cleavage of sequestosome 1/p62 by an enteroviral protease results in disrupted selective autophagy and impaired NFKB signaling. Autophagy 9, 1591–1603 [DOI] [PubMed] [Google Scholar]
- 21.Fung G., Ng C. S., Zhang J., Shi J., Wong J., Piesik P., Han L., Chu F., Jagdeo J., Jan E., Fujita T., Luo H. (2013) Production of a dominant-negative fragment due to G3BP1 cleavage contributes to the disruption of mitochondria-associated protective stress granules during CVB3 infection. PLoS One 8, e79546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eulenfeld R., Schaper F. (2009) A new mechanism for the regulation of Gab1 recruitment to the plasma membrane. J. Cell Sci. 122, 55–64 [DOI] [PubMed] [Google Scholar]
- 23.Knowlton K. U. (2008) CVB infection and mechanisms of viral cardiomyopathy. Curr. Top. Microbiol. Immunol. 323, 315–335 [DOI] [PubMed] [Google Scholar]
- 24.Lim B. K., Peter A. K., Xiong D., Narezkina A., Yung A., Dalton N. D., Hwang K. K., Yajima T., Chen J., Knowlton K. U. (2013) Inhibition of Coxsackievirus-associated dystrophin cleavage prevents cardiomyopathy. J. Clin. Invest. 123, 5146–5151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamphear B. J., Yan R., Yang F., Waters D., Liebig H. D., Klump H., Kuechler E., Skern T., Rhoads R. E. (1993) Mapping the cleavage site in protein synthesis initiation factor eIF-4 gamma of the 2A proteases from human Coxsackievirus and rhinovirus. J. Biol. Chem. 268, 19200–19203 [PubMed] [Google Scholar]
- 26.Kerekatte V., Keiper B. D., Badorff C., Cai A., Knowlton K. U., Rhoads R. E. (1999) Cleavage of Poly(A)-binding protein by coxsackievirus 2A protease in vitro and in vivo: another mechanism for host protein synthesis shutoff? J. Virol. 73, 709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng Q., Langereis M. A., Lork M., Nguyen M., Hato S. V., Lanke K., Emdad L., Bhoopathi P., Fisher P. B., Lloyd R. E., van Kuppeveld F. J. (2014) Enterovirus 2Apro targets MDA5 and MAVS in infected cells. J. Virol. 88, 3369–3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J. P., Cerny A., Asher D. R., Kurt-Jones E. A., Bronson R. T., Finberg R. W. (2010) MDA5 and MAVS mediate type I interferon responses to coxsackie B virus. J. Virol. 84, 254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones N., Dumont D. J. (1999) Recruitment of Dok-R to the EGF receptor through its PTB domain is required for attenuation of Erk MAP kinase activation. Curr. Biol. 9, 1057–1060 [DOI] [PubMed] [Google Scholar]
- 30.Schroder W. A., Buck M., Cloonan N., Hancock J. F., Suhrbier A., Sculley T., Bushell G. (2007) Human Sin1 contains Ras-binding and pleckstrin homology domains and suppresses Ras signalling. Cell. Signal. 19, 1279–1289 [DOI] [PubMed] [Google Scholar]
- 31.Cunnick J. M., Dorsey J. F., Munoz-Antonia T., Mei L., Wu J. (2000) Requirement of SHP2 binding to Grb2-associated binder-1 for mitogen-activated protein kinase activation in response to lysophosphatidic acid and epidermal growth factor. J. Biol. Chem. 275, 13842–13848 [DOI] [PubMed] [Google Scholar]
- 32.Kim S. O., Loesch K., Wang X., Jiang J., Mei L., Cunnick J. M., Wu J., Frank S. J. (2002) A role for Grb2-associated binder-1 in growth hormone signaling. Endocrinology 143, 4856–4867 [DOI] [PubMed] [Google Scholar]