Abstract
Peripheral neuropathy is a major dose-limiting side effect of paclitaxel and cisplatin chemotherapy. In the current study, we tested the involvement of a novel class of neurotoxic sphingolipids, the 1-deoxysphingolipids. 1-Deoxysphingolipids are produced when the enzyme serine palmitoyltransferase uses l-alanine instead of l-serine as its amino acid substrate. We tested whether treatment of cells with paclitaxel (250 nM, 1 µM) and cisplatin (250 nM, 1 µM) would result in elevated cellular levels of 1-deoxysphingolipids. Our results revealed that paclitaxel, but not cisplatin treatment, caused a dose-dependent elevation of 1-deoxysphingolipids levels and an increase in the message and activity of serine palmitoyltransferase (P < 0.05). We also tested whether there is an association between peripheral neuropathy symptoms [evaluated by the European Organization for Research and Treatment of Cancer (EORTC) QLQ-chemotherapy-induced peripheral neuropathy-20 (CIPN20) instrument] and the 1-deoxysphingolipid plasma levels (measured by mass spectrometry) in 27 patients with breast cancer who were treated with paclitaxel chemotherapy. Our results showed that there was an association between the incidence and severity of neuropathy and the levels of very-long-chain 1-deoxyceramides such as C24 (P < 0.05), with the strongest association being with motor neuropathy (P < 0.001). Our data from cells and from patients with breast cancer suggest that 1-deoxysphingolipids, the very-long-chain in particular, play a role as molecular intermediates of paclitaxel-induced peripheral neuropathy.—Kramer, R., Bielawski, J., Kistner-Griffin, E., Othman, A., Alecu, I., Ernst, D., Kornhauser, D., Hornemann, T., Spassieva, S. Neurotoxic 1-deoxysphingolipids and paclitaxel-induced peripheral neuropathy.
Keywords: chemotherapy, 1-deoxyceramide, sphingolipid, serine palmitoyltransferase
Chemotherapy-induced peripheral neuropathy is a common, disabling, and, in the most severe cases, dose-limiting side effect of paclitaxel and cisplatin therapies (1). Both paclitaxel and cisplatin are effective drugs used to treat a variety of cancers, and the development of peripheral neuropathy reduces their effectiveness. The mechanism of chemotherapy-induced peripheral neuropathy is unknown, and there are no preventive therapies or mechanism-based treatments available. Patients in whom this toxicity develops are treated symptomatically or by limiting the total drug dose, reducing individual doses, or discontinuing therapy (2, 3). A recent study in patients with breast cancer who were receiving taxane therapy estimated that, on average, dose reduction or termination because of peripheral neuropathy lowered the received cumulative dose by 9.4 and 28.4%, respectively (4).
The neuropathy caused by paclitaxel or cisplatin is primarily a sensory neuropathy, with patients experiencing numbness, tingling, and, less commonly, burning pain in the feet and hands (1). Paclitaxel may also cause motor neuropathy, which is typically mild and presents as muscle weakness with difficulty climbing stairs. Fine motor skills, such as buttoning a shirt also may be diminished (5). These symptoms are distinct from the subacute aches and pains associated with paclitaxel (6). Careful study of this complex of symptoms indicates that rather than being an arthralgia or myalgia, it is related to nerve pathology and may predict the development of sensory neuropathy (7).
The incidence of peripheral neuropathy associated with paclitaxel can be influenced by total dose, individual treatment dose, and treatment schedule [e.g., 22.0% of patients receiving weekly therapy experienced neuropathy (grade 2–4) compared with 17.5% of patients receiving therapy every 3 wk (8)]. Patient-related risk factors for neuropathy include patient’s age, postmenopausal status, African-American race, and pre-existing diabetes mellitus, obesity, or hyperglycemia (8, 9). The development of paclitaxel-induced neuropathy is not predictive of outcomes with adjuvant taxane therapy in breast cancer (8).
Abnormalities in sphingolipid metabolism have been correlated with neurologic disorders, such as neural tube defects (10) and lysosomal storage diseases [recent review series in Journal of Lipid Research (11)]. Sphingolipids constitute a diverse class of lipids that are important in membrane biology and cell function and are highly abundant in nervous tissue (12). Notably, the levels of a minor sphingolipid subclass, the 1-deoxysphingolipids, mediate the neurodegeneration in hereditary sensory and autonomic neuropathy (HSAN)-1 (13). HSAN1 is a length-dependent axonal neuropathy associated with the loss of sensation, ulcers, and autonomic dysfunctions and results from mutations of the SPTLC1 or -2 genes which encode 2 of the major subunits of serine palmitoyltransferase (SPT) (13–15). SPT catalyzes the first rate-limiting step in the de novo synthesis of sphingolipids (Fig. 1). The mutations induce a shift in the substrate specificity of SPT, from l-serine to l-alanine, leading to the formation of atypical 1-deoxysphingoid bases. These atypical 1-deoxygsphingosines lack the functional OH group at the C1 position and cannot be converted to complex sphingolipids or degraded (12). When produced in excess, the 1-deoxysphingolipids accumulate. The neurotoxicity of 1-deoxysphingolipids was shown in dorsal root ganglion (DRG) neurons in vitro (13) and in phase I clinical trials, where 1-deoxysphinganine (spisulosine, E-285) was tested for its anticancer potential; the clinical trials were terminated because some patients had severe, and in some cases fatal, neurotoxicity (16–18). Individuals with HSAN1 primarily experience a loss of sensation distributed in the distal parts of the upper and lower limbs (19), and most have neuropathic pain. Degeneration of motor neurons frequently occurs and is accompanied by atrophy and weakness of the distal limb muscles. Autonomic features including sweating disturbances are also seen. Many of these signs and symptoms are experienced by patients in whom neuropathy develops while they are receiving chemotherapy (see above). In addition, it has been shown that 1-deoxysphingoid bases are elevated in the plasma of diabetic patients, as compared with healthy controls, and it has been hypothesized that 1-deoxysphingolipids contribute to the progression of diabetic neuropathy (20).
Figure 1.
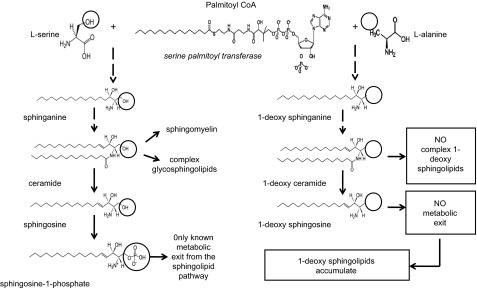
The sphingolipid pathway.
The similarities between the symptoms of diabetic neuropathy, HSAN1, and chemotherapy-induced neuropathy led to our hypothesis that changes in sphingolipid metabolism and accumulation of the neurotoxic 1-deoxysphingolipids may play a role in the development of chemotherapy-induced peripheral neuropathy. We tested this hypothesis, both in cells and in patients with early-stage breast cancer who were receiving paclitaxel therapy.
MATERIALS AND METHODS
Cell culture and treatment
U87 human astroglioma cells and human embryonic kidney (HEK) 293 cells were cultured in high-glucose DMEM (with l-glutamine, supplemented with 10% fetal bovine serum, 100 IU/ml penicillin, and 100 IU/ml streptomycin) and incubated at 37°C and 5% CO2. Cell culture medium and supplements were from Life Technologies-Invitrogen (Carlsbad, CA, USA). Paclitaxel (stock solution 10 mM in DMSO; working solutions in DMSO/ethanol; Sigma-Aldrich, St. Louis, MO, USA) and cisplatin (working solutions in water; Calbiochem, San Diego, CA, USA) were added to the growth medium at various concentrations for 24 h or 7 d.
Lipid extraction
For lipid extraction from cells, we followed a previously described procedure (21); in brief, lipid from the cell pellets corresponding to about ∼1 to 3 × 106 cells was extracted twice with 2 ml ethyl acetate, isopropanol, and water (60:30:10 v/v/v) solvent. For extraction from plasma, 100 µl plasma was extracted twice with 2 ml ethyl acetate and isopropanol (85:15 v/v). Subsequently, lipid extracts were dried under a stream of nitrogen and resuspended in 150 μl 1 mM ammonium formate in 0.2% formic acid in methanol. To control for variation during the extraction, we fortified the samples with internal standards (C17 base d-erythro-sphingosine, C17 sphingosine-1-phosphate, N-palmitoyl-d-erythro-C13 sphingosine, and N-docosanoyl-d-erythro-C13 sphingosine).
Liquid chromatography tandem mass spectrometry measurements of sphingolipids
Lipid extracts fortified with internal standards were subjected to liquid chromatography tandem mass spectrometry (LC/MS/MS) analyses performed on a TSQ Quantum triple quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), operating in a multiple-reaction–monitoring positive-ionization mode with modifications (22). Extracts were injected on the HP1100/TSQ Quantum LC/MS/MS system and gradient eluted from the BDS Hypersil C8, 150 × 3.2 mm, 3 μm particle size column, with 1 mM methanolic ammonium formate and 2 mM aqueous ammonium formate mobile phase system. Peaks corresponding to the target analytes and internal standards were collected and processed using the Xcalibur software system (Thermo Fisher Scientific). Quantitative analyses of l-serine-derived sphingolipids were based on the calibration curves generated by spiking an artificial matrix with the known amount of the target analyte, a synthetic standard, and an equal amount of the internal standards. The target analyte and internal standard peak area ratio was plotted against the analyte concentration. The target analyte and internal standard peak area ratios from the samples were similarly normalized to their respective internal standards and compared to the calibration curves, using a linear regression model. For developing the 1-deoxysphingolipid method, 1-deoxysphingosine and 1-deoxyceramides C16 and C24:1 (Avanti Polar Lipids, Alabaster, AL, USA) were used as standards. 1-Deoxysphingoid bases and 1-deoxyceramides, for which no synthetic standards are available, were quantitated by using calibration curves of their sphingoid counterparts, taking advantage of the conserved fragmentation pattern, for the long-chain bases and their ceramide derivatives. Specifically, C14, C18, C18:1, C20, and C20:1 1-deoxyceramides were quantified by using C16 1-deoxyeoxyceramide calibration. Similarly, C22, C22-1, C24, C26, and C26:1 1-deoxyceramides were quantified by using the C24:1 deoxyceramide calibration curve. Alternatively, if no authentic 1-deoxyceramide standards are available, applying consistent mass spectral conditions of collision assistant dissociation (35 eV) and electron spray ionization causes all sphingoid bases and related ceramides to undergo uniform transition from initial molecular ion (M+1) to the respective sphingoid backbone secondary ions. Consequently, calibration curves generated from authentic standards can be used for quantitation of the metabolites for which standards are not available. Results from cells were normalized to cellular lipid phosphate. Lipid phosphate was measured with a standard curve analysis and a colorimetric assay of ashed phosphate (23). Results from plasma were normalized to volume.
Free amino acid measurements
Amino acids were analyzed on an LC2010 HPLC system (Shimadzu, Kyoto, Japan) on a Zorbax Eclypse AAA column (150 × 4.5 mm, 5 µM; Agilent Technologies, Santa Clara, CA, USA) according to the manufacturers’ protocols, with fluorescence detector (Hewlett-Packard, Palo Alto, CA, USA).
RNA isolation, cDNA synthesis, and RT-PCR
Total RNA isolation was performed with the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The concentration and quality of total RNA preparations were evaluated spectrophotometrically. cDNA was synthesized from 1 μg of total RNA with the Superscript II Kit for first-strand synthesis (Life Technologies-Invitrogen). RT-PCR was performed on an iCycler detection system using iQ SYBR Green Supermix (BioRad, Hercules, CA, USA). The standard reaction volume was 25 μl containing 12.5 μl iQ SYBR Green Supermix, 9.5 μl distilled H2O, 0.4 μM specific oligonucleotide primers (Table 1), and 50 ng cDNA template. Initial steps of RT-PCR were 2 min at 50°C, followed by a 3 min hold at 95°C, and 40 cycles consisting of a 10 s melt at 98°C, followed by 45 s annealing at a temperature (Ta) specific for the primer pair and 45 s extension at 72°C. All reactions were performed in triplicate and threshold for cycle of threshold (Ct) analysis of all samples was set at 0.15 relative fluorescence units. The data were normalized to an internal control gene, GAPDH.
TABLE 1.
Primer sequences used in RT-PCR
| Name | Sequence (5′–3′) | Ta (°C) |
|---|---|---|
| SPTLC1 (F) | AAGAAGCCATTATATACTCATAT | 58 |
| SPTLC1 (R) | GGCACTGATAAGATCAATA | 58 |
| SPTLC2 (F) | GAGTGTGTACAACAGTTAGCTG | 58 |
| SPTLC2 (R) | TGGCT CACAAAGGCCAC | 58 |
| GAPDH (F) | AGG TCG GAG TCA ACG GAT TTG | 53 |
| GAPDH (R) | ATG GGT GGA ATC ATA TTG GAA CAT G | 53 |
F, forward; R, reverse.
Isotope labeling of de novo formed sphingoid bases
Two hundred thousand HEK293 cells were seeded and cultured as described above. After 3 d, the medium was exchanged with l-serine- and l-alanine–deficient DMEM (Genaxxon BioScience, Ulm, Germany). After a preincubation of 2 h, isotope labeled (2,3,3)-d3 l-serine (1 mM), together with 2 mM (3C13)-labeled l-alanine (Cambridge Isotope Laboratories, Inc., Tewksbury, MA, USA) was added to the medium. After incubation for 24 h, the cells were washed twice with PBS, harvested, and counted (Z2 Coulter Counter; Beckman Coulter, Nyon, Switzerland). The cells were centrifuged (800 g, 5 min at 4°C), and pellets were stored at −20°C upon lipid extraction and analysis. Isotope-labeled sphingoid bases were extracted and analyzed (24).
Clinical trial and patient recruitment
Women with early-stage breast cancer receiving neoadjuvant or adjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by paclitaxel, were enrolled in a clinical trial. They were at least 18 yr old, were able to provide written informed consent and to complete study questionnaires. All participants had to have a life expectancy of >6 mo. The patients began paclitaxel treatment after completion of 4 cycles of concurrent administration of doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2). Paclitaxel therapy was given either weekly for 12 wk (80 mg/m2) or biweekly for 8 wk (175 mg/m2), with pegfilgrastim support. Neuropathy symptoms were assessed at the following time points: before paclitaxel therapy began, two-thirds of the way through therapy (wk 8 for those receiving weekly and wk 6 for those receiving dose-dense therapies), and at the end of the treatment. Patients donated blood for measurement of sphingolipid species at time points of peripheral neuropathy assessment. The plasma from donated blood was separated from the blood cells by centrifugation (700 g; 15 min; 4°C), divided into aliquots, and stored at −80°C before it was used for lipid analyses.
Peripheral neuropathy assessment
Sensory, motor, and autonomic neuropathies were assessed by using the European Organization for Research and Treatment of Cancer (EORTC) quality-of-life questionnaire focused on chemotherapy-induced peripheral neuropathy (CIPN-20) (25). The EORTC QLQ-CIPN-20 is a 20-item CIPN-specific questionnaire that includes 3 scales for assessing sensory (9 items), motor (8 items), and autonomic (3 items) symptoms; functioning with each item is measured on a scale of 1 to 4 (1, not at all; 4, very much). As this study enrolled only women, one of the questions assessing autonomic function (erectile function) was omitted. The EORTC QLQ-CIPN-20 has been tested in patients with cancer and has been shown to have internal consistency reliability on the basis of Cronbach α coefficients of 0.82, 0.73, and 0.76 for the 3 scales, respectively (25).
Statistical analyses
Tests of association between baseline characteristics and baseline total 1-deoxyceramide and 1-deoxysphingosine, change in total 1-deoxyceramide and 1-deoxysphingosine, as well as baseline total neuropathy and change in total neuropathy were conducted by using a linear model. In addition, tests of associations between baseline neuropathies (total, sensory, motor, and autonomic) and baseline 1-deoxysphingolipid species, change in 1-deoxysphingolipid species, and change in neuropathies were conducted by using the same approach. Tests of changes over paclitaxel treatment in 1-deoxyceramide levels, 1-deoxysphingosine levels, and peripheral neuropathy were conducted by modeling differences in results of baseline to midtreatment to end-of-treatment evaluations as the endpoints in a mixed-effects model, allowing for a random subject-specific effect. In the same mixed-model framework, associations between baseline 1-deoxyceramide species levels and changes in neuropathy over time were tested, as well as tests of differences in 1-deoxyceramide species levels and changes in total and motor neuropathy over time. Body mass index and hyperlipidemia are included as covariates in all models of total, sensory, and motor neuropathies over treatment. Tests for 1-deoxysphinganine and 1-deoxy-dihydroceramides were performed with the same statistical methods as for 1-deoxysphingosine and 1-deoxyceramides. ANOVA and Student's t test from Sigma Plot (Systat, San Jose, CA, USA) were used for statistical analyses of the results obtained in the cell culture experiments.
Protection of human subjects
Before human subjects were recruited for the study, a clinical trial was approved by the institutional review board at the Medical University of South Carolina. A signed informed consent was obtained from each participant before inclusion in the study.
RESULTS
Cells treated with paclitaxel, but not with cisplatin, have increased cellular levels of 1-deoxysphingolipids
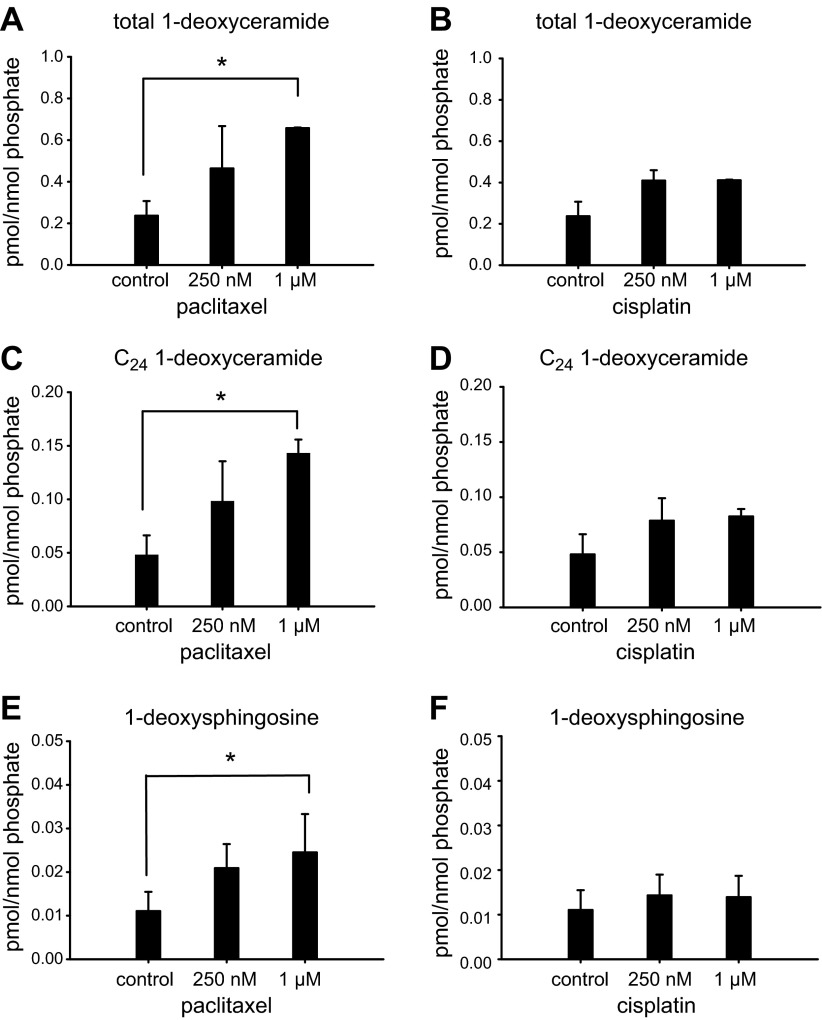
In our initial proof-of-concept experiments, we tested whether treatment of cells with paclitaxel or cisplatin, both antineoplastic drugs known to cause peripheral neuropathy, alters cellular levels of 1-deoxysphingolipids, which have been demonstrated to be neurotoxic in vitro and in an HSAN1 mouse model (13). We treated U87 human glioblastoma cells and nontransformed HEK293 cells with paclitaxel (250 nM and 1 µM) and cisplatin (250 nM and 1 µM) for 24 h. The cells were collected, subjected to 1-phase lipid extraction (see Materials and Methods) and 1-deoxysphingolipid levels were measured by LC/MS/MS. Our data show that treatment of cells with increased concentrations of paclitaxel resulted in increased levels of 1-deoxysphingolipids (Fig. 2A, C, E; Supplemental Fig. 1A, C). In contrast, treatment with cisplatin did not result in increased levels of 1-deoxysphingolipids (Fig. 2B, D, F; Supplemental Fig. 1B, D), suggesting that 1-deoxysphingolipids can play a role in paclitaxel- but not cisplatin-induced peripheral neuropathy. The most significant increase occurred for very-long-chain (VLC) 1-deoxyceramides, C24 1-deoxyceramide in particular (P < 0.02) (Fig. 2C; Supplemental Fig. 1A).
Figure 2.
Paclitaxel treatment increased the levels of 1-deoxysphingolipids in cells. U87 astroglioma cells were treated with increasing concentrations of paclitaxel (A, C, E) or cisplatin (B, D, F) for 24 h. The cells were collected, and cellular lipid extracts were subjected to LC/MS/MS for determining the concentration of: total 1-deoxyceramide (A, B), C24 1-deoxyceramide (C, D), and 1-deoxysphingosine (E, F). The levels of 1-deoxysphingolipids were normalized to the levels of the cellular lipid phosphate. Error bar: range of 2 independent experiments. *P < 0.05, by Student's t test.
Next, we tested whether the levels of 1-deoxysphingolipids in cells treated with paclitaxel increase because of higher levels of l-alanine, reduced levels of l-serine, or both. HEK293 cells were treated with increased concentrations of paclitaxel (250 nM and 1 µM), and the free cellular amino acid levels were measured by HPLC (see Materials and Methods). Our results showed that both l-alanine and l-serine levels were up-regulated to the same extent, as a result of paclitaxel treatment, keeping the change in the ratio of the 2 SPT amino acid substrates nonsignificant (Supplemental Fig. 2A). Treatment with increased concentrations of cisplatin (500 nM and 1.5 µM) also did not change the ratio of the two SPT amino acid substrates (Supplemental Fig. 2B). Similar measurements in U87 cells treated with paclitaxel did not cause a significant change in l-alanine or l-serine levels (data not shown). In addition, we tested whether the transcription level of d-3-phosphoglycerate dehydrogenase (3PGDH) was affected by paclitaxel or cisplatin treatment. 3PGDH is the enzyme of the first committed step of l-serine biosynthesis and has been shown in a rat model to decrease as a result of paclitaxel treatment (26). Our results in cells showed that 3PGDH message levels did not change as a result of paclitaxel or cisplatin treatment (Supplemental Fig. 2C, D). Our data suggest that in cells treated with paclitaxel the levels of 1-deoxysphingolipids most likely do not increase because of higher availability of the l-alanine substrate over the l-serine substrate.
Paclitaxel, but not cisplatin, results in up-regulation of SPT and increased production of 1-deoxysphingolipids
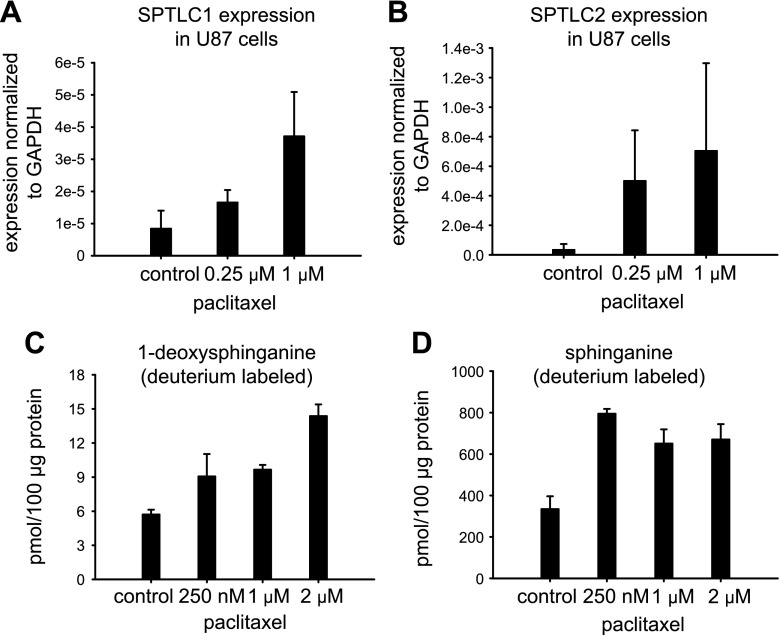
Next, we tested whether paclitaxel has an effect on SPT expression level and activity. U87 and HEK293 cells were treated with increasing concentrations of paclitaxel for 24 h; total RNA was isolated; and the expression levels of the 2 major subunits of SPT, SPTLC1 and -2 were measured by RT-PCR. In both cell lines, paclitaxel treatment resulted in increased levels of SPTLC1 (Fig. 3A; Supplemental Fig. 3A). The message levels of SPTLC2 increased only in U87 cells (Fig. 3B; Supplemental Fig. 3B), suggesting that the paclitaxel effect on the SPTLC2 expression is likely to be cell-type dependent. In contrast, cisplatin treatment did not affect the mRNA levels of SPTLC1 and -2 in both tested cell lines (data not shown).
Figure 3.
Paclitaxel treatment increased the expression level of 2 major SPT subunits and the production of 1-deoxysphingolipids in a dose-dependent manner. U87 astroglioma cells were treated with increasing concentrations of paclitaxel (A, B) for 24 h. The cells were collected and expression levels of SPTLC1 (A) and SPTLC 2 (B) were determined on RNA extracts by RT-PCR. Expression levels of the studied genes were normalized to GAPDH. Error bars: 2 independent experiments (3 technical repeats within each experiment). HEK293 cells were metabolically labeled with deuterated l-alanine (C) or l-serine (D) for 24 h. FB1 (30 µM) was used simultaneously with the metabolic label to block the conversion of deuterated 1-deoxysphinganine or deuterated sphinganine to 1-deoxyceramide or ceramide and complex sphingolipids. Increasing concentrations of paclitaxel were used to treat the cells in (C) and (D). Error bars: sd of 3 independent experiments. Overall significance in (C), P < 0.05, by ANOVA.
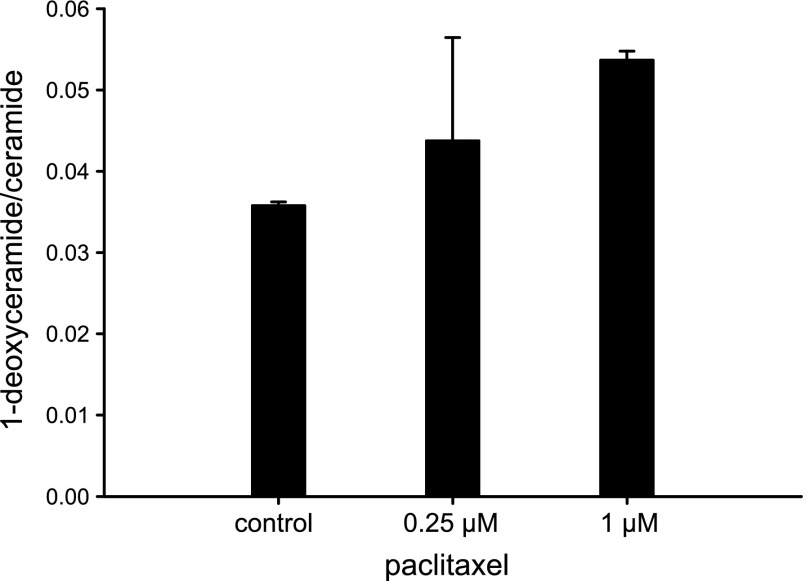
Next, we tested whether the increase in SPT transcription after paclitaxel treatment causes increased activity. For that, we used an isotope labeling assay with deuterated l-alanine (2 mM) and l-serine (1 mM) in the presence of Fumonisin B1 (FB1, 30 µM). FB1 is an inhibitor of ceramide synthase and blocks the conversion of the labeled products of the SPT reaction, deuterated sphinganine (when l-serine was used as a substrate) and deuterated 1-deoxysphinganine (when l-alanine was used as a substrate), to labeled dihydroceramide and 1-deoxy-dihydroceramide, respectively. We observed a statistically significant dose-dependent increase in the de novo production of both 1-deoxysphinganine and sphinganine, when the cells were treated with paclitaxel (Fig. 3C, D). These results indicated generally increased SPT activity as a result of paclitaxel treatment and corroborated our findings (Fig. 3A, B; Supplemental Fig. 3) of increased SPT mRNA levels induced by paclitaxel treatment. Although, both metabolic arms of the sphingolipid pathway (Fig. 1) were activated with paclitaxel, the ratio 1-deoxyceramide/ceramide increased as a result of the treatment (Fig. 4), suggesting an accumulation of 1-deoxysphingolipids over time. In contrast, cisplatin treatment did not increase 1-deoxysphinganine or sphinganine de novo synthesis (data not shown), further indicating that SPT and de novo sphingolipid metabolism, including de novo 1-deoxysphingolipid, were not affected by cisplatin treatment in cells.
Figure 4.
Paclitaxel increases the ratio of l-alanine–derived 1-deoxyceramide over l-serine–derived ceramide in cells. U87 astroglioma cells were treated with paclitaxel for 24 h. The cells were collected and cellular lipid extracts were subjected to LC/MS/MS for determining the concentration of total 1-deoxyceramide and total ceramide. The plot represents the ratios of the absolute amounts of total 1-deoxyceramide and total ceramide normalized to lipid phosphate. Error bar: the range of 2 independent experiments.
Demographics and comorbidities of patients with breast cancer receiving paclitaxel therapy included in a study testing the correlation of plasma 1-deoxysphingolipid levels and neuropathy
Our results in cells revealed that of the 2 tested anticancer drugs with proven peripheral neurotoxicity, paclitaxel and cisplatin, only paclitaxel induced increased production of 1-deoxysphingolipids. These results suggest that increased 1-deoxysphingolipid levels could be involved in neurotoxicity in patients treated with paclitaxel. We therefore tested whether there is a correlation between the plasma levels of 1-deoxysphingolipids and the incidence and severity of peripheral neuropathy in patients treated with paclitaxel. Twenty-seven patients with breast cancer receiving adjuvant or neoadjuvant paclitaxel treatment in 2 regimens (see Materials and Methods) were enrolled in the study. The 1-deoxysphingolipids were analyzed in plasma samples at 3 time points: before, two-thirds through, and after paclitaxel treatment. At the same time, the incidence and severity of the peripheral neuropathy was evaluated by the EORTC QLQ-CIPN20 instrument, which assessed for sensory, motor, and autonomic neuropathies (25).
Demographics including age, race, menopausal status, body mass index, breast cancer characteristics (estrogen receptor, progesterone receptor, and Her2 status) and comorbidities (presence or absence of diabetes mellitus and hyperlipidemia) are noted in Table 2. We tested the relationship between these characteristics and the 1-deoxysphingolipid plasma levels or neuropathy symptoms at baseline and with treatment (Table 2). The associations of characteristics were tested in a linear regression model. Participants with diabetes had higher 1-deoxysphingolipid baseline levels, with the strongest association for C14, C16, C18, and C24 1-deoxyceramide species (P < 0.05). The association of diabetes with increased 1-deoxysphingolipids is consistent with previous findings showing that patients with diabetes have increased plasma levels of 1-deoxysphingolipids compared with those in healthy control subjects (20, 24). No other patient characteristic or tumor characteristic affected 1-deoxysphingolipid levels at baseline or after treatment.
TABLE 2.
Patient characteristics at baseline
| Patient characteristics | n | % | Baseline P value baseline total 1-deoxyceramide and 1-deoxysphingosine | P value baseline total neuropathy | P value change in total 1-deoxyceramide and 1-deoxysphingosine | P value change in total neuropathy |
|---|---|---|---|---|---|---|
| Age (yr) | ||||||
| <45 | 5 | 18.5 | 0.250 | 0.325 | 0.982 | 0.230 |
| 45–54 | 8 | 29.5 | ||||
| 55–64 | 8 | 29.5 | ||||
| 65–74 | 5 | 18.5 | ||||
| ≥75 | 1 | 4.0 | ||||
| Race | ||||||
| African American | 10 | 37.0 | 0.820 | 0.731 | 0.594 | 0.477 |
| White | 17 | 63.0 | ||||
| Menopause | ||||||
| Pre | 11 | 40.7 | 0.221 | 0.052* | 0.425 | 0.276 |
| Post | 16 | 59.3 | ||||
| ER/PR | ||||||
| +/+ | 15 | 55.6 | 0.933 | 0.088 | 0.330 | 0.738 |
| +/− | 3 | 11.1 | ||||
| −/− | 9 | 33.3 | ||||
| Her2 | ||||||
| Negative | 26 | 96.3 | 0.591 | 0.465 | 0.705 | 0.525 |
| Positive | 1 | 3.7 | ||||
| Dose | ||||||
| Dense | 10 | 37.0 | 0.137 | 0.190 | 0.709 | 0.384 |
| Weekly | 17 | 63.0 | ||||
| Diabetes | ||||||
| Yes | 4 | 14.8 | 0.060† | 0.823 | 0.588 | 0.602 |
| No | 23 | 85.2 | ||||
| Hyperlipidemia | ||||||
| Yes | 4 | 14.8 | 0.150 | 0.436 | 0.431 | 0.094 |
| No | 23 | 85.2 | ||||
| Body mass index (kg/m2) | ||||||
| <18.5 | 0 | 0.0 | 0.576 | 0.150 | 0.516 | 0.096 |
| 18.5–24.9 | 8 | 29.6 | ||||
| 25–29.9 | 8 | 29.6 | ||||
| 30–39.9 | 10 | 37.0 | ||||
| ≥40 | 1 | 3.7 |
ER, estrogen receptor; PR, progesterone receptor. *Significant association with motor neuropathy (P = 0.0258); †Significant associations with C14, C16, C18, C24 1-deoxyceramides (P < 0.05).
Postmenopausal patients often had motor weakness before the initiation of paclitaxel chemotherapy (P = 0.0258). This demographic, however, did not correlate significantly with total neuropathy at the completion of therapy (Table 2) and was not taken into account in our further analyses. Hyperlipidemia and increased body mass index showed trending association with the changes in total peripheral neuropathy symptoms over the course of paclitaxel treatment (Table 2) and were therefore included as covariates in our statistical analyses.
None of the patients enrolled in our study had a pre-existing diagnosis of neuropathy. However, at baseline, some patients reported mild neuropathy symptoms (Table 3). Those patients typically experienced more severe symptoms as a result of paclitaxel chemotherapy, especially motor neuropathy (Table 3). All statistical models of neuropathy at the end of treatment were adjusted for baseline neuropathy by using the change in neuropathy over treatment as the endpoint of interest. Neuropathy at baseline did not associate with 1-deoxysphingolipid plasma level changes because of paclitaxel, suggesting that the pre-existing neuropathy is distinctive from 1-deoxysphingolipid levels, their downstream neurotoxic effect, or both.
TABLE 3.
Self-reported symptoms of peripheral neuropathy at baseline
| Type of peripheral neuropathy | n | % | P value baseline total 1-deoxyceramide and 1-deoxysphingosine | P value change in total 1-deoxyceramide and 1-deoxysphingosine | P value change in total neuropathy |
|---|---|---|---|---|---|
| Total neuropathy | |||||
| Yes (>19)a | 19 | 70.4 | 0.896 | 0.332 | < 0.001 |
| No | 8 | 29.6 | |||
| Sensory neuropathy | |||||
| Yes (>9)a | 11 | 40.7 | 0.814 | 0.569 | 0.095 |
| No | 16 | 59.3 | |||
| Motor neuropathy | |||||
| Yes (>8)a | 15 | 55.6 | 0.668 | 0.385 | < 0.001 |
| No | 12 | 44.4 | |||
| Autonomic neuropathy | |||||
| Yes (>2)a | 8 | 29.6 | 0.406 | 0.473 | 0.050 |
| No | 19 | 70.4 |
The neuropathy score above which the patients report any peripheral neuropathy symptom.
Increasing levels of the VLC 1-deoxyceramides C22:1 and C24 associated with development of paclitaxel-induced neuropathy
To test the changes of peripheral neuropathy symptoms and 1-deoxysphingolipid plasma levels over the course of paclitaxel treatment in patients with breast cancer, mixed statistical models were constructed allowing for a random subject-specific effect (see Materials and Methods). Body mass index and hyperlipidemia, as discussed above, were included as covariates in models of total, sensory, and motor neuropathies over the treatment period. Total and sensory neuropathies increased during treatment (P < 0.001), as did motor neuropathy (P = 0.024) (Table 4). Autonomic neuropathy showed a tendency to increase during treatment (P = 0.138). Data for autonomic neuropathy were less significant because of the smaller size of the studied population (27 patients) and the limited number of questions for autonomic neuropathy in the EORTC QLQ-CIPN20 instrument. Our statistical analyses also showed that total plasma 1-deoxyceramide and 1-deoxysphingosine levels, and the levels of the individual 1-deoxyceramide species did not significantly change in the breast cancer population treated with paclitaxel (Table 4 and data not shown). However, individual patients showed an increase in 1-deoxysphingolipid levels (n = 11).
TABLE 4.
Tests of changes during treatment
| Deoxysphingolipids/type of neuropathy | Mean difference (±sd) | P |
|---|---|---|
| Total 1-deoxyceramide | −0.017 (±0.124) | 0.406 |
| 1-Deoxysphingosine | 0.001 (±0.002) | 0.115 |
| Total neuropathy | 12.4 (±10.6) | <0.001 |
| Sensory neuropathy | 8.4 (±6.1) | <0.001 |
| Motor neuropathy | 3.3 (±4.4) | 0.024 |
| Autonomic neuropathy | 0.7 (±1.7) | 0.138 |
In analysis of peripheral neuropathy, differences from baseline are modeled as the endpoint; P values are from mixed models). P values for effect of time are shown for midtreatment and end-of-treatment evaluations. Mean ± sd differences between end of treatment and baseline are presented.
Therefore, we addressed the question of whether there were associations between the increase in the plasma levels of 1-deoxysphingolipids from baseline and the increase in peripheral neuropathy over time in patients receiving paclitaxel. Our analyses represented main effects of plasma 1-deoxysphingolipid levels after subtracting their baseline plasma levels. The total-neuropathy models were adjusted for hyperlipidemia and body mass index, as discussed above. We observed a significant association with VLC C24 1-deoxyceramide with changes in total, motor, and autonomic neuropathy (Table 5; Supplemental Table 1). In addition, the changes in plasma levels of another VLC 1-deoxyceramide, C22:1, were significantly associated with changes in motor neuropathy. The observation that increased plasma levels of the VLC 1-deoxyceramide species C24 and C22:1 are associated with worsening motor and total peripheral neuropathy symptoms with paclitaxel corroborates our observation that C24 1-deoxyceramide was also the most elevated when cells were treated with paclitaxel (Fig. 2C).
TABLE 5.
Associations between changes in plasma 1-deoxysphingolipid levels from baseline and changes in neuropathy over time
| Deoxysphingolipid species/type of neuropathy | Sensory | Motor | Autonomic | Total |
|---|---|---|---|---|
| Total 1-deoxyceramide | 0.803 | 0.120 | 0.118 | 0.319 |
| 1-Deoxysphingosine | 0.840 | 0.492 | 0.408 | 0.770 |
| Total 1-deoxyceramide and 1-deoxysphingosine | 0.802 | 0.124 | 0.122 | 0.323 |
| C14 1-deoxyceramide | 0.686 | 0.445 | 0.237 | 0.410 |
| C16 1-deoxyceramide | 0.420 | 0.579 | 0.093 | 0.593 |
| C18 1-deoxyceramide | 0.471 | 0.532 | 0.983 | 0.787 |
| C18:1 1-deoxyceramide | 0.167 | 0.366 | 0.243 | 0.457 |
| C20:1 1-deoxyceramide | 0.388 | 0.061 | 0.746 | 0.087 |
| C20:4 1-deoxyceramide | 0.301 | 0.406 | 0.417 | 0.319 |
| C22 1-deoxyceramide | 0.347 | 0.084 | 0.394 | 0.753 |
| C22:1 1-deoxyceramide | 0.615 | 0.001* | 0.151 | 0.126 |
| C24 1-deoxyceramide | 0.650 | 0.000* | 0.035* | 0.042* |
| C24:1 1-deoxyceramide | 0.715 | 0.094 | 0.337 | 0.261 |
| C26 1-deoxyceramide | 0.986 | 0.630 | 0.367 | 0.908 |
| C26:1 1-deoxyceramide | 0.323 | 0.299 | 0.131 | 0.589 |
Main effects of plasma 1-deoxysphingolipid levels after subtracting baseline levels. total neuropathy models were adjusted for hyperlipidemia and body mass index. P values from mixed models. *P < 0.05.
Higher levels of the C22:1 1-deoxy-dihydroceramide are associated with development of paclitaxel-induced motor neuropathy
Next, we tested whether there is an association between the increase in the plasma levels of the precursors of 1-deoxysphingolipids, 1-deoxysphinganine, and 1-deoxy-dihydroceramides (Fig. 1) from the baseline and the incidence and severity of neuropathy over time in the patients with breast cancer who are receiving paclitaxel chemotherapy. 1-Deoxysphinganine and 1-deoxy-didroceramides were implicated in HSAN1, as well (13). The data are summarized in Table 6. Note that C22:1 1-deoxy-dihydroceramide showed association with motor neuropathy, which is in line with our finding that its desaturated metabolite, C22:1 1-deoxyceramide, was also associated with motor neuropathy (Table 5; Supplemental Table 1). In addition, our results showed that C18:1 1-deoxy-dihydroceramide is associated with autonomic neuropathy.
TABLE 6.
Associations between differences in plasma 1-deoxydihydrosphingolipid levels from baseline and changes in neuropathy over time
| Deoxydihydrosphingolipid species/type of neuropathy | Sensory | Motor | Autonomic | Total |
|---|---|---|---|---|
| Total 1-deoxydihydroceramide | 0.264 | 0.095 | 0.967 | 0.133 |
| 1-Deoxysphinganine | 0.205 | 0.170 | 0.479 | 0.721 |
| C14 1-deoxydihydroceramide | 0.050 | 0.352 | 0.858 | 0.237 |
| C16 1-deoxydihydroceramide | 0.280 | 0.086 | 0.958 | 0.132 |
| C18 1-deoxydihydroceramide | 0.196 | 0.074 | 0.656 | 0.077 |
| C18:1 1-deoxydihydroceramide | 0.659 | 0.659 | 0.014* | 0.785 |
| C20 1-deoxydihydroceramide | 0.543 | 0.405 | 0.896 | 0.814 |
| C20:1 1-deoxydihydroceramide | 0.903 | 0.073 | 0.164 | 0.219 |
| C22 1-deoxydihydroceramide | 0.427 | 0.155 | 0.773 | 0.869 |
| C22:1 1-deoxydihydroceramide | 0.796 | 0.016* | 0.168 | 0.233 |
| C24 1-deoxydihydroceramide | 0.508 | 0.101 | 0.392 | 0.791 |
| C24:1 1-deoxydihydroceramide | 0.607 | 0.054 | 0.368 | 0.546 |
| C26 1-deoxydihydroceramide | 0.215 | 0.409 | 0.439 | 0.162 |
| C26:1 1-deoxydihydroceramide | 0.191 | 0.585 | 0.526 | 0.228 |
Main effects of plasma 1-deoxydihydrosphingolipid levels are shown after subtraction from baseline levels. Total neuropathy models were adjusted for hyperlipidemia and body mass index. P values are from mixed models. *P < 0.05.
Taken together, the results in our patients suggest that particular 1-deoxysphingolipids, such as C24 and C22:1 1-deoxyceramides and C22:1 and C18:1 1-deoxy-dihydroceramides can be molecular intermediates of paclitaxel-induced peripheral neuropathy.
Our statistical analyses also revealed that a higher baseline level of 1-deoxysphinganine was associated with developing motor and autonomic neuropathy as a result of paclitaxel treatment (P = 0.045 and P = 0.003, respectively); however, 1-deoxysphinganine plasma levels did not change as a result of treatment (P = 0.4), or, as shown in Table 6, there was no association between its increase over baseline with any type of neuropathy. These results suggest that 1-deoxysphinganine, by itself, may not be an intermediate of paclitaxel-induced neuropathy, but it has the potential to be used as a diagnostic marker for paclitaxel-induced motor and autonomic neuropathies.
DISCUSSION
The molecular mechanism of chemotherapy-induced peripheral neuropathy is largely unknown. We conducted preclinical studies and a small clinical trial to address the question of whether 1-deoxysphingolipids, already shown to be neurotoxic (13, 16–18), are involved in this process. Of the 2 antineoplastic drugs, paclitaxel and cisplatin, only paclitaxel increased the formation of 1-deoxysphingolipids, suggesting that 1-deoxysphingolipids are involved in paclitaxel- but not in cisplatin-induced peripheral neuropathy. This variation between the 2 drugs could be related to their different intracellular targets and cytotoxic mechanisms. Paclitaxel binds to β-tubulin, thereby affecting microtubule dynamics, which leads to arrest of mitosis (27). Cisplatin, in contrast, forms DNA cross-links that lead to apoptosis. Moreover, our results showed that paclitaxel had a broad effect on sphingolipid metabolism that was not seen with cisplatin. SPT message, protein levels, and activity, as well as cellular sphingolipid and 1-deoxysphingolipid levels, were increased in paclitaxel-treated cells (Figs. 2, 3). The effect of paclitaxel on SPT expression is likely the primary trigger, which is associated with increased 1-deoxysphingolipid levels. However, future studies are needed to reveal the underlying mechanism by which paclitaxel leads to increased SPT expression. Increased expression of SPT in response to paclitaxel treatment resulted in increased SPT activity, which led to higher levels of both l-alanine–derived 1-deoxysphingolipids and l-serine–derived sphingolipids. We noted that the increase in 1-deoxyceramides was greater than that in l-serine–derived ceramides (Fig. 4), indicating an increased affinity of SPT to l-alanine. As an alternative possibility, this difference could be caused by a slower degradation of 1-deoxysphingolipids compared with the l-serine–derived ones. Regardless of the underlying cellular mechanism, the increase of 1-deoxysphingolipids over time is in agreement with the observed side effect in patients. Peripheral neuropathy symptoms worsen with progression of the treatment and still can be persistent months after the chemotherapy is discontinued (1, 9). This finding could be explained by the accumulation of a toxic agent with slower turnover rate.
Neurotoxicity of the 1-deoxy long-chain bases 1-deoxysphingosine and 1-deoxysphinganine has been demonstrated earlier in cultured DRG neurons but also in an HSAN1 mouse model and in patients (13, 16–18). Phase 1 clinical trials, which tested 1-deoxysphinganine (spisulosine, E-285) as an experimental anticancer treatment, revealed that 1-deoxysphinganine has severe neuropathy side effects. However, it is not clear yet whether 1-deoxysphinganine itself or one of its downstream products (e.g., 1-deoxy-dihydroceramide or 1-deoxyceramide) is responsible for the neurotoxic effects. Our results from cells (Fig. 1C; Supplemental Fig. 1A) and from patients’ plasma (Tables 5, 6) revealed that the C24 and C22:1 1-deoxyceramides and C22:1 and C18:11-deoxy-dihydroceramides increased with paclitaxel treatment and correlated with the incidence and severity of the neuropathies. These plasma 1-deoxysphingolipid species could be directly involved in neurotoxicity or, upon reaching the peripheral nervous system, they could first require hydrolysis to produce the true neurotoxic molecules, the 1-deoxy long-chain bases (Fig. 1). Although it is not clear why only specific plasma 1-deoxysphingolipid species are associated with paclitaxel-induced neuropathy, it is notable that plasma 1-deoxysphingolipods with the C22:1 fatty acid moiety associated only with motor neuropathy, suggesting specificity of the C22:1 fatty acid moiety for that form of neuropathy. It is well established which ceramide synthase is responsible for the generation of which ceramides with a particular chain-length fatty acid moiety (29). The specificity of ceramide synthases for the generation of the particular 1-deoxyceramides have not been tested so far. It will be interesting to investigate in future research how plasma 1-deoxyceramide/sphingolipid metabolism is regulated and whether it played a role in our findings that only specific 1-deoxysphingolipid species were associated with paclitaxel-induced neuropathy.
A recently published work by Janes et al. (30) revealed a possible role of sphingosine-1-phosphate (S1P) and its signaling in paclitaxel-induced painful neuropathy in rats. Although, as stated above, our data show that generation of l-serine–derived sphingolipids also increased in cells treated with paclitaxel (Fig. 3D), we have not observed an increase in the levels of cellular S1P (data not shown) or an association of plasma S1P with changes in neuropathy during treatment (P > 0.10). This difference may have been caused by the different approaches that were used to address paclitaxel-induced neuropathy in our study and in the study by Janes et al. (30). We investigated the effect of paclitaxel on sphingolipid metabolism in the plasma of patients with breast cancer and in cell culture models. The plasma sphingolipids are localized on the lipoprotein particles, which are linked to liver metabolism. The sources of S1P in the plasma are platelets, liver, and vascular epithelia (32). Therefore, the effects of paclitaxel (a systemic drug) on the liver, blood cells, and epithelia sphingolipid metabolism are direct. The same is true of the cell culture models used in our study. The other study was an investigation of the effect of paclitaxel on sphingolipid metabolism in the CNS (spinal cord issue) in rats. Paclitaxel is a chemotherapy drug that does not easily permeate the blood–brain barrier, and when applied systemically, has a poor distribution in the CNS (33, 34). Therefore, the effect of paclitaxel on the sphingolipid metabolism in the spinal cord is probably indirect, making comparison of the S1P level or the level of any other sphingolipid between the two studies difficult. Taken together, the results of our study and the work of Janes et al. (30) suggest that systemic treatment with paclitaxel can affect both the peripheral and central nervous systems, although the effects are different and would be likely to require different intervention strategies.
It has been demonstrated that patients with diabetes have increased levels of 1-deoxysphingolipids (20, 24). Our study in patients with breast cancer confirmed these observations (Table 2); patients with comorbid diabetes mellitus presented higher plasma levels of 1-deoxyceramides at baseline. However, diabetes itself was not a contributing factor to a larger change in plasma 1-deoxysphingolipid levels when patients were treated with paclitaxel chemotherapy. This result suggests that the mechanisms of 1-deoxysphingolipid formation because of diabetes and paclitaxel treatment are different. Paclitaxel treatment (our data) resulted in an increased message level of SPT and increased SPT activity in cells (Fig. 3), while not significantly affecting the ratio of l-alanine/l-serine (Supplemental Fig. 2A). Diabetes, on the other hand, was shown to alter the plasma l-serine/l-alanine ratio and is therefore likely to affect the amino acid substrate availability of SPT in favor of 1-deoxysphingolipid generation (20).
Our result that a higher baseline plasma level of 1-deoxysphinganine is associated with increased motor and autonomic neuropathy in patients receiving paclitaxel chemotherapy is an important finding that indicates that the 1-deoxysphinganine plasma level could be used as a diagnostic marker. This possibility is significant, considering that not all patients develop neuropathy because of paclitaxel or manifest motor or autonomic neuropathy symptoms. Our finding can be used in developing a test based on 1-deoxysphinganine plasma levels for screening patients before starting their paclitaxel chemotherapy and in selecting candidates for targeted prevention strategy.
In a previous study in rats, it has been shown that paclitaxel treatment resulted in a transient decrease in 3PGDH expression and l-serine levels in the DRG (26). These data appear to be at odds with our results, which showed that there was no significant change in 3PGDH expression levels after paclitaxel treatment (Supplemental Fig. 2C). However, this difference could be because of the different length of the period of paclitaxel treatment in the 2 experimental settings. The study in rats showed that l-serine supplementation improved the neuropathy symptoms that were caused by paclitaxel (26). In that study, the authors did not provide evidence of the mechanism of the rescue. l-Serine supplementation also has been shown to effectively suppress 1-deoxysphingolipid formation and was successfully tested as a therapeutic approach in an HSAN1 animal model and in HSAN1 patients (35). Although our results revealed that paclitaxel did not change the ratio of l-alanine to l-serine (Supplemental Fig. 2A) it is likely, that the addition of l-serine will decrease the ratio and will suppress 1-deoxysphingolipid production. Therefore, oral l-serine supplementation may be applied as a cotreatment with paclitaxel to counterbalance the production of 1-deoxysphingolipids and to reduce the neuropathy in patients with cancer. We therefore argue that 1-deoxysphingolipids are molecular intermediates in paclitaxel-induced peripheral neuropathy and that modulating SPT substrate specificity could be a novel therapeutic approach in paclitaxel chemotherapy.
Acknowledgments
The authors thank the Clinical Trials office at the Hollings Cancer Center (Medical University of South Carolina; MUSC) for providing help with administering the clinical trial in patients with breast cancer who were receiving paclitaxel chemotherapy; Drs. Brescia and Christiansen from the Department of Medicine (MUSC) for assisting in recruiting patients for the clinical trial; and the Lipidomics Core Facility (MUSC) for processing the lipid samples. The research was supported in part by pilot research funding from U.S. National Institutes of Health (NIH) National Cancer Institute Support Grant P30 CA138313 to the Hollings Cancer Center and Specialized Centers of Research (SCOR) Grant 5P50DA016511 from the NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (to R.K. and S.S.); the Zurich Center of Integrated Human Physiology (ZIHP; University of Zurich); the 7th Framework Program of the European Commission (“RESOLVE”, Project 305707); and Rare Disease Initiative Zurich (RADIZ; University of Zurich) (to T.H.). A.O., I.A., D.E., D.K., and S.S. performed experiments and analyzed data; R.K. and S.S. designed and supervised the clinical trial with patients with breast cancer; R.K. recruited patients and evaluated neuropathy symptoms; J.B. developed the MS methods for quantifying individual deoxysphingolipid species; J.B. and A.O. performed MS analyses of sphingolipids; E.K.G. provided statistical analyses; and R.K., T.H., and S.S. designed the study and wrote the manuscript. The authors declare no conflicts of interest.
Glossary
- 3DPGH
D-3-phosphoglycerate dehydrogenase
- CIPN-20
20-item questionnaire focused on chemotherapy-induced peripheral neuropathy
- DRG
dorsal root ganglion
- EORTC
European Organization for Research and Treatment of Cancer
- FB1
fumonisin B1
- HEK
human embryonic kidney
- Her
human epidermal growth factor receptor
- HSAN1
hereditary sensory neuropathy type 1
- LC/MS/MS
liquid chromatography tandem mass spectrometry
- S1P
sphingosine-1-phosphate
- SPT
serine palmitoyltransferase
- VLC
very-long-chain
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Miltenburg N. C., Boogerd W. (2014) Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat. Rev. 40, 872–882 [DOI] [PubMed] [Google Scholar]
- 2.Hershman D. L., Lacchetti C., Dworkin R. H., Lavoie Smith E. M., Bleeker J., Cavaletti G., Chauhan C., Gavin P., Lavino A., Lustberg M. B., Paice J., Schneider B., Smith M. L., Smith T., Terstriep S., Wagner-Johnston N., Bak K., Loprinzi C. L.; American Society of Clinical Oncology (2014) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 32, 1941–1967 [DOI] [PubMed] [Google Scholar]
- 3.Kaley T. J., Deangelis L. M. (2009) Therapy of chemotherapy-induced peripheral neuropathy. Br. J. Haematol. 145, 3–14 [DOI] [PubMed] [Google Scholar]
- 4.Speck R. M., Sammel M. D., Farrar J. T., Hennessy S., Mao J. J., Stineman M. G., DeMichele A. (2013) Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J. Oncol. Pract. 9, e234–e240 [DOI] [PubMed] [Google Scholar]
- 5.Rowinsky E. K., Chaudhry V., Cornblath D. R., Donehower R. C. (1993) Neurotoxicity of Taxol. J. Natl. Cancer Inst. Monogr. (15), 107–115 [PubMed] [Google Scholar]
- 6.Loprinzi C. L., Reeves B. N., Dakhil S. R., Sloan J. A., Wolf S. L., Burger K. N., Kamal A., Le-Lindqwister N. A., Soori G. S., Jaslowski A. J., Novotny P. J., Lachance D. H. (2011) Natural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1. J. Clin. Oncol. 29, 1472–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeves B. N., Dakhil S. R., Sloan J. A., Wolf S. L., Burger K. N., Kamal A., Le-Lindqwister N. A., Soori G. S., Jaslowski A. J., Kelaghan J., Novotny P. J., Lachance D. H., Loprinzi C. L. (2012) Further data supporting that paclitaxel-associated acute pain syndrome is associated with development of peripheral neuropathy: North Central Cancer Treatment Group trial N08C1. Cancer 118, 5171–5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider B. P., Zhao F., Wang M., Stearns V., Martino S., Jones V., Perez E. A., Saphner T., Wolff A. C., Sledge G. W. Jr., Wood W. C., Davidson N. E., Sparano J. A. (2012) Neuropathy is not associated with clinical outcomes in patients receiving adjuvant taxane-containing therapy for operable breast cancer. J. Clin. Oncol. 30, 3051–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J. J., Swain S. M. (2006) Peripheral neuropathy induced by microtubule-stabilizing agents. J. Clin. Oncol. 24, 1633–1642 [DOI] [PubMed] [Google Scholar]
- 10.Missmer S. A., Suarez L., Felkner M., Wang E., Merrill A. H. Jr., Rothman K. J., Hendricks K. A. (2006) Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ. Health Perspect. 114, 237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shayman J. A. (2014) Thematic review series: Recent advances in the treatment of lysosomal storage diseases. J. Lipid Res. 55, 993–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merrill A. H., Jr (2011) Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 111, 6387–6422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penno A., Reilly M. M., Houlden H., Laurá M., Rentsch K., Niederkofler V., Stoeckli E. T., Nicholson G., Eichler F., Brown R. H. Jr., von Eckardstein A., Hornemann T. (2010) Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J. Biol. Chem. 285, 11178–11187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rotthier A., Auer-Grumbach M., Janssens K., Baets J., Penno A., Almeida-Souza L., Van Hoof K., Jacobs A., De Vriendt E., Schlotter-Weigel B., Löscher W., Vondráček P., Seeman P., De Jonghe P., Van Dijck P., Jordanova A., Hornemann T., Timmerman V. (2010) Mutations in the SPTLC2 subunit of serine palmitoyltransferase cause hereditary sensory and autonomic neuropathy type I. Am. J. Hum. Genet. 87, 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotthier A., Penno A., Rautenstrauss B., Auer-Grumbach M., Stettner G. M., Asselbergh B., Van Hoof K., Sticht H., Lévy N., Timmerman V., Hornemann T., Janssens K. (2011) Characterization of two mutations in the SPTLC1 subunit of serine palmitoyltransferase associated with hereditary sensory and autonomic neuropathy type I. Hum. Mutat. 32, E2211–E2225 [DOI] [PubMed] [Google Scholar]
- 16.Baird R. D., Kitzen J., Clarke P. A., Planting A., Reade S., Reid A., Welsh L., López Lázaro L., de las Heras B., Judson I. R., Kaye S. B., Eskens F., Workman P., deBono J. S., Verweij J. (2009) Phase I safety, pharmacokinetic, and pharmacogenomic trial of ES-285, a novel marine cytotoxic agent, administered to adult patients with advanced solid tumors. Mol. Cancer Ther. 8, 1430–1437 [DOI] [PubMed] [Google Scholar]
- 17.Massard C., Salazar R., Armand J. P., Majem M., Deutsch E., García M., Oaknin A., Fernández-García E. M., Soto A., Soria J. C. (2012) Phase I dose-escalating study of ES-285 given as a three-hour intravenous infusion every three weeks in patients with advanced malignant solid tumors. Invest. New Drugs 30, 2318–2326 [DOI] [PubMed] [Google Scholar]
- 18.Schöffski P., Dumez H., Ruijter R., Miguel-Lillo B., Soto-Matos A., Alfaro V., Giaccone G. (2011) Spisulosine (ES-285) given as a weekly three-hour intravenous infusion: results of a phase I dose-escalating study in patients with advanced solid malignancies. Cancer Chemother. Pharmacol. 68, 1397–1403 [DOI] [PubMed] [Google Scholar]
- 19.Auer-Grumbach M. (2008) Hereditary sensory neuropathy type I. Orphanet J. Rare Dis. 3, 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertea M., Rütti M. F., Othman A., Marti-Jaun J., Hersberger M., von Eckardstein A., Hornemann T. (2010) Deoxysphingoid bases as plasma markers in diabetes mellitus. Lipids Health Dis. 9, 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bielawski J., Pierce J. S., Snider J., Rembiesa B., Szulc Z. M., Bielawska A. (2010) Sphingolipid analysis by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Adv. Exp. Med. Biol. 688, 46–59 [DOI] [PubMed] [Google Scholar]
- 22.Bielawski J., Pierce J. S., Snider J., Rembiesa B., Szulc Z. M., Bielawska A. (2009) Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol. Biol. 579, 443–467 [DOI] [PubMed] [Google Scholar]
- 23.Van Veldhoven P. P., Bell R. M. (1988) Effect of harvesting methods, growth conditions and growth phase on diacylglycerol levels in cultured human adherent cells. Biochim. Biophys. Acta 959, 185–196 [DOI] [PubMed] [Google Scholar]
- 24.Othman A., Rütti M. F., Ernst D., Saely C. H., Rein P., Drexel H., Porretta-Serapiglia C., Lauria G., Bianchi R., von Eckardstein A., Hornemann T. (2012) Plasma deoxysphingolipids: a novel class of biomarkers for the metabolic syndrome? Diabetologia 55, 421–431 [DOI] [PubMed] [Google Scholar]
- 25.Postma T. J., Aaronson N. K., Heimans J. J., Muller M. J., Hildebrand J. G., Delattre J. Y., Hoang-Xuan K., Lantéri-Minet M., Grant R., Huddart R., Moynihan C., Maher J., Lucey R.; EORTC Quality of Life Group (2005) The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur. J. Cancer 41, 1135–1139 [DOI] [PubMed] [Google Scholar]
- 26.Kiya T., Kawamata T., Namiki A., Yamakage M. (2011) Role of satellite cell-derived L-serine in the dorsal root ganglion in paclitaxel-induced painful peripheral neuropathy. Neuroscience 174, 190–199 [DOI] [PubMed] [Google Scholar]
- 27.Rowinsky E. K., Cazenave L. A., Donehower R. C. (1990) Taxol: a novel investigational antimicrotubule agent. J. Natl. Cancer Inst. 82, 1247–1259 [DOI] [PubMed] [Google Scholar]
- 28.Cepeda V., Fuertes M. A., Castilla J., Alonso C., Quevedo C., Pérez J. M. (2007) Biochemical mechanisms of cisplatin cytotoxicity. Anticancer. Agents Med. Chem. 7, 3–18 [DOI] [PubMed] [Google Scholar]
- 29.Pewzner-Jung Y., Ben-Dor S., Futerman A. H. (2006) When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? Insights into the regulation of ceramide synthesis. J. Biol. Chem. 281, 25001–25005 [DOI] [PubMed] [Google Scholar]
- 30.Janes K., Little J. W., Li C., Bryant L., Chen C., Chen Z., Kamocki K., Doyle T., Snider A., Esposito E., Cuzzocrea S., Bieberich E., Obeid L., Petrache I., Nicol G., Neumann W. L., Salvemini D. (2014) The development and maintenance of paclitaxel-induced neuropathic pain require activation of the sphingosine 1-phosphate receptor subtype 1. J. Biol. Chem. 289, 21082–21097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babin P. J., Gibbons G. F. (2009) The evolution of plasma cholesterol: direct utility or a “spandrel” of hepatic lipid metabolism? Prog. Lipid Res. 48, 73–91 [DOI] [PubMed] [Google Scholar]
- 32.Ksiazek, M., Chacinska, M., Chabowski, A., and Baranowski, M. (2015) Sources, metabolism and regulation of circulating sphingosine-1-phosphate [E-pub ahead of print]. J. Lipid Res. doi:10.1194/jlr.R059543 [DOI] [PMC free article] [PubMed]
- 33.Eiseman J. L., Eddington N. D., Leslie J., MacAuley C., Sentz D. L., Zuhowski M., Kujawa J. M., Young D., Egorin M. J. (1994) Plasma pharmacokinetics and tissue distribution of paclitaxel in CD2F1 mice. Cancer Chemother. Pharmacol. 34, 465–471 [DOI] [PubMed] [Google Scholar]
- 34.Seewaldt V. L., Figge D. C., Greer B. E., Tamimi H. K., Brown W. S., Cain J. M. (1994) Primary central nervous system recurrence after paclitaxel therapy for epithelial ovarian malignancy. Gynecol. Oncol. 55, 456–458 [DOI] [PubMed] [Google Scholar]
- 35.Garofalo K., Penno A., Schmidt B. P., Lee H. J., Frosch M. P., von Eckardstein A., Brown R. H., Hornemann T., Eichler F. S. (2011) Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J. Clin. Invest. 121, 4735–4745 [DOI] [PMC free article] [PubMed] [Google Scholar]