Abstract
The bioactive sphingolipid sphingosine-1-phosphate (S1P) mediates cellular proliferation, mitogenesis, inflammation, and angiogenesis. These biologies are mediated through S1P binding to specific GPCRs [sphingosine-1-phosphate receptor (S1PR)1–5] and some other less well-characterized intracellular targets. Ezrin-radixin-moesin (ERM) proteins, a family of adaptor molecules linking the cortical actin cytoskeleton to the plasma membrane, are emerging as critical regulators of cancer invasion via regulation of cell morphology and motility. Recently, we identified S1P as an acute ERM activator (via phosphorylation) through its action on S1PR2. In this work, we dissect the mechanism of S1P generation downstream of epidermal growth factor (EGF) leading to ERM phosphorylation and cancer invasion. Using pharmacologic inhibitors, small interfering RNA technologies, and genetic approaches, we demonstrate that sphingosine kinase (SK)2, and not SK1, is essential and sufficient in EGF-mediated ERM phosphorylation in HeLa cells. In fact, knocking down SK2 decreased ERM activation 2.5-fold. Furthermore, we provide evidence that SK2 is necessary to mediate EGF-induced invasion. In addition, overexpressing SK2 causes a 2-fold increase in HeLa cell invasion. Surprisingly, and for the first time, we find that this event, although dependent on S1PR2 activation, does not generate and does not require extracellular S1P secretion, therefore introducing a potential novel model of autocrine/intracrine action of S1P that still involves its GPCRs. These results define new mechanistic insights for EGF-mediated invasion and novel actions of SK2, therefore setting the stage for novel targets in the treatment of growth factor-driven malignancies.—Adada, M. M., Canals, D., Jeong, N., Kelkar, A. D., Hernandez-Corbacho, M., Pulkoski-Gross, M. J., Donaldson, J. C., Hannun, Y. A., Obeid, L. M. Intracellular sphingosine kinase 2–derived sphingosine-1-phosphate mediates epidermal growth factor–induced ezrin-radixin-moesin phosphorylation and cancer cell invasion.
Keywords: Spns2, cell adhesion, alkaline ceramidase 2
Sphingolipids are a class of molecules that have important functions in maintaining membrane structure and integrity (1). With the identification that sphingosine exerts regulatory roles on PKC (2), sphingolipids have emerged as important bioactive molecules that exert regulatory functions on important signaling pathways, therefore affecting a myriad of cellular processes, including cell survival, proliferation, motility, inflammation, and differentiation (3). An interlinked network of metabolizing enzymes tightly regulates sphingolipid levels. Of particular interest are the sphingosine kinases (SKs) SK1 and SK2, not only because they can be modulated by external stimuli (TNF, IL-1, and platelet-derived growth factor), but also changes in their activity have significant effects on the levels of their product sphingosine-1-phosphate (S1P) (1). SK1 has been by far the most studied isoform, and there is a large body of literature supporting its role in promoting cell proliferation, regulating inflammation, as well as driving neoplastic transformation and carcinogenesis. This enzyme has been well targeted by several pharmacologic inhibitors that proved effective in reducing tumor growth, metastasis, and inflammation (4), although results with some high-potency inhibitors recently developed have questioned some of these roles (5).
On the other hand, SK2 has been less well studied. Human SK2 is localized on chromosome 19, with peak expression during later stages of embryonic development especially in the liver and kidneys (6). It possesses an N-terminal region that is absent in SK1, making this isoform significantly larger (6). It is shown that it possesses several unique phosphorylation sites on its N terminus that are believed to regulate the activity of the catalytic subunit (7). This isoform also possesses a central proline-rich sequence that coincides with the substrate-binding site, making this enzyme more promiscuous in its substrate specificity (8).
SK2 plays an important role in modulating inflammation. Whereas SK1 has been mostly proposed to promote inflammation, the role of SK2 has been less clear. Knocking down SK2 in a murine mouse model of arthritis exacerbated the disease and increased cytokines production (9). A similar effect on cytokine production was seen in a xenograft murine model using SK2−/− MCF7 cells (10). On the other hand, inhibition of SK2, albeit with low-potency inhibitors of unclear specificity, ameliorated the disease progression of murine inflammatory bowel disease models such as ulcerative colitis (11) and Crohn’s disease (12). The studies on SK2 extend to its described roles in tumorigenesis. Early studies have implicated SK2 in proapoptotic functions owing to its BH3 domain (13) or via regulation of cytochrome c release from mitochondria following TNF stimulation, using small interfering RNA (siRNA) technology in mouse embryonic fibroblasts (14). More recently, it has also been implicated in inducing cell cycle arrest (15). On the other hand, more recent studies have emerged demonstrating a protumorigenic role for SK2. For example, it has been shown that SK2-derived S1P exacerbates colon cancer by acting as an antagonist to the retinoic acid receptor β and that its overexpression reversed all trans-retinoic acid-induced growth inhibition (16). Furthermore, the pharmacologic inhibition of SK2 has been proven successful in a multitude of malignancies, including hepatocellular carcinoma (17), renal cell carcinoma, and pancreatic adenocarcinomas (18). More importantly, SK2 has been shown to play a crucial role in promoting epidermal growth factor (EGF)-driven chemotaxis of MDA-MB-453 breast cancer cells using siRNA and overexpression techniques (19); however, the mechanism of SK2-induced motility has not been described. In addition, SK2’s effect on adhesion and invasion, the 2 other crucial steps involved in cancer progression, is still unknown.
Ezrin-radixin-moesin (ERM) proteins are a family of molecules that link the plasma membrane to the cortical actin cytoskeleton in a well-regulated manner that consists of a 2-step process (20). In its inactive conformation, the protein is closed due to the interaction between the N-terminal domain (4.1 protein, ezrin, radixin, moesin) and the C-terminal domain (carboxy-terminal ERM associated domain). This conformation locks the protein in the cytosol. After binding phosphatidylinositol 4,5-bisphosphate, the phosphorylation of a C-terminal threonine residue (ezrin Thr567) creates a steric hindrance between the N terminus and the C terminus opening up the protein. Now exposed, the 4.1 protein, ezrin, radixin, moesin domain binds to the plasma membrane, whereas the actin-binding site on the carboxy-terminal ERM associated domain is readily available to bind cortical actin (20). Their localization near the plasma membrane allows them to provide a stage where different molecules can interact [such as cluster of differentiation (CD)95 (21), CD44 (22), CD43 (23), and NHE1 (24)], facilitating signal transduction between the extracellular matrix and the cytosol. Furthermore, these proteins play an important role in dictating cell shape (25) and motility (26–28) because they are heavily enriched in specialized cellular protrusions such as filopodia and lamellipodia. That being said, they have emerged as important regulators of cancer progression by enhancing adhesion, and invasion of malignant cells, leading to metastasis (29).
More recently, our group identified sphingolipids as regulators of ERM proteins through both ceramide and S1P. Whereas ceramide generation on the plasma membrane caused rapid ERM dephosphorylation (30) via activation of protein phosphatase 1α (31), S1P treatment resulted in an acute and potent ERM activation that was dependent on sphingosine-1-phosphate receptor (S1PR)2 signaling (32). In addition, we have previously shown that EGF-mediated ERM activation, and subsequent lamellipodia formation and invasion, is dependent on the S1P/SP1R2 axis (33). However, several questions remain unanswered including the mechanism of S1P generation following EGF stimulation and its site of action. Answering these questions will unveil new targets in the pathway of EGF-driven invasion; also, it will uncover new modes of actions for the bioactive sphingolipid S1P.
Here, we have explored the mechanism by which SK regulates ERM phosphorylation and its downstream biologies following EGF treatment. Using cervical cancer HeLa cells as a model system, we demonstrate that SK2, and not SK1, is essential for EGF-mediated ERM phosphorylation. In addition, increased intracellular S1P production achieved by overexpression of either SK2 or the alkaline ceramidase (ACER)2 is sufficient in promoting ERM activation. Moreover, we identify SK2 as a novel and potent target in the pathway of EGF-driven invasion. As such, down-regulation of SK2 prevents EGF-mediated adhesion and subsequent extracellular matrix invasion. We also show that SK2 overexpression increases EGF-mediated adhesion and invasion via activation of the ERM proteins. Surprisingly, and for the first time, we demonstrate that this event, although dependent on S1PR2 activation, does not require extracellular S1P secretion, defining a new model for intracellular S1P signaling. We identify spinster homolog 2 (Spns2) as a potential transporter of S1P from the cytosolic side to the vicinity of S1PR2. Taken together, these studies define a new role for SK2 that depends on production of S1P, and an intracellular action for S1P on the S1PR2 with a critical role in regulating growth factor–induced invasion.
MATERIALS AND METHODS
Materials
High-glucose DMEM, fetal bovine serum (FBS), Lipofectamine 2000, Lipofectamine RNAiMax, SuperScript III First-Strand Synthesis kit, and 488- and 647-conjugated secondary antibodies were purchased from Life Technologies (Grand Island, NY, USA). Monoclonal anti–β-actin antibody and MK-571 were from Sigma-Aldrich (St. Louis, MO, USA). Anti-pERM (phosphorylated ezrin-radixin-moesin), anti-EGFR (epidermal growth factor receptor), anti-ErbB2, and anti-pERK antibodies and EGF were from Cell Signaling Technology (Danvers, MA, USA). Anti-total Ezrin, Protein A/G agarose, horseradish peroxidase–labeled secondary antibodies, and Probenecid were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). BCA kit, Pierce ECL, and SuperSignal West Dura Chemiluminescent Substrate were from Thermo Scientific (Suwanee, GA, USA). Anti-SK2 antibody was from Abcam Inc. (Cambridge, MA, USA). PF-543, Ski-II, erlotinib, ErbB2 inhibitor, lapatinib, EMD476485, bisindoylmaleimide I, G06976, EMD124017, U016, and JTE-013 were from EMD Millipore (Billerica, MA, USA). Sphingomab was obtained from Lpath Inc. (San Diego, CA, USA). ABC294640 was purchased from Active Biochemical Co. (Hong Kong, China).
Cell culture
HeLa cells were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were grown in DMEM supplemented with 10% FBS under standard cell culture conditions: 37°C and 5% CO2. When starved, DMEM without FBS was added for at least 4 h. Cells were tested for mycoplasma infection once per month.
RNA interference
Gene silencing was carried out using siRNA directed against human SK2 (Hs_SPHK2_5 FlexiTube siRNA SI00288561; Qiagen, Germantown, MD, USA), human ErbB1 (Hs_EGFR_11 FlexiTube siRNA SI02660147; Qiagen), human ErbB2 (Hs_ERBB2_14 FlexiTube siRNA SI02223571; Qiagen), human Spns2 (AM16708; Life Technologies), and with Qiagen AllStar (AStar) siRNA as a negative control. Transfections were carried out using Lipofectamine RNAiMax according to the manufacturer’s protocol.
Plasmid constructs and DNA transfection
Full-length untagged SK2, transcript variant 1 (SC113181) was obtained from OriGene (Rockville, MD, USA). The MYC tag was introduced at the C terminus of SK2 by subcloning the wild-type (WT) SK2 obtained from OriGene into a pcDNA3.1(-)myc/his/Amp vector using Xho/HindIII-flanked primers. Single mutations on WT SK2-MYC were generated using the QuikChange Site-Directed Mutagenesis Kit from Stratagene (La Jolla, CA, USA). The sequences of all plasmids were confirmed in the DNA Sequencing Facility (Stony Brook, NY, USA). Cells growing in 12-well plates were transfected with 1 μg plasmid DNA using Lipofectamine 2000 transfection reagent according to the manufacturer’s protocol.
Immunoprecipitation and immunoblotting
For immunoprecipitation studies, cells were treated with PBS or EGF (10 ng/ml). The reaction was stopped by adding 3.75% formaldehyde in DMEM. Cells were then lysed using a buffer that contains 20 nM Tris-HCl, 100 mM NaCl, 1% Triton X-100, 1 mM EDTA, protease, and phosphatase inhibitors from Sigma-Aldrich. Cell lysates were then sonicated, centrifuged at 10,000 g for 10 min to remove cell debris. Cells were then precleared for 1 h using Protein A/G agarose beads in a circular rotator at 4°C. Antibodies (1 μg/500 μg lysate) were then incubated with the supernatants overnight at 4°C. Immune complexes were then precipitated by centrifugation at 12,000 rpm for 1 min, washed 3 times, then 2× Laemmli buffer was added. Proteins were separated via SDS-PAGE (4–20% Tris-HCl) using the Bio-Rad criterion system (Hercules, CA, USA). Proteins were then transferred into a nitrocellulose membrane, blocked for 1 h with 5% milk in PBS with 0.1% Tween. Primary antibodies were then added to the membranes at 4°C overnight. The membranes were then incubated with the secondary antibodies at room temperature for 1 h. Proteins were visualized by chemiluminescence after several washes with PBS with Tween. Protein bands were quantified using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence and confocal microscopy
Laser microscopy and immunofluorescence were carried out using similar techniques as previously published. Briefly, HeLa cells plated on 35 mm confocal dishes were fixed with warmed paraformaldehyde solution (3.7% in PBS) for 20 min. Cells were then washed 3 times with PBS, then permeabilized with 0.1% Triton X-100 in PBS for 15 min. Cells were then blocked with 6% human serum in PBS for 20 min at room temperature. Cells were then incubated with the primary antibodies diluted at 1:100 in 6% human serum overnight at 4°C. Cells were then washed 3 times and then incubated for 1 h with the secondary antibodies in the dark. DAPI was then added, and cells were examined using the Leica SP8 confocal microscopy system (Leica Microsystems, Wetzlar, Germany).
Fluorescence-based SK2 assay
The fluorescent SK2 assay was performed as previously described with minor modifications. Cell lysates were harvested after sonication in cell lysis buffer containing 20 mM Tris-HCl (pH 7.4), 20% glycerol, 1 mM 2-ME, 1 mM EDTA, and both protease and phosphatase inhibitor cocktails. SK2 assay buffer [final concentrations in 100 µl reaction volume contained 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.4), 15 mM MgCl2, 400 mM KCl, and 0.005% Triton X-100] was mixed with 10 µM NBD Sphingosine (Avanti Polar Lipids, Alabaster, AL, USA) and 1 mM ATP in 200 mM MgCl2. The reaction was started with addition of cell lysate. The reaction was incubated for 1 h at 37°C protected from light. After incubation, 100 µl of a 1 M potassium phosphate dibasic solution at pH 8.5 was added, which was immediately followed by addition of 500 µl of a 2:1 chloroform:methanol extraction buffer. The solution was vortexed, and phases were separated by centrifugation at 3000 rpm for 5 min. The aqueous phase was removed and placed into a 96-well plate, which was read in a fluorescence plate reader (Synergy HT; BioTek Instruments, Winooski, VT, USA) with excitation and emission wavelengths of 485 and 528 nm, respectively.
d-erythro-17-carbon sphingosine labeling
Cells were plated at 500,000 cells per 60 mm dish. Once 80% confluent, cells were washed with serum-free DMEM, then starved overnight. Next, cells were treated with 250 nM d-erythro-17-carbon sphingosine (C17-Sph) for 30 min before stimulating with EGF for 30 s or 5 min. Cells were then directly lysed with 2:3 70% isopropanol:ethyl acetate after medium removal. Extracted lipids were then sent to Stony Brook University lipidomics core facility for analysis.
Cellular adhesion and invasion assays
For cell adhesion, 12-well plates were coated with fibronectin according to the manufacturer’s protocol. After 4 h of starvation, cells were trypsinized, counted, and plated on the fibronectin-precoated dishes for 12 h. Medium was then washed twice with PBS; attached cells were then quantified by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to the manufacturer’s protocol (Sigma-Aldrich). As for cell invasion, it was carried out using the Corning Tumor Invasion System (Corning, NY, USA) and following the manufacturer’s protocol with minor modifications as previously described (33). Briefly, cells were starved for 4 h. Cells were then resuspended at 200,000 cells/ml, and 500 μl was added to the apical chamber of the preactivated cell invasion plate. Serum-free medium or medium with chemoattractant was added to the lower chamber at a volume of 750 μl. Cells were then allowed to invade under normal cell culture conditions. Calcein AM dye (Life Technologies) was used to stain invading cells. Fluorescence was quantified using the SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA, USA).
Cell viability assay
At the end of the treatment period, medium was removed. Cells were washed once with warm PBS, then medium containing 5 mg/ml MTT was added for 30 min at normal cell culture conditions. The insoluble formazan product of MTT was then dissolved with DMSO and quantified by measuring its absorbance at 570 nm using the SpectraMax plate reader.
TASSER structure modeling
The structure model for S1PR2 was generated using the I-TASSER server for protein structure and function predictions. I-TASSER was run using human S1PR2 (UniProt ID O95136) as the query sequence. The model prediction was built using top threading templates A2A adenosine receptor [Protein Data Bank (PDB) 3EML], S1PR1 (PDB 3V2Y), 5-HT receptor (PDB 4IAQ), and β1-adrenergic receptor (PDB 3ZPQ). All normalized Z-scores are >2.0, indicating good alignment between query protein and template. The model exhibits a C-score of −0.7, estimated template modeling score of 0.62 ± 0.14, and estimated root-mean-square deviation of 8.1 ± 4.4 Å.
Statistical analysis
The graphs are represented as the means ± se. Two-way ANOVA with Bonferroni posttest statistical analysis was implemented using software from Prism Software Corp. (Irvine, CA, USA) and GraphPad Software (La Jolla, CA, USA).
RESULTS
EGF-induced ERM phosphorylation is ErbB1 dependent
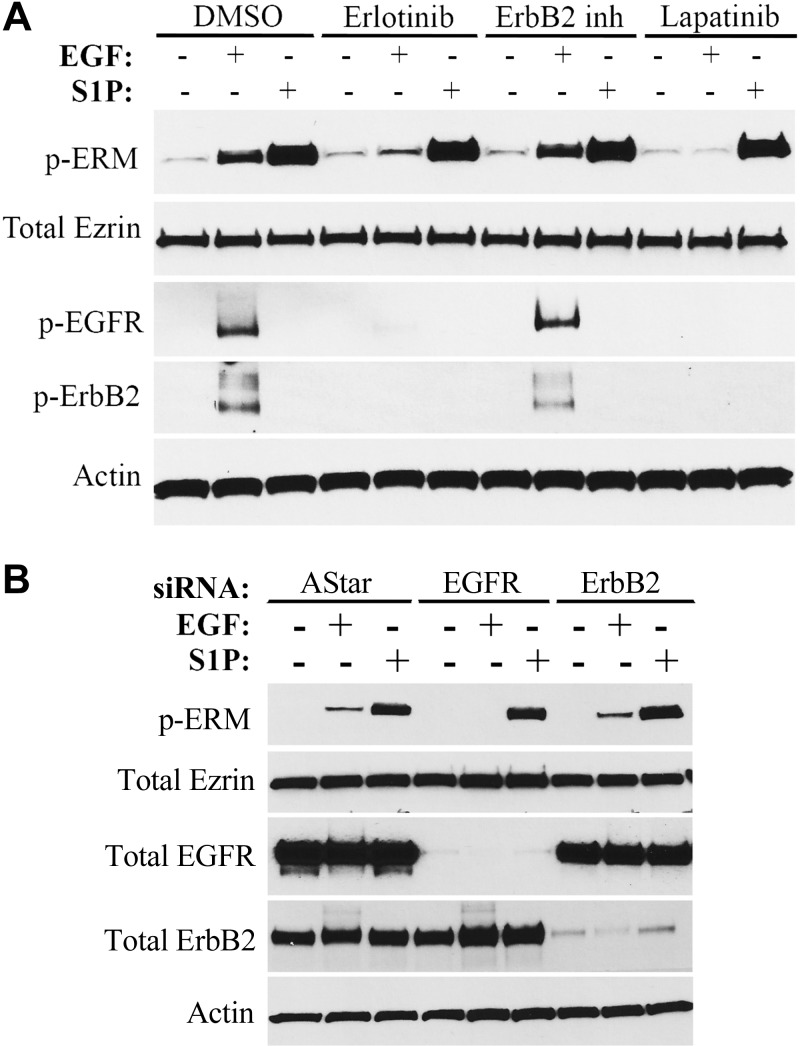
EGF exerts its functions by binding the EGFR also known as ErbB1, which subsequently forms a homodimer with another ErbB1 or a heterodimer with ErbB2 also known as Her2 (34). The homodimer regulates a myriad of signaling pathways that are different than those regulated by the heterodimer owing to the different structure of ErbB2, thus the heterogeneity of EGF responses (35). To identify the branch of the EGF pathway involved in ERM phosphorylation, erlotinib, an inhibitor of ErbB1, an ErbB2 inhibitor, and lapatinib, a well-known inhibitor of both ErbB1 and ErbB2, were used. As can be seen, inhibition of ErbB1 by erlotinib diminished ERM phosphorylation following EGF treatment (Fig. 1A). On the contrary, whereas inhibition of ErbB2 decreased its autophosphorylation and activation, it had no effect on pERM levels (Fig. 1A). As expected, inhibiting both receptors by lapatinib abrogated ERM activation (Fig. 1A). Importantly, erlotinib and lapatinib did not affect ERM phosphorylation following S1P treatment, ruling out the possibility that these inhibitors might be affecting the signaling pathway downstream of S1P (Fig. 1A). To further verify that ERM phosphorylation is strictly ErbB1 dependent, siRNA against EGFR and Her2 was used, and >90% knockdown was achieved (Fig. 1B). Again, ERM activation by EGF was only affected by EGFR knockdown (Fig. 1B). Together, these results indicate that EGF-mediated ERM phosphorylation is strictly ErbB1 dependent in HeLa cells.
Figure 1.
EGF-induced ERM phosphorylation is ErbB1 dependent. A) HeLa cells were pretreated with DMSO, erlotinib (100 nM), ErbB2 inhibitor (10 μM), or lapatinib (10 μM) for 1 h prior to treatment with EGF (10 ng/ml) or S1P (100 nM) for 5 min. pERM, phosphorylated EGFR (p-EGFR), and phosphorylated ErbB2 (p-ErbB2) levels were then assessed by immunoblotting. B) HeLa cells were treated with AStar, EGFR, or ErbB2 siRNA for 48 h. Cells were then starved for 4 h prior to treatment with EGF (10 ng/ml) for 5 min. pERM, total EGFR, and total ErbB2 levels were then assessed by immunoblotting.
SK2, and not SK1, mediates ERM phosphorylation upon EGF stimulation
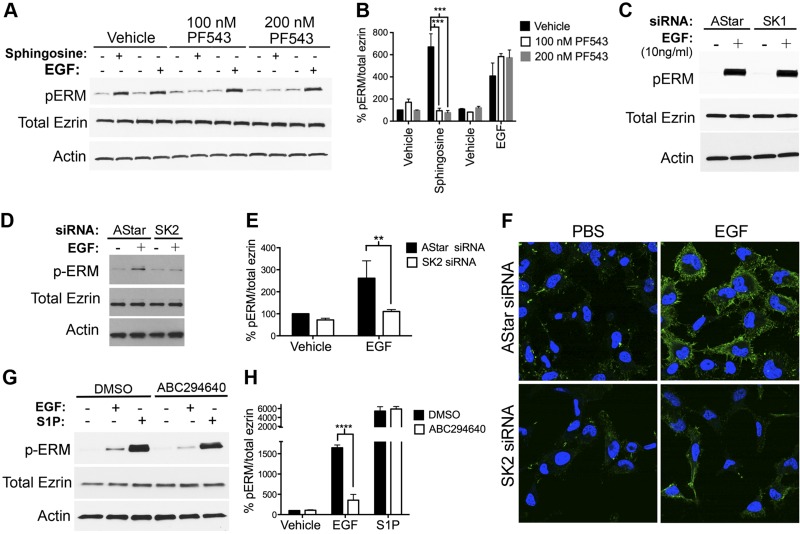
We previously reported that S1P is a potent inducer of ERM phosphorylation and that sphingosine led to ERM phosphorylation through its SK1-dependent phosphorylation to S1P (32). More recently, we showed that SK activity is required for ERM phosphorylation upon EGF stimulation (33). Nonetheless, the specific SK involved in mediating EGF action remained unknown. To address this question, a newly established compound (PF453) was utilized to acutely inhibit SK1 in cells stimulated with d-erythro-sphingosine (Sph) and EGF (Fig. 2A, B). As expected, inhibition of SK1 significantly decreased ERM phosphorylation upon sphingosine treatment, which we previously showed was SK1 dependent (32); however, PF543 did not affect EGF-mediated ERM phosphorylation compared to vehicle-treated cells (Fig. 2A, B). The role of SK1 in EGF-induced ERM phosphorylation was also ruled out by knocking it down using siRNA, also showing no effect on ERM phosphorylation (Fig. 2C). Next, and to test the role of SK2, it was knocked down in HeLa cells, and the effects on ERM phosphorylation were analyzed (Fig. 2D, E). SK2 knockdown significantly diminished ERM phosphorylation in response to EGF compared with AStar-treated cells (Fig. 2D, E). Importantly, the changes in ERM phosphorylation seen by Western blot were also observed on fixed cells by confocal microscopy (Fig. 2F), thus documenting the occurrence of these effects in cells. To show that the effects of blocking SK2 were due to the effects on S1P production, S1P was added to samples pretreated with ABC294640, a known SK2-specific inhibitor (36). Notably, this inhibitor significantly diminished EGF-mediated ERM phosphorylation (Fig. 2G, H). On the other hand, SK2 inhibition did not show a statistically significant decrease in ERM phosphorylation upon S1P treatment (Fig. 2G, H). Taken altogether, these results strongly suggest that SK2, and not SK1, is essential for EGF-mediated ERM phosphorylation.
Figure 2.
SK2, and not SK1, is essential for EGF-mediated ERM phosphorylation. A) HeLa cells were pretreated with 100 or 200 nM PF543 for 1 h prior to stimulation with EGF (10 ng/ml) or sphingosine (5 μM) for 5 min. pERM levels were then assessed by immunoblotting. Total ezrin and actin were also included as loading controls. C) HeLa cells were treated with AStar or SK1 siRNA for 48 h. Cells were then starved for 4 h prior to treatment with EGF (10 ng/ml) for 5 min. pERM levels were then assessed by immunoblotting. D) HeLa cells were treated with AStar or SK2 siRNA for 48 h. Cells were then starved for 4 h prior to treatment with EGF (10 ng/ml) for 5 min. pERM levels were then assessed by immunoblotting (C) and confocal microscopy (F), where the green color corresponds to pERM levels labeled with Alexa 488 fluorophore antibody, and the blue color corresponds to DAPI staining the nuclei (F). Total ezrin and actin were also included as loading controls (C). G) HeLa cells were pretreated with DMSO or 10 μM ABC294640 for 1 h prior to stimulation with EGF (10 ng/ml) or S1P (100 nM) for 5 min. pERM levels were then assessed by immunoblotting. Total ezrin and actin were also included as loading controls. B, E, and H) Quantification of the ratio of pERM:total ezrin in (A), (D), and (G), respectively, was performed using ImageJ software. The data represent means ± se of 3 independent experiments. **P < 0.01; ***P < 0.001; ****P < 0.0001.
Overexpression of SK2 is sufficient to promote ERM phosphorylation
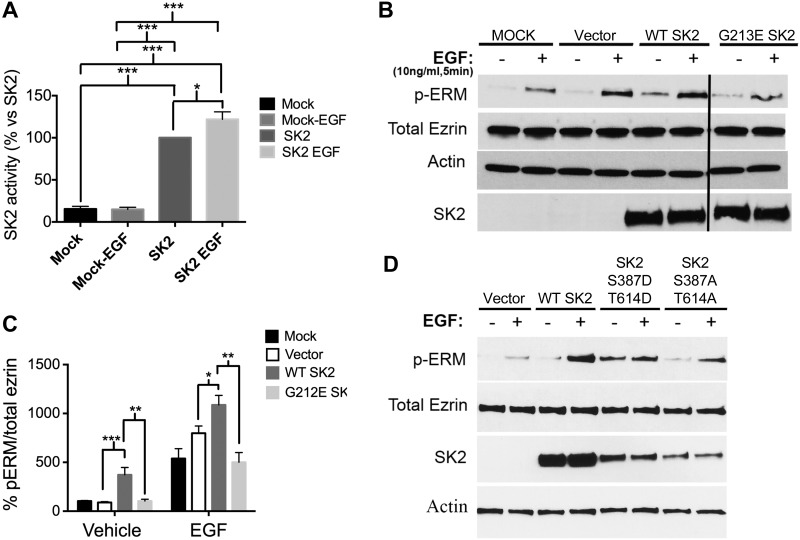
Once SK2 emerged as necessary for EGF signaling to ERM activation, it became important to determine if SK2 was sufficient to promote ERM phosphorylation. First, we assessed whether EGF stimulation affects SK2 activity. To this end, human WT MYC-tagged SK2b was transiently transfected into HeLa cells. SK2b is the longer isoform of SK2 that is widely expressed in human tissues. SK2 overexpression resulted in a 5-fold increase in SK2 activity compared to mock-transfected cells (Fig. 3A). EGF treatment increased SK2 activity by an additional 20% in SK2-overexpressing cells (Fig. 3A). Concomitantly, SK2 transfection into cells resulted in baseline increases in ERM phosphorylation in the absence of EGF stimulation (Fig. 3B, C). Moreover, EGF stimulation of SK2-transfected cells showed a statistically significant increase in ERM phosphorylation compared with mock- and vector-transfected cells that were stimulated with EGF (Fig. 3B, C). To further validate that the catalytic activity of SK2 (i.e., S1P production) is necessary for ERM phosphorylation, we generated the human catalytically inactive SK2 G213E (19). Mutating the glycine residue at position 213 in the catalytic site into glutamic acid had been previously described to abolish SK2 catalytic activity (19). Of note, G213E SK2-expressing cells failed to demonstrate the increase in ERM phosphorylation in unstimulated cells and did not enhance the EGF response (Fig. 3B, C). Taken together, these results indicate that overexpression of WT, but not the catalytically inactive SK2 (G213E), is sufficient to increase ERM phosphorylation. In addition, it has been previously reported that EGF induces phosphorylation of SK2 at Ser351 and Tyr578, which increases its activity (37). Importantly, overexpressing the phosphomimetic SK2 (Ser387D, Tyr614D) did not only increase basal ERM phosphorylation compared with WT SK2-transfected cells, but it also blunted the EGF response (Fig. 3D). On the other hand, overexpressing the nonphosphorylatable form of SK2 (Ser387A, Tyr614A) did not show any increase in basal pERM levels (Fig. 3D). Nonetheless, cells transfected with the latter construct still responded to EGF, although to a much lesser extent than the WT SK2-transfected cells (Fig. 3D). This could be due to either the presence of the endogenous SK2 or the regulation of SK2 activity by EGF at other undiscovered sites. Collectively, these results demonstrate that SK2 is sufficient to induce ERM phosphorylation, as well as they highlight the importance of its activation by EGF.
Figure 3.
SK2 is sufficient to promote ERM activation. A) HeLa cells were transfected with mock or SK2 DNA for 24 h. Cells were then starved for 4 h prior to stimulation with EGF for 30 s. Cells were then lysed, and SK2 activity was measured using an SK2-specific activity assay as described in the Materials and Methods. B) HeLa cells were transfected with vector, WT SK2, and G213E SK2 DNA for 24 h. Cells were then starved for 4 h prior to treatment with EGF (10 ng/ml) for 5 min. pERM and total SK2 levels were then assessed by immunoblotting. Total ezrin and actin were also included as loading controls. C) Quantification of the ratio of pERM:total ezrin in (D) was performed using ImageJ software. The data represent means ± se of 3 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001. D) HeLa cells were transfected with vector, WT SK2, Ser387D;Tyr614D SK2, and Ser387A;Tyr614A SK2 DNA for 24 h. Cells were then starved for 4 h prior to treatment with EGF (10 ng/ml) for 5 min. pERM and total SK2 levels were then assessed by immunoblotting. Total ezrin and actin were also included as loading controls.
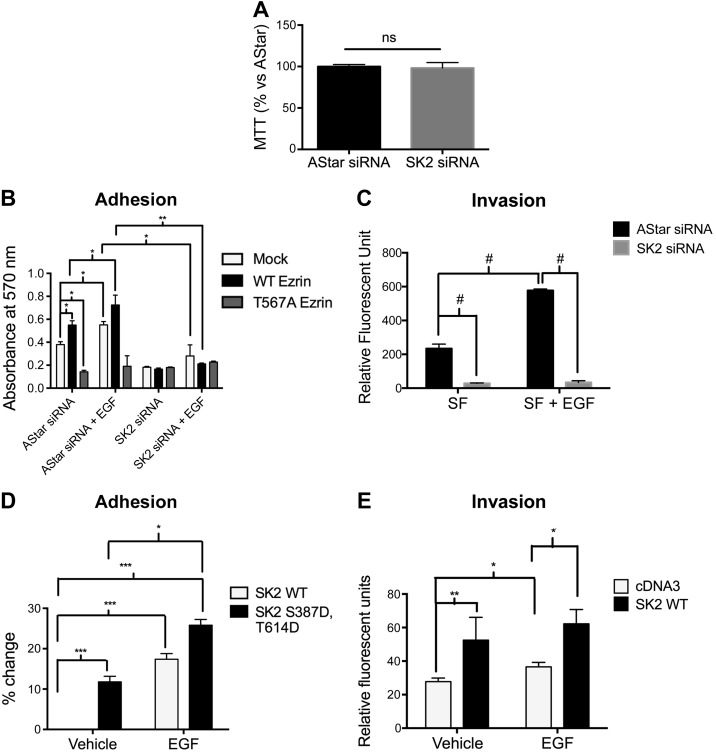
EGF-mediated cell adhesion and subsequent invasion occur in an SK2-dependent phosphorylation of ezrin Thr567
ERM proteins and EGF have both been reported to play an important role in cell adhesion, migration, and invasion (38). We have previously demonstrated that EGF-mediated lamellipodia formation and cell invasion occur via S1PR2 (33). It is also known that EGF-mediated cellular migration is dependent on SK2 in MDA-MB-453 breast cancer cells (19). Therefore, it became crucial to determine whether SK2 plays an essential role in EGF-induced cellular adhesion and invasion and whether it is ERM dependent. We first assessed whether knocking down SK2 affects cell viability. To this end, we performed an MTT assay on HeLa transfected with AStar or SK2 siRNA. As shown in Fig. 4A, knocking down SK2 had no effect on cell viability. Using fibronectin-coated plates, nontransfected HeLa cells or HeLa cells expressing VSV-G–tagged WT ezrin or the dominant-negative nonphosphorylatable ezrin mutant where threonine 567 residue is replaced with alanine (TA) were allowed to adhere for 12 h in serum-free medium. WT ezrin and AStar double-transfected cells showed a significant baseline higher adhesion levels than nontransfected cells, whereas cells overexpressing the nonphosphorylatable TA-ezrin mutant adhered significantly less than nontransfected and WT ezrin-overexpressing cells (Fig. 4B). This result consolidates the role of Thr567 ezrin phosphorylation in cellular adhesion. As reported in the literature, EGF treatment significantly increases cellular adhesion in nontransfected cells. Importantly, EGF did not increase the adhesion of TA-ezrin–transfected cells, indicating that EGF-mediated cellular adhesion is mostly mediated by ezrin Thr567 phosphorylation (Fig. 4B). To determine the role of SK2 in EGF-mediated cellular adhesion, this enzyme was knocked down before overexpressing the WT ezrin and the TA-ezrin mutant. SK2 knockdown significantly decreased baseline and EGF-mediated cell adhesion in mock- and WT ezrin-transfected cells (Fig. 4B). As expected, there was no further decrease in cellular adhesion in cells overexpressing the TA mutant upon knocking down SK2. Because the baseline adhesion of HeLa cells decreased upon knocking down SK2, these results suggest, albeit not conclusively, that EGF-mediated cell adhesion occurs through SK2-dependent phosphorylation of ezrin Thr567.
Figure 4.
SK2 is required and sufficient for cell adhesion and invasion toward EGF. A) HeLa cells were treated with AStar or SK2 siRNA for 48 h. Cells were then starved for 4 h. An MTT assay was then performed as described in the Materials and Methods. B) HeLa cells were treated with AStar or SK2 siRNA for 24 h. Cells were then transfected with mock, WT ezrin, or Thr567A ezrin DNA for another 24 h. Cells were then starved for 4 h, trypsinized, and plated on fibronectin-coated plates. Cells were then treated with vehicle (PBS) or EGF (10 ng/ml) for 12 h. MTT assay was then performed, and absorbance was measured as a quantification of cell number. C) HeLa cells were treated with AStar or SK2 siRNA for 48 h. Cells were then starved for 4 h prior to their plating in the apical chamber of Matrigel-coated transwell inserts and allowed to invade for 36 h toward serum-free or EGF-supplemented medium. Invading cells were then stained with 4 μg/μl calcein AM, and absorbance was read in a plate reader as described in the Materials and Methods. D) HeLa cells were transfected with WT SK2 or Ser387D;Tyr614D SK2 DNA for 24 h. Cell adhesion was then assessed as described in (B). E) HeLa cells overexpressing cDNA3 or WT SK2 were plated in the apical chamber of Matrigel-coated transwell inserts and allowed to invade for 36 h toward serum-free or EGF-supplemented medium. Invaded cells were then quantified as previously described. The data represent means ± se of 3 independent experiments. ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001; #P < 0.0001.
Our previous work showed that EGF-mediated cell invasion is greatly dependent on ezrin phosphorylation at Thr567 residue (33). Because adhesion is the first step necessary for subsequent cell invasion, the role of SK2 in the latter process was assessed by performing invasion assays using transwell chambers coated with Matrigel (Corning) (Fig. 4C). EGF treatment caused a 3-fold increase in cellular invasion in AStar-transfected cells. Knocking down SK2 significantly decreased cellular invasion basally and toward EGF-supplemented medium (Fig. 4C). On the other hand, knocking down SK2 did not cause a decrease in cellular invasion toward medium supplemented with 10% serum (data not shown), thus likely conferring specificity for the SK2 role toward EGF in mediating cellular invasion. In concert with the results shown above, these data suggest that EGF-mediated cell adhesion and subsequent invasion occur through SK2-dependent phosphorylation of ezrin Thr567. To further assess the role of SK2 and its product S1P in cellular adhesion, we transfected HeLa cells with the phosphomimetic mutant SK2 (Ser387D, Tyr614D). SK2 (Ser387D, Tyr614D)-overexpressing cells showed 15% higher basal adherence levels compared to WT SK2-overexpressing cells (Fig. 4D). Having proven that SK2 is partially sufficient in promoting cellular adhesion, its implication on invasion was then tested. Notably, HeLa cells overexpressing WT SK2 showed 2-fold higher basal and EGF-induced invasion than vector-transfected cells (Fig. 4E). Collectively, these results indicate that SK2 is essential and partially sufficient in promoting cellular adhesion and invasion in an ezrin-dependent manner.
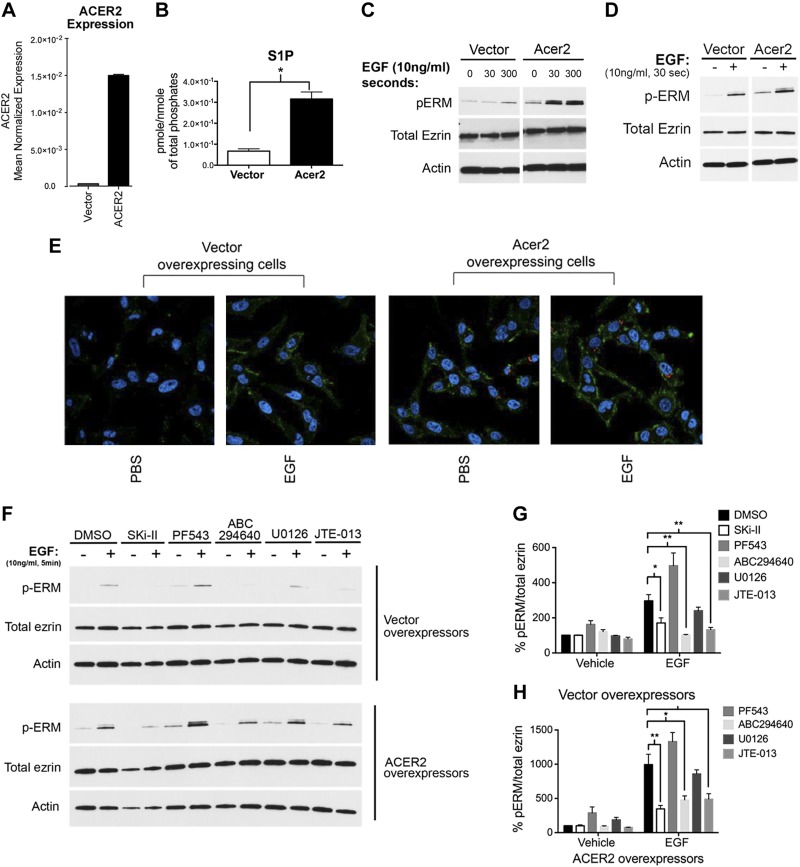
S1P production by ACER2 and SK2 is sufficient to promote ERM phosphorylation
ACER2 belongs to the family of ACERs that deacylate ceramide, thus generating sphingosine. It localizes to the endoplasmic reticulum (ER) and Golgi, and its overexpression leads to increased intracellular sphingosine and S1P levels (39). To evaluate the mechanism of S1P production in response to EGF, cells stably overexpressing ACER2 were used. ACER2-overexpressing cells showed 15-fold increase in ACER2 mRNA (Fig. 5A) and an increase in intracellular S1P levels (Fig. 5B), confirming previously published results. ACER2 stable cells showed a significant baseline increase in ERM phosphorylation compared to vector stable cells, which increased further upon EGF treatment (Fig. 5C). To rule out a chronic adaptive phenotype from the stable cell lines, HeLa cells were transiently transfected with ACER2. As found for the stable cell line, HeLa cells transiently overexpressing ACER2 also had a significant basal increase in ERM phosphorylation that was accentuated by EGF treatment compared to vector-transfected cells (Fig. 5D). Functionally, HeLa cells showed a significant increase in ERM phosphorylation upon ACER2 overexpression that was heightened after EGF treatment when examined under confocal microscopy (Fig. 5E). To test whether the higher pERM levels are due to increased S1P levels, the nonspecific SK inhibitor Ski-II, SK1-specific inhibitor PF-543 (5), and SK2-specific inhibitor ABC294640 (36) were used. Ski-II and ABC294640 significantly decreased ERM phosphorylation upon EGF treatment not only in vector-overexpressing cells but also in ACER2 stable cells (Fig. 5F–H). Notably, SK1 inhibition by PF543 slightly increased baseline and EGF-induced ERM phosphorylation (Fig. 5F–H). This could be attributed to a compensatory increase in SK2 activity upon acute inhibition of SK1. These results suggest that, upon EGF treatment, sphingosine that is generated by ACER2 is specifically channeled to SK2 for S1P conversion and subsequent ERM phosphorylation. To further validate that these effects are mediated by S1P, the specific S1PR2 antagonist JTE-013 was utilized. As expected, treatment with this receptor antagonist significantly diminished EGF-induced ERM phosphorylation in vector as well as in ACER2 stable cells (Fig. 5F–H), consolidating previous findings that establish S1PR2 as an essential player in ERM activation downstream of EGF (33). Collectively, these results suggest that ACER2 and SK2 are sufficient to promote ERM phosphorylation.
Figure 5.
Increased intracellular S1P production is sufficient to promote ERM activation. A) mRNA of HeLa cells overexpressing empty vector or ACER2 was assessed by quantitative RT-PCR. B) Cellular lipids were directly extracted in organic solvents from HeLa cells overexpressing empty vector or ACER2. S1P levels were analyzed by tandem liquid chromatography/MS. C) HeLa-ACER2-TET-ON and HeLa-vector control cells were treated with tetracycline (5 ng/ml) for 12 h. These cells were then starved for 4 h prior to treatment with EGF (10 ng/ml) for either 30 s or 5 min. pERM levels were then assessed by immunoblotting (C) and by confocal microscopy (E). The green color corresponds to pERM levels labeled with Alexa 488 fluorophore antibody, and the blue color corresponds to DAPI staining the nuclei (E). D) HeLa cells were transiently transfected with either vector control or ACER2 overexpressing DNA for 24 h. Cells were then starved for 4 h prior to treatment with EGF (10 ng/ml) for 5 min. pERM levels were then assessed by immunoblotting. Total ezrin and actin were also included as loading controls. F) HeLa cells stably overexpressing vector or ACER2 were starved overnight. These cells were then pretreated with Ski-II (1 μM), PF543 (100 nM), ABC294640 (10 μM), U0126 (10 μM), or JTE-013 (5 μM) prior to stimulation with EGF (10 ng/ml) for 5 min. G and H) Quantification of the ratio of pERM:total ezrin in (F) was performed using ImageJ software. The data represent means ± se of 3 independent experiments. *P < 0.05; **P < 0.01.
EGF-mediated ERM phosphorylation occurs by intracellular S1P generation, and not by extracellular S1P secretion
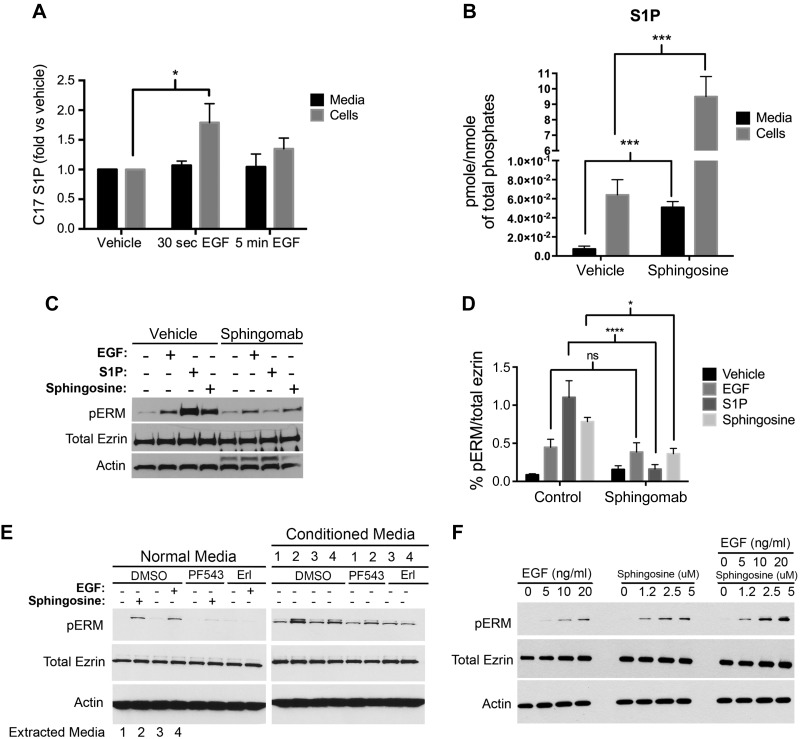
EGF has been shown to activate both SK1 and SK2 (19). However, as shown before, only SK2 is required for ERM phosphorylation upon EGF treatment, suggesting that the SK response to EGF toward ERM phosphorylation is somewhat compartmentalized. SK1 has been located to the plasma membrane, and it generates intracellular as well as extracellular S1P. The topology of SK2 and S1P generated from SK2 are more controversial. Due to the different locations of SK1 and SK2, and because both are reported to be activated by EGF, we focused on the study of a possible different location of their product, S1P. First, the localization of S1P production was assessed post-EGF treatment. For this purpose, we utilized C17-Sph, an exogenously utilized sphingosine for mass spectrometry (MS)-based measurements of cellular S1P generation (40). HeLa cells were first pretreated with C17-Sph and then stimulated with EGF. Next, HPLC/MS was used to measure C17-S1P in the medium and cells. EGF treatment caused a 40% increase in intracellular C17-S1P production that peaked in 30 s and returned to baseline levels at 5 min (Fig. 6A). However, there was no change in the C17-S1P levels in the medium upon EGF stimulation (Fig. 6A). These results suggested that EGF strictly increases intracellular and not extracellular S1P levels. The effects of sphingosine stimulation on S1P production were also assessed (Fig. 6B). Treatment of HeLa cells with sphingosine (5 μM) significantly increased intracellular as well as extracellular S1P levels (Fig. 6B).
Figure 6.
ERM phosphorylation does not require extracellular S1P production. A) HeLa cells were prelabeled for 30 min with 250 nM C17-Sph. EGF (10 ng/ml) was then added for 30 s or 5 min. C17-S1P levels from medium and cells were then analyzed via mass spectroscopy. B) HeLa cells were starved overnight, then treated with 5 μM sphingosine for 5 min. Endogenous S1P levels from medium and cells were then analyzed via mass spectroscopy. The data represent means ± se of 3 independent experiments performed in duplicates. C) HeLa cells were pretreated for 1 h with either PBS or 50 μg/well Sphingomab prior to treatment with EGF (10 ng/ml), or sphingosine (5 μM), or S1P (100 nM) for 5 min. pERM levels were then assessed by immunoblotting. Total ezrin and actin were also included as loading controls. D) Quantification of the ratio of pERM:total ezrin in (C) was performed using ImageJ software. The data represent means ± se of 2 independent experiments. E) HeLa cells were pretreated with DMSO, PF543 (100 nM), or erlotinib (Erl; 100 nM) for 1 h, then followed by treatment with either sphingosine (5 μM) or EGF (10 ng/ml) for 2 min. Media from DMSO-pretreated cells (with EGF, Sph, or vehicle treatment), named conditioned media and labeled 1–4, were then added on top of HeLa cells that are pretreated with DMSO, PF543 (100 nM), or erlotinib (100 nM). The reasoning lies in that PF543 treatment will inhibit sphingosine conversion to S1P and that erlotinib will inhibit EGF effect; thus, any effect seen after the addition of the conditioned media will be the result of S1P presence in these media. pERM levels were then assessed by immunoblotting as a marker of S1P presence. Total ezrin and actin were also included as loading controls. F) HeLa cells were plated for 24 h and then starved for 4 h prior to treatment with increasing doses of sphingosine, EGF, or both. pERM levels were then assessed by Western blotting. Total ezrin and actin were also included as loading controls. ns, not significant; *P < 0.05; ***P < 0.001; ****P < 0.0001.
To study the implications of these observations on ERM phosphorylation, the newly described S1P-neutralizing antibody Sphingomab was utilized (41). Pretreatment of HeLa cells with Sphingomab abolished the effects of exogenously applied S1P on ERM phosphorylation compared with vehicle-treated cells (Fig. 6C, D). This demonstrates the efficacy of this neutralizing antibody. Likewise, neutralizing extracellular S1P significantly decreased ERM phosphorylation upon treatment with sphingosine (5 μM) (Fig. 6C, D). However, HeLa cells stimulated with EGF to induce ERM phosphorylation were not blocked using Sphingomab. These results suggest that sphingosine treatment results in extracellular S1P production that is necessary for ERM phosphorylation, whereas EGF stimulation results in intracellular S1P production, which will induce ERM phosphorylation likely without being secreted extracellularly.
To further distinguish autocrine/intracrine from paracrine actions of EGF via S1P, we used conditioned medium from cells treated with Sph or EGF because Sph should produce extracellular S1P, whereas EGF does not. To avoid the effects of carryover of Sph and EGF in the conditioned medium, the recipient cells were treated with an SK and EGFR inhibitor, respectively; as shown above, that pretreatment of cells with PF543 inhibits sphingosine-induced ERM phosphorylation, and treatment with EGFR inhibitor erlotinib inhibits the actions of EGF. As expected, addition of the conditioned medium extracted from sphingosine-treated cells increased ERM phosphorylation of HeLa cells that were pretreated with the SK1 inhibitor (Fig. 6E). This can only be explained by the presence of S1P in the conditioned medium that was able to bypass SK1 inhibition (Fig. 6E). On the other hand, addition of the conditioned medium extracted from EGF-treated cells did not affect pERM levels of HeLa cells that were pretreated with erlotinib (Fig. 6E). This provides further evidence that EGF-mediated ERM phosphorylation does not require extracellular S1P generation. Because these results point to the fact that EGF and sphingosine act via distinct mechanisms, we tested whether their action on ERM phosphorylation is additive. HeLa cells were treated with increasing doses of EGF and/or sphingosine, and their effects on pERM were checked by Western blotting. Indeed, cells stimulated with EGF and sphingosine exhibited higher pERM levels compared with cells treated with either stimulus alone (Fig. 6F). Taken together, these results suggest that ERM phosphorylation in response to EGF treatment occurs via an intracrine S1P signaling mechanism.
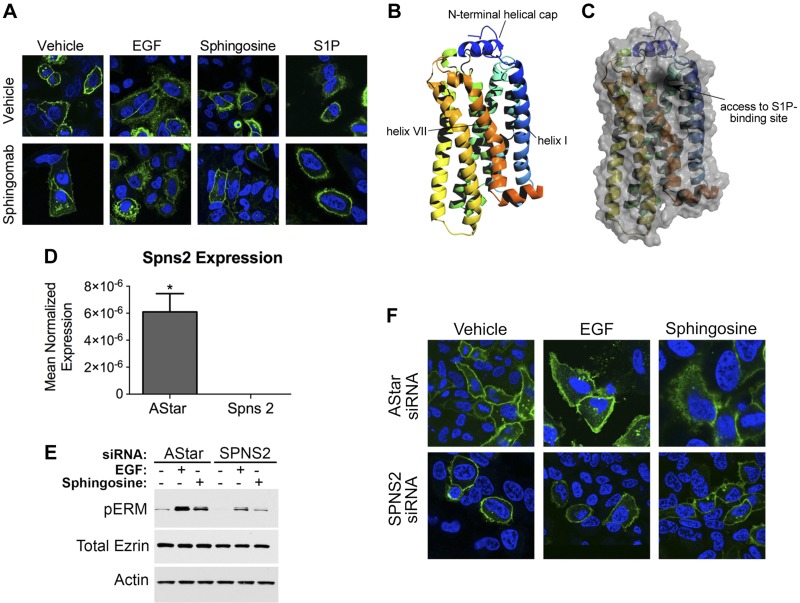
SPNS2 is required to deliver intracellular S1P to S1PR2
Because EGF-mediated ERM phosphorylation does not require extracellular S1P secretion yet is S1PR2 dependent, it became imperative to determine the mechanism of S1P transport to this receptor. This is a key step to explore because it is very unlikely for intracellular S1P to reach its receptor (oriented extracytoplasmically even in intracellular compartments) passively due to topological restraints. First, the activation status of S1PR2 was checked upon neutralizing extracellular S1P. To this end, S1PR2-green fluorescent protein (GFP) internalization was assessed upon treatment with EGF, sphingosine, and S1P in the presence or absence of Sphingomab. As expected, Sphingomab completely abolished S1PR2 internalization induced by S1P compared with vehicle-treated cells, further validating the efficacy of this antibody in binding extracellular S1P (Fig. 7A). In addition, it greatly reduced S1PR2 internalization induced by sphingosine, yet had no effect on EGF-induced S1PR2 internalization (Fig. 7A), thus strongly corroborating the notion that SK2-derived S1P activates S1PR2 without extracellular secretion. Next, it became essential to determine how SK2-derived S1P reaches its receptor. Upon cellular fractionation and confocal microscopy, we found that SK2 is mostly present in cellular membranes, including ER, Golgi, endosomes, and plasma membrane (data not shown), further confirming previously published results. The crystal structure of S1PR1 revealed that its S1P-binding site is on its lateral surface and that SK-derived S1P reaches this site via lateral diffusion on the outer leaflet of the plasma membrane (42, 43). Although the crystal structure of S1PR2 is not yet resolved, we were able to model it by superimposition with S1PR1 (Fig. 7B, C). There was great similarity between S1PR1- and S1PR2-binding pocket, where both are present on the lateral surface of the receptor (Fig. 7B, C). Furthermore, it has been previously shown that SK2 resides on the cytosolic side of cellular membranes (44); thus, there must be a transport mechanism that delivers S1P produced on the cytosolic side to the outer membranous leaflets for S1PR2 activation. SPNS2, a lipid transporter, has been recently shown to be involved in S1P export in vitro (45) as well as in vivo (46). Hence, its role in EGF-mediated ERM activation was tested. Spns2 was knocked down in HeLa cells, and the effects on Spns2 mRNA and ERM phosphorylation were analyzed (Fig. 7D, E). As can be seen, Spns2 siRNA-treated cells showed >95% knockdown of Spns2 mRNA compared with AStar negative control-treated cells (Fig. 7D). Spns2 knockdown significantly diminished ERM phosphorylation in response to sphingosine and EGF compared to AStar-treated cells (Fig. 7E). Conversely, Spns2 overexpression led to increased baseline as well as EGF-induced ERM phosphorylation compared to vector-overexpressing cells (data not shown). These results indicate that Spns2 is partly required for both sphingosine and EGF-mediated ERM phosphorylation. Next, to test for the role of Spns2 in S1PR2 activation, we knocked it down in S1PR2-GFP–overexpressing HeLa cells and checked for receptor internalization. Cells treated with Spns2 siRNA showed a partial reduction in sphingosine and EGF-induced S1PR2 internalization compared with AStar-treated cells (Fig. 7F). Therefore, these results demonstrate that Spns2 is required to transport S1P, generated from sphingosine by SK1, across the plasma membranes and to transport S1P, generated by SK2 in response to EGF, possibly across intracellular membranes to access S1PR2 and lead to ERM phosphorylation.
Figure 7.
Spns2 is partially required for EGF-mediated ERM phosphorylation and S1PR2 internalization. A) HeLa cells were transfected with S1PR2-GFP for 24 h. Following transfection, cells were starved for 4 h and then pretreated for 1 h with PBS or Sphingomab. Cells were treated with 100 nM S1P, 10 nM EGF, or 5 μM sphingosine. Cells were then fixed, and nuclei were stained with DAPI (blue). Images were taken using the Leica SP8 confocal microscope. B and C) Structure model for S1PR2 generated using the I-TASSER server (B). Cartoon with surface overlay, demonstrating the proposed point of access for S1P from the membrane into its receptor-binding site, is shown in (C). D) HeLa cells were treated with 20 nM AStar or Spns2 siRNA for 48 h, and Spns2 mRNA was then assessed by quantitative RT-PCR. *P < 0.05. E) HeLa cells were treated with AStar or Spns2 siRNA for 48 h. Cells were then starved for 4 h prior to treatment with EGF (10 ng/ml) or sphingosine (5 μM) for 5 min. pERM levels were then assessed by immunoblotting. Total ezrin and actin were also included as loading controls. F) HeLa cells were treated with AStar or Spns2 siRNA for 48 h. HeLa cells were then transfected with S1PR2-GFP, 24 h following siRNA transfection. Cells were starved for 4 h and then treated with 10 nM EGF, or 5 μM sphingosine. Cells were then fixed, and nuclei were stained with DAPI (blue). Images were taken using a Leica SP8 confocal microscope.
DISCUSSION
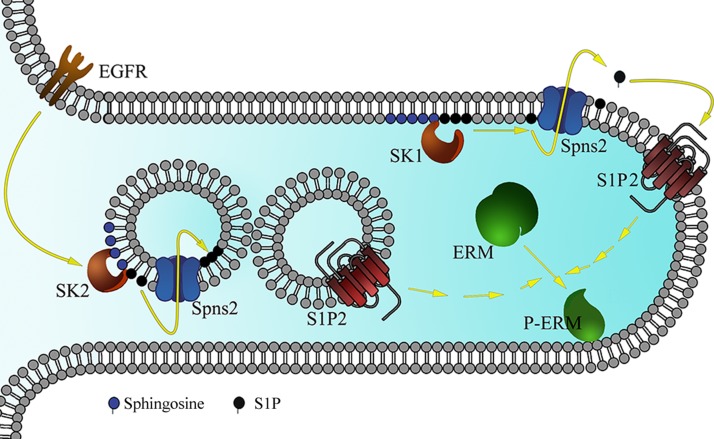
In the current study, we describe a new mechanism for EGF-driven cancer invasion by SK2-driven intracellular S1P and the activation of the ERM family of proteins. In short, EGF stimulates ERM activation in an ErbB1 and SK2-dependent manner. This is followed by intracellular S1P production that is then exported to the exocytoplasmic leaflet of endogenous membranes, where it docks on S1PR2. Once active, this receptor triggers a signaling cascade leading to ERM phosphorylation, which in turn increases cellular adhesion and subsequent invasion (Fig. 8). This mechanism is different, but probably complementary, than previously reported mechanisms of EGF-induced cellular invasion, by either inducing the MAPK pathway and/or triggering the activation of the PI3K cascade. These results have implications to several fields including novel functions pertaining to intracellular S1P signaling, a novel role for the Spns2/S1PR2 axis in cancer cell motility, revealing a novel SK2-specific function, and defining new targets for therapy in growth factor-driven cancers.
Figure 8.
Schematic representation of the proposed model for EGF-induced ERM phosphorylation. EGF treatment activates SK2 localized on the cytosolic side of intracellular vesicles. This will lead to S1P being produced and transported by Spns2 (as well as other ABC transporters) to the inner side of these vesicles. These vesicles will then fuse with S1PR2-containing vesicles or with the plasma membrane. This is followed by S1PR2 activation and ERM phosphorylation, and filopodia formation as shown in this cellular extrusion. On the other hand, sphingosine treatment causes S1P production by SK1 localized on the plasma membrane. S1P will then be exported to the extracellular milieu by Spns2, leading to S1PR2 activation and ERM phosphorylation.
The first significant finding corresponds to establishing a specific and novel function downstream of SK2 related to cell adhesion and cell invasion. There is a marked paucity in the literature about biologic functions that are mediated by a specific SK isoform. Although both SKs have been reported to be activated by EGF (19, 47), we report, in this context, only SK2 as being necessary for ERM phosphorylation in response to EGF. This is an important finding because it ascribes a completely novel function that is specific for SK2. Very few pathways have been described to be specifically mediated by SK2. These include the sequestration of the prosurvival BCL-XL protein via its BH3 domain, the activation of bcl-2 homologous antagonist killer, and the inhibition of histone deacetylase 1/2 in the nucleus. In addition, most animal studies using SK2 inhibitors fail to relate the phenotype observed to SK2 inhibition alone. This is the first report assigning a mechanistic function for SK2 (i.e., ERM activation).
The results of this study also support the emerging evidence on the procancerous roles of SK2. Previously, SK2 has been suggested to increase the migration of MDA-MB-453 cells toward EGF (19). Nonetheless, increased cell motility is not enough to confer a malignant cellular behavior because other processes, such adhesion and invasion, are crucial. In this study, we provide additional evidence of the tumorigenic role of SK2 because it is not only essential but also sufficient to augment cellular adhesion and subsequent invasion. Furthermore, there is a paucity of studies that explore the mechanisms exploited by SK2 to confer malignancy. In this study, we found that the ERM family of proteins is a potent downstream effector conducting the protumorigenic functions of SK2. These proteins have been increasingly involved in cancer metastasis and are being suggested as biomarkers for tumor aggressiveness. Therefore, targeting SK2 could constitute a novel strategy to turn off the activity of these proteins, ultimately lessening the cancer cells’ ability for adhesion and invasion.
More importantly, this study defines a new mechanism for intracellular S1P signaling. We demonstrated that EGF-mediated ERM phosphorylation does not necessitate the secretion of S1P extracellularly using several lines of evidence. Based on these results, it becomes clear that the fate of S1P is dictated by the enzyme producing it, as well as its localization. Whereas SK1-derived S1P (that is generated after sphingosine treatment) requires extracellular secretion for ERM phosphorylation, SK2-derived S1P is either maintained in the intracellular milieu or in the plasma membrane. In addition, we performed a detailed time response upon EGF treatment and measured endogenous S1P levels. To our surprise, we did not detect a significant increase in S1P levels up to 1 h following EGF stimulation (data not shown). This could be due to the generation of a small but highly localized pool of S1P produced by SK2 following EGF stimulation and that may be beyond the sensitivity of MS detection. Strikingly, S1PR2 activation was still required to mediate ERM activation. This raised the question of how this intracellular lipid can encounter its receptor. S1PR2 and SK2 are membranous proteins that are continuously trafficking between the different endosomal compartments, a phenomenon that would facilitate their interaction. Indeed, blocking intracellular trafficking by hypertonic sucrose abolished ERM-induced phosphorylation by EGF (data not shown). In addition, the possibility that S1PR2 might be signaling while localized intracellularly is very likely. This will not be the first S1PR described to have intracellular functions because recently, S1PR5 has been shown to localize to centrosomes along with both SKs to regulate cell division (48). Because sucrose is a nonspecific inhibitor for cellular trafficking, further studies are needed to test this possibility. On the other hand, one might argue that EGFR might cause S1PR2 internalization and activation regardless of SK activation, as has been previously shown with S1PR1 and platelet-derived growth factor (49). However, in our hands, knocking down SK2 significantly decreased S1PR2 activation (data not shown), thus ruling out this hypothesis. Collectively, these results describe a new mechanism for intracellular S1P signaling. Although it is thought that intracellular S1P mediates its functions by binding exclusively to specific proteins such as TNF receptor-associated factor 2 and histone deacetylases, we provide an alternative model for intracellular S1P that still requires S1PR signaling. Although this model is still nascent, it can explain the scarcity of known intracellular targets for S1P.
The current study highlights a novel function for the newly identified S1P transporter Spns2. This transporter has been shown to play an essential role in lymphocyte trafficking (50) and cardiac development (51). Although this transporter is a major regulator of S1P levels, and therefore cell motility, its role in cancer progression has been understudied as well as controversial. One study showed that knocking down this transporter increases intracellular S1P levels and consequently migration of lung cancer cells (45). In contrast, another study showed that Spns2 is an essential player in promoting colon cancer following inflammation (52). The controversy may be in part due to the dependency of tumor motility on S1PRs. Whereas migration of lung cancer cells seems to be receptor independent (45), colon cancer development is dependent on S1PR1 activation (52). This is similar to our study, where cervical cancer motility is dependent on S1PR2 signaling, and Spns2 is an essential player in transporting S1P to its receptor. Besides, we are aware that the effect of Spns2 knockdown on EGF-induced ERM phosphorylation and S1PR2 internalization are partial, but this could be due to a complementary role that is played by other S1P transporters such as AATP-binding cassette (ABC) transporters (53). In fact, inhibiting these transporters by Probenecid or MK-571 partially inhibited EGF-induced ERM activation (data not shown). Therefore, it could be that S1P transport in HeLa cells is not exclusively mediated by Spns2, but several other carriers could also play a role, including the ABC family of proteins.
Moreover, because both sphingosine and EGF treatments require Spns2, and therefore S1P transport, it became essential to explain how S1P generated upon sphingosine treatment makes it to the extracellular milieu, whereas that generated upon EGF treatment remains inside the cell. This could mostly be due to differences in SK localization (54). Our group, as well as many others, has shown that upon activation, SK1 is localized to the plasma membrane, where it phosphorylates sphingosine (55, 56). Subsequently, Spns2 and the ABC proteins transport S1P that is produced on the inner leaflet of the plasma membrane to the extracellular milieu. On the other hand, SK2 does not localize to the plasma membrane and is mainly present in intracellular membranes, mostly in the ER, Golgi, and endosomes as shown by our group (data not shown) and in other published reports (7, 57). In addition, it has been previously demonstrated that SK2 produces S1P on the cytosolic side of these intracellular vesicles (44). We suspect that Spns2 will then import S1P to the inside of these vesicles, which will then fuse with others that contain S1PR2 either intracellularly or on the plasma membrane, where activation of this receptor occurs. This activation is then also responsible for enhancing endocytosis of the receptor. This model is supported by the essential role of intracellular trafficking that we proved vital for ERM activation (data not shown). Further testing is required to validate this hypothesis and is being currently investigated in our lab.
The Spns2/S1PR2/ERM axis as defined in this work seems to be evolutionarily connected. In fact, we note that mice deficient in Spns2, S1PR2, or radixin each develop progressive hearing loss (58–60). Spns2 and S1PR2 knockout mice show degeneration of the sensory hair cells in the inner ear (58, 60). This is thought to be caused by abnormally dilated and branched stria vascularis with irregular cortical actin patterns. Furthermore, radixin knockout mice develop progressive degeneration of the stereocilia present on hair cells leading to deafness (59). On the other hand, the Spns2/S1PR2 axis has also been well established in zebrafish because it plays an essential role in vertebrate heart morphogenesis (61, 62). Spns2 and S1PR2 deletion causes the zebrafish to develop cardia bifida, a cardiac malformation characterized by the development of 2 hearts; it was attributed to a defect in the migration of myocardial precursors to the midline. Importantly, the addition of S1P to Spns2-deficient embryos restored normal heart development in an S1PR2-dependent manner (61). These observations strengthen the current results implicating the Spns2/S1PR2 axis in cell movement. Moreover, these 2 genetic mutants also share the same abnormal phenotype that is characterized by the appearance of tail blisters in zebrafish (61). In conclusion, this body of work solidifies the role of the Spns2/S1PR2 axis in cellular movement, however, in a different context that is related to cancer cell motility. Moreover, in very preliminary data, we demonstrate that Spns2 and S1PR2 likely colocalize in HeLa cells, thus underscoring the need to consolidate and extend this work in cancer cell motility and invasion.
In conclusion, these results delineate a novel pathway for growth factor–driven invasion, which is dependent on intracellular S1P signaling under EGF stimulation. This pathway could have tremendous implications in the treatment of EGF-aggravated tumors because it provides novel therapeutic targets. This is especially important for patients that become refractory to current treatment modalities due to acquired mutations in the EGFR.
Acknowledgments
The authors thank all the members of the Lipid Cancer Lab for their comments during data presentation. The authors also thank Mrs. Janet Allopenna for her help in tagging the WT sphingosine kinase 2 construct with the MYC tag, and Lpath for the generous gift of the Sphingomab antibody. The Sphingomab antibody was obtained through a material transfer agreement between LPath Inc. (San Diego, CA, USA) and the Research Foundation for the State University of New York. The authors also thank Dr. Cungui Mao and Ms. Ruijuan Xu for their generous gift of the ACER2 DNA construct, as well as the HeLa cells stably overexpressing ACER2. This work was supported by a U.S. Veterans Affairs Merit Award and U.S. National Institutes of Health Grants GM097741 (National Institute of General Medical Sciences) and PO1CA097132 (National Cancer Institute) all to L.M.O. M.M.A., D.C., Y.A.H., and L.M.O. participated in research design. M.M.A. and D.C. prepared the analytic tools. M.M.A., D.C., N.J., M.J.P.-G., M.H.-C., and A.D.K. conducted the experiments. M.M.A., D.C., Y.A.H., and L.M.O. wrote or contributed to the writing of the manuscript. The authors declare no conflicts of interest.
Glossary
- ABC
AATP-binding cassette
- ACER
alkaline ceramidase
- AStar
AllStar
- C17-Sph
d-erythro-17-carbon sphingosine
- CD
cluster of differentiation
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ER
endoplasmic reticulum
- ERM
ezrin-radixin-moesin
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- MS
mass spectrometry
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- pERM
phosphorylated ezrin-radixin-moesin
- S1P
sphingosine-1-phosphate
- S1PR
sphingosine-1-phosphate receptor
- siRNA
small interfering RNA
- SK
sphingosine kinase
- Sph
d-erythro-sphingosine
- Spns2
spinster homolog 2
- TA
threonine 567 to alanine
- WT
wild-type
REFERENCES
- 1.Hannun Y. A., Obeid L. M. (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 [DOI] [PubMed] [Google Scholar]
- 2.Hannun Y. A., Bell R. M. (1989) Regulation of protein kinase C by sphingosine and lysosphingolipids. Clin. Chim. Acta 185, 333–345 [DOI] [PubMed] [Google Scholar]
- 3.Hannun Y. A., Obeid L. M. (2011) Many ceramides. J. Biol. Chem. 286, 27855–27862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandy K. A., Obeid L. M. (2013) Regulation of the sphingosine kinase/sphingosine 1-phosphate pathway. Handb. Exp. Pharmacol. 216, 275–303 [DOI] [PubMed] [Google Scholar]
- 5.Schnute M. E., McReynolds M. D., Kasten T., Yates M., Jerome G., Rains J. W., Hall T., Chrencik J., Kraus M., Cronin C. N., Saabye M., Highkin M. K., Broadus R., Ogawa S., Cukyne K., Zawadzke L. E., Peterkin V., Iyanar K., Scholten J. A., Wendling J., Fujiwara H., Nemirovskiy O., Wittwer A. J., Nagiec M. M. (2012) Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. Biochem. J. 444, 79–88 [DOI] [PubMed] [Google Scholar]
- 6.Liu H., Sugiura M., Nava V. E., Edsall L. C., Kono K., Poulton S., Milstien S., Kohama T., Spiegel S. (2000) Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 275, 19513–19520 [DOI] [PubMed] [Google Scholar]
- 7.Neubauer H. A., Pitson S. M. (2013) Roles, regulation and inhibitors of sphingosine kinase 2. FEBS J. 280, 5317–5336 [DOI] [PubMed] [Google Scholar]
- 8.Gao P., Peterson Y. K., Smith R. A., Smith C. D. (2012) Characterization of isoenzyme-selective inhibitors of human sphingosine kinases. PLoS One 7, e44543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai W. Q., Irwan A. W., Goh H. H., Melendez A. J., McInnes I. B., Leung B. P. (2009) Distinct roles of sphingosine kinase 1 and 2 in murine collagen-induced arthritis. J. Immunol. 183, 2097–2103 [DOI] [PubMed] [Google Scholar]
- 10.Weigert A., Schiffmann S., Sekar D., Ley S., Menrad H., Werno C., Grosch S., Geisslinger G., Brüne B. (2009) Sphingosine kinase 2 deficient tumor xenografts show impaired growth and fail to polarize macrophages towards an anti-inflammatory phenotype. Int. J. Cancer 125, 2114–2121 [DOI] [PubMed] [Google Scholar]
- 11.Maines L. W., Fitzpatrick L. R., French K. J., Zhuang Y., Xia Z., Keller S. N., Upson J. J., Smith C. D. (2008) Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig. Dis. Sci. 53, 997–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maines L. W., Fitzpatrick L. R., Green C. L., Zhuang Y., Smith C. D. (2010) Efficacy of a novel sphingosine kinase inhibitor in experimental Crohn’s disease. Inflammopharmacology 18, 73–85 [DOI] [PubMed] [Google Scholar]
- 13.Liu H., Toman R. E., Goparaju S. K., Maceyka M., Nava V. E., Sankala H., Payne S. G., Bektas M., Ishii I., Chun J., Milstien S., Spiegel S. (2003) Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J. Biol. Chem. 278, 40330–40336 [DOI] [PubMed] [Google Scholar]
- 14.Chipuk J. E., McStay G. P., Bharti A., Kuwana T., Clarke C. J., Siskind L. J., Obeid L. M., Green D. R. (2012) Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 148, 988–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hait N. C., Allegood J., Maceyka M., Strub G. M., Harikumar K. B., Singh S. K., Luo C., Marmorstein R., Kordula T., Milstien S., Spiegel S. (2009) Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325, 1254–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun D. F., Gao Z. H., Liu H. P., Yuan Y., Qu X. J. (2012) Sphingosine 1-phosphate antagonizes the effect of all-trans retinoic acid (ATRA) in a human colon cancer cell line by modulation of RARβ expression. Cancer Lett. 319, 182–189 [DOI] [PubMed] [Google Scholar]
- 17.Beljanski V., Lewis C. S., Smith C. D. (2011) Antitumor activity of sphingosine kinase 2 inhibitor ABC294640 and sorafenib in hepatocellular carcinoma xenografts. Cancer Biol. Ther. 11, 524–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beljanski V., Knaak C., Zhuang Y., Smith C. D. (2011) Combined anticancer effects of sphingosine kinase inhibitors and sorafenib. Invest. New Drugs 29, 1132–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hait N. C., Sarkar S., Le Stunff H., Mikami A., Maceyka M., Milstien S., Spiegel S. (2005) Role of sphingosine kinase 2 in cell migration toward epidermal growth factor. J. Biol. Chem. 280, 29462–29469 [DOI] [PubMed] [Google Scholar]
- 20.Bretscher A., Edwards K., Fehon R. G. (2002) ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3, 586–599 [DOI] [PubMed] [Google Scholar]
- 21.Lozupone F., Lugini L., Matarrese P., Luciani F., Federici C., Iessi E., Margutti P., Stassi G., Malorni W., Fais S. (2004) Identification and relevance of the CD95-binding domain in the N-terminal region of ezrin. J. Biol. Chem. 279, 9199–9207 [DOI] [PubMed] [Google Scholar]
- 22.Tsukita S., Yonemura S., Tsukita S. (1997) ERM proteins: head-to-tail regulation of actin-plasma membrane interaction. Trends Biochem. Sci. 22, 53–58 [DOI] [PubMed] [Google Scholar]
- 23.Yonemura S., Hirao M., Doi Y., Takahashi N., Kondo T., Tsukita S., Tsukita S. (1998) Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J. Cell Biol. 140, 885–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denker S. P., Huang D. C., Orlowski J., Furthmayr H., Barber D. L. (2000) Direct binding of the Na-H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H(+) translocation. Mol. Cell 6, 1425–1436 [DOI] [PubMed] [Google Scholar]
- 25.Lamb R. F., Ozanne B. W., Roy C., McGarry L., Stipp C., Mangeat P., Jay D. G. (1997) Essential functions of ezrin in maintenance of cell shape and lamellipodial extension in normal and transformed fibroblasts. Curr. Biol. 7, 682–688 [DOI] [PubMed] [Google Scholar]
- 26.Zohar R., Suzuki N., Suzuki K., Arora P., Glogauer M., McCulloch C. A., Sodek J. (2000) Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J. Cell. Physiol. 184, 118–130 [DOI] [PubMed] [Google Scholar]
- 27.Endo K., Kondo S., Shackleford J., Horikawa T., Kitagawa N., Yoshizaki T., Furukawa M., Zen Y., Pagano J. S. (2009) Phosphorylated ezrin is associated with EBV latent membrane protein 1 in nasopharyngeal carcinoma and induces cell migration. Oncogene 28, 1725–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prag S., Parsons M., Keppler M. D., Ameer-Beg S. M., Barber P., Hunt J., Beavil A. J., Calvert R., Arpin M., Vojnovic B., Ng T. (2007) Activated ezrin promotes cell migration through recruitment of the GEF Dbl to lipid rafts and preferential downstream activation of Cdc42. Mol. Biol. Cell 18, 2935–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machesky L. M. (2008) Lamellipodia and filopodia in metastasis and invasion. FEBS Lett. 582, 2102–2111 [DOI] [PubMed] [Google Scholar]
- 30.Canals D., Jenkins R. W., Roddy P., Hernández-Corbacho M. J., Obeid L. M., Hannun Y. A. (2010) Differential effects of ceramide and sphingosine 1-phosphate on ERM phosphorylation: probing sphingolipid signaling at the outer plasma membrane. J. Biol. Chem. 285, 32476–32485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canals D., Roddy P., Hannun Y. A. (2012) Protein phosphatase 1α mediates ceramide-induced ERM protein dephosphorylation: a novel mechanism independent of phosphatidylinositol 4, 5-biphosphate (PIP2) and myosin/ERM phosphatase. J. Biol. Chem. 287, 10145–10155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gandy K. A., Canals D., Adada M., Wada M., Roddy P., Snider A. J., Hannun Y. A., Obeid L. M. (2013) Sphingosine 1-phosphate induces filopodia formation through S1PR2 activation of ERM proteins. Biochem. J. 449, 661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orr Gandy K. A., Adada M., Canals D., Carroll B., Roddy P., Hannun Y. A., Obeid L. M. (2013) Epidermal growth factor-induced cellular invasion requires sphingosine-1-phosphate/sphingosine-1-phosphate 2 receptor-mediated ezrin activation. FASEB J. 27, 3155–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Citri A., Yarden Y. (2006) EGF-ERBB signalling: towards the systems level. Nat. Rev. Mol. Cell Biol. 7, 505–516 [DOI] [PubMed] [Google Scholar]
- 35.Macdonald-Obermann J. L., Pike L. J. (2014) Different epidermal growth factor (EGF) receptor ligands show distinct kinetics and biased or partial agonism for homodimer and heterodimer formation. J. Biol. Chem. 289, 26178–26188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.French K. J., Zhuang Y., Maines L. W., Gao P., Wang W., Beljanski V., Upson J. J., Green C. L., Keller S. N., Smith C. D. (2010) Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J. Pharmacol. Exp. Ther. 333, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hait N. C., Bellamy A., Milstien S., Kordula T., Spiegel S. (2007) Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J. Biol. Chem. 282, 12058–12065 [DOI] [PubMed] [Google Scholar]
- 38.Adada M., Canals D., Hannun Y. A., Obeid L. M. (2014) Sphingolipid regulation of ezrin, radixin, and moesin proteins family: implications for cell dynamics. Biochim. Biophys. Acta 1841, 727–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu R., Jin J., Hu W., Sun W., Bielawski J., Szulc Z., Taha T., Obeid L. M., Mao C. (2006) Golgi alkaline ceramidase regulates cell proliferation and survival by controlling levels of sphingosine and S1P. FASEB J. 20, 1813–1825 [DOI] [PubMed] [Google Scholar]
- 40.Spassieva S., Bielawski J., Anelli V., Obeid L. M. (2007) Combination of C(17) sphingoid base homologues and mass spectrometry analysis as a new approach to study sphingolipid metabolism. Methods Enzymol. 434, 233–241 [DOI] [PubMed] [Google Scholar]
- 41.O’Brien N., Jones S. T., Williams D. G., Cunningham H. B., Moreno K., Visentin B., Gentile A., Vekich J., Shestowsky W., Hiraiwa M., Matteo R., Cavalli A., Grotjahn D., Grant M., Hansen G., Campbell M. A., Sabbadini R. (2009) Production and characterization of monoclonal anti-sphingosine-1-phosphate antibodies. J. Lipid Res. 50, 2245–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanson M. A., Roth C. B., Jo E., Griffith M. T., Scott F. L., Reinhart G., Desale H., Clemons B., Cahalan S. M., Schuerer S. C., Sanna M. G., Han G. W., Kuhn P., Rosen H., Stevens R. C. (2012) Crystal structure of a lipid G protein-coupled receptor. Science 335, 851–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Sullivan C., Dev K. K. (2013) The structure and function of the S1P1 receptor. Trends Pharmacol. Sci. 34, 401–412 [DOI] [PubMed] [Google Scholar]
- 44.Hait N. C., Fujita K., Lester R. L., Dickson R. C. (2002) Lcb4p sphingoid base kinase localizes to the Golgi and late endosomes. FEBS Lett. 532, 97–102 [DOI] [PubMed] [Google Scholar]
- 45.Bradley E., Dasgupta S., Jiang X., Zhao X., Zhu G., He Q., Dinkins M., Bieberich E., Wang G. (2014) Critical role of Spns2, a sphingosine-1-phosphate transporter, in lung cancer cell survival and migration. PLoS One 9, e110119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hisano Y., Kobayashi N., Yamaguchi A., Nishi T. (2012) Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One 7, e38941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Döll F., Pfeilschifter J., Huwiler A. (2005) The epidermal growth factor stimulates sphingosine kinase-1 expression and activity in the human mammary carcinoma cell line MCF7. Biochim. Biophys. Acta 1738, 72–81 [DOI] [PubMed] [Google Scholar]
- 48.Gillies L., Lee S. C., Long J. S., Ktistakis N., Pyne N. J., Pyne S. (2009) The sphingosine 1-phosphate receptor 5 and sphingosine kinases 1 and 2 are localised in centrosomes: possible role in regulating cell division. Cell. Signal. 21, 675–684 [DOI] [PubMed] [Google Scholar]
- 49.Waters C. M., Long J., Gorshkova I., Fujiwara Y., Connell M., Belmonte K. E., Tigyi G., Natarajan V., Pyne S., Pyne N. J. (2006) Cell migration activated by platelet-derived growth factor receptor is blocked by an inverse agonist of the sphingosine 1-phosphate receptor-1. FASEB J. 20, 509–511 [DOI] [PubMed] [Google Scholar]
- 50.Fukuhara S., Mochizuki N. (2013) [Lymphocytes mobilization into blood regulated by Spns2, a sphingosine 1-phosphate transporter, expressed on endothelial cells]. Seikagaku 85, 269–272 [PubMed] [Google Scholar]
- 51.Hisano Y., Ota S., Takada S., Kawahara A. (2013) Functional cooperation of spns2 and fibronectin in cardiac and lower jaw development. Biol. Open 2, 789–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Degagné E., Pandurangan A., Bandhuvula P., Kumar A., Eltanawy A., Zhang M., Yoshinaga Y., Nefedov M., de Jong P. J., Fong L. G., Young S. G., Bittman R., Ahmedi Y., Saba J. D. (2014) Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs. J. Clin. Invest. 124, 5368–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishi T., Kobayashi N., Hisano Y., Kawahara A., Yamaguchi A. (2014) Molecular and physiological functions of sphingosine 1-phosphate transporters. Biochim. Biophys. Acta 1841, 759–765 [DOI] [PubMed] [Google Scholar]
- 54.Siow D., Wattenberg B. (2011) The compartmentalization and translocation of the sphingosine kinases: mechanisms and functions in cell signaling and sphingolipid metabolism. Crit. Rev. Biochem. Mol. Biol. 46, 365–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson K. R., Becker K. P., Facchinetti M. M., Hannun Y. A., Obeid L. M. (2002) PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA). J. Biol. Chem. 277, 35257–35262 [DOI] [PubMed] [Google Scholar]
- 56.Jarman K. E., Moretti P. A., Zebol J. R., Pitson S. M. (2010) Translocation of sphingosine kinase 1 to the plasma membrane is mediated by calcium- and integrin-binding protein 1. J. Biol. Chem. 285, 483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Don A. S., Rosen H. (2009) A lipid binding domain in sphingosine kinase 2. Biochem. Biophys. Res. Commun. 380, 87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kono M., Belyantseva I. A., Skoura A., Frolenkov G. I., Starost M. F., Dreier J. L., Lidington D., Bolz S. S., Friedman T. B., Hla T., Proia R. L. (2007) Deafness and stria vascularis defects in S1P2 receptor-null mice. J. Biol. Chem. 282, 10690–10696 [DOI] [PubMed] [Google Scholar]
- 59.Kitajiri S., Fukumoto K., Hata M., Sasaki H., Katsuno T., Nakagawa T., Ito J., Tsukita S., Tsukita S. (2004) Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia. J. Cell Biol. 166, 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J., Ingham N., Kelly J., Jadeja S., Goulding D., Pass J., Mahajan V. B., Tsang S. H., Nijnik A., Jackson I. J., White J. K., Forge A., Jagger D., Steel K. P. (2014) Spinster homolog 2 (spns2) deficiency causes early onset progressive hearing loss. PLoS Genet. 10, e1004688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawahara A., Nishi T., Hisano Y., Fukui H., Yamaguchi A., Mochizuki N. (2009) The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 323, 524–527 [DOI] [PubMed] [Google Scholar]
- 62.Kupperman E., An S., Osborne N., Waldron S., Stainier D. Y. (2000) A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature 406, 192–195 [DOI] [PubMed] [Google Scholar]