Abstract
Tissue remodeling is a characteristic of many solid tumor malignancies including melanoma. By virtue of tumor lymphatic transport, remodeling pathways active within the local tumor microenvironment have the potential to be operational within lymph nodes (LNs) draining the tumor interstitium. Here, we show that lymphatic drainage from murine B16 melanomas in syngeneic, immune-competent C57Bl/6 mice is associated with LN enlargement as well as nonuniform increases in bulk tissue elasticity and viscoelasticity, as measured by the response of whole LNs to compression. These remodeling responses, which quickly manifest in tumor-draining lymph nodes (TDLNs) after tumor inoculation and before apparent metastasis, were accompanied by changes in matrix composition, including up to 3-fold increases in the abundance of soluble collagen and hyaluronic acid. Intranodal pressures were also significantly increased in TDLNs (+1 cmH2O) relative to both non-tumor-draining LNs (−1 cmH2O) and LNs from naive animals (−1 to 2 cmH2O). These data suggest that the reorganization of matrix structure, composition, and fluid microenvironment within LNs associated with tumor lymphatic drainage parallels remodeling seen in primary malignancies and has the potential to regulate the adhesion, proliferation, and signaling function of LN-resident cells involved in directing melanoma disease progression.—Rohner, N. A., McClain, J., Tuell, S. L., Warner, A., Smith, B., Yun, Y., Mohan, A., Sushnitha, M., Thomas, S. N. Lymph node biophysical remodeling is associated with melanoma lymphatic drainage.
Keywords: sentinel lymph node, cancer, tissue mechanical properties
Lymph nodes (LNs) are secondary lymphoid tissues whose primary function is to maintain mature naive lymphocytes and initiate adaptive immune response. Sentinel or tumor-draining lymph nodes (TDLNs) are thus the major immunologic sites of tumor antigen presentation and lymphocyte activation (1). TDLN metastasis is also the primary clinical diagnostic at the time of tumor resection and is the strongest prognostic indicator of patient outcome for many types of cancers (2–5). The TDLN microenvironment may thus contribute to disease progression by regulating both antitumor immunity as well as metastasis.
Physical perturbations in the tissue micro- and macroarchitecture that manifest during development of malignancies such as melanoma are now widely recognized as influencing both the intra- and intercellular signaling involved in disease progression (6); these include growth-induced solid stresses (7, 8), increased matrix stiffness (9), impaired vascular barrier and transport function (10), and elevated interstitial fluid pressures (11, 12). This paradigm has been considered almost exclusively in the context of solid tumor formation and cellular invasion from the primary tumor into the surrounding interstitial and vascular tissues. But by virtue of their proximity to growing tumors, TDLNs are exposed to a high concentration of lymph-transported molecules from the tumor interstitium (13, 14). Signaling pathways active within the local tumor microenvironment that result in tissue remodeling associated with survival (9), invasion (15–17), and immune suppression (18) therefore have the potential to be operational within TDLNs. Melanoma-associated LN remodeling, including changes in size (19), cellularity (20), and extracellular matrix composition (20, 21), has been reported but without respect to tumor stage. Moreover, evidence as to whether the physical adaptations that manifest in tumors, including increases in matrix stiffness (9) and viscoelasticity (22) as well as interstitial hypertension (11, 12), also develop within LNs during tumor disease progression is anecdotal at best (9, 11, 22), and the specific effect of LN locality (i.e., presence within or outside of the tumor-draining lymphatic basin) on these changes has not been stipulated. This limits the understanding of whether mechanotransduction pathways associated with tumor progression have the potential to function within the TDLNs (23–25). The specific contributions to this signaling by lymphatic drainage function and TDLN metastasis are also unsubstantiated.
Herein, we used a B16 murine melanoma model implanted intradermally in the left dorsal skin of immune-competent C57Bl/6 mice in order to focus tumor drainage to one side of the animal, resulting in TDLNs on the ipsilateral side and non-tumor-draining lymph nodes (NTDLNs) on the contralateral side. We have used this model previously to study the role of lymphatic drainage in tumor-induced immune suppression (18) and how immune modulatory drug delivery to TDLNs can alter antitumor immunity and tumor growth (13). Placement of the melanomas in the lateral dorsal skin results in 2 main advantages. First, this placement enables the juxtaposition of responses by LNs ipsilateral to the tumor to their subtype-matched nondraining LNs contralateral to the tumor. Second, as opposed to tumor formation in mouse limb extremities, implantation in the lateral dorsal skin results in multiple TDLNs, specifically the ipsilateral axillary and brachial LNs, which better recapitulates human cancers where several LNs are within each tumor lymphatic basin (26, 27). This syngeneic tumor model is highly invasive, forming metastases in the lung, liver, and LNs (18, 28–31), and exhibits strong immunosuppressive features that are associated with disease progression (13, 18, 32). We examined weights, cell counts, matrix composition, intranodal pressures, and hydration levels of LNs from melanoma-bearing and naive animals as well as LN tissue responses to compression at prescribed times posttumor implantation. Our findings indicate that TDLNs exhibit distinct differences in physical tissue properties relative to LNs from naive animals as well as NTDLNs signifying that local lymphatic drainage from primary melanomas directs this remodeling.
MATERIALS AND METHODS
Reagents
All reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated. Cell culture grade medium, serum, and antibiotics were from Life Technologies (Carlsbad, CA, USA) unless otherwise noted.
In vivo murine melanoma model
C57Bl/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All protocols were approved by the Institutional Animal Care and Use Committee. Isoflurane was used as anesthesia. Mice were euthanized by CO2 asphyxiation. A total of 0.5 × 106 cells were intradermally injected into the left dorsal skin on d 0. Tumor volume was calculated as ellipsoid volume from the caliper-measured melanoma width, length, and height. Animals were killed between 1 and 12 d after tumor implantation. Naive animals were used as controls.
Cell culture
B16F10 and B16 murine melanoma cells were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum and penicillin/streptomycin. Cell lines were confirmed to be negative for mycoplasma infection and animal pathogens semiannually using the Impact I Profile test (IDEXX Laboratories, Inc., Columbia, MO, USA).
In vivo fluid clearance assay
Alexa Fluor 700-dextran (500 kDa) was injected intratumorally. The fluorescent dextran conjugate was generated by conjugation of amine-dextran (Life Technologies) with Alexa Fluor 700 NHS Ester (Life Technologies) for 4 h. Fluorescent dextran conjugate was purified from free dye by Sepharose CL-6B (GE Healthcare, Pittsburgh, PA, USA) gravity chromatography and confirmed to be free of unconjugated dye. At 24 h after intratumoral injection, LNs ipsilateral and contralateral to the tumor were excised and homogenized in Dulbecco’s PBS (D-PBS; Life Technologies) using Lysing Matrix D (MP Biomedicals, Santa Ana, CA, USA) on a FastPrep-24 Automated Homogenizer (MP Biomedicals). Tissue homogenate fluorescence was measured using a Synergy H4 BioTek plate reader (BioTek Instruments, Winooski, VT, USA). Alternatively, whole-excised LNs were imaged on a Li-Cor Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE, USA) 72 h postinjection.
In vivo fluorescence lymphography
A total of 20 µl indocyanine green (ICG) (Acros Organics, Geel, Belgium) was injected intradermally into the lateral dorsal skin. Near-infrared fluorescence imaging was performed as previously described (33) at 18 h postinjection after making a ventral midline longitudinal incision and peeling the skin back to expose the draining axillary and brachial LNs. In brief, a laser diode and 20° beam diffuser (Thorlabs, Newton, NJ, USA) provided excitation at 808 nm, whereas a PIXIS 1024B back-illuminated charge-coupled device camera (Princeton Instruments, Trenton, NJ, USA) was used to capture fluorescence emission with a custom LabVIEW (National Instruments, Austin, TX, USA) code along with an Infinity K2/SC video microscope lens (Edmund Optics, Barrington, NJ, USA) and a band-pass filter centered at 840 nm with a full width at half-maximum of 15 nm (Omega Optical, Brattleboro, VT, USA).
LN harvesting
Immediately after euthanasia of naive or melanoma-bearing animals at prescribed days posttumor implantation, axillary LNs were excised using tweezers after ventral midline longitudinal incision through the dermis and bilateral skin flap peel past the mammary tissue. Brachial LNs were subsequently harvested after longitudinal midline incision through the dorsal skin, lateral peeling of the skin flaps to reveal the posterior side of each forelimb, and disruption of the muscle and connective tissue surrounding each LN. Both axillary and brachial LNs were recovered by severing both afferent and efferent lymphatics as well as connecting blood vessels via gentle pulling after insertion of tweezers underneath each LN tissue. After excision, any residual fat pad tissue was separated from the LNs by gentle pulling. Detachment of the fat pad around each LN was confirmed via visual inspection and during histologic analysis. Care was taken not to squeeze or pierce LN tissues. Histologic analysis revealed no gross disruptions such as incisions or holes in LN structure resulting from these harvest procedures. Tissues were kept in D-PBS on ice until use and in between all handling steps.
Histologic analysis
All LNs used for histologic analysis were fixed with 10% neutral buffered formalin. Paraffin-embedded LNs and tumor specimens were cut into 8 μm thick sections and stained either using Fast Green/Safranin-O or Alcian blue (both from Sigma-Aldrich) after a 1 h hyaluronidase type 1-S digestion at 37°C with an untreated control section as reference. Samples were imaged with a QImaging Retiga 1300 camera (Surrey, BC, Canada). Images were stitched using Adobe Photoshop CS6 extended version 13.0.1 (Adobe Systems Inc., San Jose, CA, USA). Hyaluronic acid abundance was determined as the difference in thresholded area fraction of Alcian blue stain per total LN cross-sectional area of untreated relative to hyaluronidase treated. Thresholds of Alcian blue signal were set based on the signal in images of Alcian blue–stained hyaluronidase-treated sections. Image analysis was performed using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Measurement of LN weights and hydration levels
Immediately after harvesting, LNs were individually placed in preweighed tubes, capped, and weighed on an analytic balance (XS105; Mettler-Toledo, Columbus, OH, USA) to determine the total weight of each freshly excised LN. LNs were then desiccated at room temperature (25°C) for 30 min, frozen for 1 min in liquid nitrogen, and subjected to lyophilization for at least 5 h to completely dehydrate the LNs. Dried LNs were then weighed on the same analytic balance. LN hydration was calculated from the difference in total initial vs. dried LN weight (converted to volume) per gram initial (wet) LN weight.
Flow cytometry
Lymphocytes were isolated from LNs harvested as previously described (13, 34, 35) from naive and tumor-bearing mice by 1 h digestion at 37°C with collagenase D (Roche Ltd., Indianapolis, IN, USA) and tissue homogenization using 70 μm pore size strainers. Cell pellets were washed in D-PBS containing 0.1% bovine serum albumin (staining buffer) and centrifuged at 300 g for 1 min. Supernatants were decanted, and cells were stained with Live/Dead Blue (Life Technologies) diluted in D-PBS for 20 min. Cells were washed with staining buffer, and fluorescently conjugated monoclonal antibodies against mouse CD45, B220, and CD11c (eBioscience, San Diego, CA, USA) appropriately diluted in staining buffer were added to cells and incubated for 30 min at 4°C in the dark. Supernatants were decanted, and cell pellets were washed 3 times and resuspended in staining buffer. Data were acquired on an LSR II flow cytometer (BD Biosciences, San Jose, CA, USA), and analysis of total live-cell counts and frequencies of LN cell subpopulations was performed using FlowJo software (version 9; Ashland, OR, USA).
LN indentation testing
Resected individual axillary and brachial LNs were placed in D-PBS and stored on ice until testing could be performed (<2 h). Each LN was measured before testing using a digital caliper to determine the thickness of the specimen at the region of indentation for use in strain and modulus calculation. Testing was performed at room temperature (25°C) utilizing the EnduraTEC Electroforce 3100 tester (Bose, EnduraTEC Systems Group, Eden Prairie, MN, USA). A 0.5 or 1.0 N load cell (Interface, Scottsdale, AZ, USA) was attached to the upper arm of the tester, and a spherical indenter tip with a 2.33 mm diameter was attached to the load cell using a coupling device. LNs were placed on the lower plate of the tester directly beneath the indenter so as to provide a uniform surface for indentation, and the force and displacement values were zeroed prior to lowering the indenter into contact with the LNs. Minimal preload (0.1–0.3 mN) was applied. Indentation was performed at the thickest portion of the LN, in an area where there are no steep surface gradients. Each LN was subjected to either a cyclic or relaxation testing regimen, each of which consisted of a single indentation at 10% strain followed by serial indentations at increasing strain levels (5, 10, 15, and 20%) with intermediate unloaded dwells to confirm baseline recovery. In relaxation testing, a single indentation relaxation dwell time of 100 s was used, whereas 30 s dwell steps were used during serial indentation relaxation testing. An indentation rate of 0.005 mm/s was used in all experiments.
The force–deflection data were analyzed to determine the modulus for each LN using the Hertz model for 2 spheres in contact (36, 37). The tissue was assumed to be incompressible and the steel indenter of sufficiently high modulus to negligibly contribute to measured force–displacement responses. Curve fitting was done using Matlab’s nonlinear least-squares regression tool (version 2010b; MathWorks, Natick, MA, USA) giving a single best-fit modulus value for each curve. Alternatively, the relative rate of viscous dissipation was determined by normalizing the force relaxation response for each LN relative to the peak load. Responses of individual LNs to compression were determined from independently performed experiments and results averaged to obtain the final values for reported modulus values and force relaxation responses for each LN type.
Measurement of LN collagen
Isolated LNs were placed in a homogenization tube on ice (OPS Diagnostics Limited Liability Company, Lebanon, NJ, USA) containing 400 μl D-PBS. LNs were homogenized 2 times consecutively using a FastPrep-24. A total of 100 µl of each homogenate slurry was placed into a 1.5 ml tube, and 200 µl of 0.5 M acetic acid was added, mixed, and incubated on ice for 4 h. Afterward, 150 µl of 1 mg/ml Picro-Sirius Red (color index 35782; Sigma-Aldrich) was added per tube, mixed, and allowed to incubate at room temperature (25°C) for 20 min. The solution was centrifuged at 8000 g for 3 min, the supernatant removed, and the pellet washed with 500 µl D-PBS. After a second centrifugation and removal of the supernatant, the pellet was resuspended in 250 µl D-PBS, of which 200 µl was measured for absorbance at 550 nm using a Synergy H4 BioTek plate reader. Sample concentrations were calculated relative to a collagen type I (Corning Inc., Life Sciences, Tewksbury, MA, USA) standard curve.
Intranodal pressure measurements
Resected intranodal pressures were measured ex vivo using a glass micropipette connected by polyvinyl chloride tubing patently interfaced with saline to a differential pressure sensor (Honeywell, Morristown, NJ, USA) that transmitted real-time data via Inter-Integrated Circuit to an Arduino Uno (chipKIT Uno32; Digilent Inc., Pullman, WA, USA) attached to a laptop utilizing PuTTY (Simon Tatham, Cambridge, United Kingdom) for data collection (38). Each isolated LN was placed on a platform at room temperature (25°C) and kept hydrated by the application of pharmacologic grade saline due to its physiologic osmolality and compatibility with our pressure sensor. Using a micromanipulator, a glass micropipette pulled in-house with a Narishige PC-10 (Narishige International USA, Inc., East Meadow, NY, USA) to an average 100 μm tip diameter was brought to the same height as the specimen, the pressure zeroed, and the micropipette tip then inserted, opposite the hilum, to half the diameter of the LN. Tissue hydration was maintained over the course of pressure measurements, and the intranodal pressure reached equilibrium within 1–5 min after insertion. The sensing time was extended to 1 h to ensure no insertion or other environmentally induced perturbations in measured equilibrium values. Upon measurement completion, the glass micropipette was inspected for clogging by return of the reading to ambient pressure upon removal from the tissue and for breakage by visual examination under a microscope.
Statistical analysis
Data are represented as means ± sem. Statistics were calculated using Prism 5 (GraphPad Software, La Jolla, CA, USA) software. Statistical significance was defined as P < 0.05 following 1-way ANOVA and post hoc analysis or by 2-way ANOVA with matching. When normality tests failed, Kruskal-Wallis tests were performed.
RESULTS
Tumor lymphatic drainage results in LN enlargement
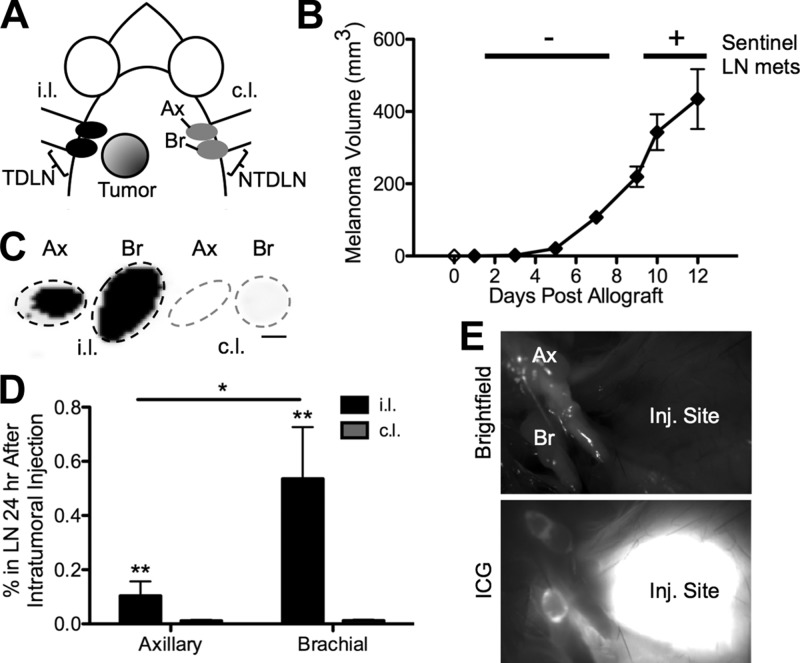
To evaluate the impact of tumor growth on TDLN remodeling, we used the well-described syngeneic and orthotopic B16 mouse model of melanoma (13, 18). Implantation of B16F10 or B16 cells in the left dorsal skin near the shoulders (Fig. 1A) results in the formation of a rapidly growing tumor within days after implantation (Fig. 1B and Supplemental Fig. 1, respectively) and lymphatic-mediated tumor drainage focused to one side of the animal. Hence, multiple subtype-matched TDLNs and NTDLNs are created because intratumorally injected fluorescent dextran accumulates appreciably in brachial and, to a lesser extent, axillary LNs ipsilateral but not contralateral to the site of tumor implantation (Fig. 1C–E). In this model, LN metastases manifest only by d 9 after tumor implantation and later, within TDLNs of ∼40 and 80% of animals killed at d 9–10 vs. 12 posttumor implantation, respectively.
Figure 1.
TDLN and NTDLN B16 murine melanoma model. Location of implanted tumor (A) and growth curve (B) of B16F10 melanoma. C–E) Ipsilateral (i.l.), but not contralateral (c.l.), axillary (Ax) and brachial (Br) LNs drain the melanoma in the dorsolateral skin. C) Black contrast is LNs accumulating 500 kDa dextran 72 h postintratumoral injection. Scale bar, 1 mm. D) *P < 0.05 and **P < 0.01 by 2-way ANOVA with post hoc Bonferroni’s tests. E) Bright-field and ICG images of ipsilateral Ax and Br LNs 18 h postdorsolateral skin injection (Inj.) of ICG.
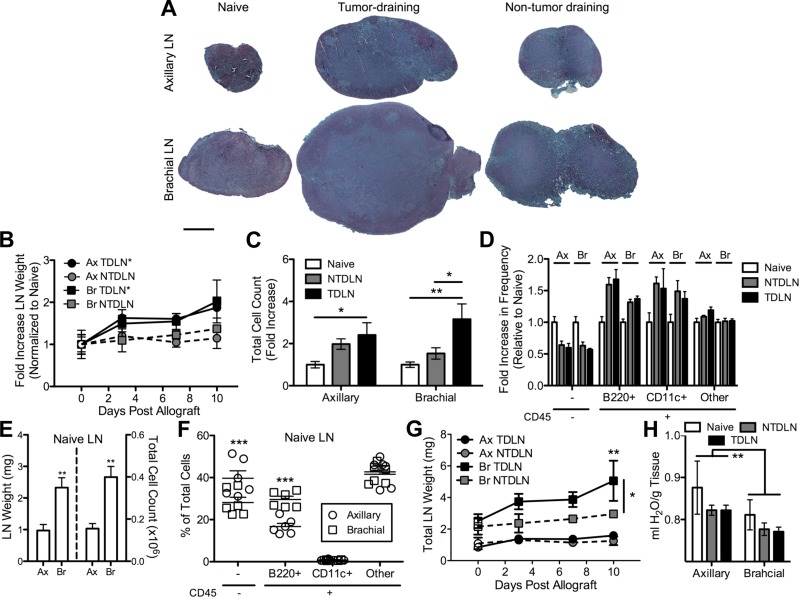
The enlargement of TDLNs, which is a prominent clinical manifestation in numerous human cancers including melanoma (39, 40), is recapitulated in this mouse melanoma model (Fig. 2A). When draining the melanoma, both axillary and brachial LN subtypes exhibited a statistically significant linear increase in weight with time post-B16F10 implantation, including a 50% increase in LN weight as early as d 3 that increased to ∼2-fold by d 10 (Fig. 2B). At d 7 postimplantation, a 2- to 3-fold increase in cell count in both axillary and brachial LNs was measured relative to naive subtype-matched LNs (2.4 ± 0.7-fold increase and 3.2 ± 0.8-fold increase, respectively), with no statistically significant difference between TDLN subtypes (Fig. 2C). On average, NTDLN cell counts were also increased relative to LNs from naive animals, but these differences were not statistically significant. B (CD45+B220+) and dendritic (CD45+CD11c+) cell frequencies in axillary and brachial LNs from animals with B16F10 melanomas d 7 postimplantation increased on average relative to LNs of naive animals with a corresponding reduction in the frequency of nonhematopoietic (CD45−) cells (Fig. 2D). Collagenase treatment used to isolate LN-resident cells was confirmed to have no effect on measured levels of B220 expression (Supplemental Fig. 2).
Figure 2.
LN enlargement is associated with tumor lymphatic drainage. A) Images of Fast Green/Safranin-O-stained histologic sections of fixed and paraffin-embedded LNs. Scale bar, 500 μm. B) Axillary and brachial LN weights from naive (plotted as open symbols on d 0) and B16F10 melanoma-bearing C57Bl/6 mice. *P < 0.05 regression line significantly different from zero. C) The number of LN-infiltrating cells is increased in axillary and brachial TDLNs d 7 post-B16F10 tumor implantation. *P < 0.05 and **P < 0.01 by 2-way ANOVA with post hoc Bonferroni’s tests. D) Fold change in frequencies of LN-infiltrating cell subtypes. E) Total weight and cell counts of LNs from naive animals. **P < 0.01 by Mann-Whitney U test. F) Frequency of LN-infiltrating cell subtypes. ***P < 0.001 by Mann-Whitney U test. G) Total LN weights with time post-B16F10 melanoma implantation. The mean weight of LNs from naive animals is plotted as open symbols on d 0. **P < 0.01 relative to naive LNs and *P < 0.05 relative to NTDLNs by 2-way ANOVA with post hoc Bonferroni’s tests. H) Hydration levels of LNs from naive animals or after B16F10 melanoma implantation. **P < 0.01 by Mann-Whitney U test. In all panels, data represent LN values from n = 3–6 individual animals.
Despite numerous similarities between measured axillary and brachial LN responses to melanoma growth, several differences in these LN subtypes were noted. First, brachial LNs from naive animals were found to be larger in size (Fig. 2A), weight (Fig. 2E), and infiltrating cell counts (Fig. 2E) than axillary LNs. The frequency of B cells in brachial LNs was also ∼2-fold higher than axillary LNs, and nonhematopoietic cell frequencies are lower by ∼25% (Fig. 2F). Accordingly, the absolute weight of brachial LNs draining the melanoma increased more significantly relative to naive or non-tumor-draining subtype-matched LNs, whereas axillary LNs exhibited a more subtle increase relative to naive or non-tumor-draining subtype-matched LNs (Fig. 2G). The fold increase in cell count relative to naive LN cell counts of brachial but not axillary TDLNs was also statistically significant relative to NTDLNs (Fig. 2C). Axillary LNs also exhibited a higher level of hydration relative to brachial LNs (Fig. 2H), though no statistically significant differences in these levels were found to be associated with tumor lymphatic drainage and time postimplantation (data not shown).
Tumor lymphatic drainage is associated with alterations in the mechanical response of LNs to compression and elevated intranodal pressures
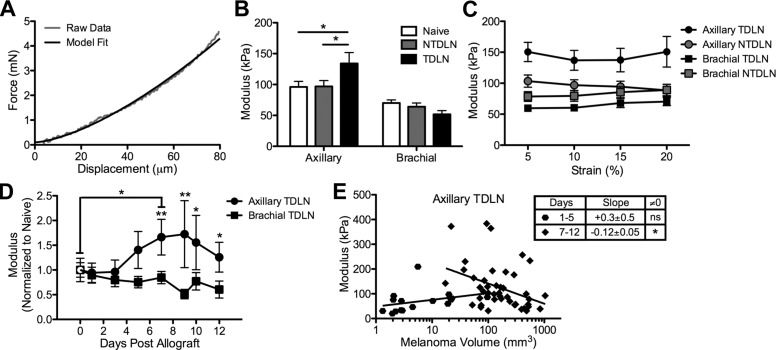
We next subjected whole LNs harvested from naive and melanoma-bearing animals to compression. During tissue compression, stress increases due to solid matrix resistance to loading and fluid pressurization because the exuding fluid is constrained by the matrix permeability (41). Indentation moduli, which are calculated from displacement versus compression force responses (Fig. 3A), are therefore suggestive of the tissue solid matrix elasticity and permeability.
Figure 3.
B16F10 melanoma growth increases indentation modulus of axillary but not brachial TDLNs. A) Representative force displacement data of LNs subjected to indentation. B) The indentation modulus of axial but not brachial TDLNs harvested from animals with melanomas 5–12 d post-B16F10 implantation subjected to 10% strain is significantly increased relative to axillary NTDLNs and LNs from naive animals. *P < 0.05 by Mann-Whitney U tests. C) LN indentation moduli are constant up to 20% strain. D) The normalized mean indentation modulus at 10% strain for axillary and brachial LNs from naive (plotted as empty symbols on d 0) and B16F10 melanoma-bearing C57Bl/6 mice. *P < 0.05 and **P < 0.01 by 2-way ANOVA and post hoc Bonferroni’s tests. The mean indentation modulus of axial but not brachial TDLNs changes with time postmelanoma implantation (D) and tumor size (E). The asterisk (*) in (E) indicates that slope of linear regression fit of measured axillary TDLN modulus vs. tumor size from animals with palpable tumors (>0 mm3) between early (1–5 d postallograft) and late (d 7–12 postallograft) is statistically different from zero (P < 0.05). ns, not significant. Data in (B–D) represent mean values of LN moduli from n ≥ 8 individual animals.
We found that axillary TDLNs but not NTDLNs harvested from animals with melanomas implanted 7–12 d prior demonstrated an ∼40% increase in indentation moduli (Fig. 3B). However, this response was not conserved between LN types because no change in brachial TDLN modulus relative to brachial NTDLNs and brachial LNs from naive animals was found (Fig. 3B). Measured moduli for each LN type were constant at each strain level (percent indentation depth of total LN depth) tested, from 5–20% strain (Fig. 3C). The selective increase in axillary but not brachial TDLN modulus relative to naive LNs was statistically significant only after 7 d postimplantation (Fig. 3D). Interestingly, however, tumor size at d 7 or later postimplantation was negatively correlated with axillary TDLN modulus (Fig. 3E), as indicated by a negative slope by linear regression analysis that is statistically nonzero. This effect was not observed however in axillary or brachial TDLNs from B16 melanoma-bearing animals (Supplemental Fig. 3A). These LN elastic moduli measurements reveal distinct changes in tissue elasticity and permeability within TDLNs resulting from both time post-B16F10 but not B16 tumor formation and size that diverge as a function of LN subtype.
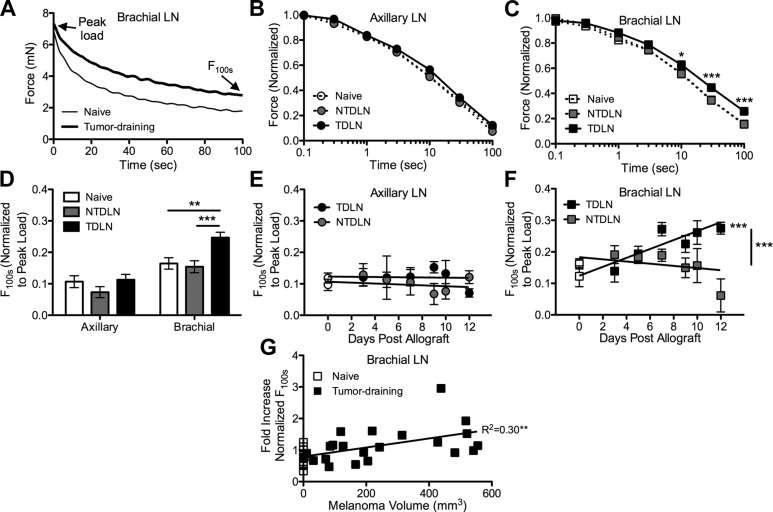
Force relaxation responses of LNs subjected to compressive indentation were next measured (Fig. 4A) to evaluate the viscoelasticity of bulk LN tissues. Tissues exhibit viscoelastic behavior as the result of fluid flow or structural interactions within the extracellular matrix of the tissue during mechanical loading (42). Force relaxation (decay) in response to sustained low-strain compression is associated with a redistribution of fluid within the solid matrix and stops when the developed compressive stress (force per contact area) equals the equilibrium stress level determined by the compressive modulus of the solid matrix.
Figure 4.
B16F10 melanoma growth delays force relaxation response of brachial but not axillary TDLNs nor NTDLNs subjected to indentation. Representative (A) and normalized (B and C) force relaxation response of LNs subjected to indentation at 10% strain. *P < 0.05 and ***P < 0.001 by 2-way ANOVA and post hoc Bonferroni’s tests. D) The average normalized force after 100 s relaxation at 10% strain in brachial but not axillary TDLNs, nor NTDLNs or LNs from naive animals, is significantly increased. A–D) LNs were harvested from melanoma-bearing animals 7–12 d postallograft. **P < 0.01 and ***P < 0.001 by 2-way ANOVA and post hoc Bonferroni’s tests. E and F) The F100s normalized to peak load for axillary and brachial LNs from naive (plotted as empty symbols on d 0) and B16F10 melanoma-bearing C57Bl/6 mice. Points represent n ≥ 8 individual LNs. ***P < 0.001 between LNs or for regression line significantly different from zero. G) Normalized F100s in brachial TDLNs increases with tumor size. **Slope of linear regression fit is statistically different from zero (P < 0.01). Data in (D) represent values from n ≥ 8 individual animals. Data in (B–F) represent means ± sem of n ≥ 8 individual LNs from individual animals.
In contrast to changes seen in measured moduli with indentation, no differences in the rate of viscous force dissipation of the peak loads required for 10% strain of axillary TDLNs harvested from animals implanted with B16F10 melanomas were observed relative to both LNs from naive animals and NTDLNs (Fig. 4B, D, E). In distinct contrast, a significant delay in the relaxation response of brachial TDLNs subjected to 10% compressive strain relative to LNs from naive animals and NTDLNs (Fig. 4C, D) was found, with the mean force after a 100 s dwell at 10% strain (F100s) normalized to peak strain increasing with time posttumor implantation (Fig. 4F) as well as tumor size (Fig. 4G). The normalized F100s d 7 and later was increased by approximately two-thirds in brachial TDLNs relative to naive brachial LNs (Fig. 4F). Brachial but not axillary TDLNs from B16 melanoma-bearing animals were similarly affected by melanoma growth (Supplemental Fig. 3B). These data reveal distinct changes in LN viscoelastic properties as the result of melanoma growth that diverge as a function of LN subtype.
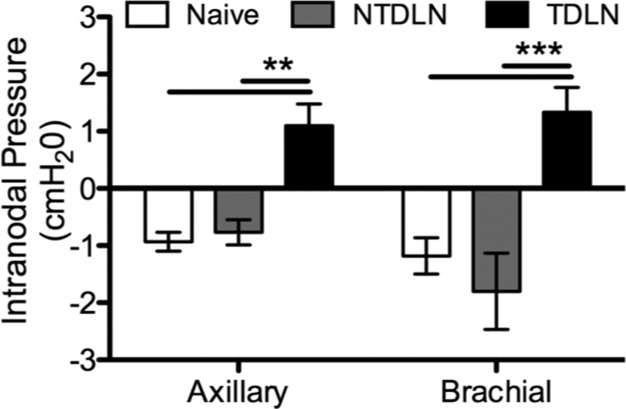
Whereas indentation moduli and force relaxation responses diverged between TDLN subtypes, we found intranodal pressures in both axillary and brachial TDLNs in animals 7 d post-B16F10 melanoma implantation to be significantly increased relative to LNs from naive animals (Fig. 5). Specifically, whereas LNs from naive animals are subatmospheric, TDLNs were elevated relative to atmospheric pressure. This increase in intranodal pressures was associated specifically with tumor lymphatic drainage because NTDLNs exhibited subatmospheric pressures equivalent to that of LNs from naive animals (Fig. 5).
Figure 5.
Elevated intranodal pressure is associated with tumor lymphatic drainage. Intranodal pressures of LNs from naive animals or animals 7 d post-B16F10 melanoma implantation are shown. Data represent n = 6 LNs from individual mice. **P < 0.01 and ***P < 0.001 by 2-way ANOVA and post hoc Bonferroni’s tests.
LN subtype-restricted increases in soluble collagen and hyaluronic acid abundance are associated with tumor lymphatic drainage
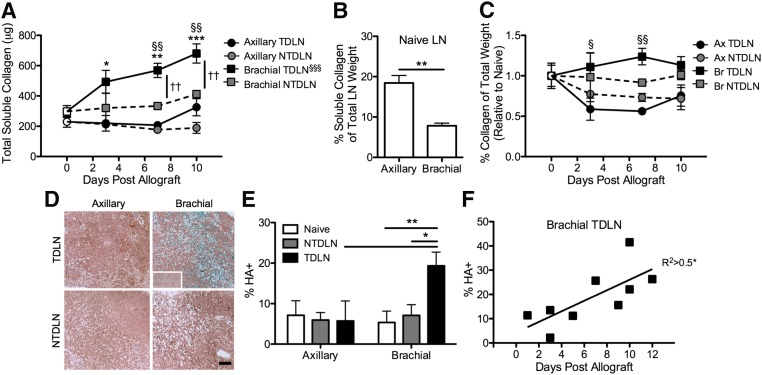
LN tissue matrix composition was next evaluated in LNs from naive and melanoma-bearing animals. Consistent with the trend of increasing absolute weight of brachial TDLNs with time posttumor implantation, B16F10 tumor lymphatic drainage was associated with a marked increase in the abundance of soluble collagen within brachial LNs (Fig. 6A). Amounts of newly synthesized collagen were elevated by 65% relative to LNs from naive animals as early as d 3 posttumor implantation and to >2-fold by d 10 (Fig. 6A). When controlling for total LN weight, axillary LNs had a higher collagen weight percentage than brachial LNs in naive animals (Fig. 6B). However, the relative abundance of soluble collagen decreased in axillary TDLNs by ∼50% as the result of the B16F10 melanoma as early as d 3 postimplantation, with little relative change occurring in brachial TDLNs (Fig. 6C).
Figure 6.
Increases in collagen and hyaluronic acid content in brachial but not axillary TDLNs are associated with tumor lymphatic drainage. A) Brachial but not axillary TDLN total soluble collagen amount increases with time post-B16F10 tumor allograft (n = 3–6 LNs from individual mice). *P < 0.05, **P < 0.01, and ***P < 0.001 relative to LNs from naive (plotted as empty symbols on d 0) animals; §§P < 0.01 relative to axillary TDLNs; ††P < 0.01 relative to NTDLNs by 2-way ANOVA with post hoc Bonferroni’s tests. §§§Slope of linear regression fit is statistically different from zero (P < 0.001). B) Soluble collagen weight fraction of whole LNs. **P < 0.01 by Mann-Whitney U tests. C) Fold change of collagen weight as a percentage of total LN weight relative to naive LNs (plotted as open symbols on d 0) vs. time post-B16F10 melanoma implantation. §P < 0.05 and §§P < 0.01 relative to axillary TDLNs by 2-way ANOVA with post hoc Bonferroni’s tests. D) Images of Alcian blue–stained histologic sections of fixed and paraffin-embedded LNs. Scale bar, 200 μm. Inset is an image of the control hyaluronidase-treated and stained section. E) The percent LN area stained positive for hyaluronic acid (Alcian blue staining, D) (n = 4–10 LNs from individual mice). *P < 0.05 and **P < 0.01 by 2-way ANOVA with post hoc Bonferroni’s tests. F) The percent LN area positive for hyaluronic acid increases with time posttumor allograft. *P < 0.05 indicates that the slope of linear regression fit is significantly different from zero. A–C) n = 3–6 LNs from individual mice.
LN glycosaminoglycan content was also evaluated because B16 and B16F10 melanomas exhibit intra- and peri-tumoral hyaluronic acid expression (43) (Supplemental Fig. 4). Hyaluronic acid levels per LN cross-sectional area, as revealed by the difference between untreated control and hyaluronidase-treated tissue sections stained with Alcian blue (Fig. 6D), were significantly elevated in brachial but not axillary TDLNs relative to NTDLNs and LNs from naive animals (Fig. 6E). Hyaluronic acid levels within brachial TDLNs also increased strongly with time posttumor implantation (Fig. 6F).
DISCUSSION
Despite both axillary and brachial TDLNs exhibiting increased size and hypertension in response to melanoma growth, we demonstrated that the compositional adaptations associated with tumor lymphatic drainage—namely, changes in collagen and hyaluronic acid content—diverged between the LN subtypes. These differences in the TDLN tissue compositions impacted LN response to compression as summarized in Table 1.
TABLE 1.
Tissue adaptations of LNs from melanoma-bearing mice relative to LNs from naive animals
| Axillary | Brachial | Axillary | Brachial | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Compositional change | TDLN | NTDLN | TDLN | NTDLN | Physical manifestation | TDLN | NTDLN | TDLN | NTDLN |
| Total weight | + | − | ++ | − | Tumor lymphatic drainage | + | − | ++ | − |
| Total cell count | + | − | ++ | − | Stiffness | + | − | − | − |
| Collagen | − | − | ++ | − | Viscoelasticity | − | − | + | − |
| Hyaluronic acid | − | − | ++ | − | Pressure | + | − | + | − |
| Hydration | − | − | − | − | |||||
+, significant change; –, no difference compared with naive LNs; ++, significant difference between brachial vs. axillary TDLNs.
Elastic and viscoelastic behaviors are integral to the structure and function of many soft tissues. Changes in the mechanical properties and associated alterations in macrostructure, such as size, and microstructure, including the distribution of collagen fiber diameter and alignment as well as glycosaminoglycan content, may reflect the effects of disease (21, 43–47). Enlarged LNs are a prominent clinical manifestation in numerous types of cancers (39, 40, 48), and a correlation between LN stiffening and LN metastasis in a small cohort of patients with lung cancer has been described (49). Matrix remodeling, including deposition and cross-linking, is characteristic of tumors (21) and lymphedema (50, 51). These adaptive changes in the tissue structure are hypothesized to compensate for increased interstitial fluid pressures associated with these pathologies as well as impact tissue mechanics and fluid transport (52–55). Additionally, abundance of glycosaminoglycans such as hyaluronic acid, which contribute to tissue viscoelasticity (56) by regulating the osmotic pressure, is elevated in malignant lesions (43, 57, 58) and predicted to lower rates of intratumoral fluid and solute transport by affecting hydraulic conductivity (59). Fluid accumulation, or increased tissue hydration, is also a hallmark of lymphedema (35, 51, 60), and hydration is a key parameter controlling interstitial transport of fluid and solutes as well as solute concentration (61).
In this work, the adaptations of mouse rather than human LNs to malignant onset were evaluated because orthotopically implanted B16 murine melanomas can be reproducibly grown as solid tumors in the skin with well-defined growth profiles (Fig. 1B). Changes in TDLN compositions that resulted in altered elastic and viscoelastic properties developed within 1 wk of melanoma implantation (Figs. 3–5). These mechanical (Figs. 3–5) features altered within TDLNs have been reported to influence malignant cell invasion (17, 62), migration (16), and proliferation (62). O’Connor et al. (63) also reported that T cells cultured on substrates of increased stiffness exhibit lower proliferative responses as well as expression of lymphoid homing receptor L-selectin and inflammatory cytokine IFN-γ. Alterations in TDLN mechanics therefore also have the potential to modulate lymphocyte function to regulate antitumor-adaptive immune response. However, because LN harvesting required separation from the connecting blood and lymphatic vessels, it should be noted that ex vivo measurements, such as those made in this work, could differ from the properties of tissues in vivo.
The effect of melanoma growth on TDLN response to compression was nonuniform with respect to LN subtype, suggesting an underlying influence of LN remodeling responses in these observed differences. First, the proportion of soluble collagen to total LN weight dropped by 50% in axillary but not brachial TDLNs as early as d 3 postimplantation and began to recover only at d 10 (Fig. 6C). Reduced relative levels of collagen might have resulted in higher prestress levels in axillary LN tissues that manifest as temporarily higher axillary but not brachial TDLN moduli upon compression. Meanwhile, the weight percentage of soluble collagen in brachial TDLNs remained relatively constant with time posttumor allograft (Fig. 6C). Given their reported role in the regulation of lymphangiogenesis-driven LN enlargement (64, 65), the 2-fold higher frequency of B cells in brachial relative to axillary LNs in naive animals (Fig. 2F) may have resulted in differential lymphangiogenic remodeling responses to the melanoma that manifest in both increased levels of tumor lymphatic drainage (Fig. 1C) as well as accumulation of hyaluronic acid (Fig. 6D, E) to increase brachial TDLN viscoelasticity. Higher levels of lymphatic flow may also have resulted in higher levels of total soluble collagen within remodeling brachial TDLNs (Fig. 6A) to sustain the collagen weight fraction (Fig. 6C) because interstitial flows, such as those through LNs, induce collagen remodeling by LN stromal cells (66). Interestingly, gross changes in the frequency of LN-resident cell subpopulations as the result of the melanoma in a manner independent from tumor lymphatic drainage were conserved between LN subtypes (Fig. 2D). Overall, these data underscore the complex tissue remodeling, but not LN-resident cell subpopulations, associated with tumor lymphatic drainage to vary significantly between TDLN subtypes.
Our data support prior reports of metastasis being preceded by remodeling of the metastatic organ microenvironment in preparation for the arrival and successful colonization by tumor cells (67). For example, studies have demonstrated increased lymphangiogenesis (68) and remodeling of high endothelial venules (69) within TDLNs. Our data indicate that LN enlargement (Fig. 2B, G) and collagen (Fig. 6A) remodeling responses are detectable as early as 6–9 d before possible metastasis. Measurable changes in tissue mechanical properties also manifest by d 7 posttumor implantation (Figs. 3D, 4F, and 5). Melanomas formed from B16 cells, the parental cell line of B16F10 cells with a reduced propensity for metastasis (70), failed to increase axillary TDLN moduli in response to compression, though exhibited similar increases in viscoelasticity of brachial TDLNs (Supplemental Fig. 2). The significance of these data, which suggest that a subclass of TDLN remodeling responses may differ in response to primary tumor burden and play an integral role in metastatic potential, remains to be elaborated in future studies.
In breast cancer, pressures of TDLNs positive for metastases have recently been reported to be elevated relative to that of TDLNs negative for metastases (71, 72). Our intranodal pressure measurements suggest that increases in intranodal pressures accompanied tumor lymphatic drainage (Fig. 5), which were measured before formation of apparent metastases, may arise earlier than appreciable tumor spread to LNs. How elevated intranodal pressures manifest as a result of tumor growth, however, remains unclear. Elevated interstitial fluid pressures in human and murine tumors (11, 12) occlude intratumoral lymphatic vessels, disrupting transport function (73). TDLN efferent and afferent lymphatic function however has yet to be reported. Despite intranodal pressure regulating tissue hydration through capillary extravasation or convection, no measurable differences in LN hydration levels were found to be associated with tumor lymphatic drainage (Fig. 2H). However, signaling via the previously reported endothelial cell and high endothelial venule remodeling responses within TDLNs (74) resulting from lymphatic-mediated vascular endothelial growth factor A–producing dendritic and B cells (64, 75) have the potential to alter transcapillary fluid exchange and hence pressure within TDLNs.
LN microstructural remodeling associated with tumor lymphatic drainage (Fig. 6) that to date has been largely overlooked mirrors microenvironmental changes of the primary lesion implicated in disease progression (76–86). For example, alterations in collagen density (76) and cross-linking (81) regulate cellular proliferation, tumor formation, and invasion. Collagens also interact with cytokines to organize lymphocyte infiltration and signaling (86). On the other hand, hyaluronic acid is implicated in the regulation of tumor growth (80, 85) and angiogenesis (77, 83, 84) as well as cell adhesion (82), motility (79), and invasion (78). Hyaluronic acid also has a well-documented role in the regulation of inflammatory signaling (60). Observations of increased TDLN collagen and hyaluronic acid content therefore suggest a role for tumor lymphatic drainage in regulating cellular signaling processes in both the antitumor-adaptive immune response as well as the establishment, development, and further spread of LN metastases.
In summary, we found that tumor lymphatic drainage is associated with LN physical remodeling, alterations that quickly develop within the time course of initial melanoma formation and before the onset of metastasis. These are the first measurements juxtaposing ex vivo samples of TDLNs to NTDLNs and LNs from naive mice that recapitulate several features seen in human malignancies. Our results highlight that whereas LN enlargement may be common in human tumors, the underlying associated tissue remodeling and resulting impact on TDLN-resident cell signaling may vary. Furthermore, the unexpected finding that TDLN intranodal pressures are elevated early in disease progression suggests that TDLN-targeted drug delivery strategies may benefit from complementary interventions that normalize vascular function as has been proposed in the context of tumor-targeted drug delivery strategies (37, 87). Overall, our findings contribute evidence that dynamic LN tissue remodeling in malignancy results in biophysical changes that are associated with tumor lymphatic drainage. These results will inform future studies on how remodeling influences the important role of TDLNs in tumor pathophysiology and therapy.
Acknowledgments
The authors thank Alex Schudel, Caitlin Powell, Monica McNerney, Karan Khendry, and Rahul Rege for technical assistance as well as Sha’aqua T. Asberry and Andrew Shaw for technical advice. This work was supported by institutional funds from the Georgia Institute of Technology; U.S. National Institutes of Health Cell and Tissue Engineering Training Grant T32 GM-008433; U.S. National Science Foundation Award 1342194; Georgia Institute of Technology Undergraduate Research Opportunities Office, Air Products; and U.S. National Science Foundation Summer Undergraduate Research in Engineering/Science Program.
Glossary
- D-PBS
Dulbecco’s PBS
- F100s
mean force after a 100 s dwell at 10% strain
- ICG
indocyanine green
- LN
lymph node
- NTDLN
non-tumor-draining lymph node
- TDLN
tumor-draining lymph node
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Marzo A. L., Lake R. A., Lo D., Sherman L., McWilliam A., Nelson D., Robinson B. W., Scott B. (1999) Tumor antigens are constitutively presented in the draining lymph nodes. J. Immunol. 162, 5838–5845 [PubMed] [Google Scholar]
- 2.Badwe R. A., Thorat M. A., Parmar V. V. (2003) Sentinel-node biopsy in breast cancer. N. Engl. J. Med. 349, 1968–1971 [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U., Paganelli G., Viale G., Luini A., Zurrida S., Galimberti V., Intra M., Veronesi P., Robertson C., Maisonneuve P., Renne G., De Cicco C., De Lucia F., Gennari R. (2003) A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N. Engl. J. Med. 349, 546–553 [DOI] [PubMed] [Google Scholar]
- 4.Phan G. Q., Messina J. L., Sondak V. K., Zager J. S. (2009) Sentinel lymph node biopsy for melanoma: indications and rationale. Cancer Control 16, 234–239 [DOI] [PubMed] [Google Scholar]
- 5.Minamiya Y., Ogawa J. (2006) Benefit of sentinel lymph node mapping in non-small cell lung cancer. Ann. Thorac. Cardiovasc. Surg. 12, 381–382 [PubMed] [Google Scholar]
- 6.Kumar S., Weaver V. M. (2009) Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 28, 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helmlinger G., Netti P. A., Lichtenbeld H. C., Melder R. J., Jain R. K. (1997) Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol. 15, 778–783 [DOI] [PubMed] [Google Scholar]
- 8.Stylianopoulos T., Martin J. D., Snuderl M., Mpekris F., Jain S. R., Jain R. K. (2013) Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse. Cancer Res. 73, 3833–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paszek M. J., Zahir N., Johnson K. R., Lakins J. N., Rozenberg G. I., Gefen A., Reinhart-King C. A., Margulies S. S., Dembo M., Boettiger D., Hammer D. A., Weaver V. M. (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 [DOI] [PubMed] [Google Scholar]
- 10.Fukumura D., Gohongi T., Kadambi A., Izumi Y., Ang J., Yun C. O., Buerk D. G., Huang P. L., Jain R. K. (2001) Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. USA 98, 2604–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutmann R., Leunig M., Feyh J., Goetz A. E., Messmer K., Kastenbauer E., Jain R. K. (1992) Interstitial hypertension in head and neck tumors in patients: correlation with tumor size. Cancer Res. 52, 1993–1995 [PubMed] [Google Scholar]
- 12.Boucher Y., Leunig M., Jain R. K. (1996) Tumor angiogenesis and interstitial hypertension. Cancer Res. 56, 4264–4266 [PubMed] [Google Scholar]
- 13.Thomas S. N., Vokali E., Lund A. W., Hubbell J. A., Swartz M. A. (2014) Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials 35, 814–824 [DOI] [PubMed] [Google Scholar]
- 14.Thomas S. N., Schudel A. (2015) Overcoming transport barriers for interstitial-, lymphatic-, and lymph node-targeted drug delivery. Curr. Opin. Chem. Eng. 7, 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shields J. D., Fleury M. E., Yong C., Tomei A. A., Randolph G. J., Swartz M. A. (2007) Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 11, 526–538 [DOI] [PubMed] [Google Scholar]
- 16.Shieh A. C., Rozansky H. A., Hinz B., Swartz M. A. (2011) Tumor cell invasion is promoted by interstitial flow-induced matrix priming by stromal fibroblasts. Cancer Res. 71, 790–800 [DOI] [PubMed] [Google Scholar]
- 17.Barcus C. E., Keely P. J., Eliceiri K. W., Schuler L. A. (2013) Stiff collagen matrices increase tumorigenic prolactin signaling in breast cancer cells. J. Biol. Chem. 288, 12722–12732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lund A. W., Duraes F. V., Hirosue S., Raghavan V. R., Nembrini C., Thomas S. N., Issa A., Hugues S., Swartz M. A. (2012) VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Reports 1, 191–199 [DOI] [PubMed] [Google Scholar]
- 19.Egger M. E., Bower M. R., Czyszczon I. A., Farghaly H., Noyes R. D., Reintgen D. S., Martin R. C. II, Scoggins C. R., Stromberg A. J., McMasters K. M. (2014) Comparison of sentinel lymph node micrometastatic tumor burden measurements in melanoma. J. Am. Coll. Surg. 218, 519–528 [DOI] [PubMed] [Google Scholar]
- 20.Mu W., Rana S., Zöller M. (2013) Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia 15, 875–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu P., Weaver V. M., Werb Z. (2012) The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Insana M. F., Pellot-Barakat C., Sridhar M., Lindfors K. K. (2004) Viscoelastic imaging of breast tumor microenvironment with ultrasound. J. Mammary Gland Biol. Neoplasia 9, 393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swartz M. A., Lund A. W. (2012) Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat. Rev. Cancer 12, 210–219 [DOI] [PubMed] [Google Scholar]
- 24.DuFort C. C., Paszek M. J., Weaver V. M. (2011) Balancing forces: architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 12, 308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathanson S. D., Shah R., Rosso K. (2015) Sentinel lymph node metastases in cancer: causes, detection and their role in disease progression. Semin. Cell Dev. Biol. 38, 106–116 [DOI] [PubMed] [Google Scholar]
- 26.Reintgen M., Murray L., Akman K., Giuliano R., Lozicki A., Shivers S., Reintgen D. (2013) Evidence for a better nodal staging system for melanoma: the clinical relevance of metastatic disease confined to the sentinel lymph nodes. Ann. Surg. Oncol. 20, 668–674 [DOI] [PubMed] [Google Scholar]
- 27.Yi M., Meric-Bernstam F., Ross M. I., Akins J. S., Hwang R. F., Lucci A., Kuerer H. M., Babiera G. V., Gilcrease M. Z., Hunt K. K. (2008) How many sentinel lymph nodes are enough during sentinel lymph node dissection for breast cancer? Cancer 113, 30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bobek V., Kolostova K., Pinterova D., Kacprzak G., Adamiak J., Kolodziej J., Boubelik M., Kubecova M., Hoffman R. M. (2010) A clinically relevant, syngeneic model of spontaneous, highly metastatic B16 mouse melanoma. Anticancer Res. 30, 4799–4803 [PubMed] [Google Scholar]
- 29.Winkelmann C. T., Figueroa S. D., Rold T. L., Volkert W. A., Hoffman T. J. (2006) Microimaging characterization of a B16-F10 melanoma metastasis mouse model. Mol. Imaging 5, 105–114 [PubMed] [Google Scholar]
- 30.Yang M., Baranov E., Jiang P., Sun F. X., Li X. M., Li L., Hasegawa S., Bouvet M., Al-Tuwaijri M., Chishima T., Shimada H., Moossa A. R., Penman S., Hoffman R. M. (2000) Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc. Natl. Acad. Sci. USA 97, 1206–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung J. I., Cho H. J., Jung Y. J., Kwon S. H., Her S., Choi S. S., Shin S. H., Lee K. W., Park J. H. (2015) High-fat diet-induced obesity increases lymphangiogenesis and lymph node metastasis in the B16F10 melanoma allograft model: roles of adipocytes and M2-macrophages. Int. J. Cancer 136, 258–270 [DOI] [PubMed] [Google Scholar]
- 32.Miteva D. O., Rutkowski J. M., Dixon J. B., Kilarski W., Shields J. D., Swartz M. A. (2010) Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ. Res. 106, 920–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiler M., Dixon J. B. (2013) Differential transport function of lymphatic vessels in the rat tail model and the long-term effects of Indocyanine Green as assessed with near-infrared imaging. Front. Physiol. 4, 215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas S. N., van der Vlies A. J., O’Neil C. P., Reddy S. T., Yu S. S., Giorgio T. D., Swartz M. A., Hubbell J. A. (2011) Engineering complement activation on polypropylene sulfide vaccine nanoparticles. Biomaterials 32, 2194–2203 [DOI] [PubMed] [Google Scholar]
- 35.Thomas S. N., Rutkowski J. M., Pasquier M., Kuan E. L., Alitalo K., Randolph G. J., Swartz M. A. (2012) Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J. Immunol. 189, 2181–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson K. L. (1985) Contact Mechanics, Cambridge University Press, Cambridge, NY, USA: [Google Scholar]
- 37.Goel S., Duda D. G., Xu L., Munn L. L., Boucher Y., Fukumura D., Jain R. K. (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 91, 1071–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kornuta J. A., Brandon Dixon J. (2014) Ex vivo lymphatic perfusion system for independently controlling pressure gradient and transmural pressure in isolated vessels. Ann. Biomed. Eng. 42, 1691–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koh Y. X., Chok A. Y., Zheng H., Xu S., Teo M. C. (2014) Cloquet’s node trumps imaging modalities in the prediction of pelvic nodal involvement in patients with lower limb melanomas in Asian patients with palpable groin nodes. Eur. J. Surg. Oncol. 40, 1263–1270 [DOI] [PubMed] [Google Scholar]
- 40.Bastiaannet E., Wobbes T., Hoekstra O. S., van der Jagt E. J., Brouwers A. H., Koelemij R., de Klerk J. M., Oyen W. J., Meijer S., Hoekstra H. J. (2009) Prospective comparison of [18F]fluorodeoxyglucose positron emission tomography and computed tomography in patients with melanoma with palpable lymph node metastases: diagnostic accuracy and impact on treatment. J. Clin. Oncol. 27, 4774–4780 [DOI] [PubMed] [Google Scholar]
- 41.Maroudas A. (1975) Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology 12, 233–248 [DOI] [PubMed] [Google Scholar]
- 42.Fung Y. C. (1993) Biomechanics: Mechanical Properties of Living Tissues, Springer-Verlag, New York: [Google Scholar]
- 43.Rudrabhatla S. R., Mahaffey C. L., Mummert M. E. (2006) Tumor microenvironment modulates hyaluronan expression: the lactate effect. J. Invest. Dermatol. 126, 1378–1387 [DOI] [PubMed] [Google Scholar]
- 44.Dunn M. G., Silver F. H. (1983) Viscoelastic behavior of human connective tissues: relative contribution of viscous and elastic components. Connect. Tissue Res. 12, 59–70 [DOI] [PubMed] [Google Scholar]
- 45.Faffe D. S., Zin W. A. (2009) Lung parenchymal mechanics in health and disease. Physiol. Rev. 89, 759–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stylianopoulos T., Martin J. D., Chauhan V. P., Jain S. R., Diop-Frimpong B., Bardeesy N., Smith B. L., Ferrone C. R., Hornicek F. J., Boucher Y., Munn L. L., Jain R. K. (2012) Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl. Acad. Sci. USA 109, 15101–15108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanter M., Bercoff J., Athanasiou A., Deffieux T., Gennisson J. L., Montaldo G., Muller M., Tardivon A., Fink M. (2008) Quantitative assessment of breast lesion viscoelasticity: initial clinical results using supersonic shear imaging. Ultrasound Med. Biol. 34, 1373–1386 [DOI] [PubMed] [Google Scholar]
- 48.Sloothaak D. A., Grewal S., Doornewaard H., van Duijvendijk P., Tanis P. J., Bemelman W. A., van der Zaag E. S., Buskens C. J. (2014) Lymph node size as a predictor of lymphatic staging in colonic cancer. Br. J. Surg. 101, 701–706 [DOI] [PubMed] [Google Scholar]
- 49.Miyaji K., Furuse A., Nakajima J., Kohno T., Ohtsuka T., Yagyu K., Oka T., Omata S. (1997) The stiffness of lymph nodes containing lung carcinoma metastases: a new diagnostic parameter measured by a tactile sensor. Cancer 80, 1920–1925 [DOI] [PubMed] [Google Scholar]
- 50.Rutkowski J. M., Moya M., Johannes J., Goldman J., Swartz M. A. (2006) Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc. Res. 72, 161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rutkowski J. M., Markhus C. E., Gyenge C. C., Alitalo K., Wiig H., Swartz M. A. (2010) Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am. J. Pathol. 176, 1122–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shieh A. C. (2011) Biomechanical forces shape the tumor microenvironment. Ann. Biomed. Eng. 39, 1379–1389 [DOI] [PubMed] [Google Scholar]
- 53.Aukland K., Reed R. K. (1993) Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol. Rev. 73, 1–78 [DOI] [PubMed] [Google Scholar]
- 54.Miserocchi G., Negrini D., Passi A., De Luca G. (2001) Development of lung edema: interstitial fluid dynamics and molecular structure. News Physiol. Sci. 16, 66–71 [DOI] [PubMed] [Google Scholar]
- 55.Wiig H., Rubin K., Reed R. K. (2003) New and active role of the interstitium in control of interstitial fluid pressure: potential therapeutic consequences. Acta Anaesthesiol. Scand. 47, 111–121 [DOI] [PubMed] [Google Scholar]
- 56.Korhonen R. K., Laasanen M. S., Töyräs J., Lappalainen R., Helminen H. J., Jurvelin J. S. (2003) Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage. J. Biomech. 36, 1373–1379 [DOI] [PubMed] [Google Scholar]
- 57.Hautmann S. H., Lokeshwar V. B., Schroeder G. L., Civantos F., Duncan R. C., Gnann R., Friedrich M. G., Soloway M. S. (2001) Elevated tissue expression of hyaluronic acid and hyaluronidase validates the HA-HAase urine test for bladder cancer. J. Urol. 165, 2068–2074 [DOI] [PubMed] [Google Scholar]
- 58.Lokeshwar V. B., Rubinowicz D., Schroeder G. L., Forgacs E., Minna J. D., Block N. L., Nadji M., Lokeshwar B. L. (2001) Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J. Biol. Chem. 276, 11922–11932 [DOI] [PubMed] [Google Scholar]
- 59.Voutouri C., Stylianopoulos T. (2014) Evolution of osmotic pressure in solid tumors. J. Biomech. 47, 3441–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiig H., Swartz M. A. (2012) Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol. Rev. 92, 1005–1060 [DOI] [PubMed] [Google Scholar]
- 61.Levick J. R. (1987) Flow through interstitium and other fibrous matrices. Q. J. Exp. Physiol. 72, 409–437 [DOI] [PubMed] [Google Scholar]
- 62.Tilghman R. W., Blais E. M., Cowan C. R., Sherman N. E., Grigera P. R., Jeffery E. D., Fox J. W., Blackman B. R., Tschumperlin D. J., Papin J. A., Parsons J. T. (2012) Matrix rigidity regulates cancer cell growth by modulating cellular metabolism and protein synthesis. PLoS One 7, e37231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Connor R. S., Hao X., Shen K., Bashour K., Akimova T., Hancock W. W., Kam L. C., Milone M. C. (2012) Substrate rigidity regulates human T cell activation and proliferation. J. Immunol. 189, 1330–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shrestha B., Hashiguchi T., Ito T., Miura N., Takenouchi K., Oyama Y., Kawahara K., Tancharoen S., Ki-I Y., Arimura N., Yoshinaga N., Noma S., Shrestha C., Nitanda T., Kitajima S., Arimura K., Sato M., Sakamoto T., Maruyama I. (2010) B cell-derived vascular endothelial growth factor A promotes lymphangiogenesis and high endothelial venule expansion in lymph nodes. J. Immunol. 184, 4819–4826 [DOI] [PubMed] [Google Scholar]
- 65.Angeli V., Ginhoux F., Llodrà J., Quemeneur L., Frenette P. S., Skobe M., Jessberger R., Merad M., Randolph G. J. (2006) B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 24, 203–215 [DOI] [PubMed] [Google Scholar]
- 66.Tomei A. A., Siegert S., Britschgi M. R., Luther S. A., Swartz M. A. (2009) Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J. Immunol. 183, 4273–4283 [DOI] [PubMed] [Google Scholar]
- 67.Peinado H., Lavotshkin S., Lyden D. (2011) The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin. Cancer Biol. 21, 139–146 [DOI] [PubMed] [Google Scholar]
- 68.Harrell M. I., Iritani B. M., Ruddell A. (2007) Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am. J. Pathol. 170, 774–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chung M. K., Do I. G., Jung E., Son Y. I., Jeong H. S., Baek C. H. (2012) Lymphatic vessels and high endothelial venules are increased in the sentinel lymph nodes of patients with oral squamous cell carcinoma before the arrival of tumor cells. Ann. Surg. Oncol. 19, 1595–1601 [DOI] [PubMed] [Google Scholar]
- 70.Fidler I. J. (1973) Selection of successive tumour lines for metastasis. Nat. New Biol. 242, 148–149 [DOI] [PubMed] [Google Scholar]
- 71.Nathanson S. D., Mahan M. (2011) Sentinel lymph node pressure in breast cancer. Ann. Surg. Oncol. 18, 3791–3796 [DOI] [PubMed] [Google Scholar]
- 72.Nathanson S. D., Shah R., Chitale D. A., Mahan M. (2014) Intraoperative clinical assessment and pressure measurements of sentinel lymph nodes in breast cancer. Ann. Surg. Oncol. 21, 81–85 [DOI] [PubMed] [Google Scholar]
- 73.Hagendoorn J., Tong R., Fukumura D., Lin Q., Lobo J., Padera T. P., Xu L., Kucherlapati R., Jain R. K. (2006) Onset of abnormal blood and lymphatic vessel function and interstitial hypertension in early stages of carcinogenesis. Cancer Res. 66, 3360–3364 [DOI] [PubMed] [Google Scholar]
- 74.Qian C. N., Berghuis B., Tsarfaty G., Bruch M., Kort E. J., Ditlev J., Tsarfaty I., Hudson E., Jackson D. G., Petillo D., Chen J., Resau J. H., Teh B. T. (2006) Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 66, 10365–10376 [DOI] [PubMed] [Google Scholar]
- 75.Webster B., Ekland E. H., Agle L. M., Chyou S., Ruggieri R., Lu T. T. (2006) Regulation of lymph node vascular growth by dendritic cells. J. Exp. Med. 203, 1903–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Provenzano P. P., Inman D. R., Eliceiri K. W., Knittel J. G., Yan L., Rueden C. T., White J. G., Keely P. J. (2008) Collagen density promotes mammary tumor initiation and progression. BMC Med. 6, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deed R., Rooney P., Kumar P., Norton J. D., Smith J., Freemont A. J., Kumar S. (1997) Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int. J. Cancer 71, 251–256 [DOI] [PubMed] [Google Scholar]
- 78.Fieber C., Baumann P., Vallon R., Termeer C., Simon J. C., Hofmann M., Angel P., Herrlich P., Sleeman J. P. (2004) Hyaluronan-oligosaccharide-induced transcription of metalloproteases. J. Cell Sci. 117, 359–367 [DOI] [PubMed] [Google Scholar]
- 79.Ichikawa T., Itano N., Sawai T., Kimata K., Koganehira Y., Saida T., Taniguchi S. (1999) Increased synthesis of hyaluronate enhances motility of human melanoma cells. J. Invest. Dermatol. 113, 935–939 [DOI] [PubMed] [Google Scholar]
- 80.Kosaki R., Watanabe K., Yamaguchi Y. (1999) Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res. 59, 1141–1145 [PubMed] [Google Scholar]
- 81.Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F., Csiszar K., Giaccia A., Weninger W., Yamauchi M., Gasser D. L., Weaver V. M. (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mummert M. E., Mummert D. I., Ellinger L., Takashima A. (2003) Functional roles of hyaluronan in B16-F10 melanoma growth and experimental metastasis in mice. Mol. Cancer Ther. 2, 295–300 [PubMed] [Google Scholar]
- 83.Savani R. C., Cao G., Pooler P. M., Zaman A., Zhou Z., DeLisser H. M. (2001) Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J. Biol. Chem. 276, 36770–36778 [DOI] [PubMed] [Google Scholar]
- 84.Trochon V., Mabilat-Pragnon C., Bertrand P., Legrand Y., Soria J., Soria C., Delpech B., Lu H. (1997) Hyaluronectin blocks the stimulatory effect of hyaluronan-derived fragments on endothelial cells during angiogenesis in vitro. FEBS Lett. 418, 6–10 [DOI] [PubMed] [Google Scholar]
- 85.Xu X. M., Chen Y., Chen J., Yang S., Gao F., Underhill C. B., Creswell K., Zhang L. (2003) A peptide with three hyaluronan binding motifs inhibits tumor growth and induces apoptosis. Cancer Res. 63, 5685–5690 [PubMed] [Google Scholar]
- 86.Yang B. G., Tanaka T., Jang M. H., Bai Z., Hayasaka H., Miyasaka M. (2007) Binding of lymphoid chemokines to collagen IV that accumulates in the basal lamina of high endothelial venules: its implications in lymphocyte trafficking. J. Immunol. 179, 4376–4382 [DOI] [PubMed] [Google Scholar]
- 87.Stylianopoulos T., Jain R. K. (2013) Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc. Natl. Acad. Sci. USA 110, 18632–18637 [DOI] [PMC free article] [PubMed] [Google Scholar]