Abstract
Background
Obesity is a chronic metabolic disorder associated with an increase in adipogenesis and often accompanied with fatty liver disease.
Objective
In this study, we investigated the anti-obesity effects of Hibiscus sabdariffa water extract (HSE) in vivo.
Method
Eight-weeks-old male mice were divided into six groups (n=8 per group) and were fed either normal feed, a high fat diet (HFD), HFD supplemented with different concentrations of HSE, or HFD supplemented with anthocyanin. After 10 weeks of feeding, all the blood and livers were collected for further analysis.
Results
Mesocricetus auratus hamster fed with a high-fat diet developed symptoms of obesity, as determined from their body weight change and from their plasma lipid levels. Meanwhile, HSE treatment reduced fat accumulation in the livers of hamsters fed with HFD in a concentration-dependent manner. Administration of HSE reduced the levels of liver cholesterol and triglycerides, which were elevated by HFD. Analysis of the effect of HSE on paraoxonase 1, an antioxidant liver enzyme, revealed that HSE potentially regulates lipid peroxides and protects organs from oxidation-associated damage. The markers of liver damage such as serum alanine aminotransferase and aspartate aminotransferase levels that were elevated by HFD were also reduced on HSE treatment. The effects of HSE were as effective as treatment with anthocyanin; therefore the anthocyanins present in the HSE may play a crucial role in the protection established against HFD-induced obesity.
Conclusions
In conclusion HSE administration constitutes an effective and viable treatment strategy against the development and consequences of obesity.
Keywords: Hibiscus sabdariffa, obesity, adipogenesis
Obesity is a chronic metabolic disorder caused by imbalance in energy intake and energy expenditure. Obesity is associated with an increase in adipogenesis, the process whereby undifferentiated preadipocytes are converted to differentiated adipocytes (1). Various metabolic syndromes such as nonalcoholic fatty liver disease (NAFLD), insulin resistance, and hypertension are known to be associated with obesity (2, 3). Obesity is most commonly accompanied by liver damage, as dietary obesity promotes liver inflammation and disease (4). Because diets containing high-fat foods are becoming common, it is necessary to find suitable alternatives, such as phytochemicals, to ameliorate the effects of a high fat diet (HFD), thereby reducing the risk associated with obesity (5, 6).
Phytochemicals are known to possess antioxidant properties with effective anti-inflammatory, antiallergic, hepatoprotective, antithrombotic, antiviral, and anticarcinogenic activities (7–9). Among them, polyphenols such as resveratrol, catechins, and anthocyanins are of great interest due to their wide distribution in plants and potent health-promoting activities (10–12). Treatments with phytochemicals obtained from various sources have been demonstrated to regulate the levels of total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) in HFD-fed rats (13–15).
Hibiscus sabdariffa L. (Malvaceae; common name “roselle”) is an attractive plant cultivated in Sudan and in Eastern Taiwan. H. sabdariffa is consumed as traditional Chinese rose tea and is used effectively against hypertension. Extracts from H. sabdariffa are known to possess effective antioxidant, antihyperlipidemic, antiatherosclerotic, and hepatoprotective properties. In our previous work, H. sabdariffa extract (HSE) was found to exhibit chemopreventive properties through mitogen-activated protein kinase (MAPK) signaling pathways (16). HSE has also been used in traditional medicines for its pharmacologic properties, which include hepatoprotection and antioxidant activities and provide protection against atherosclerosis and fatty liver disease (17–20).
Anthocyanins are widely known to be effective in controlling body weight gain, which is vital in the treatment and prevention of obesity (21). The floral extracts of H. sabdariffa are rich in anthocyanin contents, which have high therapeutic potential. Anthocyanins from blackberries (Rubus sp.), blueberries (Vaccinium angustifolium), mulberries (Morus australis Poir), and blood oranges (Citrus sinensis [L.] Osbeck) were shown to prevent obesity in mice fed HFD (22–26).
In this study, the anti-obesity activity of HSE was tested against HFD-induced obesity in Mesocricetus auratus hamster. HSE was found to be effective in controlling body weight gain and adipose deposition. Further HSE administration ameliorated lipid accumulation, lipid peroxide activities, and tissue damage in the livers of HFD-fed hamsters. The effect of HSE on HFD-induced obesity was very similar to that of anthocyanin (cyanidin-3-O-β-glucoside) used as a positive control.
Materials and methods
Preparation of HSE
The HSE extract was prepared following methods reported previously (27). H. sabdariffa was purchased from a Taitung County farmers’ association, Taitung, Taiwan, after confirming the identification by comparing with a sample in the herbarium (catalog number TAI074435) of the National Taiwan University, Pintung, Taiwan. Briefly, 20 g of H. sabdariffa was washed with distilled water and air-dried. H. sabdariffa was resuspended in 100 mL of distilled water at 4°C overnight. Next, the supernatant was filtered through two layers of gauze to remove the debris and then lyophilized. The dried HSE was stored at −20°C; before use it was reconstituted with water. Characterization of the components of HSE by high performance liquid chromatography showed that it contains 1.39% flavonoids, 2.48% anthocyanins, and 1.67% polyphenols. HSE polyphenols are composed of protocatechuic acid (8.62%), catechin (9.86%), epigallocatechin (10.11%), epigallocatechin gallate (20.34%), and caffeic acid (18.24%). The anthocyanins are composed of delphinidin (79%) and cyanidin (18%) (28).
Animals and experimental design
All animal experimental procedures followed the protocols approved by the Institutional Animal Care and Use Committee of Chung Shan Medical University (CSMU IUCAC No. 513) in Taichung, Taiwan. Male hamsters, aged 8 weeks and weighing 120 g, were purchased from the National Laboratory Animal Breeding and Research Center (Taipei, Taiwan) and housed in standard laboratory conditions (18–23°C, 55–60% humidity, and 12 h light/dark cycle) for the first 1 week of adaptation to the environment and during the experiments that followed. All hamsters were randomly divided into six groups (n=8 per group) as follows: control (standard laboratory diet, PMI Nutrition International, Brentwood, MO, USA); HFD (standard laboratory diet containing 10% coconut oil and 0.5% cholesterol); HFD supplemented with 25, 50, or 100 mg of HSE per day; or HFD supplemented with anthocyanin (cyanidin-3-O-β-glucoside, Polyphenol Laboratories AS, Sandnes, Norway). After 10 weeks of feeding on these diets, the hamsters fasted for 12–14 h and were then euthanized; all of their blood as well as their livers were collected.
Blood sample analysis
Blood samples were collected from the hamsters with EDTA tubes and immediately centrifuged at 1,500g for 10 min. The serum was then decanted and stored at 4°C. Biochemical examinations were performed within 1 h of specimen collection. Serum levels of TG, HDL-C, LDL-C, alanine transaminase (ALT), and aspartate aminotransferase (AST) were measured by using clinical chemistry reagent kits (Randox Laboratories, Antrim, UK). Serum paraoxonase 1 (PON-1) activity measurement was using EnzChek® Paraoxonase Assay Kit (Life Technologies CO., Ltd. Taipei, Taiwan). The protocol was used basically according to the manual of the kit. First 10 µl of each serum sample was added into 50 µl of reaction buffer in a plate prewarmed to 37°C. Then 50 µl of paraoxonase substrate working solution was added and the mixture was transferred to a fluorescence microplate reader set to 37°C. The plate was read using excitation at 360 nm and emission at 450 nm immediately. The collection data was calibrated and analyzed using the standard curve assay suggested by the kit manual.
Statistical analysis
All experiments were performed in triplicate. Significant differences were assessed using one-way ANOVA, and p<0.05 was considered statistically significant. The data are expressed as the mean±SEM.
Results
HSE protects hamsters from HFD-induced physiological changes
Feeding of HFD caused significant characteristic changes associated with obesity in hamsters within 10 weeks. The hamsters fed with HFD showed an increase in body weight and in the weight of gonadal fat tissue after administration (Table 1). The HSE treatment showed high efficiency in regulating the total body weight and the increased fat content. The anthocyanin treatment group showed a similar trend to that of the HSE group.
Table 1.
Effects of HSE and anthocyanin supplement on obese hamsters
| High fat diet | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Control | HSE (0 mg) | HSE (25 mg) | HSE (50 mg) | HSE (100 mg) | Anthocyanin (25 mg) | |
| Body weight before administration | 18.03±0.11 | 18.00±0.10 | 18.05±0.13 | 18.01±0.14 | 18.06±0.14 | 18.01±0.07 |
| Body weight after administration | 22.32±2.28 | 31.61±9.49# | 27.68±8.46* | 22.79±9.22* | 20.9±9.52* | 21.79±10.23* |
| Weight gain after administration | 4.29±2.36 | 13.61±9.37 | 3.63±7.65 | 4.78±8.83 | 2.84±9.11 | 3.78±10.16 |
| Gonadal fat tissue weight after administration | 0.20±0.02 | 0.29±0.05# | 0.24±0.04 | 0.22±0.05* | 0.21±0.05* | 0.22±0.06* |
HSE, Hibiscus sabdariffa water extract; HFD, high-fat diet. Each value is expressed as the mean±SD (n=10 per group). Results were statistically analyzed with one-way ANOVA.
p<0.05 compared with the control group.
p<0.05 compared with the HFD group.
HSE ameliorates the changes in serum markers induced by HFD
Obesity-associated serum markers such as the ratio of LDL-C to that of HDL-C were found to be significantly modulated in the hamster groups fed with HFD (Table 2). The treatment of HSE again proved to be very efficient in reducing the elevated serum constituents. Similarly the anthocyanin-treated groups also showed regulated levels of serum obesity markers.
Table 2.
Effects of HSE and anthocyanin supplement on plasma lipid parameters levels in hamsters
| High fat diet | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Control | HSE (0 g) | HSE (25 mg) | HSE (50 mg) | HSE (100 mg) | Anthocyanin (25 mg) | |
| LDL-C (mg/dL) | 75±9 | 311±53### | 126±9*** | 105±8*** | 99±7* | 113±9* |
| HDL-C (mg/dL) | 38±4 | 70±7### | 53±5*** | 44±6*** | 46±4* | 50±2* |
| LDL-C/HDL-C ratio | 1.97±0.23 | 4.44±0.75### | 2.38±0.17* | 2.38±0.18*** | 2.15±0.15*** | 2.26±0.18*** |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. Each value is expressed as the mean±SD (n=10 per group). Results were statistically analyzed with one-way ANOVA.
## p<0.05
p<0.001 was compared with the control group.
p<0.05
p<0.001 was compared with the HFD group.
HSE reduces fat accumulation in hamster livers
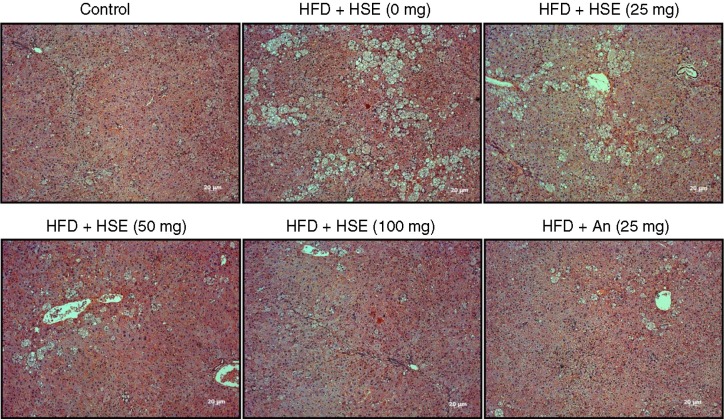
The H and E staining of the hamster liver sections show extensive accumulation of fat in the livers of hamsters fed with HFD. However, the hamsters fed with 25, 50, or 100 mg of HSE showed a dose-dependent decrease in the amount of liver fat bodies. Treatment with 100 mg of HSE exhibited a high potential for ameliorating the fatty liver conditions caused by HFD (Fig. 1). A comparative analysis of the effect of HSE and that of anthocyanin on HFD-fed hamsters shows 100 mg of HSE treatment to be superior.
Fig. 1.
Effects of Hibiscus sabdariffa water extract (HSE) supplement on body weight and adipose tissue in HFD-fed hamsters. H and E staining of liver sections shows various degrees of fat accumulation in hamsters fed a normal diet (control) and hamsters fed with HFD along with different amounts of HSE or 25 mg anthocyanin.
HSE reduces HFD feeding-related modulations in hamster livers
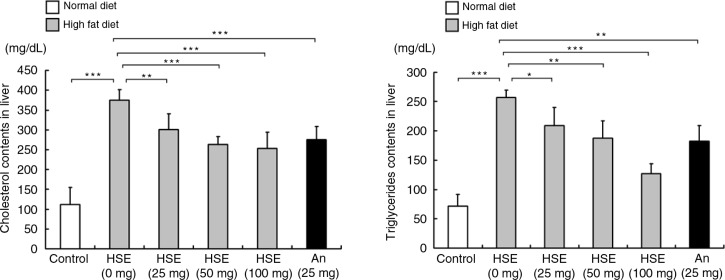
HFD increased the liver cholesterol and TG levels significantly but the levels were decreased in the hamster groups treated with HSE (Fig. 2). The effect of HSE was dose-dependent and the 100 mg HSE treatment group showed better results than the anthocyanin-treated group.
Fig. 2.
Effects of HSE supplement on serum lipid levels in HFD-fed hamsters. Serum cholesterol and triglyceride levels in hamsters fed a normal diet (control) and hamsters fed HFD along with different amounts of HSE or 25 mg anthocyanin. Data are shown as the mean±SD: *p<0.05, **p<0.01, ***p<0.001 compared with the HFD group.
HSE potentially attenuates HFD induced atherosclerosis condition
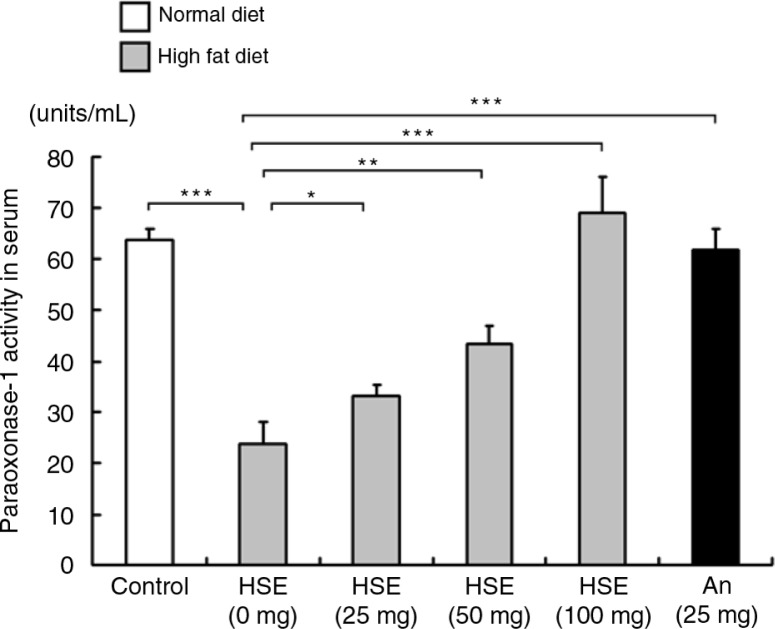
The serum activity levels of PON-1, which is known for its anti-atherosclerotic property, decreased in the HFD-fed hamster groups. However, treatment with various concentrations of HSE increased the PON-1 activity levels in the hamster serum (Fig. 3). Therefore HSE treatment reduced the risk of atherosclerosis associated with HFD feeding.
Fig. 3.
Effects of HSE supplement on paraoxonase 1 (PON-1) in HFD-fed hamsters. Serum PON-1 levels in hamsters fed a normal diet (control) and hamsters fed HFD along with different amounts of HSE or 25 mg anthocyanin. Data are shown as the mean±SD: *p<0.05, **p<0.01, ***p<0.001 compared with the HFD group.
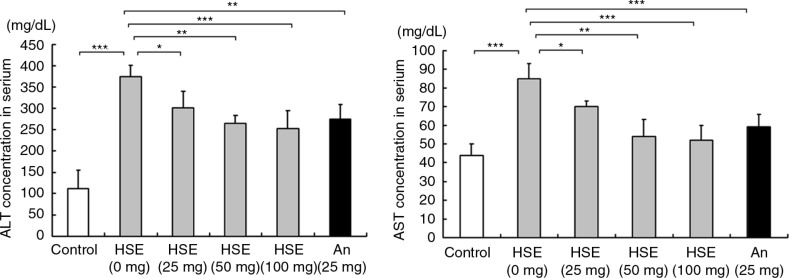
HSE regulates the ALT and AST level analysis
Analysis of the serum ALT and AST levels show that ALT and AST (Fig. 4) levels increased in hamsters fed with HFD. The ALT and AST levels were, however, reduced in the hamsters treated with various doses of HSE and the results were similar to the effects of anthocyanin.
Fig. 4.
Effects of HSE supplement on serum alanine aminotransferase and aspartate aminotransferase in HFD-fed hamsters. Serum alanine aminotransferase and aspartate aminotransferase levels in hamsters fed a normal diet (control) and hamsters fed HFD along with different amounts of HSE or 25 mg anthocyanin. Data are shown as the mean±SD: *p<0.05, **p<0.01, ***p<0.001 compared with the HFD group.
Discussion
Obesity is considered to be a major risk factor associated with various diseases, including coronary heart disease, hypertension, type 2 diabetes mellitus, cancer, respiratory complications, and osteoarthritis (29). Recent reports have outlined that administration of an efficient anti-obesity drug may decrease energy/food intake and increase energy expenditure, decrease preadipocyte differentiation and proliferation, decrease lipogenesis, and increase lipolysis and fat oxidation (30). In this study, the anti-obesity effects of HSE were determined through body weight, fat mass, serum lipid profile, and biochemical examination of liver function in HFD-fed hamsters.
Our data revealed that both HSE and anthocyanin decrease the levels of serum and hepatic lipids such as TC and TG in HFD-fed hamsters, which could be attributed to the inhibition of lipid absorption in the gut. Dietary lipids are absorbed into the bloodstream and are then digested to be transported and stored in the liver and adipose tissues in the form of TG (16).
Blood samples of the HFD-fed hamsters showed an increase in the levels of TG; the ratio of LDL-C to HDL-C increased in the hamsters. The increase in cholesterol may potentially enhance the risk of fatty liver and atherosclerosis (31, 32). Oxidized LDL has been shown to be atherogenic and inhibition of the LDL oxidation by potent dietary antioxidants effectively attenuates atherosclerosis (33, 34). HSE is rich in high antioxidant components such as anthocyanins that can decrease the oxysterols in bile acid metabolism block the absorption of lipids into the liver by the bile salt system (35, 36). Protection of lipids from oxidation can be also achieved by serum PON-1, an HDL-associated esterase that can hydrolyze and reduce specific lipid peroxides in arterial cells and in lipoproteins in coronary and carotid lesions (37). Our results further show that HSE treatment effectively enhances the serum PON-1 levels, which were found to be lower in the HFD-fed hamsters. Therefore, HSE treatment can effectively decrease the adverse effects of serum lipid peroxides in the arteries.
Obesity is known to cause inflammation of the liver (38). To have a better understanding of the HFD effects on liver function, the enzyme activities of ALT and AST were measured (39). Our results showed that HFD indeed increased serum ALT and AST levels, revealing that HFD induced liver damage in hamsters fed with HFD (40). Both ALT and AST are leakage enzymes, and their elevation in circulation indicates significant hepatocellular damage. Toxicity, inflammation, hypoxia, and tissue trauma may form the underlying reason for their elevation (41, 42). Our results revealed that HSE treatment significantly reduced ALT and AST; therefore HSE ameliorated the potential liver damage caused by HFD. The reduction of liver enzymes after HSE treatment indicated a decrease in fat deposition and necrosis in liver cells (43).
Our previous work revealed that HSE efficiently reduced serum cholesterol levels in 42 volunteers after 4 weeks of administration (20). HFD has been known to increase the liver mitochondrial reactive oxygen species (ROS) production (44). ROS causes cell damage via the mechanism involving lipid peroxidation that leads to tissue injury, especially in the liver (45). Dietary polyphenols contain a number of phenolic hydroxyl groups and have demonstrated various beneficial effects, which is mainly due to their ROS scavenging activity (46). Polyphenolics are widely distributed in vegetables, fruits, and beverages and are present as an integral part of typical human diets (47, 48). Various polyphenol-rich sources such as tea, pomegranate, grape juice, and apples have demonstrated hepatic protection against various challenges (46, 48–51). Various herbal extracts, such as Chrysanthemum morifolium extract, Morinda citrifolia L. extract, and Coix lacryma-jobi L. extract, that are abundant in polyphenols have also been shown to provide efficient liver protection against obesity-related liver damage (52–55).
Conclusions
To sum up, HSE treatment improved the HFD-induced obesity and lipid accumulation-induced damage in the liver in the animal model. Polyphenolic- and flavonoid-rich HSE treatment is also a safe therapy for HFD-induced obesity.
Acknowledgements
This work was financially supported by Chienkuo Technology University (CTU-101-RP-BS-002-048) of Taiwan (ROC).
Conflict of interest and funding
The authors declare no conflict of interest.
Authors’ contributions
ESK and JHL designed the experiments; TWH and CLC performed the experiments and analyzed the results; TWH, JHL, and ESK prepared and edited the manuscript. All authors read and approved the final manuscript.
References
- 1.Camp HS, Ren D, Leff T. Adipogenesis and fat-cell function in obesity and diabetes. Trends Mol Med. 2002;8:442–7. doi: 10.1016/s1471-4914(02)02396-1. [DOI] [PubMed] [Google Scholar]
- 2.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–79. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 4.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol. 2011;81:1343–51. doi: 10.1016/j.bcp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Kim YJ, Bae YC, Suh KT, Jung JS. Quercetin, a flavonoid, inhibits proliferation and increases osteogenic differentiation in human adipose stromal cells. Biochem Pharmacol. 2006;72:1268–78. doi: 10.1016/j.bcp.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 7.de Groot H, Rauen U. Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam Clin Pharmacol. 1998;12:249–55. doi: 10.1111/j.1472-8206.1998.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 8.Tapas AR, Sakarkar DM, Kakde RB. Flavonoids as nutraceuticals: a review. Trop J Pharm Res. 2008;7:1089–99. [Google Scholar]
- 9.Jung HS, Lim Y, Kim EK. Therapeutic phytogenic compounds for obesity and diabetes. Int J Mol Sci. 2014;15:21505–37. doi: 10.3390/ijms151121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo H, Ling W. The update of anthocyanins on obesity and type 2 diabetes: experimental evidence and clinical perspectives. Rev Endoc Metab Disord. 2015;16:1–13. doi: 10.1007/s11154-014-9302-z. [DOI] [PubMed] [Google Scholar]
- 11.Diaconeasa Z, Leopold L, Rugina D, Ayvaz H, Socaciu C. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int J Molec Sci. 2015;16:2352–65. doi: 10.3390/ijms16022352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho SJ, Park HJ, Jung UJ, Kim HJ, Moon BS, Choi MS. The beneficial effects of combined grape pomace and omija fruit extracts on hyperglycemia, adiposity and hepatic steatosis in db/db mice: a comparison with major index compounds. Int J Mol Sci. 2014;15:17778–89. doi: 10.3390/ijms151017778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Zheng L, Dong D, Xu L, Yin L, Qi Y, et al. Effects of flavonoids from Rosa laevigata Michx fruit against high-fat diet-induced non-alcoholic fatty liver disease in rats. Food Chem. 2013;141:2108–16. doi: 10.1016/j.foodchem.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Jung CH, Cho I, Ahn J, Jeon TI, Ha TY. Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother Res. 2013;27:139–43. doi: 10.1002/ptr.4687. [DOI] [PubMed] [Google Scholar]
- 15.Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322–33. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 16.Wu CH, Yang MY, Chan KC, Chung PJ, Ou TT, Wang CJ. Improvement in high-fat diet-induced obesity and body fat accumulation by a Nelumbo nucifera leaf flavonoid-rich extract in mice. J Agric Food Chem. 2010;58:7075–81. doi: 10.1021/jf101415v. [DOI] [PubMed] [Google Scholar]
- 17.Yang MY, Peng CH, Chan KC, Yang YS, Huang CN, Wang CJ. The hypolipidemic effect of Hibiscus sabdariffa polyphenols via inhibiting lipogenesis and promoting hepatic lipid clearance. J Agric Food Chem. 2010;58:850–9. doi: 10.1021/jf903209w. [DOI] [PubMed] [Google Scholar]
- 18.Lin HH, Chen JH, Wang CJ. Chemopreventive properties and molecular mechanisms of the bioactive compounds in Hibiscus sabdariffa Linne. Curr Med Chem. 2011;18:1245–54. doi: 10.2174/092986711795029663. [DOI] [PubMed] [Google Scholar]
- 19.Liu JY, Chen CC, Wang WH, Hsu JD, Yang MY, Wang CJ. The protective effects of Hibiscus sabdariffa extract on CCl4-induced liver fibrosis in rats. Food Chem Toxicol. 2006;44:336–43. doi: 10.1016/j.fct.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Lin TL, Lin HH, Chen CC, Lin MC, Chou MC, Wang CJ. Hibiscus sabdariffa extract reduces serum cholesterol in men and women. Nutr Res. 2007;27:140–5. [Google Scholar]
- 21.Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr. 2003;133:2125–30. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- 22.Titta L, Trinei M, Stendardo M, Berniakovich I, Petroni K, Tonelli C, et al. Blood orange juice inhibits fat accumulation in mice. Int J Obesity. 2010;34:578–88. doi: 10.1038/ijo.2009.266. [DOI] [PubMed] [Google Scholar]
- 23.Prior RL, Wu X, Gu L, Hager TJ, Hager A, Howard LR. Whole berries versus berry anthocyanins: interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J Agric Food Chem. 2008;56:647–53. doi: 10.1021/jf071993o. [DOI] [PubMed] [Google Scholar]
- 24.Wu T, Qi X, Liu Y, Guo J, Zhu R, Chen W, et al. Dietary supplementation with purified mulberry (Morus australis Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chem. 2013;141:482–7. doi: 10.1016/j.foodchem.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 25.Prior RL, Wilkes SE, Rogers TR, Khanal RC, Wu X, Howard LR. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J Agric Food Chem. 2010;58:3970–6. doi: 10.1021/jf902852d. [DOI] [PubMed] [Google Scholar]
- 26.Wu T, Yu Z, Tang Q, Song H, Gao Z, Chen W, et al. Honeysuckle anthocyanin supplementation prevents diet-induced obesity in C57BL/6 mice. Food Funct. 2013;4:1654–61. doi: 10.1039/c3fo60251f. [DOI] [PubMed] [Google Scholar]
- 27.Lin HH, Chen JH, Kuo WH, Wang CJ. Chemopreventive properties of Hibiscus sabdariffa L. on human gastric carcinoma cells through apoptosis induction and JNK/p38 MAPK signaling activation. Chem Biol Interact. 2007;165:59–75. doi: 10.1016/j.cbi.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Lin HH, Huang HP, Huang CC, Chen JH, Wang CJ. Hibiscus polyphenol-rich extract induces apoptosis in human gastric carcinoma cells via p53 phosphorylation and p38 MAPK/FasL cascade pathway. Mol Carcinog. 2005;43:86–99. doi: 10.1002/mc.20103. [DOI] [PubMed] [Google Scholar]
- 29.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–43. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 30.Wang YW, Jones PJH. Conjugated linoleic acid and obesity control: efficacy and mechanisms. Int J Obesity. 2004;28:941–55. doi: 10.1038/sj.ijo.0802641. [DOI] [PubMed] [Google Scholar]
- 31.Rule DC, Liebman M, Liang YB. Impact of different dietary fatty acids on plasma and liver lipids is influenced by dietary cholesterol in rats. J Nutr Biochem. 1996;7:142–9. [Google Scholar]
- 32.Onat A, Sari I, Yazici M, Can G, Hergenc G, Avci GS. Plasma triglycerides, an independent predictor of cardiovascular disease in men: a prospective study based on a population with prevalent metabolic syndrome. Int J Cardiol. 2006;108:89–95. doi: 10.1016/j.ijcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 33.Aviram M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic Res. 2000;33:S85–S97. [PubMed] [Google Scholar]
- 34.Fuhrman B, Aviram M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr Opin Lipidol. 2001;12:41–8. doi: 10.1097/00041433-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Crosignani A, Zuin M, Allocca M, Del Puppo M. Oxysterols in bile acid metabolism. Clin Chim Acta. 2011;412:2037–45. doi: 10.1016/j.cca.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 36.Lotito SB, Frei B. Dietary flavonoids attenuate tumor necrosis factor alpha-induced adhesion molecule expression in human aortic endothelial cells. Structure-function relationships and activity after first pass metabolism. J Biol Chem. 2006;281:37102–10. doi: 10.1074/jbc.M606804200. [DOI] [PubMed] [Google Scholar]
- 37.Aviram M, Rosenblat M, Gaitini D, Nitecki S, Hoffman A, Dornfeld L, et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr. 2004;23:423–33. doi: 10.1016/j.clnu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Lanthier N, Molendi-Coste O, Cani PD, van Rooijen N, Horsmans Y, Leclercq IA. Kupffer cell depletion prevents but has no therapeutic effect on metabolic and inflammatory changes induced by a high-fat diet. Faseb J. 2011;25:4301–11. doi: 10.1096/fj.11-189472. [DOI] [PubMed] [Google Scholar]
- 39.de Castro UGM, dos Santos RAS, Silva ME, de Lima WG, Campagnole-Santos MJ, Alzamora AC. Age-dependent effect of high-fructose and high-fat diets on lipid metabolism and lipid accumulation in liver and kidney of rats. Lipids Health Dis. 2013;12:136. doi: 10.1186/1476-511X-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Y, Zhao MY, An W. Increased hepatic apoptosis in high-fat diet-induced NASH in rats may be associated with downregulation of hepatic stimulator substance. J Mol Med. 2011;89:1207–17. doi: 10.1007/s00109-011-0790-y. [DOI] [PubMed] [Google Scholar]
- 41.Srivastava AR, Kumar S, Agarwal GG, Ranjan P. Blunt abdominal injury: serum ALT-A marker of liver injury and a guide to assessment of its severity. Injury. 2007;38:1069–74. doi: 10.1016/j.injury.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 42.Chapman SE, Hostutler RA. A laboratory diagnostic approach to hepatobiliary disease in small animals. Vet Clin North Am Small Anim Pract. 2013;43:1209–25. doi: 10.1016/j.cvsm.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Wang LL, Meng XJ, Zhang FQ. Raspberry ketone protects rats fed high-fat diets against nonalcoholic steatohepatitis. J Med Food. 2012;15:495–503. doi: 10.1089/jmf.2011.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vial G, Dubouchaud H, Couturier K, Cottet-Rousselle C, Taleux N, Athias A, et al. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. J Hepatol. 2011;54:348–56. doi: 10.1016/j.jhep.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 45.Tian L, Shi X, Yu L, Zhu J, Ma R, Yang X. Chemical composition and hepatoprotective effects of polyphenol-rich extract from Houttuynia cordata tea. J Agric Food Chem. 2012;60:4641–8. doi: 10.1021/jf3008376. [DOI] [PubMed] [Google Scholar]
- 46.Cui Y, Yang X, Lu X, Chen J, Zhao Y. Protective effects of polyphenols-enriched extract from Huangshan Maofeng green tea against CCl-induced liver injury in mice. Chem Biol Interact. 2014;220C:75–83. doi: 10.1016/j.cbi.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 47.Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M, et al. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agric Food Chem. 2008;56:1415–22. doi: 10.1021/jf073035s. [DOI] [PubMed] [Google Scholar]
- 48.Mukherjee S, Ghosh S, Choudhury S, Adhikary A, Manna K, Dey S, et al. Pomegranate reverses methotrexate-induced oxidative stress and apoptosis in hepatocytes by modulating Nrf2-NF-kappaB pathways. J Nutr Biochem. 2013;24:2040–50. doi: 10.1016/j.jnutbio.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Aprikian O, Busserolles J, Manach C, Mazur A, Morand C, Davicco MJ, et al. Lyophilized apple counteracts the development of hypercholesterolemia, oxidative stress, and renal dysfunction in obese Zucker rats. J Nutr. 2002;132:1969–76. doi: 10.1093/jn/132.7.1969. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues AD, Scheffel TB, Scola G, Dos Santos MT, Fank B, Dani C, et al. Purple grape juices prevent pentylenetetrazol-induced oxidative damage in the liver and serum of Wistar rats. Nutr Res. 2013;33:120–5. doi: 10.1016/j.nutres.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Heber D, Zhang Y, Yang J, Ma JE, Henning SM, Li Z. Green tea, black tea, and oolong tea polyphenols reduce visceral fat and inflammation in mice fed high-fat, high-sucrose obesogenic diets. J Nutr. 2014;144:1385–93. doi: 10.3945/jn.114.191007. [DOI] [PubMed] [Google Scholar]
- 52.Cui Y, Wang XL, Xue J, Liu JY, Xie ML. Chrysanthemum morifolium extract attenuates high-fat milk-induced fatty liver through peroxisome proliferator-activated receptor alpha-mediated mechanism in mice. Nutr Res. 2014;34:268–75. doi: 10.1016/j.nutres.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Lin YL, Chang YY, Yang DJ, Tzang BS, Chen YC. Beneficial effects of noni (Morinda citrifolia L.) juice on livers of high-fat dietary hamsters. Food Chem. 2013;140:31–8. doi: 10.1016/j.foodchem.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 54.Wang LF, Sun J, Yi QD, Wang XF, Ju XR. Protective effect of polyphenols extract of adlay (Coix lacryma-jobi L. var. ma-yuen Stapf) on hypercholesterolemia-induced oxidative stress in rats. Molecules. 2012;17:8886–97. doi: 10.3390/molecules17088886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zou YP, Lu YH, Wei DZ. Hypocholesterolemic effects of a flavonoid-rich extract of Hypericum perforatum L. in rats fed a cholesterol-rich diet. J Agric Food Chem. 2005;53:2462–6. doi: 10.1021/jf048469r. [DOI] [PubMed] [Google Scholar]