Abstract
Diabetes is a common metabolic disorder that is specified by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. The use of nonpharmacological treatments (herbal agents) is a new approach in the management of diabetes. The aim of this study was to investigate the effect of aqueous extract of alfalfa on blood glucose and serum lipids in alloxan-induced diabetic rats. In this study, 32 female rats (210–250 g) were used which were divided randomly into 4 groups including intact control group, diabetic control group, and 2 diabetic groups which received 250 and 500 mg/kg doses of aqueous extract of alfalfa, respectively. In the diabetic groups, alloxan-monohydrate was injected peritoneally to create diabetic condition. The two last groups orally received aqueous extract of alfalfa for 21 days. At the end of experiment, sugar, cholesterol, triglycerides, high-density and low-density lipoprotein, and aspartate aminotransferase (ALT) and alanine aminotransferase (AST) levels were measured in the samples. Consumption of aqueous alfalfa extract significantly reduced glucose, cholesterol, triglycerides, and low-density lipoprotein (LDL) levels in the diabetic rats but enhanced high-density lipoprotein (HDL) levels. ALT and AST liver enzyme levels were also reduced in blood. Histological examination showed that the aqueous alfalfa extract caused reconstruction of damaged liver and enhanced Langerhans islets’ diameter in pancreas. Therefore, all signs of diabetes were improved by oral administration of alfalfa in defined dose.
Keywords: aqueous extract alfalfa, diabetes, alloxan, blood sugar, Langerhans islet, insulin
Introduction
Diabetes mellitus is one of the most common chronic diseases in nearly all countries, and it continues to increase in numbers and is significant because of changes in lifestyles that have led to reduced physical activity and increased obesity. According to the 2000 statistics, there were 177 million diabetic patients in worldwide, and this number increased to 284.8 million in 2010 [1]. It is the most common disease that involves endocrine system. Its complications are hyperglycemia and impaired metabolism of carbohydrates, fats, and proteins.
Currently, the main treatment for diabetes mellitus is using insulin and hypoglycemic drugs, although there are many proven side effects for these compounds [2]. This fact has previously confirmed that herbal drugs have fewer side effects than chemically synthetic drugs, so researchers followed herbal drugs or agents to preventing and treating diabetes [3].
Alfalfa or green gold is one of the medicinal plants that are used in traditional medicine due to being high in protein, calcium, and vitamins and also its low percentage of cellulose. It contains many enzymes, including amylase, invertase, and pectinase, so it can be used as digestive aids [4]. More than 20% of dry weight of alfalfa is protein, and it is the best source of Arg, His, Asp, Phe, and Cys amino acids. Alfalfa has an extremely high nutritive value; it includes vitamins A, B1, B6, B12, C, D, E, and K, niacin, pantothenic acid, biotin, folic acid, minerals, protein, and beneficial saponins [5, 6]. Previous studies showed that adding alfalfa seed in human diet reduced triglycerides and LDL, improved HDL levels, and decreased blood glucose [7, 8]. Therefore, alfalfa leaves are traditionally used in South Africa as an effective treatment for diabetes [9, 10]. Alfalfa causes stimulation of insulin secretion. It also improves insulin function in reducing the plasma glucose, but its effects on the blood lipids have not been investigated widely [10, 11].
Insulin deficiency in diabetic patients leads to increased levels of amino acids in the blood, and it will lead to an increase in transaminase activity (enhanced level of AST and ALT levels); eventually, its result is increase of ketogenesis and gluconeogenesis [12, 13].
This paper examined the effects of different ratios which concentrate to alfalfa extraction on blood lipids and glucose in rats. Histological studies have also been conducted on pancreas tissue.
Our results like previous studies confirmed that the lack of insulin in addition to the rise of blood glucose will increase cholesterol and triglycerides levels. Increase in insulin reduces fat storage in the liver [14], and alfalfa improves this status.
Materials and Methods
This study was conducted on experimental animals, and we used adult male Wistar rats weighing 250–300 g obtained from the animal house of martyr portal. Animals with average age of 3–5.2 months were selected. Testing was carried out at temperature of 20–25 °C; day duration was 12 h and dark period was 12 h. Municipal tap water was used as drinking water and animal feed as nutrition (compressed feed), which the company that prepared feed was buying. Forty experimental animals were randomly divided into four groups (10 rats in each group) as follows: first was control group fed with usual water and food; second was diabetic control group; third was experimental group, which consists of diabetic rats administrated with 250 mg/kg dose of aqueous extract of alfalfa; and fourth group, which consists of diabetic rats treated with 500 mg/kg dose. The last three diabetic groups were created by intraperitoneal injection of 120 mg/kg dose of alloxan monohydrate for producing alloxan-induced diabetic rats [15]. Seven days after alloxan monohydrate injection for confirming the diabetic state in rats, blood glucose of rats was measured, and rats with blood glucose concentration more than 200 mg/100 cc that were diabetics have been selected (8 rats of 10 reach to diabetic state) [16]. The first control group is injected by physiologic serum for equivalency of shock obtained by intraperitoneal injection.
For preparing the treatment sample, alfalfa plants were collected after approval of agricultural experts and then were dried in the shade and grinded. The resulting powder was dissolved in physiological serum, and after 48 h, solution was filtered and used for oral administration.
Prepared aqueous solution of alfalfa was administrated orally in 250 mg/kg dose to the third diabetic group and 500 mg/kg dose to the fourth group (note that these two groups were alloxan monohydrate-induced diabetic). Alfalfa administration was repeated every day orally. This continued up to 21 days. For investigation of treatment effect after this time interval, blood samples from all animals were prepared, and blood glucose, cholesterol, and triglyceride were measured.
Alanin aminotransferase (ALT) and aspartate aminotransferase (AST) were assayed by Reitman and Frankel method [17].
The results were analyzed based on the statistical program SPSS in which ANOVA and Tukey tests were conducted to measure the significant difference in the level p < 0.05.
For histological studies at the end of experiment, rats were anesthetized by diethyl ether and their livers were removed and fixed by 10% natural formalin buffer. After tissue processing, the samples were blocked in cylindrical paraffin blockers and then stained by hematoxylin–eosin [14]. It should be noted that the samples’ diameter is 5–6 μm. Stained tissue samples were studied by light microscope.
Results
This study used alloxan monohydrate for creating diabetic status in rats except for the first control group [15]. Investigation of the feeding behavior of the treated rats showed, at early stage of experiment, daily uptake of water and food in alloxan-induced diabetic rats which increased more significantly than the first control group (p < 0.01) (Table I).
Table I.
Measurement of food and water uptake in beginning and end of experiment. As shown, usage of water and food in diabetic rats enhanced
| Groups | Uptake water
(mL) |
Uptake food (g) |
||
|---|---|---|---|---|
| Baseline | End of study | Baseline | End of study | |
| Intact control | 52.6 ± 9.4 | 53.1 ± 10.3 | 18.5 ± 4.2 | 17.8 ± 4.7 |
| Diabetic control | 148.2 ± 20.1* | 158.2 ± 37.5* | 38.4 ± 5.2* | 39.1 ± 4.8* |
| Treated by alfalfa | 151.2 ± 26.4* | 136.6 ± 33.3* | 35.2 ± 3.4* | 33.5 ± 4.5* |
| * Asterisk symbols showed significant changes by P < 0.05 | ||||
Measurement of blood glucose in rats showed that diabetic control group was treated by alloxan monohydrate and increased level of blood glucose, as expected. Glucose concentration of blood in two diabetic groups that were treated by alfalfa solution showed significant decrease (p < 0.05) as compared to diabetic controls without alfalfa treatment (Table II). As shown in Table II, decrease of blood glucose in rats treated with 500 mg/kg dose is more than in rats with 250 mg/kg dose treatment, so improving effects in alfalfa are dose dependent.
Table II.
Blood glucose and lipids measurement, which showed TG, Chol, and LDL increased in diabetic rats which decreased by alfalfa treatment. HDL improved by treatment also. Glucose increased by alloxan-induced diabetes and decreased by alfalfa treatment
| Groups | Intact control | Diabetic control | Treated by 250 mg/kg | Treated by 500 mg/kg |
|---|---|---|---|---|
| BS (mg/100 c.c.) | 107 ± 7.89 | 242.25 ± 14.15* | 182.5 ± 11.07* | 168.25 ± 16.30* |
| TG (mg/100 c.c.) | 94.87 ± 7.39 | 231.87 ± 13.37* | 169.37 ± 14.43* | 144.5 ± 11.90* |
| Chol (mg/100 c.c.) | 71.62 ± 5.65 | 120.12 ± 11.36* | 90.62 ± 3.62* | 80.75 ± 3.57 |
| HDL (mg/100 c.c.) | 16.25 ± 2.71 | 10.75 ± 1.03* | 18.75 ± 4.26 | 28.87 ± 9.50* |
| LDL (mg/100 c.c.) | 12 ± 3.02 | 21.62 ± 2.61* | 19.37 ± 3.81* | 14.25 ± 3.19 |
| VLDL (mg/100 c.c.) | 43.37 ± 7.79 | 88.87 ± 9.34* | 50.25 ± 9.11** | 37.12 ± 11.55 |
| * Asterisk symbols showed significant changes by P < 0.05 | ||||
Table II shows the LDL level of rats’ blood. Comparison of LDL concentration in all groups confirmed that diabetic rats (that were treated with alloxan monohydrate) have higher LDL level in blood than intact control group [14]. Rats that were treated with aqueous alfalfa extract showed decreased LDL level in comparison with untreated diabetic control, and in rats given higher dose of alfalfa solution, decrease in LDL is more significant (Table II).
Liver enzymes (AST and ALT) in blood serum are good indicator for liver condition because these two factors increased in serum by liver cells dysfunction due to leakage in liver cells membrane. Results showed a significant increase in AST and ALT level of serum in alloxan-induced diabetes (Table III). This enhancement showed dysfunction of liver cell membrane which is an abnormal condition. An interesting point is that treatments by alfalfa extract improved this disorder and decreased the level of these two important factors to nearly normal condition in the first control group.
Table III.
Liver enzymes measurement in blood. Results showed that treatment by alfalfa caused improvement of liver due to decrease of liver enzymes
| Enzymes | Intact control | Diabetic control | Treated by 250 mg/kg | Treated by 500 mg/kg |
|---|---|---|---|---|
| AST (IU/L) | 117 ± 4.37 | 299 ± 16.22* | 178.25 ± 7.08 | 148.75 ± 3.37 |
| ALT (IU/L) | 43.75 ± 1.83 | 94 ± 3.33* | 52.37 ± 1.59 | 50 ± 2.5 |
| * Asterisk symbols showed significant changes by P < 0.05 | ||||
Alloxan treatment showed inflammation in liver tissue, and this inflammation was improved by alfalfa treatment. Rats treated with 500 mg/kg dose showed more improvement than those treated with 250 mg/kg dose, so its therapeutic effects are dose dependent.
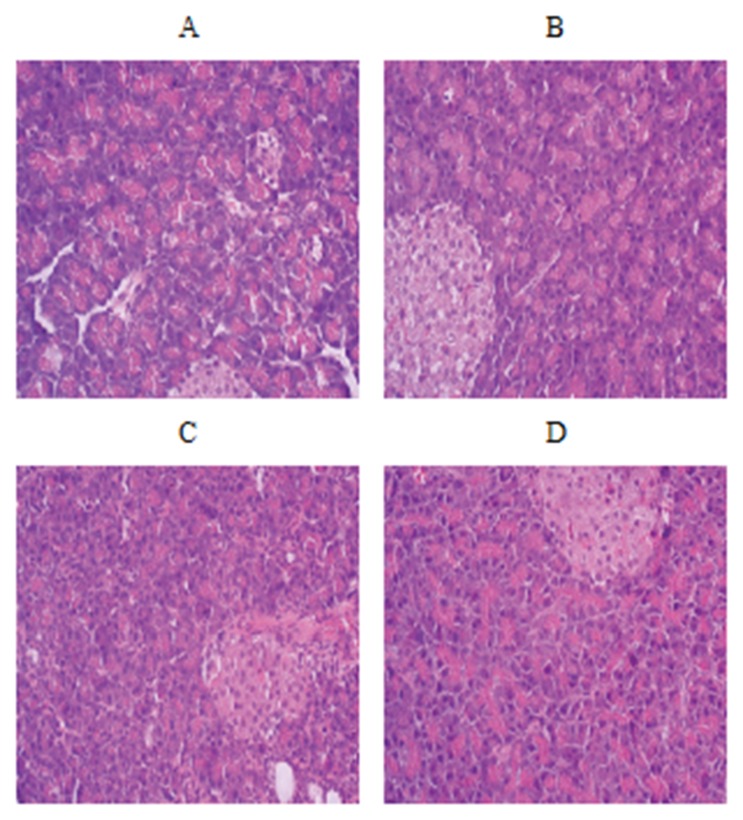
Figure 1 showed the appearance of the islets of Langerhans in rat’s pancreas. Results showed that Langerhans islets significantly reduced in alloxan-induced diabetic rats. Treatment with alfalfa improved this condition (increased number and size of Langerhans islet), so insulin secretion enhanced.
Fig. 1.
The histological studies revealed a significant reduction in the size of Langerhans islets in the diabetic group. A is pancreas slice of intact control group, B is pancreas slice of diabetic control group, C: related to rats treated by 250 mg/kg dose and D: related to rats treated by 500 mg/kg dose (×10)
Discussion
Diabetes mellitus (DM) is a group of metabolic diseases which is characterized by hyperglycemia resulting from defects in insulin secretion and/or activity. Clinical diabetes mellitus can be classified as type 1 and type 2. The alloxan-induced diabetic model mimics type 1 diabetes which is characterized by a typical autoimmune assault against the cells, inducing progressive cell death which is in line with the progressive decline in first-phase insulin secretion and results in insulin insufficiency and hyperglycemia [18].
This study investigates the effect of alfalfa solution as a possible cure for diabetes, so we used diabetic rats by alloxan monohydrate injection, which is injection of alloxan (120 mg/kg single dose) that produced a significant increase in the blood pressure, bradycardia, hyperglycemia, hypoinsulinemia, hyperlipidemia, hypothyroidism, and depression in left ventricular developed pressure (LVDP) [14]. Therefore, diabetic rats were under oral administration of aqueous extract of alfalfa in different dose. Oral administration was continued for 21 days, then triglyceride, cholesterol, and glucose level of blood was measured, and results were analyzed.
Results showed that diabetic rats treated with alfalfa solution introduced blood factors that improved as compared to diabetic control which did not receive herbal treatment. This improvement is dose dependent and enhanced with administration of high alfalfa extract dose.
Comparison of diabetic control and intact control showed an increase in cholesterol levels in diabetic control rats. Both of treatment groups compared to control diabetic group showed significant decrease in cholesterol levels, which suggests therapeutic effects of alfalfa extract. Diabetic control group showed decreased level of HDL as compared to intact control group; reduced HDL level is one of signs of diabetes disease, so application of alfalfa extract causes compensation of this decrease in treated diabetic rats by alfalfa. Overall, the results suggest that the aqueous extract of alfalfa significantly reduces blood sugar levels, triglycerides, cholesterol, LDL, and increased HDL levels [19].
Alfalfa consists of saponins that have heart protective effects due to cholesterol reduction, and these agents can absorb in digestive system in body [20]. Saponins inhibit cholesterol esterase and acetyl coenzyme and carboxylase enzymes, thus, inhibiting fatty acid synthesis, which increases the ratio of HDL cholesterol to LDL cholesterol, and it can be effective in reducing cardiovascular complications of diabetes. Saponins decrease intestinal absorption of cholesterol and increase its defecation [21]. Alfalfa is a rich source of vitamins and phytoestrogens, so it is used as a food additive in many developed countries [22]. In addition, because of its mentioned therapeutic effects, it is used as herbal drug in many countries.
The liver is one of the organs that are affected by diabetes. When liver enzyme activity increased, it reflects liver damage in serum. Possibly, increased activity of liver enzymes ALT and AST is because of leakage from the cytosol into the blood stream, leakage increased by liver damage that is caused by diabetes [23]. AST and ALT are good indicators for liver function.
Previous studies confirmed that alloxan-induced diabetes caused tissue lipid peroxidation in liver, kidney, and heart [24]. It also caused secretion and release of liver enzymes to circulation [24]. In other words, diabetes and hyperlipidemia cause destruction of cells by altering membrane structure and increase the activity of liver enzymes, and this is what happens in diabetes [13]. Oral administration of alfalfa extract with 250 mg/kg dose decreased liver enzyme concentration in blood, and this decrease is more significant in 500 mg/kg dose, so alfalfa extraction causes reduction of liver damage. It is clear that alfalfa extract causes reduction of liver enzymes that is maybe due to improved diabetes and inhibition of lipid peroxidation (Table III).
Alfalfa stimulates insulin secretion and improves insulin function in reduction of plasma glucose concentration [10, 11]. Previous study reported increase of insulin secretion up to 3-folds in the presence of alfalfa extraction [25]. High concentrations of manganese in alfalfa have been reported as a possible reason of hypoglycemia [26]. Oral administration of alfalfa seeds triggered reduction of blood lipoprotein level in diabetics that this factor is very high. Alfalfa is a phytoestrogens source [27]. Phytoestrogens are nonsteroidal plant-derived compounds with biological activity similar to estrogen [28]. Phytoestrogens increased liver lipid biosynthesis from glucose, so it decreased glucose concentration in blood and improved repair and turnover rate of cell membrane.
According to the results, water and food intake increased in alloxan-induced diabetics (with p < 0.01); this factor reduced in treatment of rats by alfalfa extraction, but this does not reach to the level of intact controls.
The histological studies revealed a significant reduction in the size of Langerhans islets in the diabetic group, so it can be a result of insulin reduction in blood and this group showed significantly increased liver inflammation as compared to intact control group. Therefore, aqueous alfalfa extract, because of all the mentioned features, caused reconstruction of damaged liver and pancreatic cells.
Inflammation that showed in the liver slice of untreated alloxan-induced diabetic rats was very slight in rats cured with alfalfa extract; this showed the effects of alfalfa in liver disease improvement in addition to metabolic disorders.
Conclusions
The results of this study indicate that, in diabetic rats treated with the alfalfa extract, the diameter and number of pancreatic islets were significantly increased as compared to diabetic controls, so production and secretion of insulin increased. Alfalfa causes reduction in cholesterol synthesis by saponins, and it can reform membrane of liver cells, thus, inhibiting leaking of liver enzymes. High dose of manganese in alfalfa and increased insulin resulted to decrease of blood glucose.
According to biochemical and histological results, we can conclude that the mechanisms of alfalfa extract for hypoglycemia are Langerhans islets repair.
Funding Statement
Funding sources: This study was supported in part by a grant from Payam Noor University. EA received research grants and honoraria and consulting fees from Payam Noor University.
Footnotes
Authors’ contribution: EA and MKF were main designers of this work. LS and ZA have done experimental works. LS and TNK wrote the manuscript and VYB is corresponding author of this paper.
Conflict of interest: None.
Contributor Information
Esmaiel Amraie,
Masome Khosravi Farsani,
Leila Sadeghi,
Tayaba Naim Khan,
Vahid Yousefi Babadi,
Zohrab Adavi,
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010 Jan;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Cole JB, Stellpflug SJ, Ellsworth H, Anderson CP, Adams AB, Engebretsen KM, Holger JS. A blinded, randomized, controlled trial of three doses of high-dose insulin in poison-induced cardiogenic shock. Clin Toxicol (Phila) 2013 May;51(4):201–207. doi: 10.3109/15563650.2013.770152. [DOI] [PubMed] [Google Scholar]
- 3.Isah AB, Ibrahim YK, Abdulrahman EM, Ibrahim MA. The hypoglycaemic activity of the aqueous extract of Stachytarpheta angustifolia (Verbanaceae) in normoglycaemic and alloxan-induced diabetic rats. Pak J Biol Sci. 2007 Jan 1;10(1):137–141. doi: 10.3923/pjbs.2007.137.141. [DOI] [PubMed] [Google Scholar]
- 4.Elakovich SD, Hampton JM. Analysis of coumestrol, a phytoestrogen, in alfalfa tablets sold for human consumption. J Agric Food Chem. 1984 Jan-Feb;32(1):173–175. doi: 10.1021/jf00121a041. [DOI] [PubMed] [Google Scholar]
- 5.Zargari A. Therapeutic Plants. 1st. Tehran: Tehran University Press; 1996. pp. 642–646. [Google Scholar]
- 6.Hong YH, Chao WW, Chen ML, Lin BF. Ethyl acetate extracts of alfalfa (Medicago sativa L.) sprouts inhibit lipopolysaccharide-induced inflammation in vitro and in vivo. J Biomed Sci. 2009 Jul 14;16:64. doi: 10.1186/1423-0127-16-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asgary S, Moshtaghian J, Hosseini M, Siadat H. Effects of alfalfa on lipoproteins and fatty streak formation in hypercholesterolemic rabbits. Pak J Pharm Sci. 2008 Oct;21(4):460–464. [PubMed] [Google Scholar]
- 8.Mehranjani MS, Shariatzadeh MA, Desfulian AR, Noori M, Abnosi MH, Moghadam ZH. Effects of Medicago sativa on nephropathy in diabetic rats. Indian J Pharm Sci. 2007;69:768–772. [Google Scholar]
- 9.Lust JB. The Herb Book. London: Bantam Books Inc.; 1986. [Google Scholar]
- 10.Gray AM, Flatt PR. The traditional plant treatment, Sambucus nigra (elder), exhibits insulin-like and insulin-releasing actions in vitro. J Nutr. 1997;78:325–334. doi: 10.1093/jn/130.1.15. [DOI] [PubMed] [Google Scholar]
- 11.Winiarska H, Dworacka M, Borowska M, Bobkiewicz-Kozłowska T, Gorecki P. The effects of plant extracts of Medicago sativa and Trigonella foenum-graceum on postprandial glucose levels in type 2 diabetic rats. Herba Polonica. 2007;53:34–44. [Google Scholar]
- 12.Gokce G, Haznedaroglu MZ. Evaluation of antidiabetic, antioxidant and vasoprotective effects of Posidonia oceanica extract. J Ethnopharmacol. 2008 Jan 4;115(1):122–130. doi: 10.1016/j.jep.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Udayakumar R, Kasthurirengan S, Mariashibu TS, Rajesh M, Anbazhagan VR, Kim SC, Ganapathi A, Choi CW. Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int J Mol Sci. 2009 May 20;10(5):2367–2382. doi: 10.3390/ijms10052367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhandapani S, Subramanian VR, Rajagopal S, Namasivayam N. Hypolipidemic effect of Cuminum cyminum L. on alloxan-induced diabetic rats. Pharmacol Res. 2002 Sep;46(3):251–255. doi: 10.1016/s1043-6618(02)00131-7. [DOI] [PubMed] [Google Scholar]
- 15.Matkovics B, Sasvári M, Kotormán M, Varga IS, Hai DQ, Varga C. Further prove on oxidative stress in alloxan diabetic rat tissues. Acta Physiol Hung. 1997-1998;85(3):183–92. [PubMed] [Google Scholar]
- 16.Takasu N, Asawa T, Komiya I, Nagasawa Y, Yamada T. Alloxan-induced DNA strand breaks in pancreatic islets. Evidence for H2O2 as an intermediate. J Biol Chem. 1991 Feb 5;266(4):2112–2114. [PubMed] [Google Scholar]
- 17.Hyder MZ. Comparative levels of ALT, AST, ALP and GGT in liver associated diseases. Am J Clin Pathol. 1957;28:56–62. [Google Scholar]
- 18.Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005 Dec;54(Suppl 2):S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 19.Devi PU, Sharada AC, Solomon FE. Anti-tumor and radiosensitizing effects of Withania somniferra (Ashwagadha) on a transplantable mouse tumor sarcoma. J Exp Biol. 1993;31:607–611. [PubMed] [Google Scholar]
- 20.Colodny LR. Alfalfa saponins may help reduce cholesterol levels. J Am Nut Ass. 2001;3:1–10. [Google Scholar]
- 21.Jackson IM. Abundance of immunoreactive thyrotropin-releasing hormone-like material in the alfalfa plant. Endocrinology. 1981 Jan;108(1):344–346. doi: 10.1210/endo-108-1-344. [DOI] [PubMed] [Google Scholar]
- 22.Barens DK, Sheaffer C. Alfalfa. Agriculture. 1995;2:205–216. [Google Scholar]
- 23.Concepción Navarro M, Pilar Montilla M, Martín A, Jiménez J, Pilar Utrilla M. Free radical scavenger and antihepatotoxic activity of Rosmarinus tomentosus. Planta Med. 1993 Aug;59(4):312–314. doi: 10.1055/s-2006-959688. [DOI] [PubMed] [Google Scholar]
- 24.Stanely P, Prince M, Menon VP. Hypoglycaemic and other related actions of Tinospora cordifolia roots in alloxan-induced diabetic rats. J Ethnopharmacol. 2000 Apr;70(1):9–15. doi: 10.1016/s0378-8741(99)00136-1. [DOI] [PubMed] [Google Scholar]
- 25.McClenaghan NH, Barnett CR, Ah-Sing E, Abdel-Wahab YH, O'Harte FP, Yoon TW, Swanston-Flatt SK, Flatt PR. Characterization of a novel glucose-responsive insulin-secreting cell line, BRIN-BD11, produced by electrofusion. Diabetes. 1996 Aug;45(8):1132–1140. doi: 10.2337/diab.45.8.1132. [DOI] [PubMed] [Google Scholar]
- 26.Rubenstein AH, Levin NW, Elliott GA. Manganese-induced hypoglycaemia. Lancet. 1962 Dec 29;2(7270):1348–1351. doi: 10.1016/s0140-6736(62)91022-x. [DOI] [PubMed] [Google Scholar]
- 27.Kurzer MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353–381. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- 28.Axelson M, Sjövall J, Gustafsson BE, Setchell KD. Soya – a dietary source of the non-steroidal oestrogen equol in man and animals. J Endocrinol. 1984 Jul;102(1):49–56. doi: 10.1677/joe.0.1020049. [DOI] [PubMed] [Google Scholar]