Abstract
Objective
The aim of the current study was to examine the relationship between clinical markers of inflammation and 8-oxo-7,8-dihydro-2′deoxyguanosine (8-oxodG), an oxidative stress marker, in middle-aged women drawn from the HANDLS study, a longitudinal epidemiologic study.
Methods and Results
We examined commonly assayed markers of inflammation, the DNA base adduct 8-oxodG, a marker of oxidative stress and cardiovascular risk factors in a cohort of women matched on age and race in three groups (n=39 per group) who had low (<3 mg/L) hsCRP, mid (>3–20 mg/L), and high (>20 mg/L) hsCRP. We found a significant relationship between hsCRP level and the oxidative stress marker, 8-oxodG. 8-oxodG was positively correlated with systolic blood pressure, pulse pressure and IL-23. hsCRP was associated with obesity variables, HDL, serum insulin levels, IL-12p70 and ICAM-1. Incubation of primary human endothelial cells with hsCRP generated reactive oxygen species in vitro. Furthermore, hsCRP specifically induced DNA base lesions, but not other forms of DNA damage including single and double strand breaks.
Conclusions
These data suggest that in women 8-oxodG is associated with hsCRP and is independently related to select cardiovascular risk factors. Our data in women suggest that hsCRP may contribute to cardiovascular disease by increasing oxidative stress.
Keywords: 8-oxodG, DNA damage, oxidative stress, C-reactive protein, inflammation, women’s health, cardiovascular disease
Introduction
Accumulating evidence indicates that the levels of high sensitivity C-reactive protein (hsCRP) are important for the assessment of cardiovascular disease (CVD) risk and for the diagnosis and treatment of CVD. Recent data suggests that hsCRP is not only a marker of inflammation but may also contribute to the pathogenesis of vascular disease 1–3. However, whether hsCRP plays a direct role in promoting atherosclerotic processes is still controversial and little is known about how hsCRP may elicit these effects 4. Nonetheless, important information has been obtained from correlating findings discovered from human populations to in vitro studies. For example, the pro-inflammatory cytokine IL-6 is known to induce hsCRP expression and in women hsCRP levels correlate directly with IL-6 5–7. In addition, hsCRP has been shown to affect the expression of various proteins that are important for atherosclerosis and thrombosis and is present in atherosclerotic lesions 1–3, 8.
Compelling data supports the idea that atherosclerosis is an inflammatory process. Furthermore, the coronary plaque environment is composed of multiple cell types including macrophages and other inflammatory cells that secrete cytokines and reaction oxygen species (ROS) that drive inflammation and oxidative stress 9. ROS generation is an important signaling mechanism in cells 10. However, it can be detrimental to cellular homeostasis by leading to DNA damage, which if left unrepaired, can lead to mutations that cause disease. Persuasive evidence suggests that oxidative stress induced DNA damage and genetic alterations may contribute to atherosclerosis 11, 12. Therefore, it is likely that both oxidative stress and inflammatory markers may suggest disease burden or risk. Although extensive research has focused on this link, it remains to be determined which markers can be used clinically to indicate both inflammatory and oxidative stress states 13.
Levels of the DNA damage base lesion, 8-oxo-7,8-dihydro-2′deoxyguanosine (8-oxodG), can be associated with increased oxidative stress as high levels of serum 8-oxodG can be detected in patients with systemic lupus erythematosus, Parkinson’s disease, end stage renal disease, and Type 2 diabetes, 14–17 and in urine and from specific tissues of various diseases 18. 8-oxodG has also been found to be increased in atherosclerosis plaques 11, 19, 20 and in lymphocytes from men with premature coronary heart disease mortality 21. Although hsCRP and serum 8-oxodG have been found to correlate in patients with end stage renal disease 17, little is known about the relationship between these two markers, particularly in community-dwelling participants.
Here we have focused on the association between inflammation and oxidative stress by examining commonly assayed inflammatory markers and the DNA base adduct 8-oxodG, as a marker of oxidative stress in a cohort of middle-aged women with low, mid-range and high hsCRP values who are also participants in the HANDLS study, an interdisciplinary epidemiologic study of health disparities in the city of Baltimore. Although traditionally in clinical settings hsCRP levels greater than 10 mg/L have been associated with acute illness, infections or autoimmune disorders not CVD risk, data from the Women’s Health study showed that hsCRP values remain predictive for cardiovascular events for the entire range of values 22, 23. In fact, women in the Women’s Health Study with hsCRP values >10 mg/L were considered to be a “very high risk” group and those with hsCRP values ≥20 mg/L were observed to be at exceptionally elevated risk for vascular disease. Moreover, women have higher levels of hsCRP than men 24, 25 and are particularly vulnerable to CVD. CVD has been cited as the challenge of the middle years for women since CVD is the primary cause of death among women in the United States and currently more woman in the United States die from CVD than men 26. We found that serum hsCRP levels correlate significantly with 8-oxodG. We have further investigated the connection between hsCRP and 8-oxodG and found that hsCRP induces ROS and DNA base lesions in vitro. Our findings suggest that hsCRP production may enhance inflammatory processes, such as atherosclerosis and thrombosis, in part by increasing oxidative stress and inducing DNA damage.
Materials and Methods
Participants
We conducted a nested-cohort study comparing inflammatory markers with the oxidative stress marker 8-oxodG on women drawn from the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study of the National Institute on Aging Intramural Research Program (NIA IRP) 27, approved by the MedStar Health Research Institute Institutional Review Board. HANDLS is an area probability sample of Baltimore City based on the 2000 Census data and this study examines the effects of race (whites and African Americans) and socioeconomic status (below or above 125% of the Federal poverty level) on health outcomes in a cohort of urban adults aged initially between 30–64. Women selected all gave written consent to store serum, had available serum for examination, and had completed HANDLS baseline assessment. We matched three groups of women (39 per group) who had low (<3 mg/L) hsCRP, mid (>3–20 mg/L), and high (>20 mg/L) hsCRP on age and race. 86 women in the total HANDLS study cohort had hsCRP values greater than 20.
Physical measurements and laboratory assays
Blood pressure was averaged for assessments in both arms while seated after a five minute rest. Body mass index (weight [kg]/height [m]2) was computed from measured height and weight. Clinical conditions were recorded based on a structured medical history interview and a physical examination. We obtained fasting blood samples the serum from which was assayed by Quest Diagnostics (Nichols Institute, Chantilly, Virginia) or stored at −80°C. Fasting glucose, insulin, cholesterol, triglycerides, LDL, HDL, creatinine, LDH and hsCRP were measured at Quest Diagnostics. Various cytokines and inflammatory markers were measured in serum using Searchlight® protein arrays from Aushon Biosystems (Billerica, MA).
Cell lines and reagents
Human umbilical vein endothelial cells (HUVEC; Clonetics®) were purchased from Lonza and were maintained in EBM®-2 media supplemented with EGM-2 SingleQuots® (Lonza). Highly purified human recombinant C-reactive protein without sodium azide and free of endotoxins was specially obtained from Trichem Resources Inc.
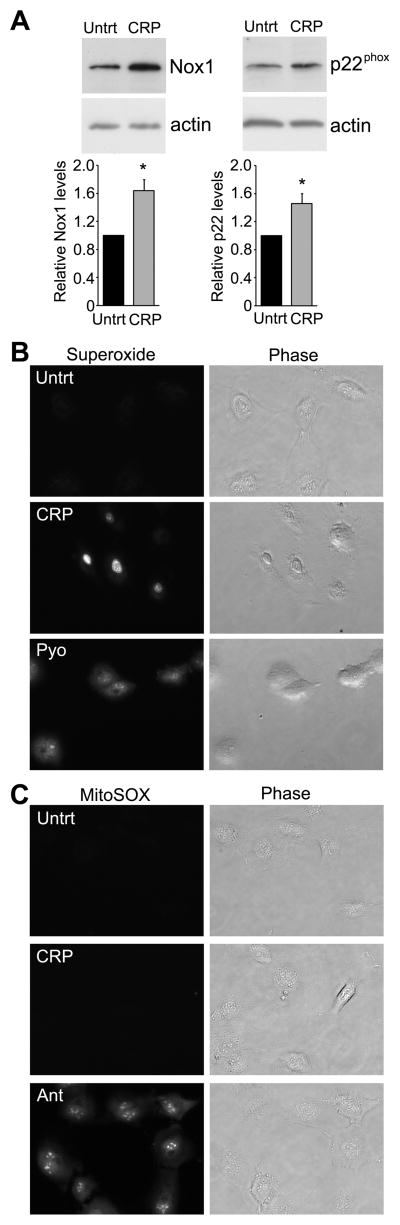
For Figure 1C, HUVECs were incubated in a 1:10 dilution of growth media for 18 hrs in the presence or absence of hsCRP (25 μg/ml). Cells were lysed in sample buffer, separated by SDS-PAGE and immunoblotted with anti-p22phox (CS9; Santa Cruz), anti-Nox1 (Sigma-Aldrich) or anti-actin antibodies (I-19; Santa Cruz) as a protein loading control.
Figure 1.
hsCRP induces superoxide in HUVECs. (A) HUVECs were untreated or treated for 18 hrs with hsCRP (25 μg/ml), lysed and probed with anti-p22phox, anti-Nox1 or anti-actin antibodies as a loading control. Relative levels of p22 phox or Nox1 were quantified from immunoblots and normalized to actin control. The histograms represent the normalized mean from 4 independent experiments + SEM. *P<0.05 by Student’s t-test.(B, C) HUVECs were incubated with either the superoxide detector dihydroethidium (B) or MitoSOX (C) to detect mitochondrial superoxide in serum-free media containing either 25 μg/ml hsCRP, 40 μM pyanocyanin (to induce superoxide) or 10 μM Antimycin A (to induce mitochondrial superoxide). Representative fluorescent or phase images are shown and similar results were obtained in three independent experiments.
8-oxodG ELISA
For 8-oxodG detection, we used the highly sensitive 8-OHdG (8-oxodG) ELISA Check kit from the Japan Institute for the Control of Aging (purchased from Genox Inc., Baltimore, MD). The specificity of the monoclonal antibody used in the ELISA assay has been previously reported 28. As suggested by the manufacturer, serum samples (0.3 ml) were centrifuged at 15,000 × g for 60 min at 4°C through a Vivaspin ultrafiltration spin column (10,000 MWCO; Sartorius-Stedim Biotech). The serum filtrate (50 μl) was used directly for the ELISA assay or frozen at −80°C. The ELISA assay was performed blind according to manufacturer’s instructions and was repeated in two independent experiments. There was a significant degree of repeatability in the two 8-oxodG assays (intraclass correlation = .45, p<.001) and the average 8-oxodG value from the two experiments was used for further analysis.
Immunofluorescence
To monitor superoxide production, HUVECs were plated on 4-well chamber slides and 18 hrs later cells were incubated in serum-free media with either the superoxide indicator dihydroethidium (3 μM; Molecular Probes) or MitoSOX (3 μM; Molecular Probes) to detect mitochondrial superoxide. In addition, either 25 μg/ml hsCRP, 40 μM pyanocyanin (to induce superoxide; Enzo Life Sciences) or 10 μM Antimycin A (to induce mitochondrial superoxide; Sigma-Aldrich) was added to the wells and incubated for 30 min. Fluorescent images of superoxide or mitochondrial superoxide and phase images were taken on a Zeiss Observer D1 microscope with an AxioCam1Cc1 camera at a set exposure time.
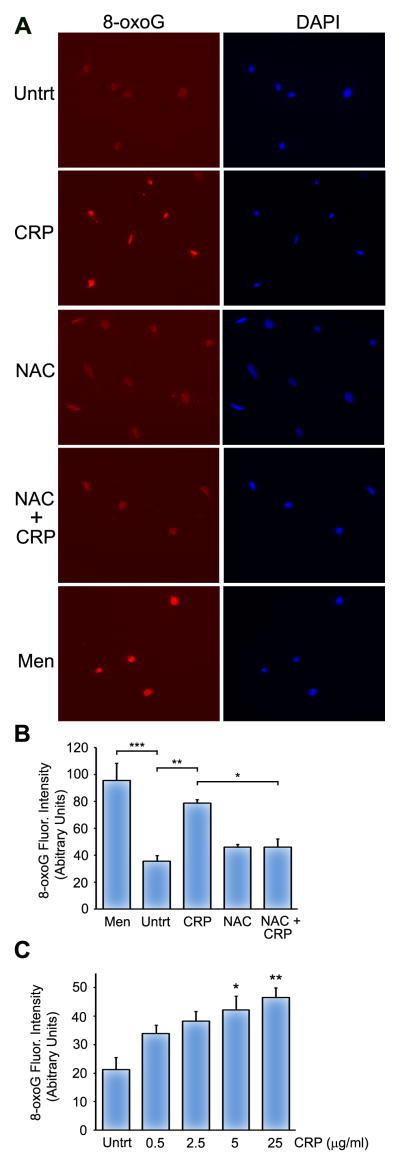
HUVECs were treated for 30 min with 10 μM menadione, 25 μg/ml hsCRP (or the indicated hsCRP concentrations), 5 mM N-Acetyl-L-cysteine (NAC; Enzo Life Sciences) or hsCRP and NAC in serum-free media. NAC was added 30 min prior to the addition of CRP (for the NAC + CRP sample). Staining for 8-oxoG and 4′,6-diamidino-2-phenylindole (DAPI) was performed as previously described 29. Pictures were taken using a Zeiss Observer D1 microscope with an AxioCam1Cc1 camera at a set exposure time and the fluorescence intensity of 8-oxoG stained nuclei were quantified from duplicate coverslips using AxioVision Rel 4.7 software. The histograms in Figure 2B and 2C represent the mean fluorescence intensity from three independent experiments.
Figure 2.
hsCRP induces 8-oxoG in HUVECs. (A) HUVECs were mock treated (Untrt) or treated for 30 min with 10 μM menadione (Men), 25 μg/ml hsCRP, 5 mM NAC or hsCRP and NAC in serum-free media. NAC was added 30 min prior to the addition of CRP. HUVECs were stained with anti-8-oxoG antibodies (left panels) and DAPI (right panels). (B) 8-oxoG fluorescent intensity was calculated as described in the Materials and Methods. (C) 8-oxoG fluorescent intensity was calculated from HUVECs that were mock treated (Untrt) or treated with the indicated concentrations of hsCRP for 30 min and subsequently stained with anti-8-oxoG antibodies. The histograms represent the mean of three independent experiments + SEM. *P<0.05, **P<0.01, ***P<0.001 for the indicated comparisons using one-way ANOVA and Tukey’s post-hoc test.
Statistical analyses
Thirty-seven women per group had statistical power of 80% to detect a difference at least as large as 0.33 SD for matched comparisons between groups with p<0.05 and power (1-β) = 0.8. We used mixed-model regressions to examine group differences (Table 1) and the effects on oxidative stress after adjusting for covariates. Categorical measures were examined with logistic regression. We included interactions in regression analyses only after they were significant following a backward elimination procedure for nonsignificant effects. We applied a log10 transform to hsCRP because the distribution was skewed. We used R 30 to perform all analyses.
Table 1.
Means and standard deviations (SD) with separate comparisons of middle and high hsCRP groups with the low hsCRP group on age, anthropometrics, blood pressure, pulse pressure, clinical laboratory assays, and inflammatory markers.
| hsCRP groups (n = 39 per group)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Low (≤3 mg/L)
|
Mid (>3–20 mg/L)
|
High (>20 mg/L)
|
||||||
| Mean | SD | Mean | SD | p | Mean | SD | p | |
|
| ||||||||
| Age (y) | 49.77 | 8.81 | 49.77 | 8.81 | 1.00 | 49.77 | 8.81 | 1.00 |
| hsCRP1 | −0.46 | 1.02 | 1.87 | 0.51 | 0.00** | 3.40 | 0.43 | 0.00** |
| 8-oxodG | 0.16 | 0.04 | 0.17 | 0.04 | 0.13 | 0.18 | 0.04 | 0.00** |
| BMI | 26.80 | 5.13 | 34.74 | 9.00 | 0.00** | 39.63 | 11.94 | 0.00** |
| Weight2 | 71.72 | 14.64 | 93.23 | 23.74 | 0.00** | 103.03 | 32.95 | 0.00** |
| Waist size3 | 92.39 | 13.48 | 106.88 | 17.43 | 0.00** | 114.97 | 23.44 | 0.00** |
| Hip size3 | 102.68 | 13.29 | 115.79 | 17.64 | 0.00** | 124.35 | 20.96 | 0.00** |
| Waist hip ratio | 0.90 | 0.08 | 0.92 | 0.07 | 0.21 | 0.92 | 0.08 | 0.44 |
| Diastolic BP4 | 71.49 | 11.68 | 75.08 | 11.28 | 0.14 | 69.89 | 8.81 | 0.95 |
| Systolic BP4 | 118.59 | 18.24 | 126.42 | 18.49 | 0.05 | 125.49 | 20.40 | 0.08 |
| Pulse pressure | 47.11 | 12.95 | 51.34 | 14.35 | 0.13 | 55.60 | 15.81 | 0.02* |
| Total cholesterol5 | 187.18 | 34.86 | 196.05 | 43.38 | 0.33 | 182.21 | 45.90 | 0.64 |
| LDL5 | 104.07 | 34.74 | 115.51 | 37.43 | 0.17 | 108.89 | 43.83 | 0.53 |
| HDL5 | 60.26 | 16.09 | 52.54 | 13.97 | 0.02* | 47.74 | 13.32 | 0.00** |
| Triglycerides5 | 114.21 | 81.17 | 139.95 | 74.63 | 0.08 | 127.82 | 61.26 | 0.34 |
| Creatinine5 | 0.96 | 0.87 | 0.87 | 0.19 | 0.39 | 0.84 | 0.22 | 0.33 |
| Glucose5 | 94.18 | 10.14 | 100.87 | 17.19 | 0.40 | 150.59 | 80.97 | 0.00** |
| Insulin6 | 10.33 | 6.87 | 12.31 | 8.75 | 0.35 | 17.23 | 12.69 | 0.01* |
| LDH7 | 160.40 | 32.25 | 178.15 | 36.33 | 0.05 | 163.08 | 34.79 | 0.68 |
| eGFR | 86.3 | 18.53 | 85.9 | 21.54 | 0.97 | 90.9 | 25.37 | 0.26 |
| IL-18 | 5.82 | 19.47 | 1.22 | 2.46 | 0.26 | 2.90 | 6.77 | 0.34 |
| IL-68 | 8.47 | 11.89 | 5.94 | 4.29 | 0.27 | 15.78 | 15.18 | 0.03* |
| IL-88 | 17.06 | 11.41 | 8.81 | 7.87 | 0.00** | 18.38 | 12.27 | 0.75 |
| IL-108 | 3.02 | 4.38 | 0.74 | 0.37 | 0.01** | 2.19 | 1.12 | 0.52 |
| IL-12p708 | 1.54 | 3.12 | 0.62 | 0.75 | 0.04* | 0.74 | 0.98 | 0.07 |
| IL-188 | 146.13 | 63.40 | 221.25 | 139.07 | 0.00** | 171.45 | 115.41 | 0.32 |
| IL-238 | 1636.76 | 7936.71 | 594.73 | 1077.08 | 0.39 | 776.51 | 2925.65 | 0.56 |
| RAGE8 | 1014.30 | 502.16 | 374.73 | 468.00 | 0.00** | 1100.76 | 575.73 | 0.57 |
| ICAM-18 | 371586.96 | 133792.62 | 532839.13 | 183161.02 | 0.00** | 479242.83 | 189260.41 | 0.01 |
| VCAM-18 | 1680988.53 | 1325971.49 | 1188016.04 | 665613.58 | 0.07 | 1290350.88 | 459042.68 | 0.11 |
| MCP-18 | 919.65 | 461.14 | 700.59 | 558.92 | 0.11 | 979.58 | 737.66 | 0.67 |
| TNFα8 | 39.29 | 122.24 | 5.98 | 15.34 | 0.20 | 22.55 | 55.16 | 0.49 |
log10(hsCRP) in mg/L
kg
cm
mm Hg
mg/dL
μIU/mL
U/L
pg/ml
p<.05;
p<.01
Results
Relationship between hsCRP and cardiovascular risk factors
To examine the relationship between hsCRP levels in woman and other variables, we studied a cohort of women with low (<3 mg/L), mid (>3–20 mg/L), or high hsCRP levels (>20 mg/L). Each group contained 39 women and the groups were age (mean age 49.7 ± 8.1 yr) and race (19 W, 20 AA) matched. There were no differences between low, mid, and high hsCRP groups on the two matching variables. Women in the mid and high hsCRP groups were significantly heavier than women in the low hsCRP group, had larger waist and hip sizes, and had greater BMI (Table 1). Women in the mid hsCRP group had significantly higher systolic blood pressure than women in the low hsCRP, but women in the high hsCRP group had greater pulse pressure suggesting that in this group increased hsCRP is associated with less compliant arteries at greater levels of inflammation. Women in the mid and high hsCRP group also had lower HDL levels and higher levels of fasting glucose and insulin.
Given the fact that hsCRP is an acute phase marker of inflammation and that atherosclerosis and atherothrombotic events are inflammatory processes, we examined the differences in the inflammatory profile of women based on hsCRP level. Surprisingly, there were few consistent significant differences in most of the inflammatory markers by hsCRP group (Table 1). Consistent with other studies, we found that IL-6 and ICAM-1 levels were higher in women with high hsCRP 5, 31. Compared with women in the low hsCRP group, women in the mid group has greater levels of IL-18 and ICAM-1, but lower levels of IL-8, IL-10, IL-12p70, and RAGE.
In addition to the inflammatory markers, we also examined whether hsCRP was associated with the oxidative stress marker, 8-oxodG. Women in the mid hsCRP group had higher levels of 8-oxodG than the low hsCRP group and women in the high hsCRP group had significantly greater 8-oxodG levels than the low hsCRP group (Table 1).
Association of hsCRP with inflammation-related conditions and diagnoses
Although participants in the HANDLS study are community dwelling, several of the individuals had preexisting chronic medical illnesses. Since some of these conditions are associated with inflammation and oxidative stress, we examined whether hsCRP levels were affected by the presence of these conditions. Inflammatory conditions and diagnoses were more prevalent in both the mid and high hsCRP groups than the low hsCRP group (Table 2). Those in the high hsCRP group had greater rates of self-reported asthma (OR = 3.63, 95% CI = 1.17–12.87), diabetes mellitus (OR = 10.29, 95% CI = 3.30–39.80), and hypertension (OR = 3.49, 95% CI = 1.36–9.40), but not rheumatoid arthritis, osteoarthritis, psoriasis, stroke, rates of cigarette smoking or statin use, coronary heart disease, metabolic syndrome or positive serology for hepatitis B and hepatitis C. Those in the mid hsCRP group had greater rates of metabolic syndrome (OR = 2.78, 95% CI = 1.09–7.40) and hypertension (OR = 2.63, 95% CI = 1.05–6.78), but not rheumatoid arthritis, osteoarthritis, psoriasis, stroke, rates of cigarette smoking or statin use, coronary heart disease, diabetes, or positive serology for hepatitis B and hepatitis C.
Table 2.
Rates of inflammatory-related conditions and diagnoses with separate comparisons of middle and high hsCRP groups with the low hsCRP group.
| hsCRP groups (n=39 per group)
|
|||||||
|---|---|---|---|---|---|---|---|
| Low (≤3 mg/L)
|
Mid (>3–20 mg/L)
|
High (>20 mg/L)
|
|||||
| Rate | Rate | OR | p | Rate | OR | p | |
|
|
|
|
|||||
| Asthma (hx) | 0.18 | 0.229 | 1.36 | 0.62 | 0.44 | 3.63 | 0.03* |
| Rheumatoid arthritis (hx) | 0.13 | 0.054 | 0.37 | 0.27 | 0.03 | 0.22 | 0.18 |
| Osteoarthritis (hx) | 0.21 | 0.216 | 1.06 | 0.92 | 0.42 | 2.77 | 0.08 |
| Psoriasis (hx) | 0.04 | 0.029 | 0.76 | 0.85 | 0.08 | 2.17 | 0.53 |
| Stroke (hx) | 0.03 | 0.056 | 1.94 | 0.59 | 0.03 | 0.97 | 0.98 |
| Current cigarette smoker | 0.47 | 0.47 | 1.00 | 1.00 | 0.33 | 0.56 | 0.23 |
| Statin use | 0.19 | 0.16 | 0.78 | 0.68 | 0.33 | 2.07 | 0.19 |
| Coronary heart disease (hx) | 0.19 | 0.211 | 1.10 | 0.86 | 0.39 | 2.64 | 0.07 |
| Metabolic syndrome1 | 0.36 | 0.611 | 2.78 | 0.03* | 0.70 | 2.12 | 0.12 |
| Diabetes2 | 0.10 | 0.256 | 3.02 | 0.08 | 0.54 | 10.29 | 0.00** |
| Hypertension3 | 0.39 | 0.632 | 2.63 | 0.04* | 0.69 | 3.48 | 0.01* |
| Hepatitis B (assay) | 0.05 | 0.000 | 0.00 | 0.99 | 0.03 | 0.51 | 0.59 |
| Hepatitis C (assay) | 0.21 | 0.132 | 0.57 | 0.36 | 0.08 | 0.34 | 0.13 |
Note: hx = by structured medical history; OR = Odds ratio
ATP-III48
Fasting glucose ≥ 140 mg/dL, medication for diabetes, or self-report
Measured blood pressure ≥ 140/90, anti-hypertensive medication, or self-report
p<.05;
p<.01
Association of 8-oxodG with age, hsCRP, anthropometrics, and inflammation-related markers
8-oxodG was associated with age, inflammatory markers, anthropometric measures in the complete sample, but the pattern of associations differed between the high, mid, and low hsCRP groups. In the complete sample, 8-oxodG was associated with age (r=.33, p<.001), hsCRP (r=.21, p=.02), BMI (r=.20, p=.03), hip size (r=.19, p=.04), systolic blood pressure (r=.32, p<.001), pulse pressure (r=.33, p<.001), and IL-23 (r=.24, p=.02). In the low hsCRP group, 8-oxodG was associated with age (r = 0.32, p=.04), diastolic blood pressure (r=.34, p=.04), systolic blood pressure (r=.53, p<.001), pulse pressure (r=.45, p=.01), creatinine (r = 0.39, p=.01), LDH (r = 0.38, p=.04), and IL-23 (r = 0.40, p=.01. In the mid-hsCRP group only age (r=.36, p=.02) was associated significantly with 8-oxodG and in the high hsCRP group only age (r=.36, p=.03) and LDH (r=.08, p=.04) were associated significantly with 8-oxodG.
8-oxodG levels are associated with cardiovascular risk factors
We examined the association of 8-oxodG, hsCRP, race, and poverty status with anthropometric measures, blood pressure, clinical laboratory assays, and inflammatory markers (Table 3). None of the four-way, three-way, or two-way interactions were significant and they were removed by backward elimination. Adjusting for hsCRP, race, and poverty status, 8-oxodG was associated with systolic blood pressure and pulse pressure, but none of the anthropometric measures, clinical laboratory assays, or inflammatory markers. Adjusting for 8-oxodG, race, and poverty status, hsCRP was associated with BMI, weight, waist and hip size, waist-hip ratio, HDL, fasting glucose, insulin, IL-12p70, and ICAM-1. Adjusting for 8-oxodG, hsCRP, and poverty status, race was associated with systolic blood pressure, pulse pressure, LDL, triglycerides, RAGE, ICAM-1, VCAM-1, and MCP-1. Adjusting for 8-oxodG, hsCRP, and race, poverty status was associated with weight, hip size, total cholesterol, LDL, HDL, and ICAM-1.
Table 3.
Regression of anthropometrics, blood pressure, pulse pressure, clinical laboratory assays, and inflammatory markers on 8-oxodG, hsCRP, race, and poverty status with separate comparisons of middle and high hsCRP groups with the low hsCRP group adjusting for matching.
| 8-oxodG
|
hsCRP1
|
Race
|
Poverty status
|
|||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | p | Coefficient | pp | Coefficient | p | Coefficient | p | |
|
|
||||||||
| BMI | 26.94 | 0.22 | 2.99 | 0.00** | −0.79 | 0.63 | −2.83 | 0.08 |
| Weight2 | 58.41 | 0.32 | 7.63 | 0.00** | −3.92 | 0.36 | −9.82 | 0.02* |
| Waist size3 | 39.96 | 0.36 | 5.71 | 0.00** | −5.27 | 0.10 | −5.64 | 0.09 |
| Hip size3 | 56.02 | 0.18 | 4.91 | 0.00** | −5.66 | 0.07 | −8.09 | 0.01* |
| Waist hip ratio | −0.11 | 0.55 | 0.01 | 0.03* | 0.00 | 0.96 | 0.02 | 0.27 |
| Diastolic BP4 | 34.34 | 0.19 | −0.37 | 0.60 | 0.43 | 0.81 | −3.24 | 0.10 |
| Systolic BP4 | 166.18 | 0.00** | 0.70 | 0.49 | 9.04 | 0.01* | −1.25 | 0.72 |
| Pulse pressure | 127.51 | 0.00** | 0.68 | 0.36 | 8.47 | 0.00** | −0.20 | 0.94 |
| Total cholesterol5 | 79.03 | 0.42 | −0.51 | 0.82 | 11.73 | 0.09 | −25.07 | 0.00** |
| LDL5 | 38.98 | 0.67 | 1.25 | 0.56 | 16.77 | 0.01* | −19.65 | 0.00** |
| HDL5 | 17.20 | 0.63 | −3.38 | 0.00** | 2.48 | 0.35 | −5.62 | 0.04* |
| Triglycerides5 | −94.78 | 0.60 | 5.65 | 0.14 | −34.20 | 0.01* | 2.31 | 0.86 |
| Creatinine5 | −0.53 | 0.32 | 0.00 | 0.86 | 0.06 | 0.10 | 0.02 | 0.69 |
| Glucose5 | 30.62 | 0.75 | 4.74 | 0.07 | −0.85 | 0.91 | 6.71 | 0.36 |
| Insulin6 | −5.87 | 0.81 | 1.58 | 0.00** | −0.68 | 0.70 | 1.44 | 0.42 |
| LDH7 | −6.58 | 0.95 | 3.40 | 0.14 | 2.37 | 0.77 | −8.45 | 0.29 |
| eGFR | −11.80 | 0.83 | 0.92 | 0.43 | 6.08 | 0.14 | 0.02 | 0.99 |
| IL-18 | 3.40 | 0.92 | −1.21 | 0.10 | 2.74 | 0.32 | 2.39 | 0.40 |
| IL-68 | 2.85 | 0.92 | 0.85 | 0.22 | 0.55 | 0.78 | 1.14 | 0.59 |
| IL-88 | 14.27 | 0.61 | −1.18 | 0.06 | −2.51 | 0.19 | 3.48 | 0.08 |
| IL-108 | −0.13 | 0.98 | 0.03 | 0.82 | −0.28 | 0.49 | −0.12 | 0.79 |
| IL-12p708 | 3.53 | 0.48 | −0.23 | 0.03* | 0.02 | 0.96 | −0.13 | 0.74 |
| IL-188 | 251.75 | 0.38 | 8.88 | 0.15 | −33.70 | 0.11 | 33.86 | 0.11 |
| IL-238 | 3522.61 | 0.40 | 229.53 | 0.29 | 44.22 | 0.89 | 79.39 | 0.83 |
| RAGE8 | 139.37 | 0.92 | −48.41 | 0.10 | −209.51 | 0.03* | 2.51 | 0.98 |
| ICAM-18 | 280653.51 | 0.52 | 31631.42 | 0.00** | −71777.77 | 0.03* | 63749.37 | 0.05 |
| VCAM-18 | 1152114.26 | 0.48 | −9018.53 | 0.86 | −339555.40 | 0.00** | 147345.65 | 0.26 |
| MCP-18 | 1488.47 | 0.31 | 9.58 | 0.77 | −373.50 | 0.00** | 67.42 | 0.54 |
| TNFα8 | 183.58 | 0.39 | −6.85 | 0.19 | 10.69 | 0.52 | 30.05 | 0.08 |
log10(hsCRP) in mg/L
kg
cm
mm Hg
mg/dL
μIU/mL
U/L
pg/ml
p<.05;
p<.01
CRP increased ROS production and DNA base lesions
The strong relationship between 8-oxodG levels and hsCRP and the fact that recent data suggests that CRP may contribute to the inflammatory disease process, led us to investigate whether hsCRP itself can increase oxidative stress and potentially induce DNA damage. We found that hsCRP induces ROS production in human umbilical vein endothelial cells (HUVECs; Suppl. Figure SI), consistent with other reports 8, 32. In vascular cells, ROS is mainly produced by the NADPH oxidases 33–35. Therefore, we examined whether longer exposure (18 hrs) to hsCRP changes expression of the different NADPH oxidase (Nox) isoforms that are known to be important for vascular generation of ROS. We found that CRP increases the protein level of Nox1, but did not affect expression of Nox2, Nox4 and Nox5 (Figure 1 and Suppl. Figure SII). Similar to previous findings, we found that hsCRP upregulated p22phox protein expression, a required subunit of NADPH oxidases 1. We also examined various antioxidant enzymes and found a modest upregulation of the cytoplasmic antioxidant enzyme, glutathione peroxidase (Suppl. Figure SII). However, hsCRP did not change the protein level of catalase, SOD1, or SOD2 (Suppl. Figure SII). Since NADPH oxidases generate superoxide and we observed an upregulation of Nox1 and p22phox, we examined whether we could detect superoxide in response to hsCRP. The well-known superoxide producer pyanocyanin and hsCRP both induced intracellular superoxide (Figure 1B). In contrast to Antimycin A, which is known to generate mitochondrial superoxide, we did not detect mitochondrial superoxide induction by hsCRP (Figure 1C). Taken together, these data suggest that CRP primarily induces intracellular ROS rather than mitochondrial ROS.
In order to examine whether hsCRP induces DNA damage, we immunostained HUVECs with antibodies against the DNA base lesion, 8-oxo-7,8-dihydro-guanine (8-oxoG), an analog of 8-oxodG that can be easily detected in tissue culture cells by immunostaining 29, 36, 37. Untreated HUVECs have a background level of 8-oxoG, which is consistent with previous reports 29, 36, 37. However, treatment with clinically relevant doses of hsCRP increased the level of 8-oxoG in a dose-dependent manner (Figure 2A–C). Furthermore, measurement of the fluorescent intensity of staining revealed that hsCRP increases 8-oxoG levels similar to the DNA damaging agent menadione (Figure 2B). To determine whether hsCRP induction of ROS contributes to 8-oxoG formation, we treated HUVECs with hsCRP in the presence of the antioxidant, N-Acetyl-L-cysteine (NAC), to inhibit ROS (Figure 2). The level of 8-oxoG was similar to untreated cells when cells were incubated with NAC alone or in combination with hsCRP, indicating that ROS is important for CRP-induced base damage.
We employed the alkaline comet assay to assess whether, in addition to 8-oxoG, hsCRP can induce other forms of DNA damage. Under these conditions the comet assay measures alkaline sensitive sites that include single strand breaks SSBs, alkaline labile sites and transient repair sites. Increased alkaline sensitive sites were observed in cells treated with H2O2, but not in HUVECs treated with hsCRP for 30 or 60 min (Suppl. Figure SIIIA). In addition, we examined whether hsCRP influences the level of double strand breaks by staining for phosphorylated H2AX (referred to as γ-H2AX), a marker of DSBs. As expected, staurosporine treatment increased γ-H2AX foci formation (Suppl. Figure SIIIB). However, hsCRP stimulation did not affect the levels γ-H2AX, suggesting that hsCRP does not substantially induce DSBs (Suppl. Figure SIIIB).
We also examined whether hsCRP can affect lipid peroxidation, another marker of oxidative stress. As measurement of malondialdehyde (MDA) has been used as a common indicator of lipid peroxidation, we analyzed MDA levels in HUVECs treated with hsCRP. hsCRP significantly increased the levels of MDA compared to untreated cells (Suppl. Figure SIV), suggesting that hsCRP can also induce products of lipid peroxidation.
Discussion
In order to gain a better understanding about the role of inflammation and oxidative stress in women at high risk for CVD, we examined a cohort of women in the HANDLS study based on three categories of hsCRP levels, low (<3 mg/L), mid (>3–20 mg/L), and high (>20 mg/L), as this represents women at low, high, and extremely elevated risk for CVD. Each of these groups contained 39 women and were age and race-matched. In this study, we found that several clinical risk factors for CVD were associated with high circulating levels of hsCRP including, obesity-related parameters, HDL, glucose, insulin, IL-12p70, and ICAM-1. As could be expected, we observed that metabolic syndrome and hypertension were higher in women with mid-range hsCRP values. Diabetes, asthma, and hypertension were all associated with high levels of hsCRP. However, hsCRP levels were also associated with the oxidative stress marker, 8-oxodG. These results lend credence to the idea that there may be close relationships between inflammation and certain forms of oxidative stress. However, a limited number of studies have investigated the relationship between hsCRP and serum 8-oxodG, especially in cohorts of community dwelling individuals not stratified by disease.
8-oxodG is an important oxidative stress marker because it is one of the most abundant oxidative base lesions and is also highly mutagenic 38. The contribution of DNA mutagenesis to cardiovascular disease is still unclear. However, emerging data indicate that DNA damage and other genetic alterations may play a pivotal role in the etiology and/or pathogenesis of atherosclerosis 11, 12. Specifically, increased levels of various DNA adducts, including 8-oxodG, have been found in atherosclerotic lesions 19, 20, 39, 40. In addition, 8-oxodG accumulates in hypertensive aged rats 41 and may be related to serum levels of antibodies against oxidized LDL, which is important because oxidation of LDL and the generation of antibodies against oxidized LDL are implicated in the progression of atherosclerosis 42. In this report, additional evidence suggests that in humans 8-oxodG levels can be reduced upon treatment with fluvastatin, indicating that cholesterol lowering therapies may reduce oxidative stress burden and DNA damage levels. Similarly, decreased levels of urinary 8-oxodG and hsCRP were also observed after treatment with rosuvastatin, as well as calcium blocking agents in hypertensive patients 43–45.
These data and the fact that circulating lymphocytes from patients with atherosclerosis have higher levels of 8-oxodG than control subjects and 8-oxodG accumulation in lymphocyte DNA is associated with higher rates of death from premature coronary artery disease 11, 21, 46, led us to examine the level of 8-oxodG in the serum rather than urinary 8-oxodG. Although a large body of work has identified urinary 8-oxodG as an informative oxidative stress marker 18, 47, detection of this biospecimen relies on careful complete collection and storage of 24-hour urine, which may be difficult to obtain. Examination of 8-oxodG in the serum has been widely used to compare 8-oxodG in normal and diseased patients 14–17. It should be noted that we used an ELISA assay to measure 8-oxodG levels. In most cases there is a consensus between chromatographic and ELISA measurements of urinary 8-oxodG, however, it is observed that higher levels of urinary 8-oxodG are obtained with the ELISA kit 47, 48, although it remains to be determined whether this is also the case for serum levels of 8-oxodG. Nevertheless, our results show that 8-oxodG levels in the serum increase with age, which is consistent with data in the literature that this lesion and other biomarkers of oxidative stress increase with age 18, 49.
Importantly, we observed that 8-oxodG levels are associated with clinical cardiovascular risk factors such as age, hsCRP, BMI, hip circumference, systolic blood pressure and pulse pressure and IL-23 levels. Interestingly, 8-oxodG correlates with creatinine levels only in the low hsCRP group and with LDH in both the low and high hsCRP groups (but not the overall group or in the mid group), although in opposite ways. These relationships are interesting and suggest that 8-oxodG and hsCRP status may influence the levels of these clinical markers, and will be important to explore further in the future. 8-oxodG levels were significantly higher in participants who had osteoarthritis or were HIV positive, which is consistent with the fact that these diseases are associated with high levels of inflammation and oxidative stress.
Surprisingly, we found a strong association of 8-oxodG levels with systolic blood pressure and with pulse pressure, independent of hsCRP level. These data indicate that although there is a strong relationship between hsCRP and 8-oxodG, these markers may give an indication of different aspects of inflammation. For example, in our cohort of woman hsCRP may reflect the inflammatory status related to metabolic factors, whereas, 8-oxodG levels may give a better indication of the properties of blood vessels and vascular health. We also found that race was associated with systolic and pulse pressure, LDL, triglycerides, RAGE, ICAM-1, VCAM-1 and MCP-1. Poverty status was related to obesity parameters, cholesterol, LDL, HDL, LDH, and ICAM-1. It is interesting to speculate that some of the associations that we observe may contribute to the health disparities related to race and poverty status.
Increased levels of 8-oxodG with high hsCRP could be due to several reasons. First, our in vitro data demonstrate that hsCRP can increase the amount of ROS and induce the DNA base lesion, 8-oxoG. These data are consistent with previous reports that treatment of human endothelial and smooth muscle cells with hsCRP in vitro generates ROS 8, 32 and may further potentiate ROS signaling by increasing the levels of Nox1 and the p22phox subunit of the NADPH oxidase, a primary source of ROS in cells 1. In particular, increased expression and activation of Nox1 has been shown to promote vascular pathologies including, hypertension, atherosclerosis, and restenosis 33–35. Therefore, we can speculate that in vivo hsCRP may also induce 8-oxodG formation. Second, inflammatory cells are recruited to atherosclerotic plaques where they produce ROS and other inflammatory cytokines that can promote both inflammation and oxidative stress, leading to increased DNA damage. It will be interesting to examine in the future whether hsCRP induces ROS and increases Nox1 and p22phox expression in other cell types other than endothelial cells. In addition, other cardiovascular risk factors such as obesity, hyperlipidemia, and hypertension, that have previously been shown to increase oxidative stress could also contribute to higher levels of 8-oxodG. Environmental factors and lifestyle could also affect levels of 8-oxodG. Furthermore, given our findings that hsCRP can increase MDA levels in vitro, hsCRP could also affect 8-oxodG levels in vivo by increasing MDA or other lipid peroxidation products that may in turn potentiate oxidative stress-induced DNA damage. It is interesting that we found that hsCRP did not induce other forms of DNA damage including SSBs and DSBs. However, we cannot exclude that prolonged exposure to hsCRP and/or the increased levels of inflammation and oxidative stress associated with vascular diseases may induce 8-oxodG, as well as, SSBs and DSBs in vivo.
In conclusion, in our cohort of woman we found that 8-oxodG and hsCRP are independently related to several cardiovascular risk factors. The present analyses were limited by a relatively small sample of participants. However, we had sufficient power to detect our anticipated effects and the sample size must be tempered by the complexity and manual labor involved in this assay. Future research lies in elucidating whether this relationship can be generalized in a larger cohort as well as in men. Nevertheless, the present sample is representative of an urban dwelling population. Our data suggest that 8-oxodG levels are associated with systolic blood pressure and pulse pressure, indicators of vascular disease, whereas, hsCRP is associated with metabolic factors. These data shed new light on the complex interplay between inflammation and oxidative stress in CVD.
Supplementary Material
Acknowledgments
The authors wish to thank HANDLS participants, HANDLS medical staff members Mary Sam-Nwoha, Catherine Sackett, and Clare Jefferson for their careful assessment of the participants and the acquisition of clinical samples and Janice Barnes and Althaf Lohani for technical assistance.
Sources of Funding: This study was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Footnotes
Disclosures: The authors declare no conflict of interest.
References
- 1.Kobayashi S, Inoue N, Ohashi Y, Terashima M, Matsui K, Mori T, Fujita H, Awano K, Kobayashi K, Azumi H, Ejiri J, Hirata K, Kawashima S, Hayashi Y, Yokozaki H, Itoh H, Yokoyama M. Interaction of oxidative stress and inflammatory response in coronary plaque instability: important role of C-reactive protein. Arterioscler Thromb Vasc Biol. 2003;23:1398–1404. doi: 10.1161/01.ATV.0000081637.36475.BC. [DOI] [PubMed] [Google Scholar]
- 2.Verma S, Devaraj S, Jialal I. Is C-reactive protein an innocent bystander or proatherogenic culprit? C-reactive protein promotes atherothrombosis. Circulation. 2006;113:2135–2150. discussion 2150. [PubMed] [Google Scholar]
- 3.Wu J, Stevenson MJ, Brown JM, Grunz EA, Strawn TL, Fay WP. C-reactive protein enhances tissue factor expression by vascular smooth muscle cells: mechanisms and in vivo significance. Arterioscler Thromb Vasc Biol. 2008;28:698–704. doi: 10.1161/ATVBAHA.107.160903. [DOI] [PubMed] [Google Scholar]
- 4.Scirica BM, Morrow DA. Is C-reactive protein an innocent bystander or proatherogenic culprit? The verdict is still out. Circulation. 2006;113:2128–2134. doi: 10.1161/CIRCULATIONAHA.105.611350. discussion 2151. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 6.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 7.Castell JV, Gomez-Lechon MJ, David M, Andus T, Geiger T, Trullenque R, Fabra R, Heinrich PC. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989;242:237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Nan B, Lin P, Yao Q. C-reactive protein increases plasminogen activator inhibitor-1 expression in human endothelial cells. Thromb Res. 2008;122:125–133. doi: 10.1016/j.thromres.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shishehbor MH, Hazen SL. Inflammatory and oxidative markers in atherosclerosis: relationship to outcome. Curr Atheroscler Rep. 2004;6:243–250. doi: 10.1007/s11883-004-0038-1. [DOI] [PubMed] [Google Scholar]
- 10.D’Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 11.Andreassi MG. Coronary atherosclerosis and somatic mutations: an overview of the contributive factors for oxidative DNA damage. Mutat Res. 2003;543:67–86. doi: 10.1016/s1383-5742(02)00089-3. [DOI] [PubMed] [Google Scholar]
- 12.Gray K, Bennett M. Role of DNA damage in atherosclerosis--bystander or participant? Biochem Pharmacol. 2011;82:693–700. doi: 10.1016/j.bcp.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Trzeciak AR, Mohanty JG, Jacob KD, Barnes J, Ejiogu N, Lohani A, Zonderman AB, Rifkind J, Evans MK. Oxidative damage to DNA and single strand break repair capacity: Relationship to other measures of oxidative stress in a population cohort. Mutat Res: Fundam Mol Mech Mutagen. 2012 doi: 10.1016/j.mrfmmm.2012.01.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin CS, Moon BS, Park KS, Kim SY, Park SJ, Chung MH, Lee HK. Serum 8-hydroxy-guanine levels are increased in diabetic patients. Diabetes Care. 2001;24:733–737. doi: 10.2337/diacare.24.4.733. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi A, Takeda A, Onodera H, Kimpara T, Hisanaga K, Sato N, Nunomura A, Castellani RJ, Perry G, Smith MA, Itoyama Y. Systemic increase of oxidative nucleic acid damage in Parkinson’s disease and multiple system atrophy. Neurobiol Dis. 2002;9:244–248. doi: 10.1006/nbdi.2002.0466. [DOI] [PubMed] [Google Scholar]
- 16.Evans MD, Cooke MS, Akil M, Samanta A, Lunec J. Aberrant processing of oxidative DNA damage in systemic lupus erythematosus. Biochem Biophys Res Commun. 2000;273:894–898. doi: 10.1006/bbrc.2000.3078. [DOI] [PubMed] [Google Scholar]
- 17.Haghdoost S, Maruyama Y, Pecoits-Filho R, Heimburger O, Seeberger A, Anderstam B, Suliman ME, Czene S, Lindholm B, Stenvinkel P, Harms-Ringdahl M. Elevated serum 8-oxo-dG in hemodialysis patients: a marker of systemic inflammation? Antioxid Redox Signal. 2006;8:2169–2173. doi: 10.1089/ars.2006.8.2169. [DOI] [PubMed] [Google Scholar]
- 18.Cooke MS, Olinski R, Evans MD. Does measurement of oxidative damage to DNA have clinical significance? Clin Chim Acta. 2006;365:30–49. doi: 10.1016/j.cca.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 19.De Flora S, Izzotti A, Walsh D, Degan P, Petrilli GL, Lewtas J. Molecular epidemiology of atherosclerosis. Faseb J. 1997;11:1021–1031. [PubMed] [Google Scholar]
- 20.Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–932. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- 21.Collins AR, Gedik CM, Olmedilla B, Southon S, Bellizzi M. Oxidative DNA damage measured in human lymphocytes: large differences between sexes and between countries, and correlations with heart disease mortality rates. Faseb J. 1998;12:1397–1400. [PubMed] [Google Scholar]
- 22.Mason PJ, Manson JE, Sesso HD, Albert CM, Chown MJ, Cook NR, Greenland P, Ridker PM, Glynn RJ. Blood pressure and risk of secondary cardiovascular events in women: the Women’s Antioxidant Cardiovascular Study (WACS) Circulation. 2004;109:1623–1629. doi: 10.1161/01.CIR.0000124488.06377.77. [DOI] [PubMed] [Google Scholar]
- 23.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 24.Ford ES, Giles WH, Mokdad AH, Myers GL. Distribution and correlates of C-reactive protein concentrations among adult US women. Clin Chem. 2004;50:574–581. doi: 10.1373/clinchem.2003.027359. [DOI] [PubMed] [Google Scholar]
- 25.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH, Jr, Grundy SM, de Lemos JA. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 26.Wenger NK. Coronary heart disease in men and women: does 1 size fit all? No! Clin Cardiol. 2011;34:663–667. doi: 10.1002/clc.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis. 2010;20:267–275. [PMC free article] [PubMed] [Google Scholar]
- 28.Toyokuni S, Tanaka T, Hattori Y, Nishiyama Y, Yoshida A, Uchida K, Hiai H, Ochi H, Osawa T. Quantitative immunohistochemical determination of 8-hydroxy-2′-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Invest. 1997;76:365–374. [PubMed] [Google Scholar]
- 29.Noren Hooten N, Kompaniez K, Barnes J, Lohani A, Evans MK. Poly(ADP-ribose) polymerase 1 (PARP-1) binds to 8-oxoguanine-DNA glycosylase (OGG1) J Biol Chem. 2011;286:44679–44690. doi: 10.1074/jbc.M111.255869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 31.Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol. 2002;22:1668–1673. doi: 10.1161/01.atv.0000029781.31325.66. [DOI] [PubMed] [Google Scholar]
- 32.Kuhlmann CR, Librizzi L, Closhen D, Pflanzner T, Lessmann V, Pietrzik CU, de Curtis M, Luhmann HJ. Mechanisms of C-reactive protein-induced blood-brain barrier disruption. Stroke. 2009;40:1458–1466. doi: 10.1161/STROKEAHA.108.535930. [DOI] [PubMed] [Google Scholar]
- 33.Ago T, Kuroda J, Kamouchi M, Sadoshima J, Kitazono T. Pathophysiological roles of NADPH oxidase/nox family proteins in the vascular system. -Review and perspective. Circ J. 2011;75:1791–1800. doi: 10.1253/circj.cj-11-0388. [DOI] [PubMed] [Google Scholar]
- 34.Streeter J, Thiel W, Brieger K, Miller FJ., Jr Opportunity Nox: The Future of NADPH Oxidases as Therapeutic Targets in Cardiovascular Disease. Cardiovasc Ther. 2012;00:1–13. doi: 10.1111/j.1755-5922.2011.00310.x. [DOI] [PubMed] [Google Scholar]
- 35.Takac I, Schroder K, Brandes RP. The Nox family of NADPH oxidases: friend or foe of the vascular system? Curr Hypertens Rep. 2012;14:70–78. doi: 10.1007/s11906-011-0238-3. [DOI] [PubMed] [Google Scholar]
- 36.Bhakat KK, Mokkapati SK, Boldogh I, Hazra TK, Mitra S. Acetylation of human 8-oxoguanine-DNA glycosylase by p300 and its role in 8-oxoguanine repair in vivo. Mol Cell Biol. 2006;26:1654–1665. doi: 10.1128/MCB.26.5.1654-1665.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Souza-Pinto NC, Maynard S, Hashiguchi K, Hu J, Muftuoglu M, Bohr VA. The recombination protein RAD52 cooperates with the excision repair protein OGG1 for the repair of oxidative lesions in mammalian cells. Mol Cell Biol. 2009;29:4441–4454. doi: 10.1128/MCB.00265-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beckman KB, Ames BN. Oxidative decay of DNA. J Biol Chem. 1997;272:19633–19636. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- 39.Binkova B, Smerhovsky Z, Strejc P, Boubelik O, Stavkova Z, Chvatalova I, Sram RJ. DNA-adducts and atherosclerosis: a study of accidental and sudden death males in the Czech Republic. Mutat Res. 2002;501:115–128. doi: 10.1016/s0027-5107(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 40.Binkova B, Strejc P, Boubelik O, Stavkova Z, Chvatalova I, Sram RJ. DNA adducts and human atherosclerotic lesions. Int J Hyg Environ Health. 2001;204:49–54. doi: 10.1078/1438-4639-00072. [DOI] [PubMed] [Google Scholar]
- 41.Ohtsubo T, Ohya Y, Nakamura Y, Kansui Y, Furuichi M, Matsumura K, Fujii K, Iida M, Nakabeppu Y. Accumulation of 8-oxo-deoxyguanosine in cardiovascular tissues with the development of hypertension. DNA Repair (Amst) 2007;6:760–769. doi: 10.1016/j.dnarep.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Inoue T, Inoue K, Maeda H, Takayanagi K, Morooka S. Immunological response to oxidized LDL occurs in association with oxidative DNA damage independently of serum LDL concentrations in dyslipidemic patients. Clin Chim Acta. 2001;305:115–121. doi: 10.1016/s0009-8981(00)00426-5. [DOI] [PubMed] [Google Scholar]
- 43.Abe M, Maruyama N, Okada K, Matsumoto S, Matsumoto K, Soma M. Effects of Lipid-Lowering Therapy with Rosuvastatin on Kidney Function and Oxidative Stress in Patients with Diabetic Nephropathy. J Atheroscler Thromb. 2011;18:1018–1028. doi: 10.5551/jat.9084. [DOI] [PubMed] [Google Scholar]
- 44.Komoda H, Inoue T, Node K. Anti-inflammatory properties of azelnidipine, a dihydropyridine-based calcium channel blocker. Clin Exp Hypertens. 2010;32:121–128. doi: 10.3109/10641960903254414. [DOI] [PubMed] [Google Scholar]
- 45.Ogawa S, Mori T, Nako K, Ito S. Combination therapy with renin-angiotensin system inhibitors and the calcium channel blocker azelnidipine decreases plasma inflammatory markers and urinary oxidative stress markers in patients with diabetic nephropathy. Hypertens Res. 2008;31:1147–1155. doi: 10.1291/hypres.31.1147. [DOI] [PubMed] [Google Scholar]
- 46.Gackowski D, Kruszewski M, Jawien A, Ciecierski M, Olinski R. Further evidence that oxidative stress may be a risk factor responsible for the development of atherosclerosis. Free Radic Biol Med. 2001;31:542–547. doi: 10.1016/s0891-5849(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 47.Cooke MS, Olinski R, Loft S. Measurement and meaning of oxidatively modified DNA lesions in urine. Cancer Epidemiol Biomarkers Prev. 2008;17:3–14. doi: 10.1158/1055-9965.EPI-07-0751. [DOI] [PubMed] [Google Scholar]
- 48.Evans MD, Olinski R, Loft S, Cooke MS. Toward consensus in the analysis of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine as a noninvasive biomarker of oxidative stress. Faseb J. 2010;24:1249–1260. doi: 10.1096/fj.09-147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simm A, Nass N, Bartling B, Hofmann B, Silber RE, Navarrete Santos A. Potential biomarkers of ageing. Biol Chem. 2008;389:257–265. doi: 10.1515/BC.2008.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.