Abstract
Background
For patients with locally advanced non-small-cell lung cancer (LA-NSCLC), the role of consolidation chemotherapy (CCT) following concurrent chemoradiotherapy (CRT) is partially defined. The aim of this study was to evaluate the efficacy and toxicity of CCT.
Methods
The characteristics of LA-NSCLC patients treated with curative concurrent CRT from 2001 to 2010 were retrospectively reviewed.
Results
Among 203 patients, 113 (55.7 %) patients received CCT. The median number of delivered CCT was 3 and 89.4 % patients completed ≥2 cycles. The OS was significantly better for patients in the CCT group compared with that in the non-CCT group (median OS, 27 months vs. 16 months; 5-year OS, 30.4 % vs. 22.5 %; p = 0.012). The median PFS were 12 months in the CCT group and 9 months in the non-CCT group (p = 0.291). The survival advantages of CCT were significant for males (HR: 0.63; 95 % CI, 0.44 − 0.90), patients with age < 60 years (HR: 0.63; 95 % CI, 0.42 − 0.95), non-squamous histology (HR: 0.44; 95 % CI, 0.25 − 0.76), pretreatment KPS ≥ 80 (HR: 0.67; 95 % CI, 0.48 − 0.93), stage IIIb (HR: 0.64; 95 % CI, 0.43 − 0.95), stable disease (HR: 0.31; 95 % CI, 0.14 − 0.65) and radiotherapy dose ≥ 60 Gy (HR: 0.69; 95 % CI, 0.48 − 1.00). There was no significant difference between the CCT group and the non-CCT group regarding treatment-related toxicities.
Conclusions
CCT might further prolong survival compared with CRT alone for LA-NSCLC without increasing treatment-related toxicities, especially for males, patients with age < 60 years, non-squamous histology, pretreatment KPS ≥ 80, stage IIIb, stable disease and radiotherapy dose ≥ 60 Gy. Large size prospective investigations that incorporate patient characteristics and treatment response are warranted to validate our findings.
Electronic supplementary material
The online version of this article (doi:10.1186/s12885-015-1710-2) contains supplementary material, which is available to authorized users.
Keywords: Locally advanced, Non-small-cell lung cancer, Consolidation chemotherapy, Efficacy, Toxicity
Background
Lung cancer remains the leading cause of cancer-related deaths worldwide [1]. Non-small-cell lung cancer (NSCLC) accounts for 80 % of all lung cancer cases and approximately 40 % of patients with NSCLC present with locally advanced non-small-cell lung cancer (LA-NSCLC) at diagnosis [2]. The standard-of-care treatment for LA-NSCLC is concurrent platinum-based chemotherapy and thoracic radiotherapy [3–5], which yields superior survival compared with either radiotherapy alone or sequential chemoradiotherapy. However, the outcome of LA-NSCLC treated with concurrent chemoradiotherapy (CRT) remains disappointing, with a median survival of 12–23.2 months [6, 7].
To improve survival, numerous studies have focused on exploring the feasibility and efficacy of consolidation chemotherapy (CCT) following concurrent CRT with discordant results. A phase II study of the Southwest Oncology Group (SWOG) 9504 [8] treated patients with concurrent CRT followed by consolidation docetaxel and achieved a promising median survival of 26 months suggesting a possible benefit of CCT. However, the Hoosier Oncology Group (HOG) [6], who published the only full article on a randomized phase III trial thus far, failed to replicate the encouraging outcome of SWOG 9504 by randomly delivering either docetaxel or observation after CRT. A recent pooled analysis [2] of 45 studies showed that CCT provided no survival benefit for LA-NSCLC patients. However, a subgroup analysis demonstrated that Asian populations (mostly from Japan and Korea) tended to benefit from CCT, although this benefit did not meet statistical significance (HR = 0.84; 95 % CI, 0.68-1.04; p = 0.105). Given the lack of substantial evidence from randomized phase III clinical trials, the definitive role of CCT in LA-NSCLC is unknown, especially in the Asian population. Therefore, our study attempted to evaluate the efficacy and toxicity of CCT after concurrent CRT at our institution.
Methods
Ethics statement
This retrospective study was approved by the ethics committee of the Cancer Hospital and Institute of Chinese Academy of Medical Sciences & Peking Union Medical College. Informed consent was exempted by the board due to the retrospective nature of this research. Patient records were anonymized and de-identified prior to analysis.
Eligibility
We retrospectively reviewed the clinical records of LA-NSCLC patients treated with concurrent CRT as an initial treatment at out institution between January 2001 and December 2010. The criteria for inclusion were defined as follows: (1) histologically or cytologically proven NSCLC; (2) clinically diagnosed as stage III disease according to the American Joint Committee on Cancer (AJCC) 2009 staging system; (3) treated with curative thoracic radiotherapy of no less than 50 Gy using intensity modulated radiotherapy (IMRT) or three-dimensional conformal radiotherapy (3D-CRT) with concurrent platinum doublet chemotherapy; (4) treatment responses evaluated 1 month after the completion of concurrent CRT in accordance with the Response Evaluation Criteria for Solid Tumors (RECIST) version 1.1 as complete response (CR), partial response (PR), and stable disease (SD).
Evaluation and follow-up
Complete blood cell counts (CBCs) and blood chemistry examinations were repeated once per week during the treatment period. The follow-up evaluations consisted of a physical examination, CBC, serum biochemistry, tumor marker, thoracic computed tomography (CT) scans, abdomen B-ultrasound examination, and other necessary imaging examinations as clinically indicated at intervals of 3 months for the first year, then every 6 months for the following 2 years, and annually thereafter. Local recurrence was defined as primary tumor recurrence, and regional recurrence was defined as recurrence in the mediastinum, hilum and supraclavicular fossa. Other sites of recurrence, including contralateral lung and metastatic lymph nodes in the neck or axilla, were defined as distant metastasis. Disease progression was determined based on a radiologic examination, histologic examination, or both. Treatment toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Data analysis
Overall survival (OS) was defined from the beginning of concurrent CRT to the time of death due to any cause or last follow-up. Cancer specific survival (CSS) was defined from the beginning of concurrent CRT to the time of death due to lung cancer or last follow-up. Progression-free survival (PFS) was defined from the beginning of concurrent CRT to the time of tumor progression or last follow-up. Local regional progression-free survival (LRPFS) was defined from the beginning of concurrent CRT to the time of local regional progression or last follow-up. Distant metastasis-free survival (DMFS) was defined from the beginning of concurrent CRT to the time of appearance of metastatic disease or last follow-up. Survival analysis was performed using the Kaplan-Meier method and log-rank test. Univariate and multivariate analyses by use of a Cox-proportional hazards model were performed to evaluate potential prognostic factors for OS and PFS. Variables with p < 0.3 in univariate analyses were entered into multivariate analyses. The Pearson χ2 test was used to compare the baseline characteristics and incidence of specific toxicities between treatment groups. Cox proportional hazards models, stratified by age, sex, histology, pretreatment Karnofsky performance score (KPS), stage, treatment response and radiotherapy dose were used to estimate HRs and 95 % confidence intervals (CIs) and test for significance for OS. A statistically significant difference was defined as p < 0.05. All data were processed with SPSS version 19.0.
Results
Patient characteristics
This retrospective study identified 261 consecutive LA-NSCLC patients who received concurrent chemotherapy and curative thoracic radiotherapy with a radiation dose ≥ 50 Gy at our institution between January 2001 and December 2010. We excluded 17 patients whose response assessments were unavailable, 13 patients who experienced disease progression within a month after concurrent CRT, 18 patients whose concurrent chemotherapy did not consist of platinum doublet regimens and 10 patients who were treated with conventional two-dimensional radiotherapy; thus, a total of 203 patients were available for analysis. The characteristics of the 203 patients are presented in Table 1. The median follow-up time was 23 months (range, 2–130 months) for the entire study population and 58.5 months (range, 10–130 months) for censored patients. The median age of the patients was 56 years (range, 31–73 years). The majority of patients were male (83.7 %) and younger than 60 years old (64 %) with no significant (<5 %) weight loss (82 %) and a smoking index > 400 (60.6 %). 94.6 % of patients had a pretreatment KPS ≥ 80, and 66.5 % of patients presented with stage IIIb disease. Most patients had normal hemoglobin (95.6 %) and carcinoembryonic antigen (CEA) (67.1 %) levels at diagnosis. The most common histology subtype was squamous cell carcinoma (SCC) (65.5 %). Only 26.1 % of patients had positron emission tomography (PET) scan staging.
Table 1.
Patient characteristics
| Characteristic | Non-CCT (%) | CCT (%) | p-value |
|---|---|---|---|
| Gender | |||
| Male | 83 (92.2) | 87 (77.0) | 0.003 |
| Female | 7 (7.8) | 26 (23.0) | |
| Age | 0.005 | ||
| < 60 years | 48 (53.3) | 82 (72.6) | |
| ≥ 60 years | 42 (46.7) | 31 (27.4) | |
| Weight loss | 0.941 | ||
| < 5 % | 74 (82.2) | 90 (81.8) | |
| ≥ 5 % | 16 (17.8) | 20 (18.2) | |
| Smoking indexa | 0.031 | ||
| ≤ 400 | 28 (31.1) | 52 (46.0) | |
| > 400 | 62 (68.9) | 61 (54.0) | |
| Pretreatment hemoglobin | 0.306 | ||
| < 120 g/L | 2 (2.2) | 7 (6.2) | |
| ≥ 120 g/L | 88 (97.8) | 106 (93.8) | |
| Pretreatment KPS | 0.697 | ||
| < 80 | 6 (6.7) | 5 (4.4) | |
| ≥ 80 | 84 (93.3) | 108 (95.6) | |
| Stage | 0.146 | ||
| IIIa | 35 (38.9) | 33 (29.2) | |
| IIIb | 55 (61.1) | 80 (70.8) | |
| Histology subtype | 0.545 | ||
| SCC | 61 (67.8) | 72 (63.7) | |
| Non-SCC | 29 (32.2) | 41 (36.3) | |
| Pretreatment CEA | 0.729 | ||
| < 5 ng/ml | 50 (68.5) | 62 (66.0) | |
| ≥ 5 ng/ml | 23 (31.5) | 32 (34.0) | |
| PET scan staging | |||
| Yes | 25 (27.8) | 28 (24.8) | 0.629 |
| No | 65 (72.2) | 85 (75.2) | |
| Radiotherapy technique | 0.010 | ||
| 3D-CRT | 26 (28.9) | 16 (14.2) | |
| IMRT | 64 (71.1) | 97 (85.8) | |
| Radiotherapy dose | 0.342 | ||
| ≥ 60 Gy | 71 (78.9) | 95 (84.1) | |
| < 60 Gy | 19 (21.1) | 18 (15.9) | |
| Concurrent chemotherapy | 0.010 | ||
| EP | 36 (40.0) | 63 (55.8) | |
| PC | 49 (54.4) | 38 (33.6) | |
| others | 5 (5.6) | 12 (10.6) | |
| Response | 0.559 | ||
| CR + PR | 72 (80.0) | 94 (83.2) | |
| SD | 18 (20.0) | 19 (16.8) |
CCT consolidation chemotherapy, CEA carcinoembryonic antigen, KPS Karnofsky performance status, PET positron emission tomography, SCC squamous cell carcinoma
aSmoking index is the number of cigarettes smoked per day × the number of cigarette-years
Of all 203 patients, 161 (79.3 %) were treated with IMRT and 42 (20.7 %) were treated with 3D-CRT. The radiation area only included the involved fields. The median radiation dose was 60 Gy in 30 fractions (range, 50–74 Gy in 25–37 fractions). For the concurrent chemotherapy regimen, 99 (48.8 %) patients were administered EP (etoposide plus cisplatin), 87 (42.8 %) patients received PC (paclitaxel plus carboplatin) and 17 (8.4 %) patients were treated with other platinum-doublet regimens. The responses of CR, PR, and SD were observed in 5 (2.5 %) patients, 161 (79.3 %) patients and 37 (18.2 %) patients, respectively. After concurrent CRT, 113 (55.7 %) patients received CCT, including 88 patients with platinum-based doublet chemotherapy regimens, and 25 patients with single-agent regimens. Among 113 patients who received CCT, the median number of delivered CCT was 3 and 101 (89.4 %) patients completed ≥2 cycles of CCT.
As shown in Table 1, females (23 % vs. 7.8 %; p = 0.003), patients aged < 60 years (72.6 % vs. 53.3 %; p = 0.005) with a smoking index ≤ 400 (46 % vs. 31.1 %; p = 0.031) who received IMRT (85.8 % vs. 71.1 %; p = 0.010) and concurrent EP chemotherapy (55.8 % vs. 40 %; p = 0.010) were more prevalent in the CCT group than in the non-CCT group. The remaining listed clinical characteristics were comparable between the two groups.
Survival and prognostic factors
The median OS and 5-year OS for all patients were 24 months and 26.9 %, respectively. Patients in the CCT group achieved significant survival prolongation compared with those in the non-CCT group (median OS, 27 months vs. 16 months; 5-year OS, 30.4 % vs. 22.5 %; p = 0.012; Fig. 1a). The median CSS and 5-year CSS for the CCT group (28 months and 34.4 %) in our study were also superior to those for the non-CCT group (17 months and 27.9 %) (p = 0.022), which was consistent with the OS results. The median PFS and 5-year PFS were 12 months and 21.8 % in the CCT group and 9 months and 21.4 % in the non-CCT group, respectively (p = 0.291; Fig. 1b). The 5-year LRPFS were 37.3 % in the CCT group and 35.1 % in the non-CCT group (p = 0.265; Fig. 1c). The 5-year DMFS were 40.1 % in the CCT group and 42.2 % in the non-CCT group (p = 0.779; Fig. 1d).
Fig. 1.
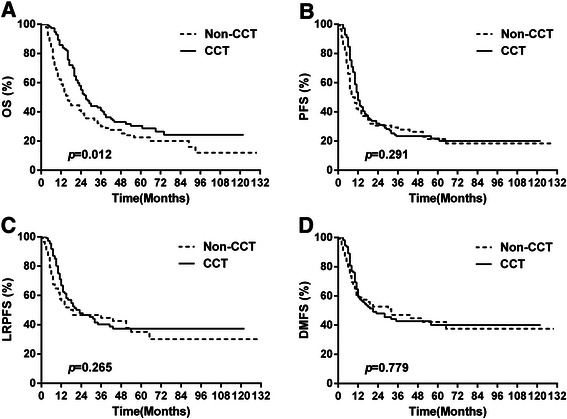
Comparison of a overall survival (OS), b progression-free survival (PFS), c local regional progression-free survival (LRPFS) and d distant metastasis-free survival (DMFS) between the consolidation chemotherapy (CCT) and non-CCT groups
The results of the univariate and multivariate analyses of potential prognostic factors for OS are shown in Table 2. Univariate analysis identified the radiotherapy dose < 60 Gy (p = 0.014), no CCT (p = 0.012) and SD (p = 0.035) as significant unfavorable prognostic factors. Multivariate analysis identified pretreatment CEA ≥ 5 ng/ml (p = 0.047), no CCT delivery (p = 0.008), and SD (p = 0.036) as predictors for poor OS. Additional file 1: Table S1 shows the results of the univariate and multivariate analyses of potential prognostic factors for PFS. The univariate analysis showed superior PFS for patients with SCC histology (p = 0.013), normal pretreatment CEA (p = 0.000), radiotherapy dose ≥ 60 Gy (p = 0.019) and CR or PR (p = 0.049). In the multivariate analysis, age < 60 years (p = 0.012), pretreatment CEA ≥ 5 ng/ml (p = 0.000), and no CCT delivery (p = 0.022) were significantly associated with unfavorable PFS.
Table 2.
Results of the univariate and multivariate analyses of prognostic factors for OS
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Characteristic | MST (mos) | 5-yr OS (%) | p-value | HR | 95 % CI | p-value |
| Gender | 0.431 | |||||
| Male | 23 | 27.4 | ||||
| Female | 28 | 24.5 | ||||
| Age | 0.834 | |||||
| < 60 years | 24 | 26.5 | ||||
| ≥ 60 years | 21 | 27.6 | ||||
| Weight loss | 0.292 | 1.01 | 0.61–1.66 | 0.977 | ||
| < 5 % | 24 | 28.7 | ||||
| ≥ 5 % | 24 | 20.2 | ||||
| Smoking index | 0.399 | |||||
| ≤ 400 | 26 | 26.5 | ||||
| > 400 | 20 | 27.4 | ||||
| Pretreatment hemoglobin | 0.580 | |||||
| < 120 g/L | 35 | - | ||||
| ≥ 120 g/L | 24 | 26.8 | ||||
| Pretreatment KPS | 0.096 | 0.68 | 0.34–1.37 | 0.285 | ||
| < 80 | 16 | 9.1 | ||||
| ≥ 80 | 24 | 28.0 | ||||
| Stage | 0.303 | |||||
| IIIa | 27 | 31.1 | ||||
| IIIb | 23 | 24.9 | ||||
| Histology subtype | 0.848 | |||||
| SCC | 24 | 28.0 | ||||
| Non-SCC | 24 | 25.1 | ||||
| Pretreatment CEA | 0.076 | 0.67 | 0.45–0.99 | 0.047 | ||
| < 5 ng/ml | 27 | 30.9 | ||||
| ≥ 5 ng/ml | 23 | 18.3 | ||||
| Radiotherapy technique | 0.128 | 1.09 | 0.66–1.79 | 0.743 | ||
| 3D-CRT | 18 | 16.2 | ||||
| IMRT | 25 | 30.1 | ||||
| Radiotherapy dose | 0.014 | 0.66 | 0.42–1.04 | 0.071 | ||
| ≥ 60 Gy | 25 | 29.5 | ||||
| < 60 Gy | 19 | 14.9 | ||||
| Concurrent chemotherapy | ||||||
| EP | 27 | 29.8 | 0.365 | |||
| PC | 19 | 24.0 | ||||
| Others | 25 | - | ||||
| Treatment modality | .012 | 0.61 | 0.42–0.88 | 0.008 | ||
| CRT + CCT | 27 | 30.4 | ||||
| CRT | 16 | 22.5 | ||||
| Response | 0.035 | 0.62 | 0.40–0.97 | 0.036 | ||
| CR + PR | 24 | 29.7 | ||||
| SD | 21 | 10.6 | ||||
In the subgroup analysis, the median OS and 5-year OS for patients receiving ≥2 cycles of CCT (27 months and 31.8 %) were better than those administered with <2 cycles of CCT (22 months and 18.7 %) (p = 0.317). The median time interval between completion of CRT to CCT was 6 weeks. The median OS and 5-year OS for patients with intervals ≤ 6 weeks (28 months and 34.4 %) were not statistically different from those with intervals > 6 weeks (25 months and 24 %) (p = 0.281). A forest plot of HRs for OS stratified by study characteristics is shown in Fig. 2. The survival advantages of CCT were statistically significant for males (HR: 0.63; 95 % CI, 0.44–0.90; p = 0.011), patients with age < 60 years (HR: 0.63; 95 % CI, 0.42–0.95; p = 0.027), non-squamous histology (HR: 0.44; 95 % CI, 0.25–0.76; p = 0.003), pretreatment KPS ≥ 80 (HR: 0.67; 95 % CI, 0.48–0.93; p = 0.017), stage IIIb (HR: 0.64; 95 % CI, 0.43–0.95; p = 0.025), SD (HR: 0.31; 95 % CI, 0.14–0.65; p = 0.002) and radiotherapy dose ≥ 60 Gy (HR: 0.69; 95 % CI, 0.48 − 1.00; p = 0.048).
Fig. 2.
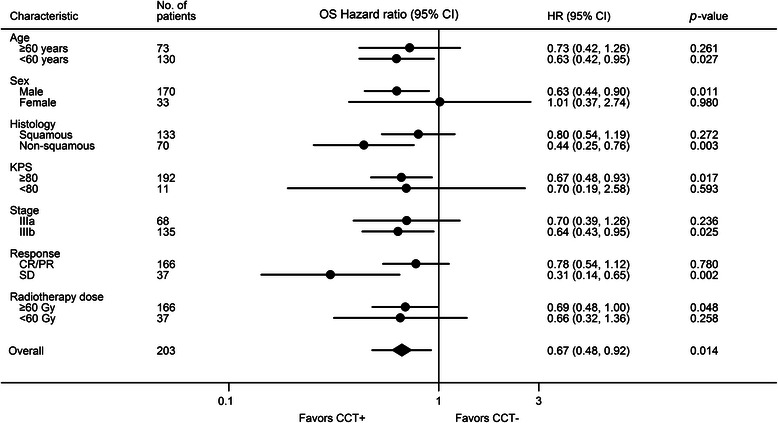
Hazard ratios of CCT to non-CCT in subgroup analysis according to study characteristics
Toxicity
The treatment-related acute toxicities during the CRT and the CCT phase are listed in Table 3. The incidence of grade ≥ 3 hematological toxicities between the CCT group and the non-CCT group was similar (30.1 % vs. 34.4 %; p = 0.509) during the CRT phase. In patients receiving CCT, 15 % experienced grade ≥ 3 hematological toxicities during the CCT phase and no patient had grade 5 hematological toxicities. The incidence of grade ≥ 3 esophagitis was comparable between the CCT group and the non-CCT group during the CRT phase (9.7 % vs. 13.3 %; p = 0.422). Grade ≥ 3 radiation pneumonitis occurred at similar rates between the CCT and the non-CCT group during the CRT (0.0 % vs. 2.2 %; p = 0.195) and CCT phase (5.3 % vs. 3.3 %; p = 0.737). A total of 4 patients died of grade 5 radiation pneumonitis, including 2 (2.2 %) in the CCT group and 2 in the non-CCT (1.8 %) group.
Table 3.
Treatment-related toxicities
| Toxicity | CRT phase | CCT phase | ||||||
|---|---|---|---|---|---|---|---|---|
| CCT (%) | Non-CCT (%) | Total | p-value | CCT (%) | Non-CCT (%) | Total | p-value | |
| Hematological | 0.509 | - | ||||||
| Grade 3/4 | 34 (30.1) | 31 (34.4) | 65 (32.0) | 17 (15.0) | - | - | ||
| Grade 1/2 | 79 (69.9) | 59 (65.6) | 138 (68.0) | 96 (85.0) | - | - | ||
| Esophagitis | 0.422 | - | ||||||
| Grade 3 | 11 (9.7) | 12 (13.3) | 23 (11.3) | - | - | - | ||
| Grade 1/2 | 102 (90.3) | 78 (86.7) | 180 (88.7) | - | - | - | ||
| Radiation pneumonitis | 0.195 | |||||||
| Grade ≥ 3 | 0 (0.0) | 2 (2.2) | 2 (1.0) | 6 (5.3) | 3 (3.3) | 9 (4.4) | 0.737 | |
| Grade 1/2 | 113 (100.0) | 88 (97.8) | 201 (99.0) | 107 (94.7) | 87 (96.7) | 194 (95.6) | ||
Discussion
The outcomes of LA-NSCLC are relatively poor, with a high possibility of residual disease after definitive CRT. Thus, many clinical trials have investigated the role of additional CCT. To date, three randomized phase III studies [6, 9, 10] have been carried out to explore the efficacy and toxicity of CCT, among which only one has been published as a full article. HOG [6] reported that consolidation docetaxel yielded no survival benefit (median OS, 21.2 months vs. 23.2 months; p = 0.883) with an increased risk for grade 3/4 pneumonitis (9.6 % vs. 1.4 %; p < 0.001), infections (11 % vs. 0 %; p = 0.003), hospitalization (28.8 % vs. 8.1 %) and treatment-related death (5.5 % vs. 0 %; p = 0.058). In the GILT [9] study, consolidation oral vinorelbine (NVBo) and cisplatin (P) after NVBo plus P failed to prolong the median PFS (6.4 months vs. 5.5 months; p = 0.630) and 4-year OS (25.3 % vs. 21.4 %). The multinational CCheIN trial [10] reported that consolidation DP (docetaxel plus cisplatin) after concurrent weekly DP resulted in a PFS (median PFS, 9.1 months vs. 8.1 months; p = 0.390) and a OS (median OS, 21.8 months vs. 20.6 months; p = 0.490) that were similar to those of the observation group. A recently reported pooled-analysis including forty-one phase II/III studies with 3479 patients also failed to provide significant survival benefit of CCT for LA-NSCLC. Unlike HOG, the GILT study and CCheIN trial observed that the addition of CCT did not increase the toxicities. Despite the negative results mentioned above, many oncologists still attempt to deliver CCT for LA-NSCLC patients with good performance status after CRT in routine clinical practice, at least partially due to a poor survival rate of less than 20 % at 5 years and a significant survival benefit achieved by CCT in stage IV disease.
The long-term results of this retrospective study suggest that CCT further prolongs survival compared with CRT alone for LA-NSCLC without increased toxicities. Although more patients in the CCT group had a positive selection factors (female, younger age and a lighter history of smoking), the multivariate analysis was able to account for those selection bias and showed that CCT was a positive prognostic factor for OS and PFS. For patients in the CCT group, the encouraging median OS and 5-year OS were 27 months and 30.4 %, respectively, which were superior to those reported in randomized clinical trials [6, 9, 10] and comparable to the survival results in SWOG 9504. The median OS and 5-year OS were 16 months and 22.5 %, respectively, in the non-CCT group, which were similar to the historical controls [4, 7]. Although there was no difference regarding LRPFS or DMFS between the CCT group and the non-CCT group, CCT prolonged survival compared with CRT alone, which may be attributed to several reasons as follows. First, the multivariate analysis for PFS showed that CCT was an independent favorable prognostic factor (HR = 0.643; 95 % CI, 0.441–0.937; p = 0.022), though we found that the LRPFS (p = 0.265) and DMFS (p = 0.779) outcomes were similar between the CCT and non-CCT group. The improvement in disease control may translate into improved survival. The improvement in disease control may translate into improved survival. The multivariate analysis for PFS showed that CCT was an independent favorable prognostic factor (HR = 0.643; 95 % CI, 0.441–0.937; p = 0.022). A second explanation is that ethnicity may affect the efficacy of CCT. Our result is consistent with a recent pooled analysis [2] that suggested that survival was better in Asian patients when CCT was delivered, though this improvement was not statistically significant. Soo et al.[11] reported that the survival and response rate to chemotherapy were better in Asian patients with lung cancer, while the treatment-related toxicities were more severe than in Caucasian patients. To date, the exact mechanisms with which ethnicity affects the efficacy of CCT are unknown. The interethnic difference may be attributable to differences in the genetic backgrounds or environment and culture. Third, it should be noted that the actually delivered cycles of CCT in most studies were relatively lower (0.7 to 3.1, average: 1.5) than those observed in our study (the median number was 3 and 89.4 % of patients completed ≥2 cycles of CCT). Last, bias may be involved in such a retrospective study. The choice of oncologists and patients may influence the administration of CCT. Treatment compliance was higher in patients in the CCT group than in those in the non-CCT group because some patients refused CCT despite the oncologists’ suggestion. Treatment compliance could impact patients’ routine follow up and motivation for salvage treatment after progression, which influences the outcome. The reason why CCT resulted in no significant increase in toxicities may be increased use of IMRT (85.8 % vs. 71.1 %; p = 0.010) and timely management of toxicity, as IMRT may decrease esophageal and pulmonary toxicity compared with 3D-CRT by increasing target conformity [12, 13].
Our study also suggested that CCT may lead to significant OS benefit for males, patients with age < 60 years, non-squamous histology, pretreatment KPS ≥ 80, stage IIIb, SD and radiotherapy dose ≥ 60 Gy. It seems plausible that fit patients with higher risk of distant metastasis would benefit from CCT. Interestingly, the fact that the HR for patients achieving SD is favoring CCT, which is contrary to Jeremic [14] holding the view that patients with a CR or a PR rather than those with a SD were likely to benefit from CCT. However, the number of patients with SD in our study was too small to draw a conclusion.
Prognostic factors are essential to understand the disease process, select treatments and design clinical trials. Numerous studies have investigated the prognostic factors for LA-NSCLC with inconsistent results. The commonly recognized favorable prognostic factors include stage IIIa, good performance status, non-significant weight loss, and female gender [15–17]. In our study, the multivariate analyses identified pretreatment CEA ≥ 5 ng/ml, no CCT, and SD after CRT as predictive of worse OS. Age < 60 years, pretreatment CEA ≥ 5 ng/ml, and no CCT were significantly associated with poor PFS. Our study did not show a significant association between OS or PFS and the widely recognized prognostic factors mentioned above, which may be the result of a relatively small sample size and under-representation of patients with pretreatment KPS < 80 (5.4 %) and weight loss ≥ 5 % (18 %).
Similar to our results, a retrospective study [18] reported that the clinical tumor response was significantly associated with OS. Kim et al. [19] found a five-fold likelihood of long term survival for responders (CR or PR) compared to non-responders (SD or PD) (p = 0.067). Because the clinical tumor response can be assessed soon after CRT, this approach may aid in the following treatment decision according to clinical tumor response to initial CRT because non-responders may need more aggressive treatment.
The prognostic role of age for LA-NSCLC is contradictory. A Radiation Therapy Oncology Group (RTOG)-based analysis [17] found that age ≤ 70 years was associated with improved survival. Nevertheless, the secondary analysis of RTOG 9410 [20] demonstrated that in patients treated with CRT, the median OS was longer for patients aged ≥ 70 years (22.4 months vs. 15.5 months, p-value not provided). Numerous recent trials [21–23] suggested that CRT yielded similar treatment outcome for fit older patients compared with younger patients, which agreed with our results that the elderly (age ≥ 60 years) were non-inferior to the young (age < 60 years) with respect to OS. The reason why age < 60 years acted as a negative predictor for PFS is unknown. The difference in the biological behavior between younger and older patients warrants further investigation.
Although our study is based on a relatively large sample size with a long follow-up period, it has some limitations. Like all other retrospective studies, our study is inevitably subject to multiple biases. Moreover, the CCT regimens were largely heterogeneous, which hindered our study from further exploring the most effective CCT regimen.
Conclusions
This retrospective study suggested that CCT further prolonged survival compared with CRT alone for LA-NSCLC without increasing treatment-related toxicities. Subgroup analysis identified that the survival advantages of CCT were more significant for males, patients with age < 60 years, non-squamous histology, pretreatment KPS ≥ 80, stage IIIb, SD and radiotherapy dose ≥ 60 Gy. Further prospective investigations that incorporate patient characteristics and treatment response are needed to validate our results.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81272616).
Abbreviations
- 3D-CRT
Three-dimensional conformal radiotherapy
- AJCC
American joint committee on cancer
- CBC
Complete blood cell count
- CCT
Consolidation chemotherapy
- CEA
Carcinoembryonic antigen
- CI
Confidence interval
- CR
Complete response
- CRT
Concurrent chemoradiotherapy
- CSS
Cancer specific survival
- CTCAE
Common terminology criteria for adverse events
- DMFS
Distant metastasis-free survival
- DP
Docetaxel plus cisplatin
- EP
Etoposide plus cisplatin
- HOG
Hoosier oncology group
- IMRT
Intensity modulated radiotherapy
- KPS
Karnofsky performance score
- LA-NSCLC
Locally advanced non-small-cell lung cancer
- LRPFS
Local regional progression-free survival
- NVBo
Oral vinorelbine
- OS
Overall survival
- P
Cisplatin
- PC
Paclitaxel plus carboplatin
- PET
Positron emission tomography
- PFS
Progression-free survival
- PR
Partial response
- RECIST
Response evaluation criteria for solid tumors
- RTOG
Radiation therapy oncology group
- SCC
Squamous cell carcinoma
- SD
Stable disease
- SWOG
Southwest oncology group
Additional file
Results of the univariate and multivariate analyses of prognostic factors for PFS. (DOCX 20 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LL participated in acquisition of data, analysis and interpretation of data, and drafting of the manuscript. NB, ZJ, J.Li, JW, XW, ZH, J.Lv, J.Liang, ZZ, YW, WY carried out the study during clinical observation, follow up, collected the clinical data for analysis. LW conceived of the study, participated in its design and coordination and revised the final manuscript. All authors have read and approved the final manuscript.
Contributor Information
Lipin Liu, Email: liulipin1988@sina.com.
Nan Bi, binan.email@yahoo.com.
Zhe Ji, Email: aschoff@163.com.
Junling Li, Email: drlijunling@vip.163.com.
Jingbo Wang, Email: wangjingbo303@yahoo.com.
Xiaozhen Wang, Email: wangxiaozhen2008@yahoo.com.
Zhouguang Hui, Email: drhuizg@163.com.
Jima Lv, Email: lvjima419@yahoo.com.
Jun Liang, Email: liang23400@163.com.
Zongmei Zhou, Email: zhouzongmei2013@163.com.
Yan Wang, Email: wangyan@csco.org.cn.
Weibo Yin, Email: wbyin05@163.com.
Luhua Wang, Phone: 86-10-87788799, Email: wlhwq@yahoo.com.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Tsujino K, Kurata T, Yamamoto S, Kawaguchi T, Kubo A, Isa S, et al. Is consolidation chemotherapy after concurrent chemo-radiotherapy beneficial for patients with locally advanced non-small-cell lung cancer? A pooled analysis of the literature. J Thorac Oncol. 2013;8(9):1181–9. doi: 10.1097/JTO.0b013e3182988348. [DOI] [PubMed] [Google Scholar]
- 3.Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–90. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 4.Curran WJ, Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452–60. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dillman RO, Herndon J, Seagren SL, Eaton WL, Jr, Green MR. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996;88(17):1210–5. doi: 10.1093/jnci/88.17.1210. [DOI] [PubMed] [Google Scholar]
- 6.Hanna N, Neubauer M, Yiannoutsos C, McGarry R, Arseneau J, Ansari R, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008;26(35):5755–60. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 7.Vokes EE, Herndon JE, 2nd, Kelley MJ, Cicchetti MG, Ramnath N, Neill H, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25(13):1698–704. doi: 10.1200/JCO.2006.07.3569. [DOI] [PubMed] [Google Scholar]
- 8.Gandara DR, Chansky K, Albain KS, Gaspar LE, Lara PN, Jr, Kelly K, et al. Long-term survival with concurrent chemoradiation therapy followed by consolidation docetaxel in stage IIIB non-small-cell lung cancer: a phase II Southwest Oncology Group Study (S9504) Clin Lung Cancer. 2006;8(2):116–21. doi: 10.3816/CLC.2006.n.039. [DOI] [PubMed] [Google Scholar]
- 9.Huber RM, Engel-Riedel W, Kollmeier J, Andreas S, Staar S, Klautke G, et al. GILT study: Oral vinorelbine (NVBo) and cisplatin (P) with concomitant radiotherapy (RT) followed by either consolidation (C) with NVBo plus P plus best supportive care (BSC) or BSC alone in stage (st) III non-small cell lung cancer (NSCLC): Final results of a phase (ph) III study. J Clin Oncol. 2012;30(15s):7001. [Google Scholar]
- 10.Park K, Ahn Y, Ahn J, Ahn M, Kim J, Cho E, et al. A multinational phase III randomized trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small cell lung cancer (CCheIN) J Clin Oncol. 2014;32(15s):7500. doi: 10.1200/JCO.2014.60.0130. [DOI] [PubMed] [Google Scholar]
- 11.Soo RA, Kawaguchi T, Loh M, Ou SH, Shieh MP, Cho BC, et al. Differences in outcome and toxicity between Asian and caucasian patients with lung cancer treated with systemic therapy. Future Oncol. 2012;8(4):451–62. doi: 10.2217/fon.12.25. [DOI] [PubMed] [Google Scholar]
- 12.Harris JP, Murphy JD, Hanlon AL, Le QT, Loo BW, Jr, Diehn M. A population-based comparative effectiveness study of radiation therapy techniques in stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;88(4):872–84. doi: 10.1016/j.ijrobp.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao ZX, Komaki RR, Thames HD, Jr, Liu HH, Tucker SL, Mohan R, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(3):775–81. doi: 10.1016/j.ijrobp.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Jeremic B. Consolidation chemotherapy after concurrent radiochemotherapy in locally advanced non-small-cell lung cancer may have been beneficial if we only knew where it have worked. J Thorac Oncol. 2014;9(1):e7. doi: 10.1097/JTO.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 15.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 16.Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst. 1980;65(1):25–32. [PubMed] [Google Scholar]
- 17.Werner-Wasik M, Scott C, Cox JD, Sause WT, Byhardt RW, Asbell S, et al. Recursive partitioning analysis of 1999 Radiation Therapy Oncology Group (RTOG) patients with locally-advanced non-small-cell lung cancer (LA-NSCLC): identification of five groups with different survival. Int J Radiat Oncol Biol Phys. 2000;48(5):1475–82. doi: 10.1016/S0360-3016(00)00801-4. [DOI] [PubMed] [Google Scholar]
- 18.Lee DS, Kim YS, Kang JH, Lee SN, Kim YK, Ahn MI, et al. Clinical Responses and Prognostic Indicators of Concurrent Chemoradiation for Non-small Cell Lung Cancer. Cancer Res Treat. 2011;43(1):32–41. doi: 10.4143/crt.2011.43.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DW, Shyr Y, Shaktour B, Akerley W, Johnson DH, Choy H. Long term follow up and analysis of long term survivors in patients treated with paclitaxel-based concurrent chemo/radiation therapy for locally advanced non-small cell lung cancer. Lung Cancer. 2005;50(2):235–45. doi: 10.1016/j.lungcan.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Langer C, Hsu C, Curran W, Komaki R, Lee J, Byhardt R, et al. Do elderly patients (pts) with locally advanced non-small cell lung cancer (NSCLC) benefit from combined modality therapy? a secondary analysis of RTOG 94–10. Int J Radiat Oncol Biol Phys. 2001;51(3):20–1. doi: 10.1016/S0360-3016(01)01860-0. [DOI] [Google Scholar]
- 21.Atagi S, Kawahara M, Yokoyama A, Okamoto H, Yamamoto N, Ohe Y, et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: a randomised, controlled, phase 3 trial by the Japan Clinical Oncology Group (JCOG0301) Lancet Oncol. 2012;13(7):671–8. doi: 10.1016/S1470-2045(12)70139-0. [DOI] [PubMed] [Google Scholar]
- 22.Jalal SI, Riggs HD, Melnyk A, Richards D, Agarwala A, Neubauer M, et al. Updated survival and outcomes for older adults with inoperable stage III non-small-cell lung cancer treated with cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel: analysis of a phase III trial from the Hoosier Oncology Group (HOG) and US Oncology. Ann Oncol. 2012;23(7):1730–8. doi: 10.1093/annonc/mdr565. [DOI] [PubMed] [Google Scholar]
- 23.Topkan E, Parlak C, Topuk S, Guler OC, Selek U. Outcomes of aggressive concurrent radiochemotherapy in highly selected septuagenarians with stage IIIB non-small cell lung carcinoma: retrospective analysis of 89 patients. Lung Cancer. 2013;81(2):226–30. doi: 10.1016/j.lungcan.2013.05.002. [DOI] [PubMed] [Google Scholar]


