Abstract
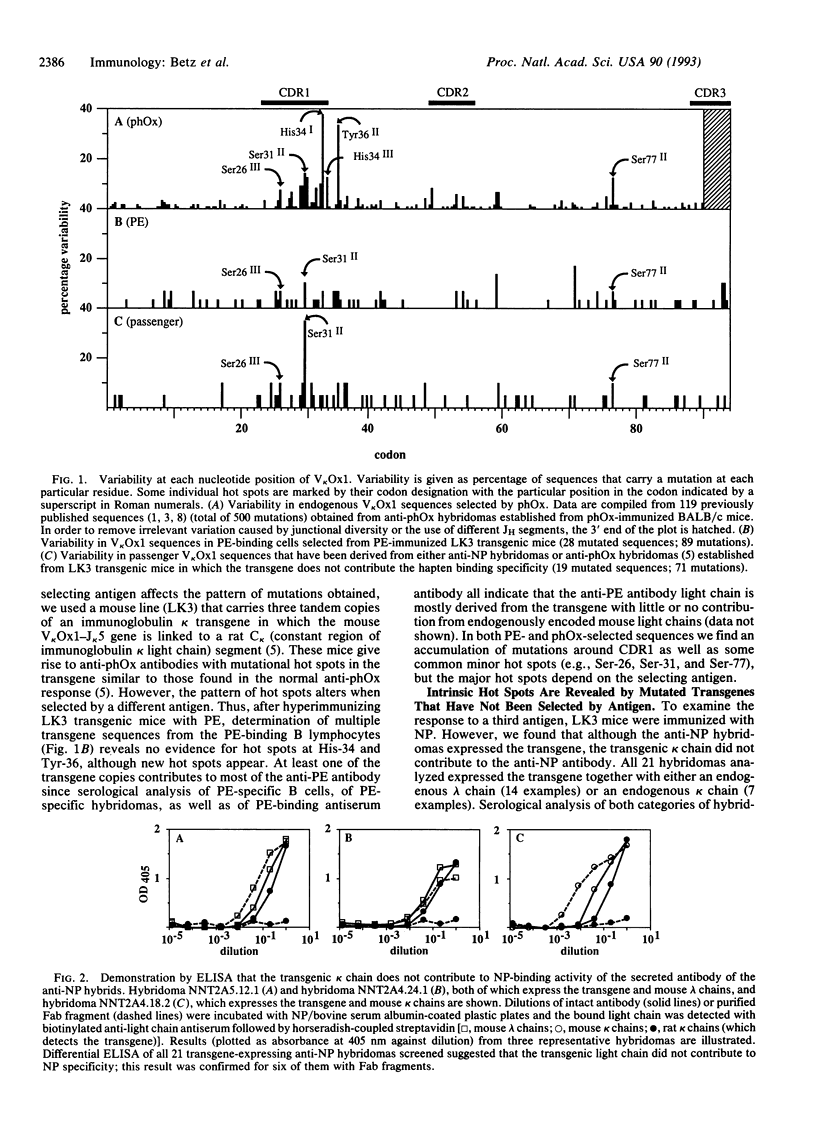
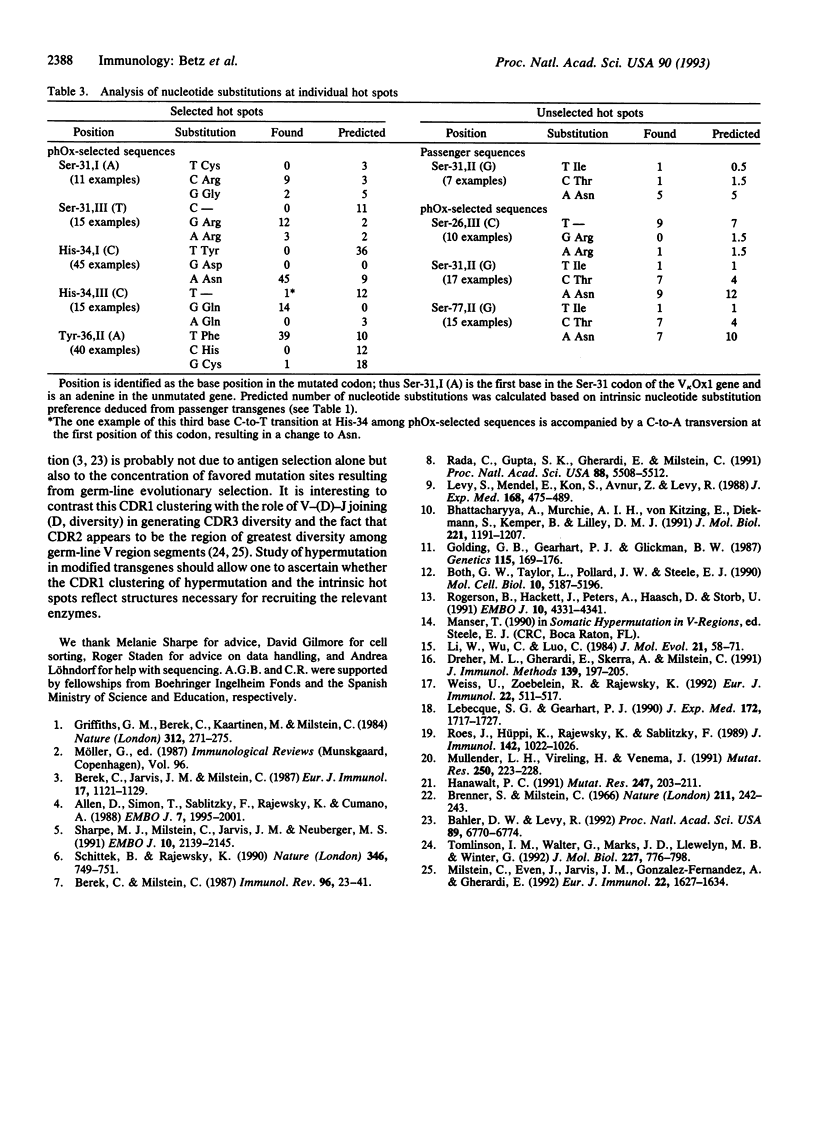
We have analyzed somatic hypermutation in mice carrying an immunoglobulin kappa transgene in order to discriminate mutations that reflect the intrinsic specificity of the hypermutation mechanism from those highlighted by antigenic selection. We have immunized animals with three different immunogens. With one immunogen, the antigen-specific B cells express a transgenic kappa chain, which does not form part of the antibody; the transgene is a passenger free to accumulate unselected mutations. With the other two immunogens, the transgenic kappa chain constitutes the light chain of the expressed antibody. A comparison of the transgene mutations obtained under these different circumstances allows us to identify common features that we attribute to the intrinsic specificity of the hypermutation process. In particular, it yields only base substitutions and leads to hot spots occurring in individual positions (e.g., the second base of the Ser-31 codon). The mutations preferentially accumulate around the first complementarity-determining region. The process exhibits specific base substitution preferences with transitions being favored over transversions. We propose that these substitution preferences can be used to discriminate intrinsic from antigen-selected hot spots. We also note that hypermutation distinguishes between the coding and noncoding strands since pyrimidines (particularly thymidines) mutate less frequently than purines.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D., Simon T., Sablitzky F., Rajewsky K., Cumano A. Antibody engineering for the analysis of affinity maturation of an anti-hapten response. EMBO J. 1988 Jul;7(7):1995–2001. doi: 10.1002/j.1460-2075.1988.tb03038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler D. W., Levy R. Clonal evolution of a follicular lymphoma: evidence for antigen selection. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6770–6774. doi: 10.1073/pnas.89.15.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek C., Jarvis J. M., Milstein C. Activation of memory and virgin B cell clones in hyperimmune animals. Eur J Immunol. 1987 Aug;17(8):1121–1129. doi: 10.1002/eji.1830170808. [DOI] [PubMed] [Google Scholar]
- Berek C., Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev. 1987 Apr;96:23–41. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A., Murchie A. I., von Kitzing E., Diekmann S., Kemper B., Lilley D. M. Model for the interaction of DNA junctions and resolving enzymes. J Mol Biol. 1991 Oct 20;221(4):1191–1207. doi: 10.1016/0022-2836(91)90928-y. [DOI] [PubMed] [Google Scholar]
- Both G. W., Taylor L., Pollard J. W., Steele E. J. Distribution of mutations around rearranged heavy-chain antibody variable-region genes. Mol Cell Biol. 1990 Oct;10(10):5187–5196. doi: 10.1128/mcb.10.10.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., Milstein C. Origin of antibody variation. Nature. 1966 Jul 16;211(5046):242–243. doi: 10.1038/211242a0. [DOI] [PubMed] [Google Scholar]
- Dreher M. L., Gherardi E., Skerra A., Milstein C. Colony assays for antibody fragments expressed in bacteria. J Immunol Methods. 1991 Jun 3;139(2):197–205. doi: 10.1016/0022-1759(91)90189-m. [DOI] [PubMed] [Google Scholar]
- Golding G. B., Gearhart P. J., Glickman B. W. Patterns of somatic mutations in immunoglobulin variable genes. Genetics. 1987 Jan;115(1):169–176. doi: 10.1093/genetics/115.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. M., Berek C., Kaartinen M., Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984 Nov 15;312(5991):271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C. Heterogeneity of DNA repair at the gene level. Mutat Res. 1991 Apr;247(2):203–211. doi: 10.1016/0027-5107(91)90016-h. [DOI] [PubMed] [Google Scholar]
- Lebecque S. G., Gearhart P. J. Boundaries of somatic mutation in rearranged immunoglobulin genes: 5' boundary is near the promoter, and 3' boundary is approximately 1 kb from V(D)J gene. J Exp Med. 1990 Dec 1;172(6):1717–1727. doi: 10.1084/jem.172.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S., Mendel E., Kon S., Avnur Z., Levy R. Mutational hot spots in Ig V region genes of human follicular lymphomas. J Exp Med. 1988 Aug 1;168(2):475–489. doi: 10.1084/jem.168.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. H., Wu C. I., Luo C. C. Nonrandomness of point mutation as reflected in nucleotide substitutions in pseudogenes and its evolutionary implications. J Mol Evol. 1984;21(1):58–71. doi: 10.1007/BF02100628. [DOI] [PubMed] [Google Scholar]
- Milstein C., Even J., Jarvis J. M., Gonzalez-Fernandez A., Gherardi E. Non-random features of the repertoire expressed by the members of one V kappa gene family and of the V-J recombination. Eur J Immunol. 1992 Jun;22(6):1627–1634. doi: 10.1002/eji.1830220642. [DOI] [PubMed] [Google Scholar]
- Mullenders L. H., Vrieling H., Venema J., van Zeeland A. A. Hierarchies of DNA repair in mammalian cells: biological consequences. Mutat Res. 1991 Sep-Oct;250(1-2):223–228. doi: 10.1016/0027-5107(91)90179-r. [DOI] [PubMed] [Google Scholar]
- Rada C., Gupta S. K., Gherardi E., Milstein C. Mutation and selection during the secondary response to 2-phenyloxazolone. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5508–5512. doi: 10.1073/pnas.88.13.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roes J., Hüppi K., Rajewsky K., Sablitzky F. V gene rearrangement is required to fully activate the hypermutation mechanism in B cells. J Immunol. 1989 Feb 1;142(3):1022–1026. [PubMed] [Google Scholar]
- Rogerson B., Hackett J., Jr, Peters A., Haasch D., Storb U. Mutation pattern of immunoglobulin transgenes is compatible with a model of somatic hypermutation in which targeting of the mutator is linked to the direction of DNA replication. EMBO J. 1991 Dec;10(13):4331–4341. doi: 10.1002/j.1460-2075.1991.tb05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittek B., Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990 Aug 23;346(6286):749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- Sharpe M. J., Milstein C., Jarvis J. M., Neuberger M. S. Somatic hypermutation of immunoglobulin kappa may depend on sequences 3' of C kappa and occurs on passenger transgenes. EMBO J. 1991 Aug;10(8):2139–2145. doi: 10.1002/j.1460-2075.1991.tb07748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol. 1992 Oct 5;227(3):776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- Weiss U., Zoebelein R., Rajewsky K. Accumulation of somatic mutants in the B cell compartment after primary immunization with a T cell-dependent antigen. Eur J Immunol. 1992 Feb;22(2):511–517. doi: 10.1002/eji.1830220233. [DOI] [PubMed] [Google Scholar]



