Abstract
Background
Intestinal macrophages are key regulators of inflammatory responses to the gut microbiome and play a central role in maintaining tissue homeostasis and epithelial integrity. However, little is known about the role of these cells in HIV infection, a disease fuelled by intestinal inflammation, a loss of epithelial barrier function and increased microbial translocation (MT).
Methods
Phenotypic and functional characterization of intestinal macrophages was performed for 23 African AIDS patients with chronic diarrhea and/or weight loss and 11 HIV-negative Africans with and without inflammatory bowel disease (IBD). AIDS patients were treated with cotrimoxazole for the prevention of opportunistic infections (OIs). Macrophage phenotype was assessed by flow cytometry and immuno-histochemistry (IHC); production of proinflammatory mediators by IHC and Qiagen PCR Arrays; in vitro secretion of cytokines by the Bio-Plex Suspension Array System. Statistical analyses were performed using Spearman’s correlation and Wilcoxon matched-pair tests. Results between groups were analyzed using the Kruskal-Wallis with Dunn’s post-test and the Mann–Whitney U tests.
Results
None of the study participants had evidence of enteric co-infections as assessed by stool analysis and histology. Compared to healthy HIV-negative controls, the colon of AIDS patients was highly inflamed with increased infiltration of inflammatory cells and increased mRNA expression of proinflammatory cytokine (tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IFN-γ, and IL-18), chemokines (chemokine (C-C motif) ligand (CCL)2 and chemokine (C-X-C) motif ligand (CXCL)10) and transcription factors (TNF receptor-associated factor (TRAF)6 and T-box (TXB)21). IHC revealed significant co-localization of TNF-α and IL-1β with CD68+ cells. As in IBD, HIV was associated with a marked increase in macrophages expressing innate response receptors including CD14, the co-receptor for lipopolysaccharide (LPS). The frequency of CD14+ macrophages correlated positively with plasma LPS, a marker of MT. Total unfractionated mucosal mononuclear cells (MMC) isolated from the colon of AIDS patients, but not MMC depleted of CD14+ cells, secreted increased levels of proinflammatory cytokines ex vivo in response to LPS.
Conclusions
Intestinal macrophages, in the absence of overt OIs, play an important role in driving persistent inflammation in HIV patients with late-stage disease and diarrhea. These results suggest intensified treatment strategies that target inflammatory processes in intestinal macrophages may be highly beneficial in restoring the epithelial barrier and limiting MT in HIV-infected patients.
Keywords: AIDS, Macrophages, Inflammation, Immune activation, Colon
Background
The human immunodeficiency virus type (HIV) induces rapid and profound damage to the gut-associated lymphoid tissue (GALT). Damage includes an early massive depletion of the T helper (Th)17 subset of CCR5+CD4+ memory T cells [1–7], changes in the frequency of CD4+CD25+FoxP3+ T regulatory (Treg) cells [8–10], an influx of activated cytotoxic CD8+ T lymphocytes [11], irreversible activation-induced fibrosis [12, 13] and a loss of epithelial integrity leading to increased microbial translocation (MT) [14]. MT is a major cause of local and systemic immune activation in HIV-infected patients, both before and during antiretroviral therapy (ART) [14–17].
Clinical manifestations of HIV infection in the gastrointestinal tract include malabsorption, diarrhea and a wasting syndrome [18–21]. Diarrhea accompanied by wasting (“slims disease”) is responsible for a significant amount of HIV-related morbidity in sub-Saharan Africa [22], even in the era of ART [23, 24]. Opportunistic infections (OIs) are a common cause of these disorders in HIV-infected individuals and include a range of viral, bacterial, fungal and parasitic pathogens [24]. Cotrimoxazole prophylaxis is a mainstay for the prevention of OIs in Africa and other resource limited settings and is recommended for severely immunocompromised HIV-infected individuals [25]. Prophylactic treatment with cotrimoxazole has been shown to improve survival and reduce morbidity in HIV patients prior to the initiation of ART [25–28].
Intestinal macrophages play a pivotal role, not only in the recognition and elimination of invading pathogens, but also in the regulation of inflammatory responses, maintaining the integrity of the epithelial barrier and in tissue remodeling and repair [29–33]. Under homeostatic conditions, stromal factors such as transforming growth factor-β (TGF-β), induce intestinal macrophages to differentiate into “inflammation anergic” cells that promote tolerance by maintaining the expression of Foxp3 in suppressive Treg cells [34]. These “resident” macrophages have avid phagocytic and bacteriocidal activity but do not express innate response receptors or co-stimulatory molecules and do not produce inflammatory cytokines in response to microbial stimulation [29, 35]. Inflammation anergy plays a central role in preventing damage to the gastrointestinal tract, an organ that is constantly exposed to a large number of commensal bacteria and other antigenic stimuli.
However, under conditions of chronic inflammation, intestinal macrophages can develop a pro-inflammatory phenotype and secrete inflammatory mediators such as TNF-α, interleukin-Iβ (IL-1β), interleukin-6 (IL6), and nitric oxide in response to invading pathogens and antigenic stimuli. Failure to downregulate this inflammatory response, as observed in patients with IBD and cirrhosis, is a major driver of intestinal disease [29, 36, 37]. Pro-inflammatory macrophages in the colon of patients with Crohn’s disease express CD14, produce inflammatory mediators in response to Toll-Like Receptor (TLR) stimulation and contribute to chronic intestinal inflammation via induction of the interleukin-23/interferon-γ (IL-23/IFN-γ)–positive feedback loop [29]. The accumulation of CD14+ macrophages in patients with Crohn’s disease has been attributed to an increased recruitment of inflammatory monocytes and a concomitant disruption of physiological, tissue-specific macrophage differentiation [38]. Consistent with these findings, a recent study of the duodenum has shown that HIV infection is associated with increased production of macrophage-related pro-inflammatory molecules (IL-1β, CCL5, CXCL9, and CXCL10) and enrichment of macrophages with low phagocytic activity [39].
In this study, we evaluated the inflammatory milieu and characterized the phenotypic and functional properties of macrophages in the colon of cotrimoxazole-treated ART-naïve African AIDS patients with unexplained diarrhea and/or weight loss who had no evidence of a confounding co-infection. Results were compared to those obtained in HIV-negative African controls with and without IBD. We detected a marked increase in pro-inflammatory macrophages in the colon of AIDS patients compared to healthy HIV seronegative controls. These cells expressed CD14 and other innate response receptors and produced inflammatory cytokines (TNF-α and IL-6), both constitutively and in response to LPS stimulation.
Methods
Study participants
The initial study cohort consisted of 23 cotrimoxazole-treated, ART-antiretroviral therapy naïve AIDS patients who were undergoing diagnostic endoscopy for unexplained diarrhea (>12 weeks duration) and weight loss (>10 % of the patient’s body weight). Five additional HIV-infected patients were subsequently sampled for ex vivo studies of LPS stimulation and cytokine/chemokine production. All patients had been receiving on Cotrimoxazole for >6 months. After obtaining written informed consent, biopsies were collected from the ascending and descending colon using a standard colonoscope and were processed for flow cytometry and histological evaluation. Blood samples were collected for routine patient management [CD4+ and CD8+ T cell counts, plasma viral load (VL)] and for quantification of plasma LPS. Patients were eligible for the study if they tested negative for Mycobacterium tuberculosis and for enteric pathogens in stool specimens. Subjects with secondary gastrointestinal infections, as determined by culture and microscopic examination of stool specimens and by histological evaluation of biopsies were excluded from the study. Clostridium difficile was excluded using three different methods - enzyme immunoassay for toxins A and B, PCR and histopathology. Healthy HIV-seronegative South Africans (n = 5) who tested pathogen negative and showed no evidence of disease during screening for colorectal cancer served as controls. These subjects were matched to HIV subjects by age and gender (median age 34, range 26–45 years; 60 % male). Six HIV-negative South Africans with inflammatory bowel disease (IBD) and symptoms of disease activity including abdominal cramps, diarrhea or bloody stools were also recruited. None of the IBD patients had received corticosteroids, azathioprine, 6-Mercaptopurine (6-MP) or anti-TNF therapy during the 6 months prior to enrollment. Following informed consent, biopsies were obtained from areas of active colitis. The study was reviewed and approved by the Research Ethics Committee of the Faculty of Health Sciences, University of Pretoria.
Specimen collection and processing
Biopsies were collected prior to ART. A total of 8–10 “pinch biopsies” were collected from each patient. Biopsies were placed on ice in tissue culture medium RPMI 1640 supplemented with 10 % fetal calf serum (FCS) and immediately processed for flow cytometry or in vitro stimulations. Biopsies for immunohistochemistry (IHC) were fixed in 10 % neutral buffered formalin and embedded in paraffin; biopsies for PCR array were snap frozen in liquid nitrogen and stored at −80 °C.
Histological analyses
Hematoxylin and eosin (H&E), periodic acid-Schiff diastase (PAS-d), Ziehl-Neelsen and Giemsa staining were performed to exclude acid fast bacilli (AFB), fungal and parasitic organisms (including Cryptosporidium and Isopora, spp., as well as Microsporidia). Cytomegalovirus, adenovirus and herpes simplex virus were excluded by careful scrutiny of H&E slides supplemented with appropriate IHC staining. Clostridial infection was excluded on morphologic grounds by means of careful evaluation of H&E sections. For T cell quantification, tissue sections were incubated with murine anti-human CD4 (clone 4B12, DAKO, Denmark) or CD8 (clone CD8/CD144B, DAKO, Denmark) monoclonal antibodies (mAbs), visualized with 3,3′-Diaminobenzidine (DAB) and counter-stained with haematoxylin. CD68 (KP1, DAKO, Denmark), CD14 (M0825 clone TUK4, DAKO), IL-1β (Abcam ab8320), IL-6 (Abcam ab9324) and TNF (PeproTech 500-M26) staining was performed on sections retrieved in PT101 Link Pre-Treatment Module (Dako), as previously described [37]. Following blocking of endogenous peroxidase and incubation with rabbit polyclonal anti-CD14 Ab or with mouse mAbs directed against CD68, IL1β, IL-6 or TNF-α, tissue sections were treated with Envision™/Horseradish peroxidase (HRP) dual link polymer, visualized with DAB+ chromogen and counter-stained with hematoxylin. IHC staining on two colonic biopsies per patient was evaluated, with the area on the slides showing the most positivity used for evaluation. The number of positive cells in 5 contiguous high power fields (HPF) of 0.80 mm2 (magnification x 400) was counted and reported as the average number of macrophages/HPF. Images were acquired, recorded and processed using a Leica DMLB 11888011 microscope and digital camera and an IM50 Image Manager (Leica Microsystems Wetzlar, GmbH, Germany). To determine if CD68+ macrophages were producing inflammatory cytokines, colonic biopsies were subjected to sequential double labeling with Abs directed against CD68 and either IL1β or TNF-α using established methods [37]. Slides were incubated with anti-human IL1β or TNF-α primary Ab, washed and incubated with Alexa Fluor-488-conjugated secondary Ab (Invitrogen). After boiling and treatment with 3 % H2O2/methanol to block the antigenicity of the first set of Abs, the staining procedure was repeated using mouse anti-human CD68 and Alexa Fluor-568-conjugated secondary Ab (Invitrogen). Nuclei were stained with DAPI. The optimal concentration of each primary antibody (either mouse anti-IL1β or anti-TNF-α, or in the case of the second primer set, mouse anti-CD68) was determined using positive (tissue sections from patients with acute intestinal inflammation) and negative (reagents lacking primary antibody) controls and a dilution series of the primary antibody of interest. Data acquisition was performed using a Zeiss Axioplan 2 fluorescent microscope equipped with a triple filter, Fluoarc fluorescent light source and Axiocam ICc1 camera. All studies were performed in a blinded fashion by a histopathologist with extensive experience in the analysis of intestinal specimens.
Phenotyping of Mucosal Mononuclear Cells (MMC)
MMC were obtained by digesting the biopsies in RPMI 1640 containing 10 % FCS and 0.5 mg/ml collagenase type IV (Sigma, St Louis, MO) for 30 min at 37 °C as previously described [40]. The resultant single cell suspensions were passed through a 70-μm cell strainer to remove debris (Becton Dickenson, Labware, NJ). The remaining tissue fragments were re-digested and pooled digests were strained, washed and processed for flow cytometry or LPS stimulation. Multi-parameter flow cytometry was performed on digested colonic MMC (0.5 x106 cells/tube) that had been stained for 30 min at 4 °C with various combinations of anti-human CD3, CD4, CD8, CD33, CD14, CD16, CD80, and CD86 mAbs (Beckman Coulter Inc., Fullerton, CA). T cell populations were identified based on forward and side-scatter characteristics and expression of CD3. Macrophage populations were identified based on forward and side scatter characteristics and the expression of CD33. CD14, CD16, CD80 and CD86 levels were determined by gating on CD33+ cells. CD33+ cells were confirmed to be positive for plasma membrane CD45 and CD68 by back gating during the initial establishment of flow cytometry protocols. Samples were analyzed on a Beckman Coulter FC500 flow cytometer with a minimum of 5000 gated events collected per tube. Results were analyzed using Flow Jo (Tree Star, Ashland, OR).
PCR array
Expression levels of 84 inflammatory genes were determined using the human Th17 Autoimmunity and Inflammation RT2 Profiler PCR array (SABioscience, Frederick, MD). Total RNA was extracted from 12 to 15 mg of colonic tissue using the Maxwell® 16 Tissue LEV Total RNA Purification Kit. cDNA was synthesized from 0.5 μg of total RNA using the RT2 PCR array first strand kit. Real time PCR was performed in 96-well plates using RT2 SYBR Green qPCR Master Mix and a CFX96 Real Time PCR Detection System (BioRad, Hercules, CA). Ten AIDS patients, 6 IBD patients and 5 HIV negative controls were analyzed in triplicate. Results were normalized using 5 housekeeping genes and analyzed by the comparative cycle threshold method.
Quantification of plasma LPS
Peripheral venous blood was collected in EDTA tubes at the time of colonoscopy. Plasma was isolated by centrifugation at 1800 rpm for 10 min at 4 °C and stored in 1.0 mL aliquots at −80 °C. LPS levels were quantified using the Limulus Amoebocyte Lysate assay QCL-1000 (Lonza, Valais Switzerland) and expressed as EU/mL as previously described [15].
LPS stimulation of CD14+ and CD14− MMC
CD14+ macrophages were depleted from colonic MMC by positive selection (CD14 Microbeads, Miltenyi Biotech). Total MMC and CD14− MMC (0.25x106 cells/well) were cultured in triplicate in RPMI/10 % FCS/5 % human AB serum in the presence or absence of LPS (1 μg/mL; Sigma) for 18 h. Cytokine and chemokine levels (pg/ml) in culture supernatants were quantified using the Bio-Plex Suspension Array System according to manufacturer’s instructions (Bio-Rad).
Statistical analyses
Prism 5 from GraphPad Software (La Jolla, CA) was used for statistical analyses. Paired observations were compared using Wilcoxon matched pair tests. The Kruskal-Wallis with Dunn’s post-test or Mann–Whitney U tests were used to compare between groups. Linear correlations were assessed using Spearman’s rank correlation coefficient. Two-tailed p values <0.05 were considered significant. PCR array fold change calculations were performed using the RT2 Profiler PCR Array Data Analysis Template v3.2 (SABioscience).
Results
Patient characteristics
Clinical characteristics of the AIDS study cohort (n = 23) are shown in Table 1. All patients had high plasma VLs (median 5.08 (4.2–6.6) log10 HIV RNA copies/mL) and low blood CD4+ T cells counts (median 69 (6–237) cells/uL). Symptoms leading to endoscopy were chronic diarrhea in 13 (56 %) and/or unexplained weight loss in 10 (44 %) patients. Five additional AIDS patients were subjected to ex vivo stimulation studies. None of the 28 patients had evidence of enteric co-infections as assessed by stool analysis and histology. HIV-negative healthy controls (n = 5) showed no evidence of structural damage or inflammatory cell infiltrates. HIV-negative IBD patients had typical well-documented Crohn’s disease (CD; n = 3), or ulcerative colitis (UC; n = 3) that included granulomatous lesions, ulcerations and gross structural damage to the colon.
Table 1.
Clinical characteristics of African AIDS patients with unexplained diarrhea and/or weight loss
| Age | Mean ± S.D. | 35 ± 11 |
| Median (Range) | 33 (21–52) | |
| Gender | Male | 12 (52 %) |
| Female | 11 (48 %) | |
| CD4 count (cells/μl) | Mean ± S.D. | 90 ± 68 |
| Median (Range) | 69 (6–237) | |
| % CD4 cells | Mean ± S.D. | 8.5 ± 5.5 |
| Median (Range) | 7.4 (0.42–22.7) | |
| log10 HIV RNA copies/ml | Mean ± S.D. | 4.89 ± 0.97 |
| Median (Range) | 5.08 (4.2–6.6) | |
| GIT complications | Diarrhea | 13 (56 %) |
| Weight loss | 10 (44 %) |
The colon of AIDS patients with unexplained diarrhea and/or wasting contains inflammatory cell infiltrates and increased levels of pro-inflammatory cytokines
Understanding the potential contribution of intestinal macrophages to HIV-induced pathogenesis requires a comprehensive analysis of the local microenvironment. The current study was performed on a relatively homogeneous population of co-pathogen negative ART-naïve patients with late-stage disease. As in previous studies [40], there was a dramatic reduction in the number CD4+ T cells and a corresponding increase in CD8+ T cell numbers in the colon of AIDS compared to HIV-negative IBD patients and healthy controls (CD4 counts: median of 13 vs. 72 vs. 63 cells/0.8 mm2, respectively; CD8 counts: median of 84 vs. 39 vs. 59 cells/0.8 mm2 respectively) (Fig. 1a and b). Mild-to-moderate non-specific colitis, as measured by the infiltration of inflammatory cells, namely CD8+ T cells, eosinophils and plasma cells, was detected in the colon of AIDS patients. IHC staining indicated that there was a marked increase in proinflammatory cells expressing IL-1β (median 174 vs. 75 cells/0.8 mm2, p = 0.04) and TNF-α (median 226 vs. 107 cells/0.8 mm2, p = 0.01) in the colon of AIDS patients compared to healthy controls (Fig. 1c and d). Thus, diarrhea and wasting were associated with increased recruitment of inflammatory cells and increased pro-inflammatory cytokine production, even in the absence of overt OIs.
Fig. 1.
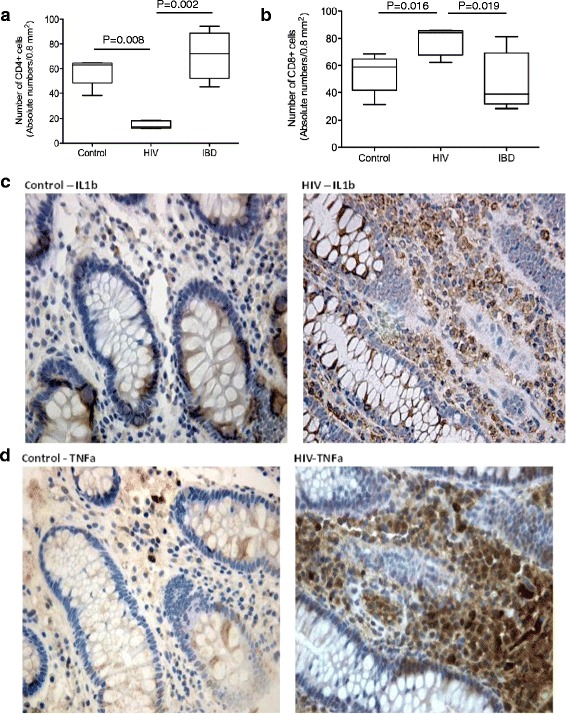
Inflammation in the colon of AIDS patients occurs in the setting of severe T cell dysregulation. a Differences in the frequency of CD4+ T in the colon of AIDS patients (n = 23) relative to uninfected healthy controls (n = 5) and IBD patients (n = 5) as determined by IHC. b Increased number of CD8+ T cells in the colon of AIDS patients relative to controls (p = 0.016) and patients with IBD (p = 0.019), as determined by IHC. Graphs show the median values and statistical significance between groups as calculated by Kruskal-Wallis with Dunn’s post-test. c Representative IHC staining showing increased levels of IL-1β in the colon of AIDS patients vs. uninfected controls (p = 0.04). d IHC staining showing increased TNF-α expression in the colon of AIDS patients compared to controls (p = 0.01)
Genes involved in macrophage activation and Th1 responses are preferentially up-regulated in the colon of AIDS patients
We next assessed changes in mRNA expression in total colonic tissue isolated from AIDS patients (n = 10), HIV-negative IBD patients (n = 6) and HIV-negative healthy controls (n = 5). Out of 84 genes tested using the Human Th17 for Autoimmunity and Inflammation PCR array (SABioscience), 23 were abnormally expressed in the colon of AIDS and/or IBD patients (Table 2). IL-18, CCL2, TRAF6 and IL-12Rβ1 were upregulated in both AIDS and IBD patients compared to controls. Consistent with IHC results, there was a significant increase in TNF-α (FC 7.11, p = 0.05) and IL-1β (FC = 4.27, p = 0.05) mRNA in AIDS patients compared to controls. HIV infection was also associated with increased expression of genes involved in chemotaxis and activation of monocytes/macrophages (CCL2 and TRAF6) and Th1 responses (IFN-γ and TBX21) as reported in Table 2. Genes associated with maintaining tissue homeostasis and an anti-inflammatory environment (ie. IL-10, TGF-β1 and suppressor of cytokine signaling (SOCS)3) were unchanged in AIDS patients, but increased in HIV-negative subjects with IBD, a finding that is suggestive of a tissue-protective response in these patients (Table 2). Thus, chronic diarrhea and/or weight loss in African AIDS patients is associated with increased levels of macrophage related cytokines (TNF-α and IL-1β) and chemokines (CCL2) compared to HIV-negative healthy controls.
Table 2.
Fold changes in mRNA levels of immune mediators altered in African AIDS patients and in patients with IBD
| HIV vs. Controls | CD vs. Controls | UC vs. Controls | |||||
|---|---|---|---|---|---|---|---|
| FC | p-value | FC | p-value | FC | p-value | ||
| Cytokines and Chemokines | IFN-γ | 5.31 | 0.001 | 4.81 | 0.037 | 1.02 | 0.667 |
| TNF-α | 7.11 | 0.005 | 14.27 | 0.001 | 13.65 | 0.001 | |
| IL-1β | 4.27 | 0.050 | 12.97 | 0.032 | 59.26 | 0.049 | |
| IL-8 | 7.24 | 0.212 | 36.84 | 0.075 | 194.5 | 0.002 | |
| IL-10 | 3.03 | 0.376 | 4.19 | 0.123 | 5.44 | 0.012 | |
| IL-18 | 26.85 | 0.031 | 32.17 | 0.004 | 28.57 | 0.001 | |
| TGF-β1 | 2.55 | 0.542 | 3.46 | 0.029 | 4.04 | 0.045 | |
| CCL2 | 10.78 | 0.008 | 16.07 | 0.007 | 59.33 | 0.009 | |
| CCL22 | 1.40 | 0.697 | 3.61 | 0.043 | 1.94 | 0.491 | |
| CXCL2 | 1.77 | 0.387 | 2.25 | 0.447 | 3.23 | 0.097 | |
| Receptors | ICAM1 | 4.31 | 0.231 | 14.17 | 0.008 | 31.75 | 0.001 |
| IL12Rβ1 | 3.69 | 0.017 | 4.06 | 0.026 | 3.42 | 0.045 | |
| TLR4 | 3.28 | 0.192 | 5.05 | 0.211 | 6.16 | 0.042 | |
| IL6R | 1.09 | 0.782 | 1.14 | 0.541 | 0.66 | 0.037 | |
| IL17RE | 0.64 | 0.521 | 0.67 | 0.245 | 0.38 | 0.041 | |
| IL17Rβ | 0.76 | 0.469 | 0.27 | 0.037 | 0.15 | 0.006 | |
| Transcription Factors | TRAF6 | 14.75 | 0.014 | 12.77 | 0.027 | 12.81 | 0.049 |
| SOCS1 | 2.29 | 0.512 | 4.24 | 0.076 | 8.27 | 0.029 | |
| SOCS3 | 1.96 | 0.341 | 5.52 | 0.023 | 20.31 | 0.005 | |
| S1PR1 | 3.12 | 0.231 | 4.29 | 0.049 | 7.15 | 0.009 | |
| TBX21 | 5.73 | 0.012 | 4.04 | 0.038 | 2.53 | 0.234 | |
| YY1 | 11.91 | 0.046 | 5.84 | 0.071 | 7.97 | 0.001 | |
| CLEC7 | 2.09 | 0.432 | 2.49 | 0.381 | 4.84 | 0.043 | |
IFN-γ Interferon-γ, TNF-α Tumour Necrosis Factor-α, IL Interleukin, TGF-β1 Transforming Growth Factor-β1, CCL Chemokine (C-C motif) ligand, CXCL chemokine (C-X-C motif) ligand, TLR Toll-like receptors, SOCS suppressor of cytokine signaling proteins, TRAF TNF receptor associated factor, TBX T-box transcription factor, YY Yin Yang, CLEC C-type lectin domain family
Macrophages in the colon of AIDS patients produce TNF-α and IL-1β and have an activated CD14+ phenotype
To determine whether macrophages were contributing to the pro-inflammatory milieu in the colon of AIDS patients, we performed double labeling of CD68+ macrophages with TNF-α and IL-1β using a dye swap method with validation by fluorescence microscopy [26]. TNF-α+ and IL-1β+ CD68-positive macrophages were present in the lamina propria of AIDS patients (Figs. 2 and 3). As in CD, there was a marked increase in CD14+ macrophages in the colon of AIDS patients compared to HIV-negative controls. The absolute number of CD14+ cells, as determined by IHC, increased from 1.8 cells/HPF in controls to 55 cells/HPF (p = 0.004) in patients with AIDS; the percentage of CD14+ cells, as assessed by flow cytometry using CD33, increased from a median 6.5 to 21.2 % (p =0.007) (Fig. 4). In addition to CD14, CD33+ macrophages in AIDS patients co-expressed the Fc receptor γ (CD16) and T cell the co-stimulatory molecules CD80 and CD86 (Table 3). Of considerable interest was the detection of a positive correlation between the percentage of CD14+CD33+ colonic macrophages and the levels of plasma LPS (R = 0.67, p = 0.002). The percentage of CD14+CD33+ colonic macrophages did not correlate with any other clinical variable or marker of inflammation (e.g., plasma or tissue HIV viral load, CD4 count, age). Collectively, these findings indicate that macrophages in the colon of African AIDS patients with diarrhea and/or weight loss have an activated CD14+ phenotype and express pro-inflammatory cytokines (TNF-α, IL-1β) that are known to be induced by LPS–mediated activation of CD14+ macrophages [41].
Fig. 2.
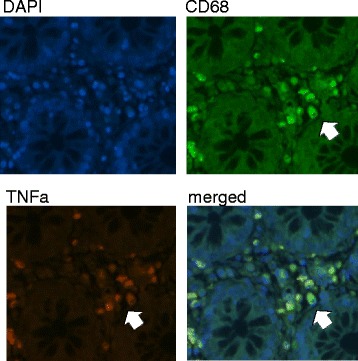
Macrophages in the colon of AIDS patients with unexplained diarrhea and/or weight loss produce TNF-α. Fluorescence microscopy of colon tissue from ART-naive AIDS patients dually stained with anti-CD68 (green) and anti-TNF-α (red) mAbs showing TNF-α+CD68+ macrophages in the lamina propria
Fig. 3.
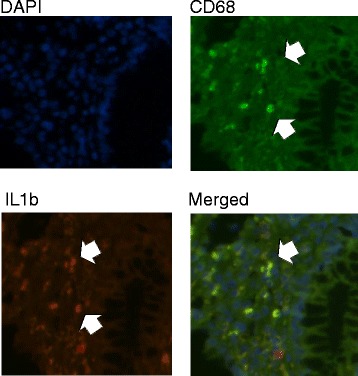
Macrophages in the colon of AIDS patients with unexplained diarrhea and/or weight loss produce IL-1β. Fluorescence microscopy of colon tissue from ART-naive AIDS patients dually stained with anti-CD68 (green) and anti-IL-1β (red) mAbs showing IL-1β+CD68+ macrophages in the lamina propria
Fig. 4.
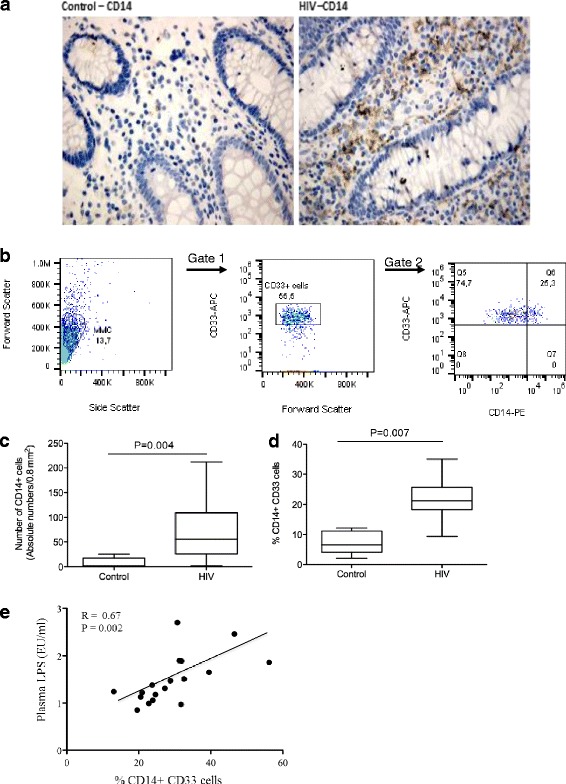
CD14+ macrophages accumulate in the colon of AIDS patients despite cotrimoxazole prophylaxis and absence of co-infections. a Representative IHC staining showing higher levels of CD14 expression on colonic macrophages of AIDS patients vs. uninfected healthy controls. b Representative histogram from flow cytometric analysis showing gating strategy and co-expression of CD14 in CD33+ cells. c Quantification of IHC analysis showing increased levels of CD14+ cells in the colon of AIDS patients relative to uninfected healthy controls; the median values and the statistical significance of the analysis between the two groups (p = 0.004) are indicated. d Summary of flow cytometric analyses showing increased expression of CD14 on CD33+ macrophages isolated from the colon of AIDS patients vs. uninfected healthy controls; the median values and the statistical significance of the analysis between the two groups are as indicated (p = 0.007). e Spearman correlations (R and p values) showing positive correlation between CD14+ macrophages in the colon of these patients and plasma levels of LPS (right panel). Results were considered significant if p < 0.05. Correlations were considered strong if r >0.6
Table 3.
Phenotypic characterization of colonic macrophages as determined by flow cytometry
| CD14+ (median % of CD33+ cells) | CD16+ (median % of CD33+ cells) | CD80+ (median % of CD33+ cells) | CD86+ (median % of CD33+ cells) | |
|---|---|---|---|---|
| HIV− | 6.5 ± 4.8 | 11.1 ± 7.6 | 9.2 ± 5.5 | 15.1 ± 7.3 |
| HIV+ | 21.2 ± 11.7 | 19.4 ± 12.5 | 25.1 ± 15.4 | 23.7 ± 18.1 |
LPS stimulation induces increased production of macrophage related pro-inflammatory cytokines in MMC isolated from the colon of AIDS patients
To assess the LPS responsiveness of colonic immune cells, total and CD14-depleted MMC isolated from the colon of 10 HIV+ patients and 5 healthy controls were cultured in vitro for 18 h in the presence or absence of LPS. Secretion of cytokines and chemokines into the culture supernatant was measured using customized Bio-Plex plates. In the absence of LPS, unstimulated total MMC from AIDS patients secreted significantly higher levels of macrophage related pro-inflammatory cytokines (TNF-α and IL-6) and chemokines (CCL2, CXCL10) compared to the MMC isolated from healthy controls (Fig. 5). LPS stimulation of total MMC from AIDS patients induced a further increase in TNF-α (median: 17.9 vs 31.8 pg/mL in unstimulated vs. stimulated MMC, respectively, p = 0.031) and IL-6 (median: 19.3 vs 45.46 pg/mL, respectively, p = 0.006) secretion. In contrast, LPS failed to increase CCL2 and CXCL10 secretion in MMC, suggesting that the upregulation of these chemokines is controlled by an LPS-independent mechanism. When MMC from AIDS patients were depleted of CD14+ cells (ie. CD14− MMC), they produced less TNF-α (median: 9.7 pg/ml, p = 0.032) and IL-6 (median 2.2 pg/mL, p = 0.002) than unfractionated MMC, suggesting that CD14+ macrophages played a major role in the expression of these pro-inflammatory cytokines (Fig. 6). Unlike CD14+ MMC, LPS had no inductive effect on cytokine production in CD14-depleted MMC. These results suggest that CD14+ macrophages in the colon of African AIDS patients presenting with diarrhea and/or weight loss may have increased responsiveness to microbial products, a finding that would be expected to perpetuate the local inflammatory response.
Fig. 5.
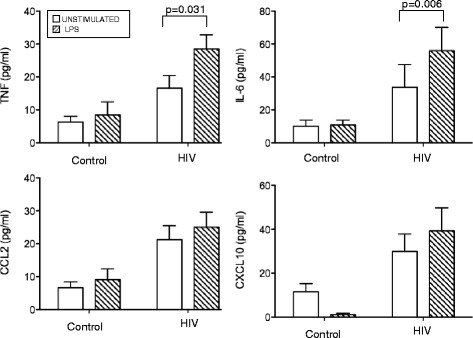
LPS induces increased pro-inflammatory cytokine production by MMC isolated from the colon of AIDS patients. Total MMC isolated from the colon of African AIDS patients (n = 10) and healthy uninfected healthy controls (n = 5) were cultured for 18 h in the presence or absence of LPS. Cytokine and chemokine levels in the culture supernatants were quantified using customized Bio-Plex plates from BioRad. The results represent the mean ± SD of two independent experiments performed in triplicate. Statistical significance between groups was calculated using the Mann–Whitney U test. Statistically significant differences (p < 0.05) are indicated by an asterisk
Fig. 6.
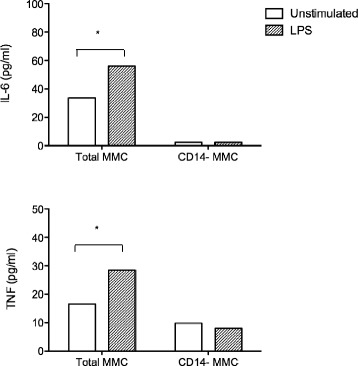
Depletion of CD14+ macrophages leads to a marked decrease in the ability of MMC to produce TNF and IL-6, both constitutively and in response to LPS stimulation. CD14+ macrophages were depleted from MMC isolated from the colon of ART-naïve AIDS patients. Total (CD14+) and CD14-depleted MMC were then cultured for 18 h in the presence or absence of LPS. Cytokine/chemokine secretion into the culture supernatant was quantified using customized Bio-Plex plates. Results represent the mean ± SD of triplicate assays performed on 10 African patients with AIDS. Statistical significance between groups was calculated using the Mann–Whitney U test. Statistically significant differences (p < 0.05) are indicated by an asterisk
Discussion
In this study, we used several different in situ (IHC) and ex vivo (flow cytometry, cytokine/chemokine production, mRNA profiling) methods to assess the phenotype and functional parameters of macrophages in the colon of ART-naïve African AIDS patients with unexplained gastrointestinal symptoms. Despite prophylactic treatment with cotrimoxazole and the apparent absence of OIs, we found that the colon of AIDS patients with chronic diarrhea and/or weight loss was highly inflamed and contained increased numbers of inflammatory cell infiltrates. The total expression of cytokines, chemokines and transcription factors associated with macrophage recruitment and activation was also significantly increased in the colon of AIDS patients compared to negative controls. The pattern of cytokine/chemokine upregulation in these patients was similar to that observed in HIV-negative subjects with CD. In addition, we detected a significant increase in activated CD14+ macrophages that produced pro-inflammatory cytokines and were responsive to LPS stimulation supporting the view that, as in CD [30, 32, 36], disrupted or abnormal differentiation of macrophages may be a major cause of the excessive inflammation and loss of tolerance to commensal bacteria that is observed in HIV-infected patients. While we did not observe any direct associations between the levels of CD14+ macrophages and tissue or plasma HIV viral loads, we speculate that high levels of HIV replication in the gastrointestinal tract during acute infection results in increased the recruitment of inflammatory monocytes. Unlike resident tissue macrophages, these newly recruited cells differentiate into macrophages that produce pro-inflammatory cytokines and chemokines in response to bacterial and viral products. Production of these pro-inflammatory mediators, together with residual viral replication and ongoing recruitment and differentiation of monocytes into pro-inflammatory macrophages, leads to further propagation of the inflammatory response a process that continues throughout the course of infection.
In the current study, we observed increased recruitment of inflammatory cell infiltrates (CD8+ T cells, eosinophils and/or plasma cells) and increased production of pro-inflammatory cytokines (including TNF-α and IL-1β) in the colon of ART-naive patients with late-stage HIV infection. At the mRNA level, we detected a significant increase in transcripts coding for pro-inflammatory cytokines and their receptors (TNF-α, IL-1β, IL-18, IFN-γ, IL-12RB1), chemokines (CCL2, CXCL10) and inflammation-related transcription factors (TRAF6, TXB21, YY1) in AIDS patients compared to HIV-negative controls. These findings are consistent with North American and European studies showing increased expression of pro-inflammatory cytokines in the rectosigmoid colon and duodenum of ART-naïve patients [39, 42, 43] with peak expression of TNF-α and IL-1β mRNA in the rectum occurring in patients with AIDS [44]. Our data is also consistent with studies showing increased expression of chemokines (CCL5, CXCL9, and CXCL10) in the duodenum of untreated HIV-infected patients [39]. Many of the immune mediators expressed at high levels in AIDS patients were also up-regulated in patients with CD (TNF-α, IL-1β, and IFN-γ) and have been implicated in the pathogenesis of CD [45]. As observed for HIV, CD is associated with systemic inflammation and increased plasma levels of markers of microbial translocation [46–48]. In CD, these changes are associated with increased secretion of IL-12 by activated intestinal macrophages. Higher levels of IL-12 drive increased production of IFN-γ in T cells, increased epithelial permeability and further enhancement of macrophage activation and microbial translocation [29]. Given the marked increase in IFN-γ observed in the colon of AIDS patients, it is conceivable that a similar feedback loop may be occurring in ART-naïve patients with advanced HIV infection.
Phenotypic and functional characterization of intestinal macrophages indicated that there was marked a increase in a unique subset of pro-inflammatory (CD14+) mononuclear phagocytes in the colon of cotrimoxazole-treated ART-naïve AIDS patients compared to uninfected controls. As in CD, these mononuclear phagocytes produced TNF-α and IL-1β and expressed innate response receptors including CD14, the co-receptor for LPS. Although these cells are believed to be macrophages based on their expression of CD33 and CD68, we cannot exclude the possibility that they may also have some properties of myeloid dendritic cell (mDC). Studies by Kamada et al. have reported that CD14+CD33+CD68+ “macrophages” in the colon of CD patients co-express the mDC markers, CD205 and CD209 (DC-SIGN) [29, 30].
Ex vivo studies of total MMC (containing CD14+ macrophages) isolated from the colon of AIDS patients indicated that these cells secreted increased levels of TNF-α, IL-6, CCL2 and CXCL10 compared to HIV-negative controls. Removal of CD14+ cells resulted in a marked decrease in the ability of MMC to produce pro-inflammatory cytokines suggesting that CD14+ macrophages are an important source of pro-inflammatory mediators in ART-naïve patients with late-stage HIV infection. Macrophages isolated from AIDS patients also had an increased ability to respond to microbial products including LPS, a TLR4 ligand, presumably due to the upregulation of CD14 and other innate response receptors. The positive correlation observed between CD14+ macrophages and plasma LPS suggests that the increased frequency of these cells in the colon of AIDS patients is linked to MT. Additional studies will be required to determine whether CD14+ macrophages are responsive to TLR-ligands other than LPS and to better define the relationship between CD14+ macrophages and microbial translocation. Studies are also needed to assess the phagocytic properties of CD14+ macrophages in the colon of African AIDS patients. A recent study by Allers et al., has shown that mononuclear cells isolated from the duodenum of HIV-infected patients have a reduced ability to phagocytose E coli BioParticles and thus, may be less likely to eliminate antigenic and microbial products that have crossed the epithelial barrier [39].
The strengths of our study relate to the homogeneity of our patient cohort with respect to treatment, stage of infection, viral load and CD4+ T cell counts. Limitations relate to the small size of our positive (IBD) and negative control groups. In addition, despite efforts to exclude patients with enteric co-infections (cotrimoxazole prophylaxis, stool culture and histological staining for acid-fast bacilli, fungal and parasitic infections), we cannot rule out the possibility that opportunistic co-infections may have affected our results. Additional studies are needed to determine whether our results can be extended to patients with early HIV infection and ART-treated patients with and without enteric co-infections. Studies exploring the relationships between increased inflammatory macrophage infiltrate and alterations in the functional gene content of intestinal microbiota are also of major importance given the vital role of the microbiota in shaping the intestinal microenvironment.
Conclusions
We have demonstrated that the colon of cotrimoxazole-treated ART-naïve AIDS patients contains increased numbers of activated CD14+ pro-inflammatory macrophages and that these cells are responsive to bacterial LPS and linked to MT. This study underscores the similarities between HIV and non-infectious IBD and highlights the need for detailed comparative studies to determine if there is a common mechanism driving these inflammatory pathologies. It will be especially important to determine whether the persistence of inflammation and immune activation in cotrimoxazole-treated patients is driven by HIV replication or by alterations in the intestinal microenvironment (such as changes in the microbiome [49, 50]; irreversible (or reversible) damage to the intestinal epithelium and/or non-specific activation of CD8+ T cells). This information is needed to design highly targeted treatments that can restore the integrity of the intestinal epithelium, normalize the intestinal microbiota and prevent MT.
Acknowledgments
This research and selected researchers (EC, TR, PM, SM and CS) were funded in part by a grant from the Delegation of the European Union to South Africa: “Drug Resistance Surveillance and Treatment Monitoring Network for the Public Sector HIV Antiretroviral Treatment Programme in the Free State – Sante 2007/147-790” and by a grant from the National Research Council of South Africa, Unlocking the Future 61509.
We owe a special thanks to the endoscopy nurses of the Unitas and Pretoria East GI-units and to Dr. Mark Theron, the study anesthetist, for their dedication and commitment to patient care. We also thank Dr. Saverio Di Palo (Department of Surgery, San Raffele Scientific Institute, Milan, Italy) for providing gut specimens from uninfected individuals used in setting up MMC isolations. Most of all, we thank our patients and volunteers. Without their participation and commitment this study would not have been possible.
Abbreviations
- 6-MP
6-Mercaptopurine
- Ab
Antibody
- AFB
Acid fast bacilli
- AIDS
Acquired Immune Deficiency Syndrome
- ART
Antiretroviral therapy
- CCL
Chemokine (C-C motif) ligand
- CD
Crohn’s disease
- CXCL
Chemokine (C-X-C motif) ligand
- DAB
3,3′-Diaminobenzidine
- EDTA
Ethylenediaminetetraacetic acid
- FCS
Fetal calf serum
- GIT
Gastrointestinal tract
- H&E
Hematoxylin and eosin
- HIV
Human Immunodeficiency Virus
- HPR
High power fields
- HRP
Horseradish peroxidase
- IBD
Inflammatory Bowel Disease
- IHC
Immuno-histochemistry
- IFN
Interferon
- IL
Interleukin
- LPS
Lipopolysaccharide
- mAbs
Monoclonal antibodie
- mDC
Myeloid dendritic cells
- MMC
Mucosal mononuclear cells
- MT
Microbial translocation
- OIs
Opportunistic infections
- PAS-d
Periodic acid-Schiff diastase
- RPMI
Roswell Park Memorial Institute medium
- SOCS
Suppressor of cytokine signaling
- TBX
T-box
- TGF
Transforming growth factor
- Th17
T helper 17 cell
- TLR
Toll like receptor
- TNF
Tumour necrosis factor
- TRAF
TNF receptor-associated factor
- Treg
T regulatory cells
- UC
Ulcerative colitis
- VL
Plasma viral load
Footnotes
Theresa Rossouw and Schalk W. van der Merwe contributed equally to this work.
Competing interests
None of the authors have any competing financial or other conflicts of interests.
Authors’ contributions
EC conceived and designed the study, performed flow cytometric, cytokine and gene expression analyses, interpreted the macrophage data and wrote the manuscript; TR, SVM, RB and JDP designed the clinical protocol, obtained specimens, supervised the collating of clinical data and cared for the patients; GP and MA provided expertise in data interpretation and critically reviewed the manuscript; TS, CJ, TR and FN performed and analyzed the immunohistchemistry and double staining experiments; SM and PM contributed to laboratory analyses; CS provided expertise in study design and database management. All authors have read and approved the final manuscript.
Contributor Information
Edana Cassol, Email: edana.cassol@carleton.ca.
Theresa Rossouw, Email: theresa.rossouw@up.ac.za.
Susan Malfeld, Email: susan.malfeld@up.ca.za.
Phetole Mahasha, Email: thakgalo03@gmail.com.
Tomas Slavik, Email: slavikt@ampath.co.za.
Chris Seebregts, Email: chris@jembi.org.
Robert Bond, Email: robertbond@mweb.co.za.
Johannie du Plessis, Email: johannie.duplessis@kuleuven.be.
Carl Janssen, Email: carljanssen@uz.leuven.be.
Tania Roskams, Email: Tania.Roskams@med.kuleuven.be.
Frederik Nevens, Email: frederik.nevens@uz.kuleuven.ac.be.
Massimo Alfano, Email: alfano.massimo@hsr.it.
Guido Poli, Email: poli.guido@hsr.it.
Schalk W. van der Merwe, Email: schalk.vandermerwe@uzleuven.be
References
- 1.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1(1):23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200(6):761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112(7):2826–35. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt PW. Th17, gut, and HIV: therapeutic implications. Curr Opin HIV AIDS. 2010;5(2):189–93. doi: 10.1097/COH.0b013e32833647d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, Tabb B, et al. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol. 2012;3(4):387–98. doi: 10.1038/mi.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77(21):11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood. 2013;121(1):29–37. doi: 10.1182/blood-2012-07-409755. [DOI] [PubMed] [Google Scholar]
- 9.Prendergast A, Prado JG, Kang YH, Chen F, Riddell LA, Luzzi G, et al. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS. 2009;24(4):491–502. doi: 10.1097/QAD.0b013e3283344895. [DOI] [PubMed] [Google Scholar]
- 10.Moreno-Fernandez ME, Presicce P, Chougnet CA. Homeostasis and function of regulatory T cells in HIV/SIV infection. J Virol. 2012;86(19):10262–9. doi: 10.1128/JVI.00993-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shacklett BL, Cox CA, Sandberg JK, Stollman NH, Jacobson MA, Nixon DF. Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J Virol. 2003;77(10):5621–31. doi: 10.1128/JVI.77.10.5621-5631.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estes J, Baker JV, Brenchley JM, Khoruts A, Barthold JL, Bantle A, et al. Collagen deposition limits immune reconstitution in the gut. J Infect Dis. 2008;198(4):456–64. doi: 10.1086/590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110(8):1133–9. doi: 10.1172/JCI0216413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 15.Cassol E, Malfeld S, Mahasha P, van der Merwe S, Cassol S, Seebregts C, et al. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 2010;202(5):723–33. doi: 10.1086/655229. [DOI] [PubMed] [Google Scholar]
- 16.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10(9):655–66. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 17.Shan L, Siliciano RF. Unraveling the relationship between microbial translocation and systemic immune activation in HIV infection. J Clin Invest. 2014;124(6):2368–71. doi: 10.1172/JCI75799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotler DP, Gaetz HP, Lange M, Klein EB, Holt PR. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;101(4):421–8. doi: 10.7326/0003-4819-101-4-421. [DOI] [PubMed] [Google Scholar]
- 19.Sharpstone D, Neild P, Crane R, Taylor C, Hodgson C, Sherwood R, et al. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut. 1999;45(1):70–6. doi: 10.1136/gut.45.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coodley GO, Loveless MO, Merrill TM. The HIV wasting syndrome: a review. J Acquir Immune Defic Syndr. 1994;7(7):681–94. [PubMed] [Google Scholar]
- 21.Kapembwa MS, Fleming SC, Sewankambo N, Serwadda D, Lucas S, Moody A, et al. Altered small-intestinal permeability associated with diarrhoea in human-immunodeficiency-virus-infected Caucasian and African subjects. Clin Sci (Lond) 1991;81(3):327–34. doi: 10.1042/cs0810327. [DOI] [PubMed] [Google Scholar]
- 22.Mayer HB, Wanke CA. Diagnostic strategies in HIV-infected patients with diarrhea. AIDS. 1994;8(12):1639–48. doi: 10.1097/00002030-199412000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Holmes CB, Wood R, Badri M, Zilber S, Wang B, Maartens G, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42(4):464–9. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 24.Slavik T. Human immunodeficiency virus-related gastrointestinal pathology: a southern Africa perspective with review of the literature (part 1: infections) Arch Pathol Lab Med. 2012;136(3):305–15. doi: 10.5858/arpa.2011-0332-RA. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization; UNAIDS Provisional WHO/UNAIDS recommendations on the use of contrimoxazole prophylaxis in adults and children living with HIV/AIDS in Africa. Afr Health Sci. 2001;1(1):30–1. [PMC free article] [PubMed] [Google Scholar]
- 26.Grimwade K, Swingler G. Cotrimoxazole prophylaxis for opportunistic infections in adults with HIV. Cochrane Database Syst Rev. 2003;3:CD003108. doi: 10.1002/14651858.CD003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anglaret X, Chene G, Attia A, Toure S, Lafont S, Combe P, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d’Ivoire: a randomised trial. Cotrimo-CI Study Group Lancet. 1999;353(9163):1463–8. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 28.Jones JL, Hanson DL, Dworkin MS, Alderton DL, Fleming PL, Kaplan JE, et al. Surveillance for AIDS-defining opportunistic illnesses, 1992–1997. MMWR CDC Surveill Summ. 1999;48(2):1–22. [PubMed] [Google Scholar]
- 29.Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118(6):2269–80. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamada N, Hisamatsu T, Okamoto S, Sato T, Matsuoka K, Arai K, et al. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol. 2005;175(10):6900–8. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
- 31.Kanai T, Watanabe M, Okazawa A, Nakamaru K, Okamoto M, Naganuma M, et al. Interleukin 18 is a potent proliferative factor for intestinal mucosal lymphocytes in Crohn’s disease. Gastroenterology. 2000;119(6):1514–23. doi: 10.1053/gast.2000.20260. [DOI] [PubMed] [Google Scholar]
- 32.Schenk M, Bouchon A, Seibold F, Mueller C. TREM-1--expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest. 2007;117(10):3097–106. doi: 10.1172/JCI30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huff KR, Akhtar LN, Fox AL, Cannon JA, Smith PD, Smythies LE. Extracellular matrix-associated cytokines regulate CD4+ effector T-cell responses in the human intestinal mucosa. Mucosal Immunol. 2011;4(4):420–7. doi: 10.1038/mi.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 35.Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206:149–59. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 36.Smith AM, Rahman FZ, Hayee B, Graham SJ, Marks DJ, Sewell GW, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J Exp Med. 2009;206(9):1883–97. doi: 10.1084/jem.20091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du Plessis J, Vanheel H, Janssen CE, Roos L, Slavik T, Stivaktas PI, et al. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol. 2013;58(6):1125–32. doi: 10.1016/j.jhep.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 38.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6(3):498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allers K, Fehr M, Conrad K, Epple HJ, Schurmann D, Geelhaar-Karsch A, et al. Macrophages accumulate in the gut mucosa of untreated HIV-infected patients. J Infect Dis. 2013;209(5):739–48. doi: 10.1093/infdis/jit547. [DOI] [PubMed] [Google Scholar]
- 40.Cassol E, Malfeld S, Mahasha P, Bond R, Slavik T, Seebregts C, et al. Impaired CD4+ T-cell restoration in the small versus large intestine of HIV-1-positive South Africans receiving combination antiretroviral therapy. J Infect Dis. 2013;208(7):1113–22. doi: 10.1093/infdis/jit249. [DOI] [PubMed] [Google Scholar]
- 41.Meng F, Lowell CA. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med. 1997;185(9):1661–70. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olsson J, Poles M, Spetz AL, Elliott J, Hultin L, Giorgi J, et al. Human immunodeficiency virus type 1 infection is associated with significant mucosal inflammation characterized by increased expression of CCR5, CXCR4, and beta-chemokines. J Infect Dis. 2000;182(6):1625–35. doi: 10.1086/317625. [DOI] [PubMed] [Google Scholar]
- 43.McGowan I, Elliott J, Fuerst M, Taing P, Boscardin J, Poles M, et al. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J Acquir Immune Defic Syndr. 2004;37(2):1228–36. doi: 10.1097/01.qai.0000131846.12453.29. [DOI] [PubMed] [Google Scholar]
- 44.Reka S, Garro ML, Kotler DP. Variation in the expression of human immunodeficiency virus RNA and cytokine mRNA in rectal mucosa during the progression of infection. Lymphokine Cytokine Res. 1994;13(6):391–8. [PubMed] [Google Scholar]
- 45.Kam LY, Targan SR. Cytokine-based therapies in inflammatory bowel disease. Curr Opin Gastroenterol. 1999;15(4):302–7. doi: 10.1097/00001574-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Funderburg NT, Stubblefield Park SR, Sung HC, Hardy G, Clagett B, Ignatz-Hoover J, et al. Circulating CD4(+) and CD8(+) T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology. 2013;140(1):87–97. doi: 10.1111/imm.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caradonna L, Amati L, Magrone T, Pellegrino NM, Jirillo E, Caccavo D. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res. 2000;6(3):205–14. [PubMed] [Google Scholar]
- 48.Pastor Rojo O, Lopez San Roman A, Albeniz Arbizu E, de la Hera Martinez A, Ripoll Sevillano E, Albillos Martinez A. Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(3):269–77. doi: 10.1002/ibd.20019. [DOI] [PubMed] [Google Scholar]
- 49.Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest. 2014;124(10):4212–8. doi: 10.1172/JCI72333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. 2013;5(193):193ra91. doi: 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]


